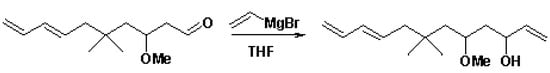
Scheme
To a stirred solution of the diene aldehyde [1] in dry THF under Ar at -78 fC, vinylmagnesium bromide in THF was added via a syringe. The reaction was stirred for 15 minutes then quenched at -78 fC with sat aq sodium potassium tartrate and stirred while warming to room temperature. The mixture was extracted with pet ether and the combined organic layers dried over Na2SO4 and concentrated to give a 1 : 1 diastereomeric mixture of the triene alcohols as a colorless oil in 69 percent yield.
1H NMR (CDCl3): d: 6.30 (dt, J = 17.0, 10.2Hz, 1H), 6.01 (bdd, J = 14.8, 10.6Hz, 1H), 5.84 (ddd, J = 17.1, 10.7, 5.8Hz, 1H), 5.70 (dt, J = 15.1, 7.5Hz, 1H), 5.26 (dd, J = 14.1, 7.6Hz, 1H), 5.1 - 5.04 (m, 2H), 4.94 (d, J = 10.0Hz, 1H), 4.38 (m, CHOH), 4.21 (m, CHOH), 3.54 (m, CHOMe), 3.44 (M, CHOMe), 3.30 (s, OMe), 3.28 (s, (OMe), 3.25 (bs, OH), 2.90 (bs, OH), 1.99 (d, J = 7.5Hz, 2H), 1.9 - 1.48 (m, 3H), 1.35 - 1.25 (m, 1H), 0.91 (s, 3H), 0.87 (s, 3H).
IR (CDCl3): 3600, 3450, 2960, 2940, 2830, 1645, 1600, 1460, 1425, 1390, 1370, 1085, 1010, 890.
MS (m/e): 238 (M+, 2), 223, 205, 198, 169, 115, 113, 108 (100), 99, 94, 93, 59.
HRMS: calc. for C14H21O: (M - CH5O): 205.1592; found: 205.1593.
Supplementary materials
Supplementary File 1Supplementary File 2References and Notes
- Smith, D. 3-Methoxy-5,5-dimethyl-7(E),9-decadienal. Molecules 1997, 2, M36. [Google Scholar]
- Sample Availability: No sample available.
© 1997 MDPI. All rights reserved