Abstract
Irradiation of benzotriazoles 1a–e at λ = 254 nm in acetonitrile solution generated the corresponding 1,3-diradicals which underwent intermolecular cycloaddition with maleimides to afford the corresponding dihydropyrrolo[3,4-b]indoles and with acetylene derivatives to afford indoles as the major products. This offers an interesting and simple access to such ring systems of potential synthetic and biological interest. The structures of the photoproducts were established spectroscopically and by single crystal X-ray crystallography.
1. Introduction
The photochemistry of benzotriazoles 1 has been extensively studied in the past [1,2,3,4,5]. Katritzky et al., has already reviewed this chemistry in several review articles [6,7]. Scheme 1 summarizes the photolytic reactions of benzotriazoles which occur through initial extrusion of molecular nitrogen and formation of the diradical intermediate 2, followed by subsequent rearrangement to: (i) cyanocyclopentadiene [8,9]; (ii) ring closure to condensed heterocyclic products [10,11,12]; (iii) reaction with solvent to yield 2-aminobiphenyl [13,14]; and (iv) intramolecularphotocycloaddtion to produce indoles and benzimidazoles [15,16].
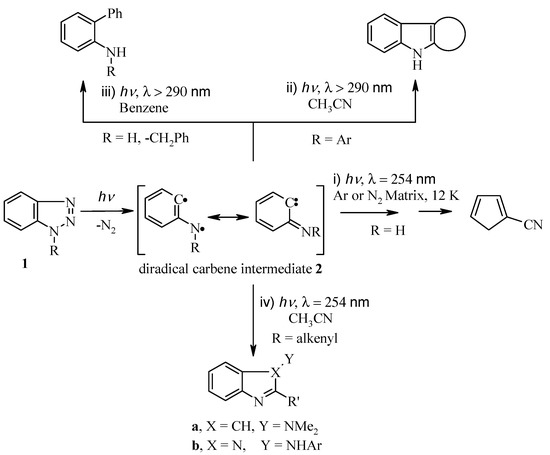
Scheme 1.
Literature photolysis of benzotriazole derivatives.
In the present work, we describe intermolecular trapping of the photochemically generated diradical intermediate 2 and its derivatives with alkenes or alkynes. To the best of our knowledge this intermolecular reaction has not been reported before and is expected to offer a new and simple route to indole and dihydropyrrolo[3,4-b]indolederivatives. These compounds are structurally similar to 1,2-dihydropyrrolo[3,4-b]indol-3(4H)-one and its derivatives which have been reported as a vital class of pharmaceutical moieties such as mG1uR1 antagonists [17], cannabinoid 2 receptor agonists [18], potent inhibitor of purified human rennin [19] and therapeutic agents for the treatment of osteoporosis [20].
2. Results and Discussion
Irradiation of acetonitrile solution of benzotriazole (1a) and N-phenylmaleimide (3a) using a 16 W low pressure mercury arc-lamp (λ = 254 nm) led to the formation of three products 4a, 6a and 7a (Scheme 2). The major product of this reaction was 2-phenyl-3a,4-dihydropyrrolo[3,4-b]indole-1,3(2H, 8bH)-dione (4a, Figure 1).
Several experiments were carried out in order to optimize the formation of 4a, and the results are summarized in Table 1. From this table it is clear that the best reaction condition for the formation of the pyrroloindole 4a is achieved by irradiating 1a and 3a (1:2 molar ratio) in acetonitrile for 16 h. The formation of 4a in the reaction mixture was monitored by the Ha and Hb proton signals, which appear as two doublet signals at δ: 4.85, 4.61 ppm (J = 9.2 Hz) in the 1H-NMR spectra as shown in Figure 1. The percent yield of 4a in the reaction mixture was calculated by 1H-NMR using DCM as calibration reference, as reported previously [21].
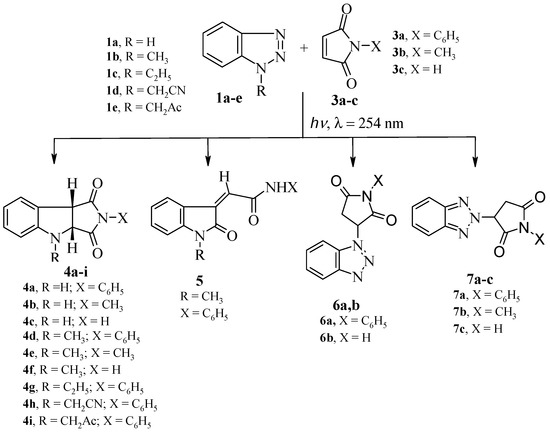
Scheme 2.
Products of irradiation of benzotriazoles 1a–e with maleimides 3a–c.
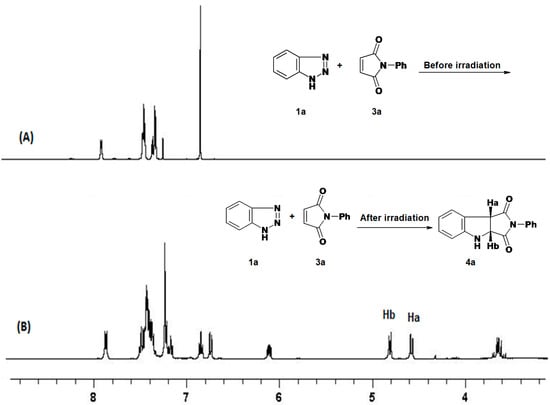
Figure 1.
1H-NMR of: (A) reaction mixture of benzotriazole (1a) with N-phenylmaleimide (3a) before irradiation, and (B) reaction mixture after irradiation using low pressure Hg-lamp (λ = 254 nm) in CH3CN.

Table 1.
Optimizing photolysis condition of benzotriazole (1a) with N-phenylmaleimide (3a).
| Run * | Solvent | Time(h) | Molar Ratio (1a):(3a) | Yield (%) |
|---|---|---|---|---|
| 4a | ||||
| 1 | MeOH | 24 | 1:1 | - |
| 2 | DCM | 24 | 1:1 | 5 |
| 3 | THF | 24 | 1:1 | 8 |
| 4 | MePh | 24 | 1:1 | 7 |
| 5 | MeCN | 24 | 1:1 | 12 |
| 6 | MeCN | 24 | 1:2 | 19 |
| 7 | MeCN | 16 | 1:2 | 19 |
| 8 | MeCN | 10 | 1:2 | 12 |
| 9 | MeCN | 36 | 1:2 | 19 |
Note: * Reaction conditions: Acetonitrile (25 mL), 1a (0.0595 g, 0.5 mmol) and 3a (0.173 g, 1 mmol) irradiated at λ = 254 nm under nitrogen.
With the optimized reaction conditions in hand, irradiation of benzotriazole (1a) with maleimides 3a–c produced the dihydropyrrolo[3,4-b]indoles 4a–c in 19%–21% yield, together with Michael adducts 6a,b in 17%–18% and 7a–c in 13%–16% yields, as shown in Scheme 2 and Table 2.

Table 2.
Products from irradiation of benzotriazoles 1a–e with maleimides 3a–c.
| Entry * | Reactant | Products (Yield %) | |||||
|---|---|---|---|---|---|---|---|
| 4 | 5 | 6a,b | 7a–c | Unreacted Bt | |||
| 1 | 1a | 3a | 4a (19) | - | 6a(17) | 7a (13) | 45 |
| 2 | 1a | 3b | 4b (21) | - | - | 7b(16) | 58 |
| 3 | 1a | 3c | 4c (19) | - | 6b(18) | 7c(15) | 43 |
| 4 | 1b | 3a | 4d(32) | (20) | - | - | 42 |
| 5 | 1b | 3b | 4e (33) | - | - | - | 60 |
| 6 | 1b | 3c | 4f(35) | - | - | - | 60 |
| 7 | 1c | 3a | 4g (38) | - | - | - | 56 |
| 8 | 1d | 3a | 4h(25) | - | - | - | 70 |
| 9 | 1e | 3a | 4i(35) | - | - | - | 60 |
Note: *: Reaction conditions: In acetonitrile (100 mL), 1a–e (2 mmol) and 3a–c (4 mmol) was irradiated at λ = 254 nm under nitrogen for 16 h.
The 1H-NMR spectrum (CDCl3) of pure 4a showed two doublet signals at δ 4.85, 4.61 (J = 9.2 Hz) corresponding to H-b, H-a respectively (Figure 1). The 13C-NMR also showed two signals at δ 60.2 and 48.9 assigned to C-b, C-a respectively (HSQC). In addition GC-MS of 4a showed a M+ peak at m/z = 264. These data support the structure of 4a. Moreover, the structure of 4c was unequivocally established by single crystal X-ray crystallography (Figure 2). The structures of compounds 6a,b and 7a–c were established by spectroscopic data and are the result of ground state Michael addition of 1a and its tautomer to 3a–c. In support of this view, heating of benzotriazole 1a and N-phenylmaleimide 3a in acetonitrile under reflux for 24 h gave a mixture of 6a (24%) and 7a (16%). On the other hand heating a mixture of 1a and 3a in a sealed tube at 230–300 °C produced only a mixture of Michael adducts 6a (80%) and 7a (10%) yields. Similar Michael adducts have also been reported recently [22].
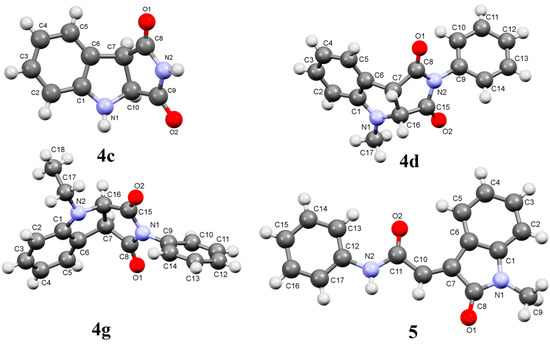
Figure 2.
X-Ray crystal structures of compounds 4c, 4d and 5.
The formation of Michael adducts 6a,b and 7a–c and the low yield of dihydropyrroloindoles 4a–c promotedus to investigate the effect of substituting N-1 of benzotriazole with various alkyl groups [23]. Thus, irradiation of 1-methyl-1H-benzotriazole (1b) with maleimides 3a–c produced dihydropyrrolo[3,4-b]indoles 4d–f in 32%–35%. Similarly, irradiation of benzotriazoles 1c–e with N-phenylmaleimide 3a produced also the corresponding dihydropyrroloindoles 4g–i in 25%–38% yields.
Irradiation of 1b with 3a was accompanied by another photoproduct in 20% yield which was identified as 2-(1-methyl-2-oxoindoline-3-ylidene)-N-phenylacetamide (5). The formation of compound 5 could be explained as shown in Scheme 3. Thus, C-H insertion of the photogenerated diradicals or its tautomeric carbene intermediates leads to the formation of the intermediate product A or its tautomer B respectively, followed by nucleophilic transamidation leading to the final product 5. The structures of 4d, 4g and 5 were established from single crystal X-ray crystallography (Figure 2).
In a similar way, irradiation of benzotriazole 1a with phenylacetylene (8a), cyclohexen-1-yl acetylene (8b) or ethyl propiolate (8c) in acetonitrile under the same conditions afforded the functionally substituted-1H-indole derivatives 9a–c in 31%–37% yield. Interestingly, the reaction took place regioselectively depending on the monosubstituted alkyne. Thus, only 2-substituted indole is formed with phenylacetylene and cyclohexen-1-ylacetylene, whereas a 3-substituted indole is formed with ethyl propiolate. In the latter case, the reaction presumably proceeds by initially formation of Michael adduct (3-benzotriazol-1-ylacrylic acid ethyl ester) followed by photolytic extrusion of nitrogen and intramolecular radical cyclization to give 9c. The latter has been previously prepared from the same Michael adducts by pyrolytic extrusion of nitrogen and intramolecular cyclization [24]. This presumption has now been further supported by the formation of the ethyl N-methylindole-2-carboxylate 9f upon irradiation of 1b with ethyl propiolate.
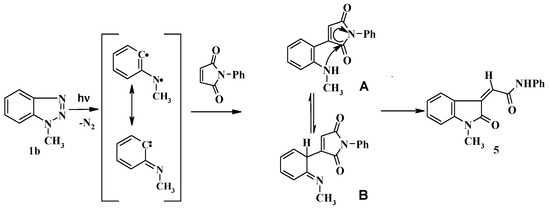
Scheme 3.
Proposed mechanism for formation of compound 5.
On the other hand, irradiation of 1a with dimethyl and diethyl acetylenedicarboxylates (DMADC) 8d and (DEADC) 8e, gave only the corresponding Michael adducts 10a,b, 11a,b and 12. However, irradiating of 1-methyl-1H-benzotriazole (1b) with 8d,e produced the corresponding indoles 9d,e Scheme 4, Table 3.
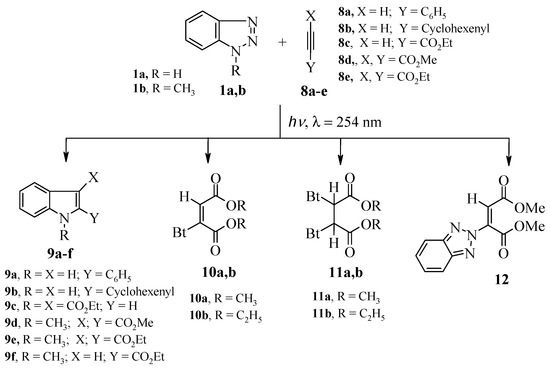
Scheme 4.
Products of irradiation of benzotriazoles 1a,b with alkynes 8a–e.

Table 3.
Products from irradiation of benzotriazoles 1a,b with alkynes 8a–e.
| Entry | Reactants | Products (Yield %) | |||||
|---|---|---|---|---|---|---|---|
| 9a–f | 10a,b | 11a,b | 12 | Unreacted Bt | |||
| 1 | 1a | 8a | 9a (31) | - | - | - | 60 |
| 2 | 1a | 8b | 9b (36) | - | - | - | 48 |
| 3 | 1a | 8c | 9c (37) | - | - | - | 50 |
| 4 | 1a | 8d | - | 10a (25) | 11a (29) | 12 (19) | 5 |
| 5 | 1a | 8e | - | 10b (31) | 11b (28) | - | 25 |
| 6 | 1b | 8d | 9d (32) | - | - | - | 55 |
| 7 | 1b | 8e | 9e (23) | - | - | - | 61 |
| 8 | 1b | 8c | 9f (19) | - | - | - | 70 |
Note: Reaction conditions: In acetonitrile (100 mL) 1a, b (2 mmol) and 8a–e (4 mmol) was irradiated at λ = 254 nm under nitrogen for 16 h.
3. Experimental Section
3.1. General Information
All melting points were recorded on a Gallenkamp apparatus and were uncorrected. IR spectra were recorded using KBr pellets on a Perkin Elmer System 2000 FT-IR spectrophotometer. 1H- and 13C-NMR spectra were recorded on a Bruker DPX 400MHz or Avance 600 MHz spectrometer with proton spectra measured at 400, 600 MHz and carbon spectra at 100, 150 MHz. Mass spectra were measured on a VG Auto-spec-Q (high resolution, high performance, tri-sector GC/MS/MS) and with LCMS using Agilent 1100 series LC/MSD with an API-ES/APCI ionization mode. Microanalyses were performed on LECO CH NS-932 Elemental Analyzer. X-ray analysis was performed using a Rigaku Rapid II and Bruker X8 Prospector diffractometer [25].
3.2. Irradiation of Benzotriazoles 1a–e with Maleimides 3a–c orAlkynes 8a–e
General procedure: A mixture of each benzotriazole derivative 1a–e (2 mmol), the appropriate maleimide 3a–c or alkyne 8a–e (4 mmol) was dissolved in acetonitrile (100 mL) in a quartz tube and purged with nitrogen for 20 min, while being irradiated for 16 h at room temperature using an annular reactor model APQ40 (Applied Photo-Physics Limited, Surrey, UK) fitted with a 16 W low pressure mercury arc-lamp (λ = 254 nm). The progress of reaction was monitored by TLC. The solvent was removed under reduced pressure and the resulting residue was subject to column chromatography on silica gel (70–230 mesh) using ethyl acetate/petroleum ether (bp. 60–80 °C) as an eluent to give the corresponding products. All yields reported in the Experimental are isolated yields.
3.3. Products from Irradiation of Benzotriazoles 1a–e with Maleimides 3a–c
2-Phenyl-3a,4-dihydropyrrolo[3,4-b]indole-1,3(2H,8bH)-dione (4a). This compound was separated as white needles by column chromatography using ethyl acetate/pet. ether (1:4, Rf = 0.56), yield (95 mg), mp.164–165 °C; LCMS (m/z) = 265 (M + 1); MS: (m/z, %) = 264 (M+, 60), 144 (15), 117 (100); IR νmax (KBr)/cm−1 3257, 3046, 2949, 1775, 1715, 1494, 1452, 1265, 1196, 739. NMR δH (600 MHz, CDCl3) 7.48–7.45 (m, 5H), 7.40 (dt, 1H, J = 8.4, 1.2), 7.26 (t, 1H, J = 8.0), 7.21 (dd, 1H, J = 8.0, 1.0), 6.89 (dt, 1H, J = 8.4, 1.2), 6.78 (d, 1H, J = 8.4), 4.85 (d, 1H, J = 9.0), 4.61 (d, 1H, J = 9.0); δC (150 MHz, CDCl3) 176.9, 174.8, 149.0, 131.5, 130.0, 129.2, 128.8, 126.3, 125.5, 122.7, 120.5, 110.8, 60.2, 48.9; HR-MS (EI) m/z [M]+ calcd for C16H12N2O2 264.0899, found = 264.0892.
2-Methyl-3a,4-dihydropyrrolo[3,4-b]indole-1,3(2H,8bH)-dione (4b). White needles (85 mg) after column chromatography using ethyl acetate/pet. ether (1:6, Rf = 0.64), mp. 148–150 °C; MS: (m/z, %) = 202 (M+, 15), 119 (100), 91 (90); IR νmax (KBr)/cm−1 3370, 2957, 2928, 1773, 1713, 1598, 1484, 1394, 1188, 750; NMR δH (400 MHz, CDCl3) 7.43 (d, 1H, J = 8.0), 7.17 (t, 1H, J = 8.4), 6.87 (dt, 1H, J = 8.4, 1.2), 6.74 (d, 1H, J = 8.0), 4.70 (d, 1H, J = 8.8), 4.47 (d, 1H, J = 8.8), 3.1 (br, 1H, NH), 3.02 (s, 3H, CH3); δC (100 MHz, CDCl3) 178.0, 176.1, 149.2, 130.0, 125.7, 123.0, 120.5, 110.9, 60.3, 49.1, 25.4; HR-MS (EI) m/z [M]+ calcd for C11H10N2O2 202.0742, found 202.0736.
3a,4-Dihydropyrrolo[3,4-b]indole-1,3(2H,8bH)-dione (4c). A white solid (72 mg) from column chromatography using ethyl acetate/pet. ether (1:4, Rf = 0.64), mp. 165–167 °C; MS: (m/z, %) = 188 (M+, 85), 118 (100), 91 (60); IR νmax (KBr)/cm−1 3380, 3360, 3087, 2958, 1765, 1711, 1600, 1480, 1344, 1187, 1051, 794; NMR δH (400 MHz, CDCl3) 8.18 (br, 2H, 2NH), 7.39 (d, 1H, J = 7.2), 7.17 (t, 1H, J = 7.6), 6.83 (t, 1H, J = 7.2), 6.72 (d, 1H, J = 7.6), 4.68 (d, 1H, J = 9.2), 4.46 (d, 1H, J = 9.2); δC (100 MHz, CDCl3) 178.0, 175.8, 149.3, 130.1, 125.6, 122.6, 120.6, 110.9, 61.5, 50.3; HR-MS (EI) m/z [M]+ calcd for C10H8N2O2 188.0586, found 188.0580.
4-Methyl-2-phenyl-3a,4-dihydropyrrolo[3,4-b]indole-1,3(2H,8bH)-dione (4d). A white solid (178 mg) from column chromatography using ethyl acetate/pet. ether (1:3, Rf = 0.62), mp. 146–148 °C; MS: (m/z, %) = 278 (M+, 100), 183 (15), 156 (100); IR νmax (KBr)/cm−1 3044, 2893, 1774, 1711, 1600, 1491, 1379, 1293, 1187, 741; NMR δH (400 MHz, CDCl3) 7.46–7.42 (m, 4H), 7.30–7.25 (m, 3H), 6.80 (t, 1H, J = 7.8), 6.54 (d, 1H, J = 7.6), 4.60 (d, 1H J = 9.6), 4.56 (d, 1H, J = 9.6), 3.16 (s, 3H); δC (100 MHz, CDCl3) 174.7, 174.6, 150.9, 131.6, 130.0, 129.1, 128.7, 126.4, 125.5, 122.1, 118.6, 107.1, 66.3, 48.1, 34.0; HR-MS (EI) m/z [M]+ calcd for C17H14N2O2 278.1055, found 278.1050.
2,4-Dimethhyl-3a,4-dihydropyrrolo[3,4-b]indole-1,3(2H,8bH)-dione (4e). A white solid (143 mg) from column chromatography using ethyl acetate/pet. ether (1:4, Rf = 0.53) mp. 98–100 °C; MS: (m/z, %) = 216 (M+, 100), 158 (45), 131 (85); IR νmax (KBr)/cm−1 3044, 2893, 1774, 1711, 1600, 1491, 1379, 1293, 1187, 741; NMR δH (400 MHz, CDCl3) 7.37 (d, 1H, J = 7.2), 7.21 (t, 1H, J = 7.6), 6.75–6.70 (m, 1H), 6.46 (d, 1H, J = 8.0), 4.42 (d, 1H, J = 9.6), 4.39 (d, 1H, J = 9.6), 3.09 (s, 3H, CH3), 2.98 (s, 3H, CH3); δC (100 MHz, CDCl3) 176.0, 175.8, 150.9, 130.1, 125.6, 122.3, 118.5, 107.1, 66.3, 48.2, 34.1, 23.9; HR-MS (EI) m/z [M]+ calcd for C12H12N2O2 216.0899, found 216.0893.
4-Methyl-3a,4-dihydropyrrolo[3,4-b]indol-1,3(2H,8bH)-dione (4f). A white solid (142 mg) from column chromatography using ethyl acetate/pet. ether (1:5, Rf = 0.44), mp. 155–157 °C; MS: (m/z, %) = 202 (M+, 95), 158 (15), 131 (100); IR νmax (KBr)/cm−1 3274, 3051, 2958, 1772, 1711, 1605, 1487, 1341, 1198, 746; NMR δH (400 MHz, CDCl3) 8.02 (br, 1H, NH), 7.35 (d, 1H, J = 7.2), 7.22 (t, 1H, J = 7.6), 6.75 (t, 1H, J = 7.8), 6.48 (d, 1H,J =8.0), 4.45 (d, 1H, J = 9.2), 4.39 (d, 1H, J = 9.2), 3.08 (s, 3H, CH3); δC (100 MHz, CDCl3) 175.7, 175.6, 151.0, 130.2, 125.6, 122.0, 118.7, 107.2, 67.5, 49.5, 34.1; HR-MS (EI) m/z [M]+ calcd for C11H10N2O2 202.0742, found 202.0735.
4-Ethyl-2-phenyl-3a,4-dihydropyrrolo[3,4-b]indol-1,3(2H,8bH)-dione (4g). A white solid (223 mg) from column chromatography using ethyl acetate/pet. ether (1:3, Rf = 0.74), mp. 155–156 °C; MS: (m/z, %) = 292 (M+, 100), 277 (65), 130 (70); IR νmax (KBr)/cm−1 3055, 2953, 1776, 1706, 1603, 1467, 1348, 1218, 756;NMR δH (600 MHz, CDCl3) 7.46–7.44 (m, 3H), 7.39 (t, 1H, J = 7.4), 7.28–7.21 (m, 2H), 7.22 (t, 1H, J = 7.2), 6.77 (t, 1H, J = 7.8), 6.55 (d, 1H, J = 7.6), 4.69 (d, 1H, J = 9.6), 4.59 (d, 1H, J = 9.6), 3.61 (m, 1H), 3.56 (m, 1H), 1.29 (t, 3H, J = 7.2); δC (150 MHz, CDCl3) 175.1, 174.8, 149.9, 131.7, 129.9, 129.1, 128.7, 126.4, 125.6, 122.3, 118.4, 107.4, 63.9, 48.1, 41.6, 11.6; HR-MS (EI) m/z [M]+ calcd for C18H16N2O2 292.1212, found 292.1207.
2-(1,3-Dioxo-2-phenyl-1,2,3,3a-tetrahydropyrrolo[3,4-b]indol-4(8bH)-yl)-acetonitrile (4h). A pale yellow solid (154 mg) from column chromatography using ethyl acetate/pet. ether (1:4, Rf = 0.55), mp. 165–167 °C; MS: (m/z, %) = 303 (M+, 100), 183 (15), 156 (100); IR νmax (KBr)/cm−1 3060, 2960, 2246, 1768, 1717, 1601, 1487, 1382, 1194, 1173, 751; NMR δH 600 MHz, CDCl3) 7.53 (d, 1H, J = 7.8), 7.47–7.44 (m, 2H), 7.41–7.38 (m, 2H), 7.34 (t, 1H, J = 7.8), 7.29–7.27 (m, 1H), 6.98 (t, 1H, J = 7.2), 6.68 (d, 1H, J = 7.8), 4.72 (d, 1H, J = 9.0), 4.67 (d, 1H, J = 9.0), 4.51 (d, 1H, J = 18.6), 4.42 (d, 1H, J = 18.6); δC (100 MHz, CDCl3) 174.2, 174.0, 147.6, 131.4, 130.5, 129.5, 129.2, 126.5, 126.4, 123.1, 121.9, 114.9, 108.7, 64.3, 48.2, 36.4; HR-MS (EI) m/z [M]+ calcd for C18H13N3O2 303.1008, found 303.1002.
4-(2-Oxopropyl)-2-phenyl-3a,4-dihydropyrrolo[3,4-b]indole-1,3(2H,8bH)-dione (4i). A white solid (224 mg) from column chromatography using ethyl acetate/pet. ether (1:2, Rf = 0.44), mp. 98–100 °C; MS: (m/z, %) = 320 (M+, 20), 277 (100), 130 (35); IR νmax (KBr)/cm−1 3052, 2957, 1777, 1714, 1600, 1489, 11245, 1168, 747; NMR δH (400 MHz, CDCl3) 7.39–7.35 (m, 4H), 7.28–7.25 (m, 2H), 7.18 (t, 1H, J = 7.2), 6.80 (t, 1H, J = 7.2), 6.33 (d, 1H, J = 7.6), 4.77 (d, 1H, J = 9.6), 4.65 (d, 1H, J = 9.6), 4.40 (d, 1H, J = 18.8), 4.31 (d, 1H, J = 18.8), 2.21 (s, 3H); δC (150 MHz, CDCl3) 204.7, 174.90, 174.86, 149.7, 131.7, 130.1, 129.4, 129.0, 126.6, 126.0, 122.3, 119.5, 106.9, 64.3, 55.7, 48.4, 27.5; HR-MS (EI) m/z [M]+ calcd for C19H16N2O3 320.1161, found 320.1155.
2-(1-Methyl-2-oxoindolin-3-ylidene)-N-phenylacetamide (5). Yellow needles (110 mg) from column chromatography using ethyl acetate/pet. ether (1:1, Rf = 0.58), mp. 225–227 °C; MS: (m/z, %) = 278 (M+, 70), 186 (100), 146 (35); IR νmax (KBr)/cm−1 3325, 3051, 2858, 1701, 1671, 1603, 1540, 1369, 1244, 1189, 741; NMR δH (400 MHz, CDCl3) 8.73 (d, 1H, J = 7.6), 8.16 (br, 1H, NH), 7.68 (d, 2H, J = 8.0), 7.41–7.35 (m, 3H), 7.18 (t, 1H, J = 7.8), 7.11–7.00 (m, 2H), 6.80 (d, 1H, J = 8.0), 3.56 (s, 3H); δC (150 MHz, CDCl3) 168.4, 162.6, 145.7, 137.9, 136.5, 132.3, 129.6, 129.4, 129.2, 126.2, 125.3, 123.3, 120.4, 108.3, 26.6. HR-MS (EI) m/z [M]+ calcd for C17H14N2O2 278.1055, found 278.1050.
3-(1H-Benzotriazol-1-yl)-1-phenylpyrrolidine-2,5-dione (6a). A colorless powder (100 mg) from column chromatography using ethyl acetate/pet. ether (1:3, Rf = 0.65), mp. 146–148 °C (145–147 °C, [22]); MS: (m/z,%) = 292 (M+, 80), 236 (15), 173 (100); νmax (KBr)/cm−1 2980, 2936, 2852, 1717, 1595, 1499, 1395, 1186, 840, 748; NMR δH (400 MHz, CDCl3) 8.14 (d, 1H, J = 8.4), 7.63–7.57 (m, 2H), 7.53–7.42 (m, 4H), 7.38 (dd, 2H, J = 8.4, 1.6), 5.98 (dd, 1H, J = 9.6, 5.6), 3.86 (dd, 1H, J = 18.4, 5.6), 3.65 (dd, 1H, J = 18.4, 5.6); δC (100 MHz, CDCl3) 172.2, 170.9, 146.5, 133.2, 131.3, 129.6, 129.5, 128.8, 126.5, 124.9, 120.9, 109.2, 55.8, 35.1; HR-MS (EI) m/z [M]+ calcd for C16H12N4O2 292.0960), found 292.0951.
3-(1H-Benzotriazol-1-yl)-pyrrolidine-2,5-dione (6b). A colorless powder (78 mg) from column chromatography using ethyl acetate/pet. ether (1:2, Rf = 0.58), mp. 216–218 °C; MS: (m/z, %) = 216 (M+, 80), 159 (40), 103 (100); IR νmax (KBr)/cm−1 2989, 2926, 1722, 1598, 1496, 1398, 1187, 852, 739; NMR δH (400 MHz, DMSO-d6) 11.95 (br, 1H, NH), 8.12 (d, 1H, J = 8.4), 7.83 (d, 1H, J = 8.4), 7.64 (t, 1H, J = 8.6), 7.48 (t, 1H, J = 8.6), 6.35 (dd, 1H, J = 9.6, 5.2), 3.43 (dd, 1H, J = 18.4, 9.2), 3.32 (dd, 1H, J = 18.4, 9.2); δC (150 MHz, DMSO-d6) 175.8, 174.8, 145.6, 133.4, 128.6, 125.0, 120.0, 110.8, 57.3, 36.7; HR-MS (EI) m/z [M]+ calcd for C10H8N4O2 216.0647, found 216.0641.
3-(2H-Benzotriazol-2-yl)-1-phenylpyrrolidine-2,5-dione (7a). A white solid (76 mg) from column chromatography using ethyl acetate/pet. ether (1:2, Rf = 0.54), mp. 218–219 °C; MS: (m/z, %) = 292 (M+, 80), 173 (80), 145 (100); IR νmax (KBr)/cm−1 2990, 2966, 2852, 1722, 1595, 1499, 1395, 1216, 1184, 840, 748; NMR δH (400 MHz, CDCl3) 7.91–7.88 (m, 2H), 7.53 (dt, 2H, J = 8.2, 1.6), 7.49–7.42 (m, 5H), 6.13 (dd, 1H, J = 9.6, 5.6), 3.66 (dd, 2H, J = 12.4, 9.2); δC (100 MHz, CDCl3) 172.3, 170.2, 145.1, 131.4, 129.6, 129.4, 127.6, 126.6, 118.5, 63.1, 36.3; HR-MS (EI) m/z [M]+ calcd for C16H12N4O2 292.0960, found 292.0954.
3-(2H-Benzotriazol-2-yl)-1-methylpyrrolidine-2,5-dione (7b). A white solid (74 mg) from column chromatography using ethyl acetate/pet. ether (1:1, Rf = 0.58), mp. 155–157 °C; MS: (m/z, %) = 230 (M+, 60), 145 (100), 91 (15); IR νmax (KBr)/cm−1 2980, 2916, 2854, 1720, 1585, 1479, 1395, 1216, 840, 768; NMR δH (400 MHz, CDCl3) 7.88–7.85 (m, 2H), 7.44–7.41 (m, 2H), 5.98 (dd, 1H, J = 9.2, 5.6), 3.54 (dd, 1H, J = 18.4, 5.6), 3.44 (dd, 1H, J = 18.4, 5.6), 3.17 (s, 3H, CH3); δC (150 MHz, CDCl3) 173.2, 171.3, 145.1, 127.6, 118.5, 63.1, 36.2, 25.9; HR-MS (EI) m/z [M]+ calcd for C11H10N4O2 230.0804, found 230.0799.
3-(2H-Benzotriazol-2-yl)-pyrrolidine-2,5-dione (7c). A white solid (65 mg) from column chromatography using ethyl acetate/pet. ether (1:1, Rf = 0.58), mp. 168–170 °C; MS (m/z, %) = 216 (M+, 85), 145 (30), 103 (100); IR νmax (KBr)/cm−1 3236, 2959, 2930, 2861, 1795, 1727, 1595, 1462, 1276, 1125, 1072, 744; NMR δH (600 MHz, CDCl3) 8.14 (br, 1H, NH), 7.87 (dd, 2H, J = 7.2, 3.0), 7.46 (dd, 2H, J = 6.6, 3.0), 6.02 (dd, 1H, J = 11.4, 6.0), 3.61 (dd, 1H, J = 18.6, 5.6), 3.50 (dd, 1H, J = 18.6, 5.6); δC (150 MHz, CDCl3) 172.2, 170.5, 145.1, 127.7, 118.5, 64.0, 37.2; HR-MS (EI) m/z [M]+ calcd for C10H8N4O2 216.0647, found 216.0641.
3.4. Photoproducts from Irradiation of Benzotriazoles 1a,b with Alkynes 8a–e
2-Phenyl-1H-indole (9a). White solid (120 mg), mp. 189–190 °C (187–188 °C, [26]); MS: (m/z,%) = 193 (M+, 100), 165 (25), 96 (15); NMR δH (400 MHz, CDCl3) 8.35 (br, 1H, NH), 7.66 (d, 2H, J = 7.6), 7.63 (d, 1H, J = 7.6), 7.46–77.40 (m, 2H), 7.35 (t, 1H, J= 8.0), 7.20 (dt, 1H, J = 8.0, 1.2), 7.17 (dt, 1H, J = 8.0, 1.2), 7.13 (t, 1H, J = 8.0), 6.84 (s, 1H); δC (100 MHz, CDCl3) 138.1, 137.0, 132.6, 129.5, 129.3, 127.9, 125.4, 122.6, 120.9, 120.5, 111.1, 100.2; HR-MS (EI) m/z [M]+ calcd for C14H11N 193.0891, found 193.0886.
2-Cyclohexen-1-yl-1H-indole (9b). White solid (142 mg), mp. 138–140 °C (137–139 °C, [26]); MS: (m/z, %) = 197 (M+, 100), 168 (65); NMR δH (600 MHz, CDCl3) 8.09 (br, 1H, NH), 7.55 (d, 1H, J = 7.8), 7.30 (d, 1H, J = 7.8), 7.14 (t, 1H, J = 7.4), 7.06 (t, 1H, J = 7.4), 6.44 (s, 1H), 6.14 (s, 1H), 2.47 (br, 2H), 2.26–2.25 (br, 2H), 1.82–1.78 (m, 2H), 1.72–1.69 (m, 2H); δC (150 MHz, CDCl3) 139.7, 136.4, 129.4, 129.2, 122.8, 122.2, 120.6, 120.0, 110.6, 98.9, 26.3, 25.8, 22.8, 22.4; HR-MS (EI) m/z [M]+ calcd for C14H15N 197.1204, found 197.1199.
Ethyl 1H-indole-3-carboxylate (9c). White solid (142 mg), mp. 124–126 °C (125–127 °C, [24,26]); MS: (m/z, %) = 189 (M+, 50), 144 (100); NMR δH (400 MHz, CDCl3): 8.73 (br, 1H, NH), 8.23 (m, 1H), 7.94 (d, 1H, J = 2.8), 7.45–7.41 (m, 1H), 7.32–7.27 (m, 2H), 4.42 (q, 2H, J = 7.4), 1.45 (t, 3H, J = 7.4); δC (150 MHz, CDCl3) 165.6, 136.3, 131.2, 126.0, 123.4, 122.2, 121.8, 111.7, 109.3, 60.1, 14.8; HR-MS (EI) m/z [M]+ calcd for C11H11NO2 189.0790, found 189.0784.
Dimethyl 1-methyl-1H-indole-2,3-dicarboxylate (9d). Yellow solid (122 mg), mp. 38–40 °C (37–40 °C, [27]); MS: (m/z, %) = 247 (M+, 60), 216 (100), 149 (50); NMR δH (400 MHz, CDCl3): 8.12 (d, 1H, J = 8.0), 7.38–7.36 (m, 2H), 7.32–7.26 (m, 1H), 4.02 (s, 3H, CH3), 3.93 (s, 3H, CH3), 3.84 (s, 3H, CH3); δC (100 MHz, CDCl3): 164.8, 163.5, 137.0, 134.9, 129.7, 125.5, 124.7, 122.9, 122.6, 110.4, 53.3, 51.7, 31.6; HR-MS (EI) m/z [M]+ calcd for C13H13NO4 247.0845, found 247.0839.
Diethyl 1-methyl-1H-indole-2,3-dicarboxylate (9e). A yellow oil (126 mg) from column chromatography using ethyl acetate/pet. ether (1:1, Rf = 0.58), MS: (m/z, %) = 275 (M+ , 80), 230 (35), 202 (95); NMR δH (400 MHz, CDCl3) 8.14 (d, 1H, J = 7.6), 7.38–7.36 (m, 2H), 7.32–7.28 (m, 1H), 4.48 (q, 2H, J = 7.2), 4.38 (q, 2H, J = 7.2), 3.84 (s, 3H), 1.44 (t, 3H, J = 7.2), 1.41 (t, 3H, J = 7.2); δC (100 MHz, CDCl3) 164.3, 163.1, 136.9, 135.2, 125.6, 124.5, 122.7, 122.5, 110.3, 108.1, 62.5, 60.4, 31.6, 14.6, 14.3; HR-MS (EI) m/z [M]+ calcd for C15H17NO4 275.1158, found 275.1153 [28].
Ethyl N-methylindole-2-carboxylate (9f). A white solid (80 mg) from column chromatography using ethyl acetate/pet. ether (1:3, Rf = 0.68), mp. 58–60 °C (59–60 °C, [29]); MS: (m/z, %) = 203 (M+, 40), 178 (70), 89 (85); NMR δH (400 MHz, CDCl3) 7.71 (d, 1H, J = 7.6), 7.40–7.33 (m, 2H), 7.31 (s, 1H), 7.15 (dt, 1H, J = 7.0, 1.2), 4.38 (q, 2H, J = 7.2), 4.09 (s, 3H), 1.42 (t, 3H, J = 7.2); δC (100 MHz, CDCl3) 162.3, 139.6, 128.8, 125.9, 124.9, 122.5, 120.5, 110.21, 110.07, 60.5, 31.6, 14.4; HR-MS (EI) m/z [M]+ calcd for C12H13NO2 203.0946, found 203.0941.
Dimethyl 2-(1H-benzotriazol-1-yl)maleate (10a). A white solid (130 mg) from column chromatography using ethyl acetate/pet. ether (1:3, Rf = 0.68), mp. 84–86 °C; MS: (m/z, %) = 261 (M+, 100), 202 (50), 175 (65); IR νmax (KBr)/cm−1 3040, 2954, 1746, 1718, 1638, 1434, 1360, 1263, 1159, 1056, 746; NMR δH (400 MHz, CDCl3) 8.16 (d, 1H, J = 8.4), 7.634–7.62 (m, 2H), 7.52–7.48 (m, 1H), 6.68 (s, 1H), 4.07 (s, 3H), 3.86 (s, 3H); δC (100 MHz, CDCl3) 164.9, 162.5, 146.9, 140.6, 131.4, 130.0, 125.8, 121.4, 111.1, 110.1, 54.0, 52.8; HR-MS (EI) m/z [M]+ calcd for C12H11N3O4 261.0750, found 261.0745.
Diethyl 2-(1H-benzotriazol-1-yl)maleate (10b). A colorless oil (180 mg) from column chromatography using ethyl acetate/pet. ether (1:3 Rf = 0.58); MS: (m/z, %) = 289 (M+, 40), 187 (90), 159 (100); νmax (KBr)/cm−1 3040, 2954, 1746, 1718, 1638, 1434, 1360, 1263, 1159, 1056, 746; NMR δH (600 MHz, CDCl3) 8.16 (dd, 1H, J = 7.8, 1.6), 7.67–7.62 (m, 2H), 7.60–7.48 (dd, 1H, J = 8.4, 1.2), 6.66 (s, 1H), 4.53 (q, 2H, J = 7.2), 4.32 (q, 2H, J = 7.2), 1.39 (t, 3H, J = 7.2) 1.35 (t, 3H, J = 7.2); δC (100 MHz, CDCl3) 164.3, 162.0, 146.9, 140.5, 131.5, 129.8, 125.7, 121.3, 111.1, 110.8, 63.4, 61.8, 14.35, 14.05; HR-MS (EI) m/z [M]+ calcd for C14H15N3O4 289.1063, found 289.1058.
Dimethyl 2,3-Di(1H-benzotriazol-1-yl)succinate (11a). A white solid (220 mg) from column chromatography using ethyl acetate/pet. ether (1:2, Rf = 0.72), mp.147–149 °C; MS: (m/z, %) = 380 (M+, 15), 293 (40), 190 (95); IR νmax (KBr)/cm−1 3051, 2991, 1759, 1730, 1613, 1556, 1452, 1303, 1278, 1169, 1001, 778, 750; NMR δH (400 MHz, CDCl3) 7.73 (d, 2H, J =8.4), 7.43 (t, 4H, J = 8.0), 7.25–7.23 (m, 2H), 6.63 (s, 2H), 3.85 (s, 6H, 2CH3); δC (100 MHz, CDCl3) 166.5, 145.3, 133.1, 128.6, 124.6, 120.0, 109.3, 58.9, 53.9; HR-MS (EI) m/z [M]+ calcd for C18H16N6O4 380.1233, found 380.1228.
Diethyl 2,3-di(1H-benzotriazol-1-yl)succinate (11b). A white solid (230 mg) from column chromatography using ethyl acetate/pet. ether (1:1, Rf = 0.58), mp. 124–126 °C; MS: (m/z, %)= 408 (M+, 5), 289 (45), 187 (95); IR νmax (KBr)/cm−1 3051, 2991, 1759, 1730, 1613, 1556, 1452, 1303, 1278, 1169, 1001, 778, 750; NMR δH (400 MHz, CDCl3) 7.77 (d, 2H, J = 8.4), 7.43–7.36 (m, 4H), 7.23–7.19 (m, 2H), 6.60 (s, 2H), 4.37–4.26 (m, 4H, 2CH2), 1.23 (t, 6H, J = 7.2, 2CH3); δC (100 MHz, CDCl3) 166.0, 145.3, 133.2, 128.5, 124.5, 120.0, 109.0, 63.4, 60.3, 14.0; HR-MS (EI) m/z [M]+ calcd for C20H20N6O4 408.1546, found 408.1540.
Dimethyl 2-(2H-benzotriazol-2-yl)maleate (12). A white solid (100 mg) from column chromatography using ethyl acetate/pet. ether (1:2, Rf = 0.59), mp. 147–149 °C; MS: (m/z, %) = 261 (M+, 45), 230 (30), 71 (65); IR νmax (KBr)/cm−1 3010, 2957, 1748, 1714, 1643, 1437, 1357, 1259, 1201, 1155, 1053, 744; NMR: δH (400 MHz, CDCl3) 7.87–7.84 (m, 2H), 7.45–7.26 (m, 2H), 7.13 (s, 1H), 4.11 (s, 3H), 3.85 (s, 3H); δC (150 MHz, CDCl3) 164.5, 162.0, 145.7, 143.9, 128.9, 118.7, 110.7, 53.8, 52.6; HR-MS (EI) m/z [M]+ calcd for C12H11N3O4 261.0750 found 261.0744.
4. Conclusions
For the first time intermolecular trapping of the diradical intermediates, formed by irradiation of benzotriazoles with a 16 W low pressure mercury arc-lamp (λ = 254 nm) in the presence of electron poor alkenes and alkynes has been achieved. The present study offers an interesting simple access to dihydropyrrolo[3,4-b]indoles and functionally substituted indoles of potential synthetic and biological interest.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/12/20695/s1.
Acknowledgments
The support of the University of Kuwait received through research grant # SC 03/12 and the facilities of ANALAB/SAF (grants no. GS01/01, GS02/01, GS03/08) are gratefully acknowledged.
Author Contributions
Nader Al-Jalal, Nouria Al-Awadi and Mohamed Elnagdi designed the research; Maher Ibrahim performed chemical reactions and purified the products, Nader Al-Jalal, Mohamed Elnagdi and Yehia Ibrahim interpreted the results and prepared the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Burgess, E.M.; Caithers, R.; McCullagh, L. Photochemical decomposition of 1H-1,2,3-triazole derivatives. J. Am. Chem. Soc. 1968, 90, 1923–1924. [Google Scholar]
- Hubert, A.J. Photochemistry of benzotriazoles. J. Chem. Soc. 1969, 10, 1334–1336. [Google Scholar]
- Wender, P.A.; Cooper, C.B. The Photochemistry of 1-Alkenylbenzotriazoles: Methodology for the synthesis of indoles. Tetrahedron 1986, 42, 2985–2991. [Google Scholar]
- Marky, M.; Schmid, H.; Hansen, H. Photoreaktionen von 1-Alkylbenztriazolen. Helv. Chim. Acta 1979, 62, 2129–2153. [Google Scholar]
- Döpp, D.; Orlewska, C.; Saczewski, F. The photolysis of 1-(4,5-dihydro-1H-imidazol-2-yl)benzotriazole. J. Heterocycl. Chem. 1993, 30, 833–834. [Google Scholar]
- Katritzky, A.R.; Lan, X.; Yang, J.; Denisko, O. Properties and synthetic utility of N-substituted benzotriazoles. Chem. Rev. 1998, 98, 409–548. [Google Scholar]
- Katritzky, A.R.; Rachwal, S. Synthesis of Heterocyclesmediated by benzotriazole. 2. Bicyclic systems. Chem. Rev. 2011, 111, 7063–7120. [Google Scholar]
- Kizka, M.; Dunkin, I.R.; Gebicki, J.; Wang, H.; Wirz, J. The photochemical transformation and tautomeric composition of matrix isolated benzotriazole. J. Chem. Soc. Perkin Trans. 2 2000, 2420–2426. [Google Scholar]
- Wang, H.; Burda, C.; Persy, G.; Wirz, J. Photochemistry of 1H-Benzotriazole in Aqueous Solution: A Photolatent Base. J. Am. Chem. Soc. 2000, 122, 5849–5855. [Google Scholar]
- Ohashi, M.; Tsujimoto, K.; Yonezawa, T. Nature of the intermediates in the photolysis of benzotriazoles. J. Chem. Soc. (Sec. D) Chem. Commun. 1970, 1089–1090. [Google Scholar]
- Kulagowski, J.J.; Mitchell, G.; Moody, C.J.; Rees, C.W. Preparation and rearrangement of 6a-methyl-6aH-benzo[a]carbazole and 11b-methyl-11bH-benzo[c]carbazole. J. Chem. Soc. Chem. Commun. 1985. [Google Scholar] [CrossRef]
- Kulagowski, J.J.; Moody, C.J.; Rees, C.W. Preparation and rearrangement of 6a-methyl-6aH-benzo[a]carbazole and 11b-methyl-11bH-benzo[c]carbazole. J. Chem. Soc. Perkin Trans. 1 1985. [Google Scholar] [CrossRef]
- Razmara, F.; Behjati, B.; Afandilians, N. Zur photolyse des benzotriazols. J. Heterocycl. Chem. 1979, 16, 1641. [Google Scholar]
- Tsujimoto, K.; Ohashi, M.; Yonezawa, T. The Photochemical Decomposition of Benzotriazoles. Bull. Chem. Soc. Jpn. 1972, 45, 515–519. [Google Scholar]
- Al-Jalal, N.A.; Al-Awadi, N.A.; Ibrahim, M.R.; Elnagdi, M.H. The photochemistry of 1-alkenyl-substituted-1,2,3-benzotriazolesleading to formation of indole and fused indole derivatives. Arkivoc 2011, X, 288–297. [Google Scholar]
- Al-Jalal, N.A.; Ibrahim, M.R.; Al-Awadi, N.A.; Elnagdi, M.H. The Photochemistry of benzotriazole derivatives. Part 2: Photolysis of 1-substituted benzotriazolearylhydrazones: New route to phenanthridin-6-yl-2-phenyldiazines. Molecules 2011, 16, 10256–10268. [Google Scholar]
- Di Fabio, R.; Micheli, F.; Alvvaro, G.; Cavanni, P.; Donati, D.; Gagliardi, T.; Fontana, G.; Giovannini, R.; Maffeis, M.; Mingardi, A.; et al. From pyrroles to 1-oxo-2,3,4,9-tetrahydro-1H-β-carbolines: A new class of orally bioavailable mGluR1 antagonists. Bioorg. Med. Chem. Lett. 2007; 17, 2254–2259. [Google Scholar]
- Cichero, E.; Cesarini, S.; Mosti, L.; Fossa, P. CoMFA and CoMSIA analyses on 1,2,3,4-tetrahydropyrrolo[3,4-b]indole and benzimidazole derivatives as selective CB2 receptor agonists. J. Mol. Mod. 2010, 16, 1481–1498. [Google Scholar]
- Kempf, D.J.; Condon, S.L. Synthesis of rigid, heterocyclic dipeptide analogs. J. Org. Chem. 1990, 55, 1390–1394. [Google Scholar]
- Bratton, L.D.; Roth, B.D.; Trivedi, B.K.; Unangst, P.C. Synthesis of novel 3- and 5-substituted indole-2-carboxamides. J. Heterocycl. Chem. 2000, 37, 1103–1108. [Google Scholar]
- Behbehani, H.; Ibrahim, M.R.; Ibrahim, Y.A. Efficient atom economic approaches towards macrocyclic crown diamides via ring-closing metathesis. Tetrahedron Lett. 2002, 43, 6421–6426. [Google Scholar]
- Farion, I.A.; Khaltarov, Z.M.; Kushnarev, D.F.; Rokhin, A.V. Synthesis of benzotriazolylsuccinimides in melt. Russ. Chem. Bull. 2008, 57, 409–411. [Google Scholar]
- Novak, I.; Abu-Iznied, T.; Kovac, B.; Klasine, L. Electronic Structure and Stability of Benzotriazoles. J. Phys. Chem. A 2009, 113, 9751–9756. [Google Scholar]
- Cox, M.; Heidarizadeh, F.; Prager, R. Flash vacuum pyrolysis of N-alkenylbenzotriazolesand N-alkenylisoxazolones. Aust. J. Chem. 2000, 53, 665–671. [Google Scholar]
- Crystallographic data of (excluding structure factor) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Center as supplementary publication nos. CCDC 1012453 (4c), CCDC 101142 (4d), 1011041 (4g), CCDC 1011694 (5), 1012536 (6a) and 1012450 (11a). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax: +44-(0)1223–336033 or e-mail: deposit@ccdc.cam.uk.
- Ymane, Y.; Liu, X.; Hamasaki, A.; Ishida, T.; Haruta, M.; Yokoyama, T.; Tokunaga, M. One-Pot synthesis of indolesand aniline derivatives from nitroarenesunder hydrogenation condition with supported gold nanoparticles. Org. Lett. 2009, 11, 5162–5165. [Google Scholar]
- Liu, Y.; Gribble, G.W. Generation and reactions of 2,3-dilithio-N-methylindole. Synthesis of 2,3-disubstituted indoles. Tetrahedron Lett. 2001, 42, 2949–2951. [Google Scholar]
- Sayyed, I.A.; Alex, K.; Tillack, A.; Schwarz, N.; Michalik, D.; Beller, M. A Convenient and General Method for the Synthesis of Indole-2,3-dicarboxylates and 2-Arylindole-3-carboxylates. Eur. J. Org. Chem. 2007, 4525–4528. [Google Scholar]
- Eggers, M.E.; Jog, P.V.; Bates, D.K. Intramolecularsulfoxide electrophilic sulfenylation in 2- and 3-indoleanilides. Tetrahedron 2007, 63, 12185–12194. [Google Scholar]
- Sample Availability: Samples of the compoundsare available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).