Abstract
Starting from pinane-, apopinane- and carane-based 1,3-amino alcohols obtained from monoterpene-based β-amino acids, a library of monoterpene-fused 2-imino-1,3-thiazines as main products and 2-thioxo-1,3-oxazines as side-products were prepared via two- or three-step syntheses. When thiourea adducts prepared from 1,3-amino alcohols and aryl isothiocyanates were reacted with CDI under mild conditions, O-imidazolylcarbonyl intermediates were isolated which could be transformed to the desired 1,3-thiazines under microwave conditions. 1,3-Thiazines and 2-thioxo-1,3-oxazine side-products could also be prepared in one-step reactions through the application of CDI and microwave irradiation. The ring-closure process was extended to cycloalkane-based γ-hydroxythioureas. The carane- and apopinane-based derivatives exhibited marked antiproliferative activity against a panel of human adherent cancer cell lines (HeLa, A2780, MCF7 and A431).
1. Introduction
In the past decade, alicyclic 1,3-amino alcohols have proved to be versatile building blocks. They have been applied as useful starting materials in the stereoselective syntheses of compounds of pharmacological interest and they have served as chiral ligands and auxiliaries in enantioselective transformations [1,2,3].
Several natural chiral terpenes, including (+)-pulegone [4,5,6] α- and β-pinene [7,8,9] and fenchone-camphor [10,11,12], have proved to be excellent sources for the production of various amino alcohols, which have been successfully applied in enantioselective syntheses. The transformation of enantiomerically pure α-pinene to β-amino acid derivatives such as 1,3-amino alcohols was recently reported [9,13]. These synthons served as chiral auxiliaries in the enantioselective synthesis of secondary alcohols or pharmacons, e.g., esomeprasol [14,15,16,17,18].
Besides their value in enantioselective catalysis, 1,3-amino alcohols are good starting points for the synthesis of various heterocyclic ring systems, such as 1,3-oxazines, 1,3-thiazines or 1,4-oxazepams [2,19]. The 2-imino-1,3-thiazine and 2-iminothiazolidine ring systems can be found as moieties in biologically relevant compounds, including antifungicidal and antimicrobial agents [20], BACE1 inhibitors [21], or cannabinoid receptor agonists [22,23,24]. New spiro derivatives of 2-imino-1,3-thiazines have been synthetized and shown to be potential neuroprotectors [25].
In recent years, we have devised novel pathways to synthetize new monoterpene-based chiral β-lactams and β-amino acid derivatives derived from (–)- and (+)-α-pinene, (–)-3-carene, (–)- and (+)-apopinene and myrtenic acid [9,13,26,27,28,29,30]. These amino acid derivatives have proved to be excellent building blocks for the syntheses of compounds with multidrug resistance (MDR) antagonist activity [29].
We also found that monoterpene-based 1,3-amino alcohols prepared from the abovementioned β-amino acid derivatives are excellent building blocks for the synthesis of 2-imino-1,3-oxazines which possess marked anti-cancer activity [31]. The analogous pinane- or apopinane-based 2-imino-1,3-thiazines could not be prepared by the methods applied earlier for the synthesis of cycloalkane- or norbornane-fused analogue 1,3-thiazines [32,33].
The aim of the present work was to synthetize new chiral pinane-, apopinane- and carane-fused 2-imino-1,3-thiazines, analogues of 1,3-oxazines with noteworthy cytoselective toxicity on multiple cancer cell lines.
2. Results and Discussion
2.1. Syntheses of Alicyclic and Monoterpene-Based 1,3-Amino Alcohols
The synthetic routes applied for the preparation of 1,3-amino alcohols 9–12 and 16–18 followed literature methods (Figure 1) [9,13,27,28,29,31,33]. The corresponding β-lactams were prepared by the stereoselective cycloaddition of chlorosulfonyl isocyanate to cyclopentene, cycloxene, α-pinene, 3-carene and apopinene, followed by ring opening, which resulted in cis-fused β-amino esters 5–8 and 14. Under alkaline conditions, the cis-amino ester 14 underwent fast and complete isomerization at the carboxylic function, resulting in the trans-amino ester 15 in excellent yield [28]. Reduction of 5–8, 14 and 15 with LAH led to the primary amino alcohols 9–12, 16 and 17. From 17, N-benzyl derivative 18 was prepared by reductive alkylation with benzaldehyde and NaBH4 in EtOH [31].
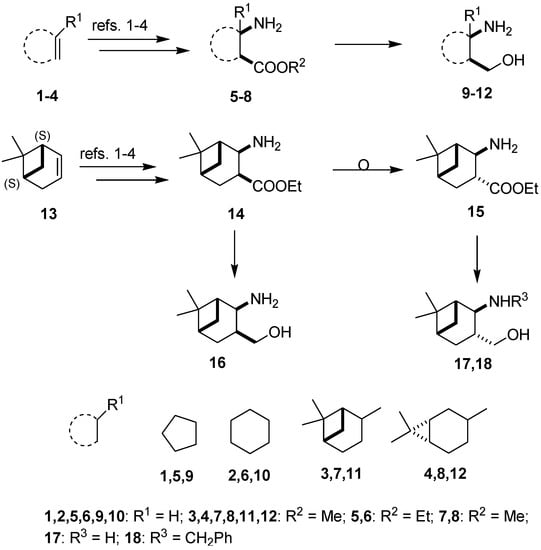
Figure 1.
Synthesis of 1,3-amino alcohol starting materials.
2.2. Syntheses of 2-Imino-1,3-thiazine Derivatives
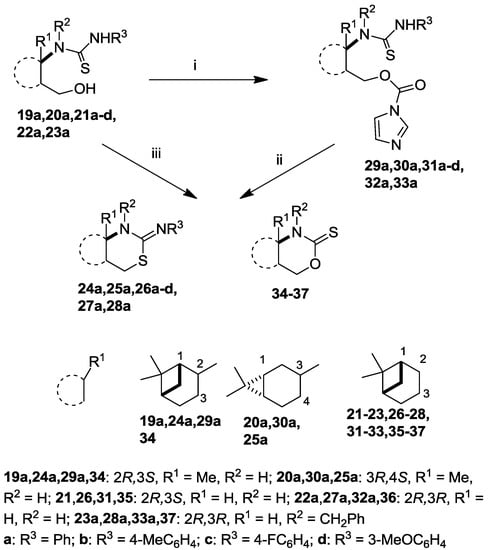
The intermediate thiourea adducts 19–23 were prepared in good to excellent yields by the reaction of the appropriate aryl isothiocyanates and 1,3-amino alcohols 10, 11 and 16–18 (Scheme 1, Table 1) [13,27,31].
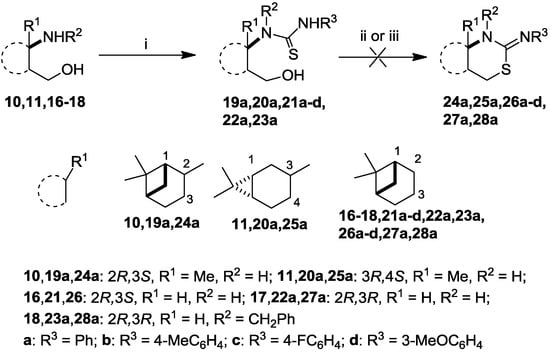
Scheme 1.
Synthesis and attempted ring closure of thioureas 19–23.

Table 1.
Thioureas 19–23 and O-imidazolylcarbonyl intermediates 29–33.
| General Structure | R1 | R2 | R3 | Compound No. |
|---|---|---|---|---|
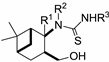 | Me | H | Ph | 19a |
| H | H | Ph | 21a | |
| H | H | 4-MeC6H4 | 21b | |
| H | H | 4-FC6H4 | 21c | |
| H | H | 3-MeOC6H4 | 21d | |
 | H | H | Ph | 22a |
| H | CH2Ph | Ph | 23a | |
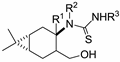 | Me | H | Ph | 20a |
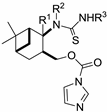 | Me | H | Ph | 29a |
| H | H | Ph | 31a | |
| H | H | 4-MeC6H4 | 31b | |
| H | H | 4-FC6H4 | 31c | |
| H | H | 3-MeOC6H4 | 31d | |
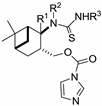 | H | H | Ph | 32a |
| H | CH2Ph | Ph | 33a | |
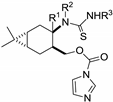 | H | H | H | 30a |
Although a number of methods are known for the conversion of 3-hydroxypropylthioureas to the corresponding 2-imino-1,3-thiazines, e.g., acid-promoted dehydrative cyclization [32,33] or Mitsubobu conditions [34], in the cases of the highly constrained acid-sensitive bicyclic pinane and carane skeletons the expected 2-imino-1,3-thiazine systems were not obtained.
Bernacki et al. recently reported that they also identified 2-aminothiazoline cyclization products besides the expected main product thioureas in the reactions of 1,2-amino alcohols and 3-aminobenzonitrile in the presence of thio-CDI [35]. Finally, they devised an excellent protocol for the cyclization of 1,2-amino alcohol-based thiourea derivatives to yield 2-phenylaminothiazolines under mild conditions (rt, THF, CDI or thio-CDI).
When we applied the above-mentioned mild conditions, we observed the formation of O-imidazolylcarbonyl intermediates 29–33 (similar intermediates were presumed, but could not be isolated in Bernacki’s work) as single products even when the reaction mixture was subjected to conventional heating from room temperature to reflux (Table 1). The structures of intermediates 31c and 31d were determined by X-ray crystallography (Figure 2) and NMR measurements.
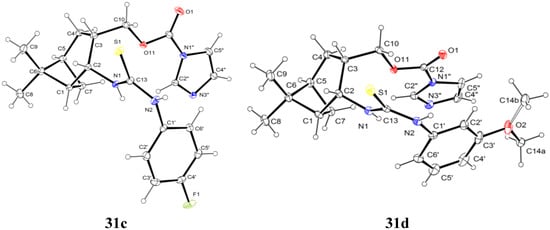
Figure 2.
ORTEP plots of the configurations of the major diastereoisomers 31c and 31d (the C atom of the methoxy group (C14) is disordered in a 1:1 ratio).
However, when microwave irradiation was applied to the isolated intermediates 29–33 in THF, we obtained the desired 2-imino-1,3-thiazines 25–28 as main products in acceptable yields in a short time (60 min) (Scheme 3, Table 2).

Table 2.
2-Phenylimono-1,3-thiazines 24–28.
| General Structure | R1 | R2 | R3 | Compound No. |
|---|---|---|---|---|
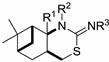 | Me | H | Ph | 24a |
| H | H | Ph | 26a | |
| H | H | 4-MeC6H4 | 26b | |
| H | H | 4-FC6H4 | 26c | |
| H | H | 3-MeOC6H4 | 26d | |
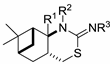 | H | H | Ph | 27a |
| H | CH2Ph | Ph | 28a | |
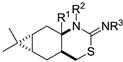 | Me | H | Ph | 25a |
The ring-closure process could be carried out in one step by applying CDI in THF under microwave conditions. Moreover, we observed that 2-thioxo-1,3-oxazines 34–37 were also formed as minor products in 10%–24% yields. The transformation of 20a was an exception, when thiazine 25a was isolated as a single product. Besides NMR assignments, the structures of 35 and 36 were proved by independent synthesis starting from the corresponding amino alcohols and thiophosgene.
The above procedure was subsequently extended to 1-(2-hydroxymethylcycloalkyl)thioureas. Starting from cycloalkane-based 1,3-amino alcohols 9 and 10, thioureas 38 and 39 were prepared by a literature method [33]. When 38 and 39 were treated with CDI in THF, rapid conversion to intermediates 40 and 41 was observed and in this case the ring-closure proceeded under conventional heating to yield 1,3-thiazines 42 and 43 (Scheme 2). We observed that the developed method could be applied more easily in the case of thioureas with sterically less hindered structures.
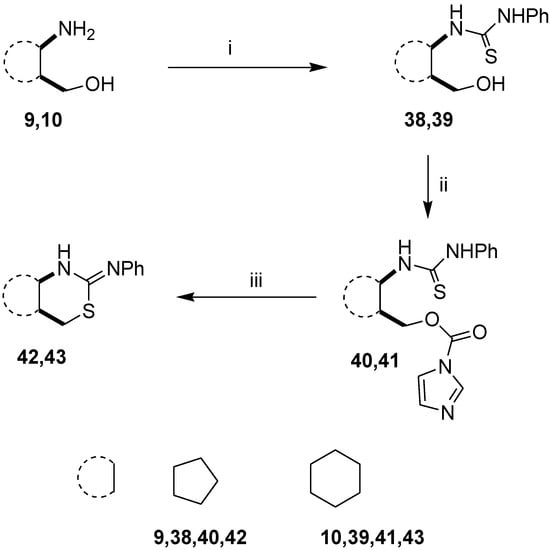
Scheme 2.
Extension of the ring-closure process.
2.3. Antiproliferative Activities
The novel 2-imino-1,3-thiazines 24–28 (Scheme 3) and some of their analogues were subjected to in vitro pharmacological studies in order to investigate their antiproliferative properties on a panel of human adherent cancer cell lines. The results of MTT assays are presented in Table 3. Carane-based compound 25a proved to be the most potent of the tested compounds, exhibiting a cell growth-inhibiting capacity comparable to that of the reference agent cisplatin. Compounds with a pinane ring were generally less potent, while the introduction of a methyl group at position 2 (Scheme 3) favoured the action (24a). Substitution of the N-phenyl ring had a limited and inconsequential impact on the efficacy (26a–d), while the introduction of a N-benzyl function onto the 1,3-thiazine skeleton was disadvantageous (28a). Since no substantial difference was observed between the effects of 26a and 27a, the configuration of C-3 (cis or trans ring fusion, Scheme 3) also seems irrelevant. On the other hand, the 2-thioxo-1,3-oxazine analogue (35) was completely ineffective, indicating that the arylimino substituent is an essential part of the molecule. Replacement of the pinane or carane ring system with cyclopentane (42) or cyclohexane (43) also led to ineffective congeners, demonstrating the crucial role of the bicyclic monoterpene as a building block for the design and synthesis of novel antiproliferative agents.
Scheme 3.
CDI mediated ring closure of thioureas 19–23.

Table 3.
Antiproliferative effects of 2-imino-1,3-thiazines 24–28, 42, 43 and 2-thioxo-1,3-oxazine 35 on human cancer cell lines.
| Compnd. | Conc. | Growth Inhibition, % ± SEM a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HeLa | A2780 | MCF7 | A431 | |||||||
| 24a | 10 µM | - | 67.45 ± 0.83 | 39.40 ± 2.41 | 73.01 ± 1.52 | |||||
| 30 µM | 25.59 ± 1.93 | 86.02 ± 0.27 | 62.08 ± 1.78 | 83.15 ± 1.25 | ||||||
| 25a | 10 µM | - | 65.24 ± 1.65 | 65.91 ± 0.96 | 60.54 ± 1.79 | |||||
| 30 µM | 96.40 ± 0.28 | 96.42 ± 0.15 | 86.09 ± 1.20 | 94.17 ± 0.51 | ||||||
| 26a | 10 µM | - | - | 26.52 ± 2.79 | 67.70 ± 1.49 | |||||
| 30 µM | 22.03 ± 1.57 | 68.93 ± 0.97 | 50.45 + 1.79 | 85.68 ± 0.74 | ||||||
| 26b | 10 µM | - | 45.45 ± 2.01 | - | 65.20 ± 1.47 | |||||
| 30 µM | 37.02 ± 2.20 | 58.67 ± 1.29 | 43.66 ± 2.32 | 74.49 ± 1.02 | ||||||
| 26c | 10 µM | 44.90 ± 0.88 | 40.02 ± 0.88 | 21.55 ± 0.99 | 32.93 ± 1.20 | |||||
| 30 µM | 53.60 ± 1.07 | 56.86 ± 1.17 | 30.93 ± 1.29 | 34.20 ± 1.02 | ||||||
| 26d | 10 µM | 27.40 ± 0.52 | 20.87 ± 1.87 | - | 34.92 ± 2.66 | |||||
| 30 µM | 33.67 ± 2.61 | 92.17 ± 0.51 | 63.84 ± 2.36 | 82.46 ± 1.11 | ||||||
| 27a | 10 µM | - | 37.26 ± 2.35 | 43.83 ± 0.99 | 77.32 ± 0.94 | |||||
| 30 µM | 21.57 ± 0.92 | 42.80 ± 2.78 | 47.15 ± 2.93 | 80.05 ± 1.15 | ||||||
| 28a | 10 µM | 46.83 ± 1.42 | 23.10 ± 1.00 | - | - | |||||
| 30 µM | 42.24 ± 2.48 | 50.23 ± 0.52 | 23.84 ± 1.08 | - | ||||||
| 35 | 10 µM | - | - | - | - | |||||
| 30 µM | - | - | - | - | ||||||
| 42 | 10 µM | - | - | - | - | |||||
| 30 µM | - | 41.23 ± 1.30 | - | - | ||||||
| 43 | 10 µM | - | - | - | - | |||||
| 30 µM | 24.07 ± 2.96 | - | 32.59 ± 1.49 | - | ||||||
| Cisplatin | 10 µM | 42.61 ± 2.33 | 83.57 ± 1.21 | 53.03 ± 2.29 | 88.54 ± 0.50 | |||||
| 30 µM | 99.93 ± 0.26 | 95.02 ± 0.28 | 86.90 ± 1.24 | 90.18 ± 1.78 | ||||||
a Substances eliciting less than 20% inhibition of cell proliferation were regarded as ineffective and the results are not presented.
3. Experimental Section
3.1. General Information
1H- and 13C-NMR spectra were recorded on a Bruker Avance DRX 400 spectrometer (400 MHz, δ = 0 (TMS)), in an appropriate solvent. Chemical shifts are expressed in ppm (δ) relative to TMS as internal reference. J values are given in Hz. FT-IR spectra were recorded on a Perkin-Elmer Spectrum 100 instrument. Microanalyses were performed on a Perkin-Elmer 2400 elemental analyser. Microwave reactions were carried out by heating at 125 °C and 200 W for 1 h in a CEM Discover LabMate microwave reactor. Optical rotations were obtained with a Perkin-Elmer 341 polarimeter. Melting points were determined on a Kofler apparatus and are uncorrected. Chromatographic separations were carried out on Merck Kieselgel 60 (230–400 mesh ASTM). Reactions were monitored with Merck Kieselgel 60 F254-precoated TLC plates (0.25 mm thickness). All the chemicals and solvents were used as supplied.
Compounds 5–18 and thioureas 19a, 20a, 38 and 39 were prepared by literature methods; all their spectroscopic data and physical properties were similar to those reported therein [9,13,27,28,29,31,33].
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of Thioureas 21–23
Amino alcohol 16, 17 or 18 (1.08 mmol) and the appropriate isothiocyanate (1.14 mmol) were dissolved in toluene (80 mL) and the mixture was stirred at room temperature for 6 h, except that in the case of N-benzylamino alcohol 18, heating at 50 °C for 6 h was indicated. The resulting mixtures were then evaporated to dryness, filtered and washed with n-hexane. The purities of the products obtained were determined via NMR to be >97%.
(1R,2R,3S,5R)-1-(3-Hydroxymethyl-6,6-dimethylbicyclo[3.1.1]hept-2-yl)-3-phenylthiourea (21a): 0.31 g (95%); mp 152–155 °C,  = +52.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (1H, d, J = 9.9 Hz), 1.01 (3H, s), 1.23 (3H, s), 1.63–1.70 (1H, m), 1.72–1.79 (1H, m), 1.88–1.94 (1H, m), 1.98–2.18 (3H, m), 2.54–2.65 (1H, m), 3.48-3.63 (2H, m), 5.15 (1H, t, J = 8.2 Hz), 7.16 (1H, d, J = 8.5 Hz), 7.19-7.44 (5H, m), 7.68 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.5, 26.6, 26.8, 30.0, 32.6, 39.3, 40.7, 46.4, 57.0, 65.1, 125.7, 127.6, 130.3, 136.5, 180.2. Anal. Calcd for C17H24N2OS (304.45): C, 67.07; H, 7.95; N, 9.20; S, 10.53%; Found: C, 67.39; H, 8.13; N, 9.01; S, 10.42%.
= +52.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (1H, d, J = 9.9 Hz), 1.01 (3H, s), 1.23 (3H, s), 1.63–1.70 (1H, m), 1.72–1.79 (1H, m), 1.88–1.94 (1H, m), 1.98–2.18 (3H, m), 2.54–2.65 (1H, m), 3.48-3.63 (2H, m), 5.15 (1H, t, J = 8.2 Hz), 7.16 (1H, d, J = 8.5 Hz), 7.19-7.44 (5H, m), 7.68 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.5, 26.6, 26.8, 30.0, 32.6, 39.3, 40.7, 46.4, 57.0, 65.1, 125.7, 127.6, 130.3, 136.5, 180.2. Anal. Calcd for C17H24N2OS (304.45): C, 67.07; H, 7.95; N, 9.20; S, 10.53%; Found: C, 67.39; H, 8.13; N, 9.01; S, 10.42%.
(1R,2R,3S,5R)-1-(4-Tolyl)-3-(3-hydroxymethyl-6,6-dimethylbicyclo[3.1.1]hept-2-yl)thiourea (21b): 0.32 g (92%); mp 98–101 °C,  = +63.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.92 (1H, d, J = 10.1 Hz), 1.01 (3H, s), 1.22 (3H, s), 1.62–1.68 (1H, m), 1.73–1.86 (1H, m), 1.87–1.93 (1H, m), 1.98–2.16 (3H, m), 2.35 (3H, s), 2.54–2.64 (1H, m), 3.47–3.61 (2H, m), 5.15 (1H, br s), 7.05 (1H, d, J = 8.5 Hz), 7.09 (2H, d, J = 8.2 Hz), 7.20 (2H, d, J = 8.2 Hz). 13C-NMR (CDCl3) δ (ppm) 21.2, 21.5, 26.8, 26.9, 30.0, 32.8, 39.4, 40.8, 46.5, 57.0, 65.3, 126.0, 131.0, 132.8, 137.7, 151.9, 180.1. Anal. Calcd for C18H26N2OS (318.48): C, 67.88; H, 8.23; N, 8.80; S, 10.07%; Found: C, 68.13; H, 8.35; N, 8.69; S, 9.94%.
= +63.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.92 (1H, d, J = 10.1 Hz), 1.01 (3H, s), 1.22 (3H, s), 1.62–1.68 (1H, m), 1.73–1.86 (1H, m), 1.87–1.93 (1H, m), 1.98–2.16 (3H, m), 2.35 (3H, s), 2.54–2.64 (1H, m), 3.47–3.61 (2H, m), 5.15 (1H, br s), 7.05 (1H, d, J = 8.5 Hz), 7.09 (2H, d, J = 8.2 Hz), 7.20 (2H, d, J = 8.2 Hz). 13C-NMR (CDCl3) δ (ppm) 21.2, 21.5, 26.8, 26.9, 30.0, 32.8, 39.4, 40.8, 46.5, 57.0, 65.3, 126.0, 131.0, 132.8, 137.7, 151.9, 180.1. Anal. Calcd for C18H26N2OS (318.48): C, 67.88; H, 8.23; N, 8.80; S, 10.07%; Found: C, 68.13; H, 8.35; N, 8.69; S, 9.94%.
(1R,2R,3S,5R)-1-(4-Fluorophenyl)-3-(3-hydroxymethyl-6,6-dimethylbicyclo[3.1.1]hept-2-yl)thiourea (21c): 0.32 g (93%); mp 162–163 °C,  = +55.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (1H, d, J = 9.8 Hz), 1.00 (3H, s), 1.22 (3H, s), 1.62–1.72 (1H, m), 1.87–1.94 (1H, m), 1.98–2.16 (4H, m), 2.51–2.63 (1H, m), 3.51 (1H, dd, J = 4.6, 10.5 Hz), 3.61 (1H, dd, J = 3.2, 10.7 Hz), 5.11 (1H, br s), 7.01–7.26 (4H, m). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.6, 26.7, 29.9, 32.4, 39.3, 40.7, 46.3, 57.0, 65.0, 117.1 (d, J = 23.7 Hz), 128.1 (d, J = 8.5 Hz), 162.6, 180.1. Anal. Calcd for C17H23FN2OS (322.44): C, 63.32; H, 7.19; N, 8.69; S, 9.94%; Found: C, 63.49; H, 7.29; N, 8.48; S, 9.77%.
= +55.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (1H, d, J = 9.8 Hz), 1.00 (3H, s), 1.22 (3H, s), 1.62–1.72 (1H, m), 1.87–1.94 (1H, m), 1.98–2.16 (4H, m), 2.51–2.63 (1H, m), 3.51 (1H, dd, J = 4.6, 10.5 Hz), 3.61 (1H, dd, J = 3.2, 10.7 Hz), 5.11 (1H, br s), 7.01–7.26 (4H, m). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.6, 26.7, 29.9, 32.4, 39.3, 40.7, 46.3, 57.0, 65.0, 117.1 (d, J = 23.7 Hz), 128.1 (d, J = 8.5 Hz), 162.6, 180.1. Anal. Calcd for C17H23FN2OS (322.44): C, 63.32; H, 7.19; N, 8.69; S, 9.94%; Found: C, 63.49; H, 7.29; N, 8.48; S, 9.77%.
(1R,2R,3S,5R)-1-(3-Methoxyphenyl)-3-(3-hydroxymethyl-6,6-dimethylbicyclo[3.1.1]hept-2-yl)thiourea (21d): 0.33 g (90%); mp 118–120 °C,  = +57.0 (c 0.20, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.99 (1H, d, J = 8.8 Hz), 1.01 (3H, s), 1.23 (3H, s), 1.65–1.74 (1H, m), 1.78–1.92 (2H, m), 1.99–2.19 (3H, m), 2.55–2.66 (1H, m), 3.53 (1H, dd, J = 4.5, 10.7 Hz), 3.61 (1H, br d, J = 10.5 Hz), 3.79 (3H, s), 5.17 (1H, br s), 6.72–6.83 (3H, m), ), 7.23–7.34 (2H, m). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.7, 26.8, 29.9, 32.6, 39.3, 40.8, 46.4, 55.8, 57.1, 65.1, 110.9, 113.1, 117.3, 128.8, 131.1, 137.6, 161.1, 179.9. Anal. Calcd for C18H26N2O2S (334.48): C, 64.64; H, 7.84; N, 8.39; S, 9.59%; Found: C, 64.73; H, 7.99; N, 8.11; S, 9.38%.
= +57.0 (c 0.20, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.99 (1H, d, J = 8.8 Hz), 1.01 (3H, s), 1.23 (3H, s), 1.65–1.74 (1H, m), 1.78–1.92 (2H, m), 1.99–2.19 (3H, m), 2.55–2.66 (1H, m), 3.53 (1H, dd, J = 4.5, 10.7 Hz), 3.61 (1H, br d, J = 10.5 Hz), 3.79 (3H, s), 5.17 (1H, br s), 6.72–6.83 (3H, m), ), 7.23–7.34 (2H, m). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.7, 26.8, 29.9, 32.6, 39.3, 40.8, 46.4, 55.8, 57.1, 65.1, 110.9, 113.1, 117.3, 128.8, 131.1, 137.6, 161.1, 179.9. Anal. Calcd for C18H26N2O2S (334.48): C, 64.64; H, 7.84; N, 8.39; S, 9.59%; Found: C, 64.73; H, 7.99; N, 8.11; S, 9.38%.
(1R,2R,3R,5R)-1-(3-Hydroxymethyl-6,6-dimethylbicyclo[3.1.1]hept-2-yl)-3-phenylthiourea (22a): 0.31 g (94%); mp 151–155 °C,  = −19.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (3H, s), 1.23 (3H, s), 1.30 (1H, d, J = 10.2 Hz), 1.57–1.64 (1H, m), 1.71–1.88 (2H, m), 1.92–2.00 (1H, m), 2.06–2.13 (1H, m), 3.42–3.50 (1H, m), 3.66–3.83 (2H, m), 4.91 (1H, t, J = 7.7 Hz), 6.16 (1H, d, J = 9.4 Hz), 7.18 (2H, d, J = 7.7 Hz), 7.29 (1H, t, J = 7.0 Hz), 7.43 (2H, t, J = 7.5 Hz), 7.82 (1H, s). 13C-NMR (CDCl3) δ (ppm) 19.8, 23.8, 27.1, 27.3, 39.3, 40.4, 41.1, 46.6, 56.6, 64.4, 125.3, 127.7, 130.7, 135.8, 159.6, 179.5. Anal. Calcd for C17H24N2OS (304.45): C, 67.07; H, 7.95; N, 9.20; S, 10.53%; Found: C, 67.31; H, 7.80; N, 9.30; S, 10.41%.
= −19.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (3H, s), 1.23 (3H, s), 1.30 (1H, d, J = 10.2 Hz), 1.57–1.64 (1H, m), 1.71–1.88 (2H, m), 1.92–2.00 (1H, m), 2.06–2.13 (1H, m), 3.42–3.50 (1H, m), 3.66–3.83 (2H, m), 4.91 (1H, t, J = 7.7 Hz), 6.16 (1H, d, J = 9.4 Hz), 7.18 (2H, d, J = 7.7 Hz), 7.29 (1H, t, J = 7.0 Hz), 7.43 (2H, t, J = 7.5 Hz), 7.82 (1H, s). 13C-NMR (CDCl3) δ (ppm) 19.8, 23.8, 27.1, 27.3, 39.3, 40.4, 41.1, 46.6, 56.6, 64.4, 125.3, 127.7, 130.7, 135.8, 159.6, 179.5. Anal. Calcd for C17H24N2OS (304.45): C, 67.07; H, 7.95; N, 9.20; S, 10.53%; Found: C, 67.31; H, 7.80; N, 9.30; S, 10.41%.
(1R,2R,3R,5R)-1-Benzyl-1-(3-hydroxymethyl-6,6-dimethylbicyclo[3.1.1]hept-2-yl)-3-phenylthiourea (23a): 0.36 g (85%); mp 177–178 °C,  = +7.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.07 (3H, s), 1.26 (3H, s), 1.65 (1H, d, J = 10.2 Hz), 1.75–1.86 (1H, m), 1.89–2.01 (3H, m), 2.09–2.15 (1H, m), 2.23–2.31 (1H, m), 3.55–3.63 (1H, m), 3.92–3.99 (1H, m), 4.73–4.90 (2H, m), 6.01 (1H, br s), 7.03–7.45 (10H, m). 13C-NMR (CDCl3) δ (ppm) 19.8, 25.6, 27.3, 27.5, 35.6, 40.0, 42.8, 45.8, 49.0, 59.6, 62.1, 126.4, 126.5, 126.6, 128.5, 128.9, 129.7, 139.3, 139.8, 183.1. Anal. Calcd for C24H30N2OS (394.57): C, 73.06; H, 7.66; N, 7.10; S, 8.13%; Found: C, 73.40; H, 7.86; N, 7.05; S, 8.38%.
= +7.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.07 (3H, s), 1.26 (3H, s), 1.65 (1H, d, J = 10.2 Hz), 1.75–1.86 (1H, m), 1.89–2.01 (3H, m), 2.09–2.15 (1H, m), 2.23–2.31 (1H, m), 3.55–3.63 (1H, m), 3.92–3.99 (1H, m), 4.73–4.90 (2H, m), 6.01 (1H, br s), 7.03–7.45 (10H, m). 13C-NMR (CDCl3) δ (ppm) 19.8, 25.6, 27.3, 27.5, 35.6, 40.0, 42.8, 45.8, 49.0, 59.6, 62.1, 126.4, 126.5, 126.6, 128.5, 128.9, 129.7, 139.3, 139.8, 183.1. Anal. Calcd for C24H30N2OS (394.57): C, 73.06; H, 7.66; N, 7.10; S, 8.13%; Found: C, 73.40; H, 7.86; N, 7.05; S, 8.38%.
3.2.2. General Procedure for the Reactions of Thioureas 19–23 with CDI to Yield Intermediates 29–33
To the respective thiourea (1.25 mmol) 19–23, 38 or 39 in THF solution (12 mL), CDI (0.306 g, 1.88 mmol) was added at room temperature. The reaction was stirred for 2–6 h at room temperature (TLC monitoring), followed by careful evaporation of the solvent at 35 °C. The crude products obtained were purified by flash column chromatography on silica gel.
(1R,2R,3S,5R)-[2,6,6-Trimethyl-2-(3-phenylthioureido)bicyclo[3.1.1]hept-3-yl]methyl imidazole-1-carboxylate (29a): 0.27 g (52%); oil,  = +32.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.92 (3H, s), 1.07 (1H, d, J = 10.1 Hz), 1.22 (3H, s), 1.42 (3H, s), 1.60–1.76 (1H, m), 1.92–2.20 (3H, m), 2.90–3.00 (1H, m), 4.35 (2H, d, J = 5.2 Hz), 5.26 (1H, t, J = 9.4 Hz), 6.25 (1H, d, J = 9.0 Hz), 7.05–7.39 (7H, m), 7.80 (1H, br s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.3, 22.5, 26.7, 26.6, 29.6, 30.1, 39.5, 40.8, 46.5, 55.6, 71.5, 117.5, 125.3, 128.5, 130.0, 130.4, 131.2, 135.5, 148.7, 181.5. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 63.89; H, 6.91; N, 13.73; S, 7.65%.
= +32.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.92 (3H, s), 1.07 (1H, d, J = 10.1 Hz), 1.22 (3H, s), 1.42 (3H, s), 1.60–1.76 (1H, m), 1.92–2.20 (3H, m), 2.90–3.00 (1H, m), 4.35 (2H, d, J = 5.2 Hz), 5.26 (1H, t, J = 9.4 Hz), 6.25 (1H, d, J = 9.0 Hz), 7.05–7.39 (7H, m), 7.80 (1H, br s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.3, 22.5, 26.7, 26.6, 29.6, 30.1, 39.5, 40.8, 46.5, 55.6, 71.5, 117.5, 125.3, 128.5, 130.0, 130.4, 131.2, 135.5, 148.7, 181.5. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 63.89; H, 6.91; N, 13.73; S, 7.65%.
(1R,2R,3S,5R)-[2,6,6-Trimethyl-2-(3-phenylthioureido)bicyclo[3.1.1]hept-3-yl]methyl imidazole-1-carboxylate (30a): 0.32 g (62%); oil,  = –140.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.58 (1H, t, J = 8.5 Hz), 0.76–0.85 (2H, m), 0.93 (3H, s), 1.05 (3H, s), 1.28–1.55 (3H, m), 1.67 (3H, s), 1.72–1.82 (1H, m), 3.36 (1H, dd, J = 2.7, 11.1 Hz), 3.75 (1H,dd, J = 7.1, 14.0 Hz), 3.84 (1H, dd, J = 11.3, 16.7 Hz), 3.95 (1H, dd, J = 2.7, 11.2 Hz), 7.19 (1H, br s), 7.22–7.46 (7H, m). 13LC-NMR (CDCl3) δ (ppm) 15.6, 19.1, 19.3, 24.0, 29.0, 30.1, 31.1, 45.4, 63.0, 69.1, 122.5, 126.6, 126.9, 127.6, 128.8, 130.1, 137.0, 143.0, 180.0. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 64.21; H, 6.97; N, 13.23; S, 7.71%.
= –140.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.58 (1H, t, J = 8.5 Hz), 0.76–0.85 (2H, m), 0.93 (3H, s), 1.05 (3H, s), 1.28–1.55 (3H, m), 1.67 (3H, s), 1.72–1.82 (1H, m), 3.36 (1H, dd, J = 2.7, 11.1 Hz), 3.75 (1H,dd, J = 7.1, 14.0 Hz), 3.84 (1H, dd, J = 11.3, 16.7 Hz), 3.95 (1H, dd, J = 2.7, 11.2 Hz), 7.19 (1H, br s), 7.22–7.46 (7H, m). 13LC-NMR (CDCl3) δ (ppm) 15.6, 19.1, 19.3, 24.0, 29.0, 30.1, 31.1, 45.4, 63.0, 69.1, 122.5, 126.6, 126.9, 127.6, 128.8, 130.1, 137.0, 143.0, 180.0. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 64.21; H, 6.97; N, 13.23; S, 7.71%.
(1R,2R,3S,5R)-[6,6-Dimethyl-2-(3-phenylthioureido)bicyclo[3.1.1]hept-3-yl]methyl imidazole-1-carboxylate (31a): 0.39 g (78%); oil,  = +39.0 (c 0.32, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.86 (1H, d, J = 10.3 Hz), 1.03 (3H, s), 1.26 (3H, s), 1.65–1.72 (1H, m), 1.95–2.26 (4H, m), 2.90–3.00 (1H, m), 4.39 (2H, d, J = 5.4 Hz), 5.29 (1H, t, J = 9.2 Hz), 6.26 (1H, d, J = 9.0 Hz), 7.00–7.30 (7H, m), 7.85 (1H, br s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.5, 26.6, 29.6, 30.1, 39.5, 40.7, 46.5, 55.8, 71.5, 117.3, 125.5, 128.1, 130.1, 130.5, 131.0, 135.9, 148.9, 181.0. Anal. Calcd for C21H26N4O2S (398.52): C, 63.29; H, 6.58; N, 14.06; S, 8.05%; Found: C, 63.54; H, 6.60; N, 13.84; S, 7.91%.
= +39.0 (c 0.32, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.86 (1H, d, J = 10.3 Hz), 1.03 (3H, s), 1.26 (3H, s), 1.65–1.72 (1H, m), 1.95–2.26 (4H, m), 2.90–3.00 (1H, m), 4.39 (2H, d, J = 5.4 Hz), 5.29 (1H, t, J = 9.2 Hz), 6.26 (1H, d, J = 9.0 Hz), 7.00–7.30 (7H, m), 7.85 (1H, br s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.1, 26.5, 26.6, 29.6, 30.1, 39.5, 40.7, 46.5, 55.8, 71.5, 117.3, 125.5, 128.1, 130.1, 130.5, 131.0, 135.9, 148.9, 181.0. Anal. Calcd for C21H26N4O2S (398.52): C, 63.29; H, 6.58; N, 14.06; S, 8.05%; Found: C, 63.54; H, 6.60; N, 13.84; S, 7.91%.
(1R,2R,3S,5R)-{6,6-Dimethyl-2-[3-(4-tolyl)thioureido]bicyclo[3.1.1]hept-3-yl}methyl imidazole-1-carboxylate (31b): 0.36 g (69%); mp 148–150 °C,  = +81.0 (c 0.265, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (3H, s), 1.19 (1H, d, J = 10.0 Hz), 1.24 (3H, s), 1.66–1.75 (1H, m), 1.91–1.99 (2H, m), 2.06–2.16 (1H, m), 2.19–2.30 (1H, m), 2.24 (3H, s), 2.79–2.91 (1H, m), 4.34–4.44 (2H, m), 5.14 (1H, t, J = 8.8 Hz), 7.01–7.09 (3H, m), 7.25 (1H, d, J = 8.2 Hz), 7.57 (1H, s), 7.72 (1H, d, J = 8.9 Hz), 8.23 (1H, s), 9.28 (1H, s). 13C-NMR (DMSO–d6) δ (ppm) 21.3, 21.5, 27.1, 27.2, 29.9, 30.4, 39.3, 47.0, 53.7, 59.0, 71.8, 118.4, 124.1, 129.7, 131.0, 134.2, 137.2, 137.7, 149.3, 181.6. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 64.39; H, 6.93; N, 13.27; S, 7.56%.
= +81.0 (c 0.265, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (3H, s), 1.19 (1H, d, J = 10.0 Hz), 1.24 (3H, s), 1.66–1.75 (1H, m), 1.91–1.99 (2H, m), 2.06–2.16 (1H, m), 2.19–2.30 (1H, m), 2.24 (3H, s), 2.79–2.91 (1H, m), 4.34–4.44 (2H, m), 5.14 (1H, t, J = 8.8 Hz), 7.01–7.09 (3H, m), 7.25 (1H, d, J = 8.2 Hz), 7.57 (1H, s), 7.72 (1H, d, J = 8.9 Hz), 8.23 (1H, s), 9.28 (1H, s). 13C-NMR (DMSO–d6) δ (ppm) 21.3, 21.5, 27.1, 27.2, 29.9, 30.4, 39.3, 47.0, 53.7, 59.0, 71.8, 118.4, 124.1, 129.7, 131.0, 134.2, 137.2, 137.7, 149.3, 181.6. Anal. Calcd for C22H28N4O2S (412.55): C, 64.05; H, 6.84; N, 13.58; S, 7.77%; Found: C, 64.39; H, 6.93; N, 13.27; S, 7.56%.
(1R,2R,3S,5R)-{6,6-Dimethyl-2-[3-(4-fluorophenyl)thioureido]bicyclo[3.1.1]hept-3-yl}methyl imidazole-1-carboxylate (31c): 0.39 g (75%); mp 201–203 °C,  = +44.0 (c 0.35, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.87 (1H, d, J = 10.5 Hz), 1.03 (3H, s), 1.24 (3H, s), 1.65–1.72 (1H, m), 1.96–2.26 (4H, m), 2.91–3.01 (1H, m), 4.37–4.46 (2H, m), 5.26 (1H, t, J = 9.9 Hz), 6.20 (1H, br s), 6.94–7.15 (5H, m), 7.30 (1H, s), 7.78 (1H, br s), 8.14 (1H, br s). 13C-NMR (DMSO–d6) δ (ppm) 21.0, 26.5, 26.6, 29.7, 30.1, 39.4, 40.6, 46.6, 55.4, 71.6, 117.2, 117.3, 117.4, 127.7, 127.8, 148.8, 160.4, 162.9, 181.3. Anal. Calcd for C21H25FN4O2S (416.51): C, 60.56; H, 6.05; N, 13.45; S, 7.70%; Found: C, 60.79; H, 6.41; N, 13.08; S, 7.53%.
= +44.0 (c 0.35, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.87 (1H, d, J = 10.5 Hz), 1.03 (3H, s), 1.24 (3H, s), 1.65–1.72 (1H, m), 1.96–2.26 (4H, m), 2.91–3.01 (1H, m), 4.37–4.46 (2H, m), 5.26 (1H, t, J = 9.9 Hz), 6.20 (1H, br s), 6.94–7.15 (5H, m), 7.30 (1H, s), 7.78 (1H, br s), 8.14 (1H, br s). 13C-NMR (DMSO–d6) δ (ppm) 21.0, 26.5, 26.6, 29.7, 30.1, 39.4, 40.6, 46.6, 55.4, 71.6, 117.2, 117.3, 117.4, 127.7, 127.8, 148.8, 160.4, 162.9, 181.3. Anal. Calcd for C21H25FN4O2S (416.51): C, 60.56; H, 6.05; N, 13.45; S, 7.70%; Found: C, 60.79; H, 6.41; N, 13.08; S, 7.53%.
(1R,2R,3S,5R)-{6,6-Dimethyl-2-[3-(3-methoxyphenyl)thioureido]bicyclo[3.1.1]hept-3-yl}methyl imidazole-1-carboxylate (31d): 0.39 g (73%); mp 128–130 °C,  = +9.0 (c 0.26, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.91 (1H, d, J = 10.6 Hz), 1.03 (3H, s), 1.26 (3H, s), 1.66–1.72 (1H, m), 1.97–2.02 (1H, m), 2.06–2.28 (3H, m), 2.90–3.00 (1H, m), 3.73 (3H, s), 4.34–4.44 (2H, m), 5.30 (1H, t, J = 9.8 Hz), 6.41 (1H, d, J = 9.7 Hz), 6.60–6.69 (3H, m), 7.02 (1H, s), 7.17 (1H, t, J = 8.1 Hz), 7.22 (1H, s), 7.79 (1H, s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.2, 26.6, 26.7, 29.6, 30.2, 39.6, 40.7, 46.6, 55.8, 55.9, 71.5, 110.6, 111.2, 113.4, 117.1, 117.3, 131.0, 131.3, 137.0, 148.9, 161.4, 180.8. Anal. Calcd for C22H28N4O3S (428.55): C, 61.66; H, 6.59; N, 13.07; S, 7.48%; Found: C, 61.83; H, 6.84; N, 12.89; S, 7.53%.
= +9.0 (c 0.26, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.91 (1H, d, J = 10.6 Hz), 1.03 (3H, s), 1.26 (3H, s), 1.66–1.72 (1H, m), 1.97–2.02 (1H, m), 2.06–2.28 (3H, m), 2.90–3.00 (1H, m), 3.73 (3H, s), 4.34–4.44 (2H, m), 5.30 (1H, t, J = 9.8 Hz), 6.41 (1H, d, J = 9.7 Hz), 6.60–6.69 (3H, m), 7.02 (1H, s), 7.17 (1H, t, J = 8.1 Hz), 7.22 (1H, s), 7.79 (1H, s), 7.92 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.2, 26.6, 26.7, 29.6, 30.2, 39.6, 40.7, 46.6, 55.8, 55.9, 71.5, 110.6, 111.2, 113.4, 117.1, 117.3, 131.0, 131.3, 137.0, 148.9, 161.4, 180.8. Anal. Calcd for C22H28N4O3S (428.55): C, 61.66; H, 6.59; N, 13.07; S, 7.48%; Found: C, 61.83; H, 6.84; N, 12.89; S, 7.53%.
(1R,2R,3R,5R)-[6,6-Dimethyl-2-(3-phenylthioureido)bicyclo[3.1.1]hept-3-yl]methyl imidazole-1-carboxylate (32a): 0.33 g (67%); oil,  = −15.0 (c 0.30, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.26 (3H, s), 1.31 (1H, d, J = 9.5 Hz), 1.61–1.72 (1H, m), 1.94–2.19 (5H, m), 4.54 (1H, dd, J = 6.6, 10.8 Hz), 4.65 (1H, dd, J = 6.6, 10.7 Hz), 5.04 (1H, t, J = 8.7 Hz), 6.16 (1H, d, J = 9.2 Hz), 7.06 (1H, s), 7.13 (2H, d, J = 7.8 Hz), 7.24–7.29 (1H, m), 7.40 (2H, t, J = 7.9 Hz), 7.46 (1H, br s), 8.13 (1H, br s), 8.16 (1H, s). 13C-NMR (CDCl3) δ (ppm) 19.9, 23.6, 26.7, 27.5, 35.6, 40.2, 40.646.2, 55.7, 70.4, 117.6, 125.2, 127.5, 130.6, 131.0, 136.5, 149.2, 180.3. Anal. Calcd for C21H26N4O2S (398.52): C, 63.29; H, 6.58; N, 14.06; S, 8.05%; Found: C, 63.43; H, 6.75; N, 13.85; S, 8.16%.
= −15.0 (c 0.30, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.26 (3H, s), 1.31 (1H, d, J = 9.5 Hz), 1.61–1.72 (1H, m), 1.94–2.19 (5H, m), 4.54 (1H, dd, J = 6.6, 10.8 Hz), 4.65 (1H, dd, J = 6.6, 10.7 Hz), 5.04 (1H, t, J = 8.7 Hz), 6.16 (1H, d, J = 9.2 Hz), 7.06 (1H, s), 7.13 (2H, d, J = 7.8 Hz), 7.24–7.29 (1H, m), 7.40 (2H, t, J = 7.9 Hz), 7.46 (1H, br s), 8.13 (1H, br s), 8.16 (1H, s). 13C-NMR (CDCl3) δ (ppm) 19.9, 23.6, 26.7, 27.5, 35.6, 40.2, 40.646.2, 55.7, 70.4, 117.6, 125.2, 127.5, 130.6, 131.0, 136.5, 149.2, 180.3. Anal. Calcd for C21H26N4O2S (398.52): C, 63.29; H, 6.58; N, 14.06; S, 8.05%; Found: C, 63.43; H, 6.75; N, 13.85; S, 8.16%.
(1R,2R,3R,5R)-[6,6-Dimethyl-2-(1-benzyl-3-phenylthioureido)bicyclo[3.1.1]hept-3-yl]methyl imidazole-1-carboxylate (33a): 0.37 g (60%); oil,  = −15.0 (c 0.30, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.09 (3H, s), 1.32 (3H, s), 1.68 (1H, d, J = 10.1 Hz), 1.80–1.89 (1H, m), 1.95–2.11 (2H, m), 2.20 (1H, t, J = 5.4 Hz), 2.33–2.50 (2H, m), 4.65 (1H, dd, J = 7.3, 11.6 Hz), 4.80–4.89 (3H, m), 6.50 (1H, br d, J = 10.6 Hz), 7.03–7.55 (12H, m), 8.20 (1H, s). 13C-NMR (CDCl3) δ (ppm) 20.1, 25.7, 27.1, 28.1, 33.3, 39.9, 42.5, 45.8, 49.1, 58.9, 70.3, 117.7, 126.3, 126.5, 128.7, 128.9, 129.2, 130.9, 131.8, 136.1, 137.1, 140.0, 149.2, 184.2. Anal. Calcd for C28H32N4O2S (488.22): C, 68.82; H, 6.60; N, 11.47; S, 6.56%; Found: C, 68.63; H, 6.49; N, 11.72; S, 6.83%.
= −15.0 (c 0.30, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.09 (3H, s), 1.32 (3H, s), 1.68 (1H, d, J = 10.1 Hz), 1.80–1.89 (1H, m), 1.95–2.11 (2H, m), 2.20 (1H, t, J = 5.4 Hz), 2.33–2.50 (2H, m), 4.65 (1H, dd, J = 7.3, 11.6 Hz), 4.80–4.89 (3H, m), 6.50 (1H, br d, J = 10.6 Hz), 7.03–7.55 (12H, m), 8.20 (1H, s). 13C-NMR (CDCl3) δ (ppm) 20.1, 25.7, 27.1, 28.1, 33.3, 39.9, 42.5, 45.8, 49.1, 58.9, 70.3, 117.7, 126.3, 126.5, 128.7, 128.9, 129.2, 130.9, 131.8, 136.1, 137.1, 140.0, 149.2, 184.2. Anal. Calcd for C28H32N4O2S (488.22): C, 68.82; H, 6.60; N, 11.47; S, 6.56%; Found: C, 68.63; H, 6.49; N, 11.72; S, 6.83%.
2-[(3-Phenylthioureido)cyclopent-3-yl]methyl imidazole-1-carboxylate (40): 0.28 g (65%); oil, 1H-NMR (CDCl3) δ (ppm) 1.36–1.49 (2H, m), 1.54–1.75 (2H, m), 1.92–2.02 (1H, m), 2.08–2.18 (1H, m), 2.73–2.84 (1H, m), 4.31 (1H, dd, J = 5.3, 11.0 HZ), 4.39 (1H, dd, J = 6.1, 11.1 Hz), 4.96–5.04 (1H, m), 5.96 (1H, d, J = 7.9 Hz), 7.03 (1H, s), 7.10–7.15 (2H, m), 7.19–7.25 (1H, m), 7.30–7.37 (3H, m), 7.99 (1H, br s), 8.03 (1H, s). 13C-NMR (CDCl3) δ (ppm) 22.5, 27.7, 33.0, 39.9, 57.9, 69.0, 117.5, 125.6, 128.1, 130.7, 131.1, 136.2, 137.5, 148.9, 181.3. Anal. Calcd for C17H20N4O2S (344.43): C, 59.28; H, 5.85; N, 16.27; S, 9.39%; Found: C, 59.28; H, 5.85; N, 16.27; S, 9.39%.
2-[(3-Phenylthioureido)cyclohex-3-yl]methyl imidazole-1-carboxylate (41): 0.26 g (57%); oil, 1H-NMR (CDCl3) δ (ppm) 0.86–1.00 (1H, m), 1.06–1.20 (1H, m), 1.32–1.45 (1H, m), 1.59–1.79 (4H, m), 1.86–1.97 (1H, m), 2.24–2.36 (1H, m), 4.23–4.35 (2H, m), 4.96–5.04 (1H, m), 6.23 (1H, d, J = 9.0 Hz), 7.06–7.09 (1H, m), 7.23–7.28 (2H, m), 7.37 (1H, t, J = 7.5 Hz), 7.47–7.53 (3H, m), 8.20 (1H, s). 13C-NMR (CDCl3) δ (ppm) 21.0, 24.7, 26.4, 32.2, 32.6, 34.3, 52.5, 122.8, 123.5, 125.6, 125.7, 129.3, 135.0, 146.1, 156.4, 171.2. Anal. Calcd for C18H22N4O2S (358.46): C, 60.31; H, 6.19; N, 15.63; S, 8.95%; Found: C, 60.47; H, 6.23; N, 15.12; S, 8.69%.
3.2.3. General Procedure for the Synthesis of 2-Imino-1,3-thiazines 24–28 and 2-Thioxo-1,3-oxazines 34–37
Method A: A CEM Discover 10 mL vial containing the respective thiourea (1.25 mmol) 19–23 in THF solution (12 mL) and CDI (0.306 g, 1.88 mmol) and sealed with a Teflon cap was irradiated at 125 °C (200 W) for 60 min with a ramp time of 5 min. Next, the solvent was evaporated off under reduced pressure and the residue was purified by flash column chromatography on silica gel; elution with n-hexane/EtOAc (4:1) gave 2-imino-1,3-thiazines and 2-thioxo-1,3-oxazines.
Method B: A CEM Discover 10 mL vial containing the respective intermediates (1.25 mmol) 29–33 in THF solution (12 mL) and sealed with a Teflon cap was irradiated at 125 °C (200 W) for 60 min with a ramp time of 5 min. Next, the solvent was evaporated off under reduced pressure and the residue was purified by flash column chromatography on silica gel; elution with n-hexane/EtOAc (4:1) gave 2-imino-1,3-thiazines and 2-thioxo-1,3-oxazines.
(1R,2R,7S,9R)-(10,10-Dimethyl-5-thia-3-azatricyclo[7.1.1.02,7]undec-4-ylidene)phenylamine (26a): Method A: 0.197 g (55%), method B: 0.204 g (57%); mp 124–128 °C,  = +142.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.24 (3H, s), 1.30 (1H, d, J = 10.5 Hz), 1.50–1.58 (1H, m), 1.92–1.99 (2H, m), 2.13–2.30 (2H, m), 2.61–2.73 (2H, m), 2.87 (1H, t, J = 13.4 Hz), 3.92 (1H, t, J = 8.9 Hz), 6.88–7.30 (5H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 33.1, 34.1, 35.7, 39.0, 41.1, 47.7, 54.9, 122.9, 123.7, 129.3, 137.7, 160.4. Anal. Calcd for C17H22N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19%; Found: C, 71.39; H, 8.00; N, 9.21; S, 11.11%.
= +142.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.24 (3H, s), 1.30 (1H, d, J = 10.5 Hz), 1.50–1.58 (1H, m), 1.92–1.99 (2H, m), 2.13–2.30 (2H, m), 2.61–2.73 (2H, m), 2.87 (1H, t, J = 13.4 Hz), 3.92 (1H, t, J = 8.9 Hz), 6.88–7.30 (5H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 33.1, 34.1, 35.7, 39.0, 41.1, 47.7, 54.9, 122.9, 123.7, 129.3, 137.7, 160.4. Anal. Calcd for C17H22N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19%; Found: C, 71.39; H, 8.00; N, 9.21; S, 11.11%.
(1R,2R,7S,9R)-10,10-Dimethyl-5-oxa-3-azatricyclo[7.1.1.02,7]undecane-4-thione (35): Method A: 0.053 g (20%), method B: 0.058 g (22%); this compound was also isolated from transformations of thioureas 26c–d, in 18%–24% yield; mp 160–161 °C,  = +112.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.91 (3H, s), 1.26 (3H, s), 1.28 (1H, d, J = 11.8 Hz), 1.45–1.52 (1H, m), 1.97–2.24 (4H, m), 2.64–2.75 (1H, m), 3.85–3.88 (1H, m), 3.91 (1H, dd, J = 10.2, 21.1 Hz), 4.24 (1H, dd, J = 6.1, 10.8 Hz), 7.53 (1H, br s). 13C-NMR (CDCl3) δ (ppm) 20.4, 25.3, 26.6, 26.7, 28.0, 39.6, 40.5, 46.1, 54.2, 72.6, 192.5. Anal. Calcd for C11H17NOS (211.32): C, 62.52; H, 8.11; N, 6.63; S, 15.17%; Found: C, 62.77; H, 8.36; N, 6.41; S, 15.00%.
= +112.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.91 (3H, s), 1.26 (3H, s), 1.28 (1H, d, J = 11.8 Hz), 1.45–1.52 (1H, m), 1.97–2.24 (4H, m), 2.64–2.75 (1H, m), 3.85–3.88 (1H, m), 3.91 (1H, dd, J = 10.2, 21.1 Hz), 4.24 (1H, dd, J = 6.1, 10.8 Hz), 7.53 (1H, br s). 13C-NMR (CDCl3) δ (ppm) 20.4, 25.3, 26.6, 26.7, 28.0, 39.6, 40.5, 46.1, 54.2, 72.6, 192.5. Anal. Calcd for C11H17NOS (211.32): C, 62.52; H, 8.11; N, 6.63; S, 15.17%; Found: C, 62.77; H, 8.36; N, 6.41; S, 15.00%.
(1R,2R,7S,9R)-(10,10-Dimethyl-5-thia-3-azatricyclo[7.1.1.02,7]undec-4-ylidene)-p-tolylamine (26b): Method A: 0.199 g (53%), method B: 0.203 g (54%); mp 199–203 °C,  = +170.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (3H, s), 1.23 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.57 (1H, m), 1.88–1.99 (2H, m), 2.11–2.29 (2H, m), 2.31 (3H, s), 2.59–2.71 (2H, m), 2.87 (1H, t, J = 13.4 Hz), 3.91 (1H, d, J = 8.3 Hz), 5.01 (1H, br s), 6.80 (1H, d, J = 8.1 Hz), 7.08 (1H, d, J = 8.1 Hz). 13C-NMR (CDCl3) δ (ppm) 20.9, 21.3, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.8, 122.5, 129.9, 133.0, 146.2, 159.7. Anal. Calcd for C18H24N2S (300.46): C, 71.95; H, 8.05; N, 9.32; S, 10.67%; Found: C, 71.99; H, 8.21; N, 9.10; S, 10.70%.
= +170.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.94 (3H, s), 1.23 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.57 (1H, m), 1.88–1.99 (2H, m), 2.11–2.29 (2H, m), 2.31 (3H, s), 2.59–2.71 (2H, m), 2.87 (1H, t, J = 13.4 Hz), 3.91 (1H, d, J = 8.3 Hz), 5.01 (1H, br s), 6.80 (1H, d, J = 8.1 Hz), 7.08 (1H, d, J = 8.1 Hz). 13C-NMR (CDCl3) δ (ppm) 20.9, 21.3, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.8, 122.5, 129.9, 133.0, 146.2, 159.7. Anal. Calcd for C18H24N2S (300.46): C, 71.95; H, 8.05; N, 9.32; S, 10.67%; Found: C, 71.99; H, 8.21; N, 9.10; S, 10.70%.
(1R,2R,7S,9R)-(10,10-Dimethyl-5-thia-3-azatricyclo[7.1.1.02,7]undec-4-ylidene)-3-Methoxyphenylamine (26c): Method A: 0.225 g (57%), method B: 0.233 g (59%); mp 154–155 °C,  = +234.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.25 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.59 (1H, m), 1.90–2.02 (2H, m), 2.13–2.32 (2H, m), 2.61–2.75 (2H, m), 2.88 (1H, t, J = 12.5 Hz), 3.91 (1H, d, J = 9.1 Hz), 6.81–7.00 (4H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.9, 55.6, 108.3, 109.6, 115.2, 129.8, 129.9, 150.6, 160.6. Anal. Calcd for C18H24N2OS (316.46): C, 68.32; H, 7.64; N, 8.85; S,10.13%; Found: C, 68.45; H, 7.93; N, 8.50; S,9.89%.
= +234.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.25 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.59 (1H, m), 1.90–2.02 (2H, m), 2.13–2.32 (2H, m), 2.61–2.75 (2H, m), 2.88 (1H, t, J = 12.5 Hz), 3.91 (1H, d, J = 9.1 Hz), 6.81–7.00 (4H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.9, 55.6, 108.3, 109.6, 115.2, 129.8, 129.9, 150.6, 160.6. Anal. Calcd for C18H24N2OS (316.46): C, 68.32; H, 7.64; N, 8.85; S,10.13%; Found: C, 68.45; H, 7.93; N, 8.50; S,9.89%.
(1R,2R,7S,9R)-(10,10-Dimethyl-5-thia-3-azatricyclo[7.1.1.02,7]undec-4-ylidene)-4-fluorophenylamine (26d): Method A: 0.221 g (58%), method B: 0.232 g (61%); mp 201–205 °C,  = +272.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.25 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.59 (1H, m), 1.90–2.02 (2H, m), 2.13–2.32 (2H, m), 2.61–2.75 (2H, m), 2.88 (1H, t, J = 12.5 Hz), 3.91 (1H, d, J = 9.1 Hz), 6.81–7.00 (4H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.9, 55.6, 108.3, 109.6, 115.2, 129.9, 150.6, 160.6. Anal. Calcd for C17H21FN2S (304.43): C, 67.07; H, 6.95; N, 9.20; S, 10.53%; Found: C, 67.41; H, 7.13; N, 9.01; S 10.27%.
= +272.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.95 (3H, s), 1.25 (3H, s), 1.30 (1H, d, J = 10.6 Hz), 1.49–1.59 (1H, m), 1.90–2.02 (2H, m), 2.13–2.32 (2H, m), 2.61–2.75 (2H, m), 2.88 (1H, t, J = 12.5 Hz), 3.91 (1H, d, J = 9.1 Hz), 6.81–7.00 (4H, m). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.9, 27.0, 34.2, 35.8, 39.1, 41.2, 47.8, 54.9, 55.6, 108.3, 109.6, 115.2, 129.9, 150.6, 160.6. Anal. Calcd for C17H21FN2S (304.43): C, 67.07; H, 6.95; N, 9.20; S, 10.53%; Found: C, 67.41; H, 7.13; N, 9.01; S 10.27%.
(1R,2R,7R,9R)-(10,10-Dimethyl-5-thia-3-azatricyclo[7.1.1.02,7]undec-4-ylidene)phenylamine (27a): Method A: 0.186 g (52%), method B: 0.197 g (55%); mp 192–195 °C,  = +165.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.82 (3H, s), 1.25 (3H, s), 1.43–1.53 (1H, m), 1.67 (1H, d, J = 10.6 Hz), 1.91–2.16 (5H, m), 2.91–3.03 (2H, m), 3.44 (1H, d, J = 8.1 Hz), 6.95–7.28 (5H, m). 13C-NMR (CDCl3) δ (ppm) 20.1, 23.8, 27.3, 29.9, 33.8, 41.1, 41.5, 46.6, 58.1, 121.5, 123.0, 129.2, 145.2, 160.8. Anal. Calcd for C17H22N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19%; Found: C, 71.35; H, 7.83; N, 9.65; S, 11.17%.
= +165.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.82 (3H, s), 1.25 (3H, s), 1.43–1.53 (1H, m), 1.67 (1H, d, J = 10.6 Hz), 1.91–2.16 (5H, m), 2.91–3.03 (2H, m), 3.44 (1H, d, J = 8.1 Hz), 6.95–7.28 (5H, m). 13C-NMR (CDCl3) δ (ppm) 20.1, 23.8, 27.3, 29.9, 33.8, 41.1, 41.5, 46.6, 58.1, 121.5, 123.0, 129.2, 145.2, 160.8. Anal. Calcd for C17H22N2S (286.43): C, 71.28; H, 7.74; N, 9.78; S, 11.19%; Found: C, 71.35; H, 7.83; N, 9.65; S, 11.17%.
(1R,2R,7R,9R)-10,10-Dimethyl-5-oxa-3-azatricyclo[7.1.1.02,7]undecane-4-thione (36): Method A: 0.053 g (20%), method B: 0.055 g (21%); mp 176–179 °C,  = +331.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.83 (3H, s), 1.33 (3H, s), 1.54 (1H, t, J = 12.5 Hz), 1.78 (1H, d, J = 10.7 Hz), 1.87–1.95 (1H, m), 2.03–2.12 (1H, m), 2.23–2.31 (1H, m), 2.40–2.53 (1H, m), 3.62 (1H, d, J = 10.2 Hz), 4.31 (1H, dd, J = 10.1, 12.5 Hz), 4.52 (1H, dd, J = 4.7, 10.0 Hz), 8.29 (1H, br s). 13C-NMR (DMSO–d6) δ (ppm) 19.6, 23.2, 23.5, 27.5,32.6, 41.6, 42.4, 43.1, 54.9, 75.6, 188.2. Anal. Calcd for C11H17NOS (211.32): C, 62.52; H, 8.11; N, 6.63; S, 15.17%; Found: C, 62.73; H, 8.46; N, 6.52; S, 15.01%.
= +331.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.83 (3H, s), 1.33 (3H, s), 1.54 (1H, t, J = 12.5 Hz), 1.78 (1H, d, J = 10.7 Hz), 1.87–1.95 (1H, m), 2.03–2.12 (1H, m), 2.23–2.31 (1H, m), 2.40–2.53 (1H, m), 3.62 (1H, d, J = 10.2 Hz), 4.31 (1H, dd, J = 10.1, 12.5 Hz), 4.52 (1H, dd, J = 4.7, 10.0 Hz), 8.29 (1H, br s). 13C-NMR (DMSO–d6) δ (ppm) 19.6, 23.2, 23.5, 27.5,32.6, 41.6, 42.4, 43.1, 54.9, 75.6, 188.2. Anal. Calcd for C11H17NOS (211.32): C, 62.52; H, 8.11; N, 6.63; S, 15.17%; Found: C, 62.73; H, 8.46; N, 6.52; S, 15.01%.
(1R,2R,7R,9R)-(3-Benzyl-10,10-dimethyl-5-thia-3-azatricyclo-[7.1.1.02,7]undec-4-ylidene)phenylamine (28a): Method A: 0.221 g (47%), method B: 0.231 g (49%); mp 108–109 °C,  = +567.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.77 (3H, s), 1.20 (3H, s), 1.47 (1H, dt, J = 5.1, 13.6 Hz), 1.52 (1H, d, J = 10.8 Hz), 1.91–1.97 (1H, m), 2.04–2.14 (2H, m), 2.25–2.42 (2H, m), 2.88–2.99 (2H, m), 3.81 (1H, d, J = 8.1 Hz), 4.51 (1H, d, J = 16.4 Hz), 5.31 (1H, d, J = 16.6 Hz), 6.78–6.83 (2H, m), 6.94–6.99 (1H, m), 7.15–7.32 (7H, m). 13C-NMR (CDCl3) δ (ppm) 20.1, 24.2, 27.5, 31.1, 34.0, 35.8, 40.3, 40.4, 42.5, 49.0, 60.2, 122.7, 122.9, 126.7, 12.8, 128.7, 129.1, 140.2, 150.5, 152.6. Anal. Calcd for C24H28N2S (376.56): C, 76.55; H, 7.49; N, 7.44; S, 8.52%; Found: C, 76.86; H, 7.63; N, 7.21; S, 8.30%.
= +567.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.77 (3H, s), 1.20 (3H, s), 1.47 (1H, dt, J = 5.1, 13.6 Hz), 1.52 (1H, d, J = 10.8 Hz), 1.91–1.97 (1H, m), 2.04–2.14 (2H, m), 2.25–2.42 (2H, m), 2.88–2.99 (2H, m), 3.81 (1H, d, J = 8.1 Hz), 4.51 (1H, d, J = 16.4 Hz), 5.31 (1H, d, J = 16.6 Hz), 6.78–6.83 (2H, m), 6.94–6.99 (1H, m), 7.15–7.32 (7H, m). 13C-NMR (CDCl3) δ (ppm) 20.1, 24.2, 27.5, 31.1, 34.0, 35.8, 40.3, 40.4, 42.5, 49.0, 60.2, 122.7, 122.9, 126.7, 12.8, 128.7, 129.1, 140.2, 150.5, 152.6. Anal. Calcd for C24H28N2S (376.56): C, 76.55; H, 7.49; N, 7.44; S, 8.52%; Found: C, 76.86; H, 7.63; N, 7.21; S, 8.30%.
(1R,2R,7R,9R)-3-Benzyl-10,10-dimethyl-5-oxa-3-azatricyclo[7.1.1.02,7]undecane-4-thione (37): Method A: 0.053 g (14%), method B: 0.060 g (16%); mp 94–97 °C,  = +132.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.71 (3H, s), 1.23 (3H, s), 1.42–1.57 (2H, m), 1.83–1.91 (1H, m), 1.95–2.06 (2H, m), 2.20 (1H, t, J = 5.14), 2.43–2.51 (1H, m), 3.64 (1H, d, J = 9.6 Hz), 4.17 (1H, dd, J = 9.6, 12.2 Hz), 4.36 (1H, dd, J = 4.9, 9.6 Hz), 4.49 (1H, d, J = 16.2 Hz), 4.68 (1H, d, J = 16.2 Hz), 7.19–7.32 (5H, m). 13C-NMR (CDCl3) δ (ppm) 19.5, 23.5, 24.0, 27.8, 34.1, 40.6, 41.5, 41.6, 48.1, 57.1, 72.2, 127.3, 127.4, 128.9, 138.7, 155.8. Anal. Calcd for C18H23NOS (301.45): C, 71.72; H, 7.69; N, 4.65; s, 10.64%; Found: C, 71.98; H, 7.83; N, 4.39; S, 10.39%.
= +132.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.71 (3H, s), 1.23 (3H, s), 1.42–1.57 (2H, m), 1.83–1.91 (1H, m), 1.95–2.06 (2H, m), 2.20 (1H, t, J = 5.14), 2.43–2.51 (1H, m), 3.64 (1H, d, J = 9.6 Hz), 4.17 (1H, dd, J = 9.6, 12.2 Hz), 4.36 (1H, dd, J = 4.9, 9.6 Hz), 4.49 (1H, d, J = 16.2 Hz), 4.68 (1H, d, J = 16.2 Hz), 7.19–7.32 (5H, m). 13C-NMR (CDCl3) δ (ppm) 19.5, 23.5, 24.0, 27.8, 34.1, 40.6, 41.5, 41.6, 48.1, 57.1, 72.2, 127.3, 127.4, 128.9, 138.7, 155.8. Anal. Calcd for C18H23NOS (301.45): C, 71.72; H, 7.69; N, 4.65; s, 10.64%; Found: C, 71.98; H, 7.83; N, 4.39; S, 10.39%.
(1R,2R,7S,9R)-Phenyl-(2,10,10-trimethyl-5-thia-3-azatricyclo[7.1.1.02,7]undec-4-ylidene)amine (24a): Method A: 0.240 g (64%), method B: 0.252 g (67%); mp 182–186 °C,  = –123.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.05 (3H, s), 1.28 (3H, s), 1.38 (1H, d, J = 12.5 Hz), 1.41 (3H,s), 1.69–1.98 (2H, m), 2.10–2.23 (2H, m), 2.42–2.53 (1H, m), 2.63 (1H, dd, J = 8.0, 12.8 Hz), 2.97 (1H, dd, J = 3.9, 12.9 Hz), 5.04 (1H, br s), 6.87–7.06 (3H, m), 7.22–7.30 (2H, m). 13C-NMR (CDCl3) δ (ppm) 24.1, 27.8, 28.2, 31.7, 33.4, 33.9, 40.3, 40.6, 55.3, 60.9, 122.7, 123.4, 129.2, 148.6, 158.4. Anal. Calcd for C18H24N2S (300.46): C, 71.95; H, 8.05; N, 9.32; S, 10.67%; Found: C, 72.19; H, 8.33; N, 9.11; S, 10.60%.
= –123.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 1.05 (3H, s), 1.28 (3H, s), 1.38 (1H, d, J = 12.5 Hz), 1.41 (3H,s), 1.69–1.98 (2H, m), 2.10–2.23 (2H, m), 2.42–2.53 (1H, m), 2.63 (1H, dd, J = 8.0, 12.8 Hz), 2.97 (1H, dd, J = 3.9, 12.9 Hz), 5.04 (1H, br s), 6.87–7.06 (3H, m), 7.22–7.30 (2H, m). 13C-NMR (CDCl3) δ (ppm) 24.1, 27.8, 28.2, 31.7, 33.4, 33.9, 40.3, 40.6, 55.3, 60.9, 122.7, 123.4, 129.2, 148.6, 158.4. Anal. Calcd for C18H24N2S (300.46): C, 71.95; H, 8.05; N, 9.32; S, 10.67%; Found: C, 72.19; H, 8.33; N, 9.11; S, 10.60%.
(1R,2R,7S,9R)-2,10,10-Trimethyl-5-oxa-3-azatricyclo[7.1.1.02,7]undecane-4-thione (34): Method A: 0.028 g (10%), method B: 0.023 g (8%); mp 120–123 °C,  = +36.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (1H, d, J = 11.1 Hz), 1.06 (3H, s), 1.31 (3H, s), ), 1.35 (3H, s), 1.79–1.87 (1H, m), 1.95–2.03 (2H, m), 2.14–2.42 (3H, m), 4.08 (1H, d, J = 11.0 Hz), 4.18 (1H, d, J = 10.7 Hz), 7.43 (1H, br s). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.7, 27.2, 33.1, 34.1, 35.5, 39.3, 40.2, 41.2, 47.4, 55.1, 158.2. Anal. Calcd for C12H19NOS (225.35): C, 63.96; H, 8.50; N, 6.22; S, 14.23%; Found: C, 64.25; H, 8.79; N, 6.01; S, 13.88%.
= +36.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.97 (1H, d, J = 11.1 Hz), 1.06 (3H, s), 1.31 (3H, s), ), 1.35 (3H, s), 1.79–1.87 (1H, m), 1.95–2.03 (2H, m), 2.14–2.42 (3H, m), 4.08 (1H, d, J = 11.0 Hz), 4.18 (1H, d, J = 10.7 Hz), 7.43 (1H, br s). 13C-NMR (CDCl3) δ (ppm) 20.9, 26.7, 27.2, 33.1, 34.1, 35.5, 39.3, 40.2, 41.2, 47.4, 55.1, 158.2. Anal. Calcd for C12H19NOS (225.35): C, 63.96; H, 8.50; N, 6.22; S, 14.23%; Found: C, 64.25; H, 8.79; N, 6.01; S, 13.88%.
(1aR,2aR,6aS,7aS)-Phenyl-(1,1,6a-trimethyloctahydro-4-thia-6-azacyclopropa[b]naphthalen-5-ylidene)amine (25a): Method A: 0.225 g (60%), method B: 0.233 g (62%); mp 157–160 °C,  = −18.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.71–0.83 (2H, m), 0.98 (3H, s), 1.07 (3H, s), 1.22 (1H, dd, J = 4.5, 15.8 Hz), 1.28 (3H, s), 1.53–1.71 (2H, m), 1.89–2.01 (2H, m), 2.65 (1H, d, J = 3.4, 12.4 Hz), 3.29 (1H, d, J = 3.5, 11.7 Hz), 7.02–7.12 (3H, m), 7.28–7.34 (2H, m). 13C-NMR (CDCl3) δ (ppm) 15.6, 17.6, 18.2, 19.6, 21.8, 28.8, 28.9, 31.6, 35.4, 52.1, 123.2, 123.4, 123.9, 129.3, 155.0. Anal. Calcd for C18H24N2OS (300.46): C, 71.95; H, 8.05; N, 9.32; S,10.67%; Found: C, 72.20; H, 8.19; N, 9.02; S, 10.54%.
= −18.0 (c 0.25, MeOH), 1H-NMR (CDCl3) δ (ppm) 0.71–0.83 (2H, m), 0.98 (3H, s), 1.07 (3H, s), 1.22 (1H, dd, J = 4.5, 15.8 Hz), 1.28 (3H, s), 1.53–1.71 (2H, m), 1.89–2.01 (2H, m), 2.65 (1H, d, J = 3.4, 12.4 Hz), 3.29 (1H, d, J = 3.5, 11.7 Hz), 7.02–7.12 (3H, m), 7.28–7.34 (2H, m). 13C-NMR (CDCl3) δ (ppm) 15.6, 17.6, 18.2, 19.6, 21.8, 28.8, 28.9, 31.6, 35.4, 52.1, 123.2, 123.4, 123.9, 129.3, 155.0. Anal. Calcd for C18H24N2OS (300.46): C, 71.95; H, 8.05; N, 9.32; S,10.67%; Found: C, 72.20; H, 8.19; N, 9.02; S, 10.54%.
3.2.4. Alternative Procedure for the Synthesis of 2-Thioxo-1,3-oxazines 35 and 36
The respective amino alcohol 16 or 17 (1.18 mmol) in dry toluene solution (20 mL), TEA (3.54 mmol) and thiophosgene (0.181 mL, 2.36 mmol) was stirred at room temperature for 6 h. Next, the solvent was evaporated off under reduced pressure and the residue was purified by flash column chromatography on silica gel; elution with n-hexane/EtOAc (4:1) gave 2-thioxo-1,3-oxazine 35 or 36.
3.2.5. General Procedure for the Synthesis of 2-Imino-1,3-thiazines 42 and 43
Method A: The respective thiourea (1.25 mmol) 38 or 39 in THF solution (12 mL) and CDI (0.306 g, 1.88 mmol) was heated to reflux for 3 h. Next, the solvent was evaporated off under reduced pressure and the residue was purified by flash column chromatography on silica gel; elution with n-hexane/EtOAc (4:1) gave the pure 2-imino-1,3-thiazines 42 or 43.
Method B: A CEM Discover 10 mL vial containing the respective intermediates (0.63 mmol) 40 or 41 in THF solution (12 mL) was heated to reflux for 3 h. Next, the solvent was evaporated off under reduced pressure and the residue was purified by flash column chromatography on silica gel; elution with n-hexane/EtOAc (4:1) gave the pure 2-imino-1,3-thiazines 42 or 43.
(Hexahydrocyclopenta[d][1,3]thiazin-2-ylidene)phenylamine (42): 0.218 g (75%); mp 180–181 °C (Lit. [33] 180–181 °C), 1H-NMR (CDCl3) δ (ppm): 1.47–1.71 (3H, m), 1.74–2.10 (3H, m), 2.52–2.66 (1H, m), 2.77 (1H, dd, J = 9.3, 12.5 Hz), 2.92 (1H, dd, J = 4.5, 12.5 Hz), 3.81–3.93 (1H, m), 6.95–7.10 (3H, m), 7.26–7.34 (2H, m). 13C-NMR (CDCl3) δ (ppm): 24.0, 31.4, 31.7, 35.7, 39.7, 57.3, 122.5, 123.4, 129.2, 148.5, 157.6. Anal. Calcd for C13H16N2S (232.34): C, 67.20; H, 6.94; N, 12.06; S, 13.80%; Found: C, 67.47; H, 6.81; N, 12.30; S, 13.42%.
(Octahydrobenzo[d][1,3]thiazin-2-ylidene)phenylamine (43): 0.222 g (72%); mp 183–186 °C (Lit.[33] 187–188 °C), 1H-NMR (CDCl3) δ (ppm): 1.30–1.81 (7H, m), 1.83–1.97 (1H, m), 2.05–2.15 (1H, m), 2.81 (1H, dd, J = 4.8, 12.4 Hz), 3.12 (1H, dd, J = 4.1, 12.5 Hz), 3.57–3.65 (1H, m), 6.98–7.10 (3H, m), 7.21–7.30 (2H, m). 13C-NMR (CDCl3) δ (ppm): 21.0, 24.7, 26.4, 32.2, 32.6, 34.3, 52.5, 122.8, 123.5, 129.3, 146.1, 156.4. Anal. Calcd for C14H18N2S (246.34): C, 68.25; H, 7.36; N, 11.37%; Found: C, 68.31; H, 7.51; N, 11.02%.
3.2.6. Determination of Antiproliferative Activities
Antiproliferative effects against four human cancer cell lines were determined as published recently [36]. Briefly, HeLa (cervix adenocarcinoma), MCF7 (breast adenocarcinoma), A2780 (ovarian carcinoma) and A431 (skin epidermoid carcinoma; all cell lines purchased from ECACC; Salisbury, UK) cells were cultivated in minimal essential medium (Sigma-Aldrich, Budapest, Hungary) supplemented with 10% foetal bovine serum, 1% non-essential amino acids and an antibiotic-antimycotic mixture.
Near-confluent cells were seeded into a 96-well plate (5000 cells/well) and, after overnight standing, the medium (200 µL) containing the tested compound (at 10 or 30 µM) was added. Following a 72-h incubation in a humidified atmosphere of 5% CO2 at 37 °C, the living cells were assayed by the addition of 20 µL of 5 mg/mL MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] solution [37]. During a 4-h contact period, the MTT was converted by intact mitochondrial reductase and precipitated as blue crystals. The medium was then removed, the precipitated formazan crystals were solubilized in DMSO (100 µL) during a 60-min period of shaking at 25 °C, and the absorbance was read at 545 nm with a microplate reader. Wells with untreated cells were utilized as controls. All in vitro experiments were carried out on two microplates with at least five parallel wells. Stock solutions of the tested substances (10 mM) were prepared with DMSO. The DMSO concentration (0.3%) of the medium did not have any significant effect on cell proliferation. Cisplatin was used as reference compound.
3.2.7. X-ray Crystallographic Studies
Crystallographic data were collected at 123 K for 31c and 31d by using a Bruker Nonius-Kappa CCD diffractometer with an APEXII area detector and graphite-monochromatized Mo-Kα radiation (λ = 0.71073 Å), as reported earlier [38].
Crystal data for 31c: C21H25FN4O2S, Mr = 416.51, triclinic, space group P-1 (no. 2), a = 8.5831(5), b = 10.7338(6), c = 11.9244(5) Å, α = 86.414(3)°, β = 69.835(3)°, γ = 85.729(3)°, V = 1027.59(9) Å3, T = 123 K, Z = 2, μ(Mo-Kα) = 0.192 mm−1, 6132 unique reflections (Rint = 0.0211) which were used in calculations. The final R1 (for the data with F2 > 2δ(F2) was 0.0426 and wR2(F2) (all data) was 0.1034.
Crystal data for 31d: C22H28N4O3S, Mr = 428.54, monoclinic, space group C2/c (no. 15), a = 23.1162(6), b = 8.7978(3), c = 21.4232(5) Å, β =92.4232(5)°, V = 4352.8(2) Å3, T = 123 K, Z = 8, μ(Mo-Kα) 0.180 mm−1, 7979 unique reflections (Rint = 0.0279) which were used in calculations. The final R1 (for the data with F2 > 2δ(F2) was 0.0454 and wR2(F2) (all data) was 0.1063.
The structures were solved by direct methods by use of the SHELXS-97 program [39], and full-matrix, least-squares refinements on F2 were performed by use of the SHELXL-97 program [39]. The CH hydrogen atoms were included at fixed distances from their host atoms with the fixed displacement parameters. The NH hydrogen atom positions were refined with the fixed displacement parameters. In 31d, the methoxy C atom has two different orientations in a 1:1 ratio (due to packing reasons). The graphics were drawn with ORTEP3 for Windows [40]. The depositions numbered CCDC 1000179 (for 31c) and CCDC 1000178 (for 31d) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html (or from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif).
4. Conclusions
In conclusion, we have developed a mild and efficient method for the synthesis of 2-imino-1,3-thiazines by the ring closure of thiourea adducts of 1,3-amino alcohols in the presence of CDI under microwave condition. Besides the main product 1,3-thiazines, the formation of 2-thioxo-1,3-oxazines as side-products was observed. The ring-closure process was extended to cycloalkane-based γ-hydroxythioureas and the method developed for the synthesis of 2-imino-1,3-thiazines containing an acid-sensitive structure proved to be comparable to those reported in the literature. The resulting 1,3-thiazines exert marked antiproliferative action on a panel of human cancer cell lines.
Acknowledgments
We are grateful for financial support from the Hungarian Research Foundation (OTKA NK81371, K112442 and K109293) and from the European Union for cofunding by the European Social Fund (TÁMOP-4.2.2/A-11/1/KONV-2012-0035).
Author Contributions
The listed authors contributed to this work as described in the following. FF designed, planed research and interpreted the results. ZS planed research, interpreted the results and carried out of the synthetic work. IZ performed and interpreted the biological experiments. RS performed X-ray experiments. All authors discussed the results, prepared and commented on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lait, S.M.; Rankic, D.A.; Keay, B.A. 1,3-Amino alcohols and their derivatives in asymmetric organic synthesis. Chem. Rev. 2007, 107, 767–796. [Google Scholar]
- Lázár, L.; Fülöp, F. 1,3-Oxazines and their benzo derivatives. In Comprehensive Heterocyclic Chemistry. III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; pp. 373–459. [Google Scholar]
- Szakonyi, Z.; Fülöp, F. Monoterpene-based chiral β-amino acid derivatives prepared from natural sources: Syntheses and applications. Amino Acids 2011, 41, 597–608. [Google Scholar]
- Andrés, C.; Gonzáles, I.; Nieto, J.; Rosón, C.D. Lewis acid mediated diastereoselective keto-ene cyclization on chiral perhydro-1,3-benzoxazines: Synthesis of enantiopure cis-3,4-disubstituted 3-hydroxypyrrolidines. Tetrahedron 2009, 65, 9728–9736. [Google Scholar]
- Andrés, C.; Infante, R.; Nieto, J. Perhydro-1,3-benzoxazines derived from (−)-8-aminomenthol as ligands for the catalytic enantioselective addition of diethylzinc to aldehydes. Tetrahedron: Asymmetry 2010, 21, 2230–2237. [Google Scholar]
- Jaworska, M.; Blocka, E.; Kozakiewicz, A.; Welniak, M. α-Pinene-type chiral schiff bases as tridentate ligands in asymmetric addition reactions. Tetrahedron: Asymmetry 2011, 22, 648–657. [Google Scholar]
- Evans, P.A.; Brandt, T.A. Enantioselective allylic substitution using a novel (phosphino-1,3-oxazine)palladium catalyst. Tetrahedron Lett. 1996, 37, 9143–9146. [Google Scholar]
- Evans, P.A.; Brandt, T.A. Enantioselective palladium-catalyzed allylic alkylation using E- and Z-vinylogous sulfonates. Org. Lett. 1999, 1563–1565. [Google Scholar]
- Szakonyi, Z.; Balázs, Á.; Martinek, T.A.; Fülöp, F. Enantioselective addition of diethylzinc to aldehydes catalyzed by γ-amino alcohols derived from (+)- and (−)-α-pinene. Tetrahedron: Asymmetry 2006, 17, 199–204. [Google Scholar]
- Li, X.; Lou, R.; Yeung, C.-H.; Chan, A.S.C.; Wong, W.K. Asymmetric hydrogenation of dehydroamino acid derivatives catalyzed by a new aminophosphine phosphinite ligand derived from ketopinic acid. Tetrahedron: Asymmetry 2000, 11, 2077–2082. [Google Scholar]
- De las Casas Engel, T.; Maroto, B.L.; García Martínez, A.; de la Moya Cerero, S. N/N/O versus N/O/O and N/O amino isoborneols in the enantioselective ethylation of benzaldehyde. Tetrahedron: Asymmetry 2008, 19, 269–272. [Google Scholar]
- Sánches-Carnerero, E.M.; de las Casas Engel, T.; Maroto, B.L.; de la Moya Cerero, S. Polyoxygenated ketopinic-acid-derived γ-amino alcohols in the enantioselective diethylzinc addition to benzaldehyde. Tetrahedron: Asymmetry 2009, 20, 2655–2657. [Google Scholar]
- Szakonyi, Z.; Martinek, T.A.; Hetényi, A.; Fülöp, F. Synthesis and transformations of enantiomeric 1,2-disubstituted monoterpene derivatives. Tetrahedron: Asymmetry 2000, 11, 4571–4579. [Google Scholar]
- Koneva, E.A.; Volcho, K.P.; Korchagina, D.V.; Komarova, N.I.; Kochnev, A.I.; Salakhutdinov, N.F.; Tolstikov, A.G. New chiral Schiff bases derived from (+)- and (−)-α-pinenes in the metal complex catalyzed asymmetric oxidation of sulfides. Russ. Chem. Bull. 2008, 57, 108–117. [Google Scholar]
- Koneva, E.A.; Volcho, K.P.; Korchagina, D.V.; Salakhutdinov, N.F.; Tolstikov, G.A. Synthesis of new chiral schiff bases from (+)-3-carene and their use in asymmetric oxidation of sulfides catalyzed by metal complexes. Russ. J. Org. Chem. 2009, 815–824. [Google Scholar]
- Koneva, E.A.; Suslov, E.V.; Korchagina, D.V.; Genaev, A.M.; Volcho, K.P.; Salakhutdinov, N.F. Catalytic asymmetric addition of diethylzinc to benzaldehyde using α-pinene-derived ligands. Open Catal. J. 2011, 4, 107–112. [Google Scholar]
- Koneva, E.A.; Khomenko, T.M.; Kurbakova, S.Y.; Komarova, N.I.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F.; Tolstikov, A.G.; Tolstikov, G.A. Synthesis of optically active omeprazole by catalysis with vanadyl complexes with chiral Schiff bases. Russ. Chem. Bull. Int. Ed. 2008, 57, 1680–1685. [Google Scholar]
- Koneva, E.A.; Korchagina, D.V.; Gatilov, Y.V.; Genaev, A.M.; Krysin, A.P.; Volcho, K.P.; Tolstikov, A.G.; Salakhutdinov, N.F. New chiral ligands based on (+)-α-pinene. Russ. J. Org. Chem. 2010, 46, 1109–1115. [Google Scholar]
- Fülöp, F.; Bernáth, G.; Pihlaja, K. Synthesis, stereochemistry and transformations of cyclopentane-, cyclohexane-, cycloheptane-, and cyclooctane-fused 1,3-oxazines, 1,3-thiazines, and pyrimidines. Adv. Heterocycl. Chem. 1998, 69, 349–477. [Google Scholar]
- Xu, X.; Qian, X.; Li, Z.; Song, G.; Chen, W. Synthesis and fungicidal activity of fluorine-containing phenylimino-thiazolidines derivatives. J. Fluorine Chem. 2005, 126, 297–300. [Google Scholar]
- Woltering, T.J.; Wostl, W.; Hilpert, H.; Rogers-Evans, M.; Pinard, E.; Maywega, A.; Göbel, M.; Banner, D.W.; Benz, J.; Travagli, M.; et al. BACE1 inhibitors: A head group scan on a series of amides. Bioorg. Med. Chem. Lett. 2013, 23, 4239–4243. [Google Scholar]
- Kai, H.; Morioka, Y.; Murashi, T.; Morita, K.; Shinonome, S.; Nakazato, H.; Kawamoto, K.; Hanasaki, K.; Takahashi, F.; Mihara, S.; et al. 2-Arylimino-5,6-dihydro-4H-1,3-thiazines as a new class of cannabinoid receptor agonists. Part 1: Discovery of CB2 receptor selective compounds. Bioorg. Med. Chem. Lett. 2007, 17, 4030–4034. [Google Scholar]
- Kai, H.; Morioka, Y.; Tomida, M.; Takahashi, T.; Hattori, M.; Hanasaki, K.; Koike, K.; Chiba, H.; Shinohara, S.; Kanemasa, T.; et al. 2-Arylimino-5,6-dihydro-4H-1,3-thiazines as a new class of cannabinoid receptor agonists. Part 2: Orally bioavailable compounds. Bioorg. Med. Chem. Lett. 2007, 17, 3925–3929. [Google Scholar]
- Kai, H.; Morioka, Y.; Koriyama, Y.; Okamoto, K.; Hasegawa, Y.; Hattori, M.; Koike, K.; Chiba, H.; Shinohara, S.; Iwamoto, Y.; et al. 2-Arylimino-5,6-dihydro-4H-1,3-thiazines as a new class of cannabinoid receptor agonists. Part 3: Synthesis and activity of isosteric analogs. Bioorg. Med. Chem. Lett. 2008, 18, 6444–6447. [Google Scholar]
- Blokhina, S.V.; Volkova, T.V.; Ol’khovich, M.V.; Sharapova, A.V.; Proshin, A.N.; Bachurin, S.O.; Perlovich, G.L. Synthesis, biological activity, distribution and membrane permeability of novel spiro-thiazines as potent neuroprotectors. Eur. J. Med. Chem. 2014, 77, 8–17. [Google Scholar]
- Szakonyi, Z.; Fülöp, F. Mild and efficient ring opening of monoterpene-fused β-lactam enantiomers. Synthesis of novel β-amino acid derivatives. Arkivoc 2003, 2003, 225–232. [Google Scholar]
- Gyónfalvi, S.; Szakonyi, Z.; Fülöp, F. Synthesis and transformation of novel cyclic β-amino acid derivatives from (+)-3-carene. Tetrahedron: Asymmetry 2003, 14, 3965–3972. [Google Scholar]
- Szakonyi, Z.; Martinek, T.A.; Sillanpää, R.; Fülöp, F. Regio- and stereoselective synthesis of constrained enantiomeric β-amino acid derivatives. Tetrahedron: Asymmetry 2008, 19, 2296–2303. [Google Scholar]
- Fülöp, F.; Szakonyi, Z. Chiral cyclic β-amino acids and their derivates, pharmaceutical compositions containing them and the use of such compounds. WO2008059299 A1, 22 May 2008. [Google Scholar]
- Szakonyi, Z.; Balázs, Á.; Martinek, T.A.; Fülöp, F. Stereoselective synthesis of pinane-based β- and γ-amino acids via conjugate addition of lithium amides and nitromethane. Tetrahedron: Asymmetry 2010, 21, 2498–2504. [Google Scholar]
- Fülöp, F.; Szakonyi, Z.; Pallai, P.V. 1,3-Heterocycles Condensed with Monoterpene Skeleton, Their Use and Pharmaceutical Compositions Comprising Such Compounds. WO 2010070365 A1, 24 June 2010. [Google Scholar]
- Sohár, P.; Stájer, G.; Szabó, A.; Fülöp, F.; Szúnyog, J.; Bernáth, G. Stereochemical studies. Part 89. Saturated heterocycles. Part 84. Preparation and nuclear magnetic resonance study of norbornane-norbornene-fused 2-phenylimino-1,3-oxazines and -thiazines. J. Chem. Soc. Perkin Trans. 2 1987. [Google Scholar] [CrossRef]
- Fülöp, F; Csirinyi, G.; Bernáth, G. Saturated heterocycles, 135. Cyclic amino alcohols and related compounds, 29. Synthesis of condensed-skeleton cis- and trans-2-phenylimino- and 2-methyliminotetrahydro-1,3-thiazines and 1,3-oxazines. Acta Chim. Hung. 1988, 125, 193–199. [Google Scholar]
- Kim, T.H.; Cha, M.-H. Efficient synthesis of 2-methylaminothiazolines via Mitsunobu reaction of N-(2-hydroxyethyl)-N'-methyl-thioureas. Tetrahedron Lett. 1999, 40, 3125–3128. [Google Scholar]
- Bernacki, A.L.; Zhu, L.; Hennings, D.D. A selective and convenient method for the synthesis of 2-phenylaminothiazolines. Org. Lett. 2010, 12, 5526–5529. [Google Scholar]
- Berényi, A.; Minorics, R.; Iványi, Z.; Ocsovszki, I.; Ducza, E.; Thole, H.; Messinger, J.; Wölfling, J.; Mótyán, G.; Mernyák, E.; et al. Synthesis and investigation of the anticancer effects of estrone-16-oxime ethers in vitro. Steroids 2013, 78, 69–78. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 6, 55–63. [Google Scholar]
- Kanizsai, I.; Szakonyi, Z.; Sillanpää, R.; D’Hooghe, M.; de Kimpe, N.; Fülöp, F. Synthesis of chiral 1,5-disubstituted pyrrolidinones via electrophile-induced cyclization of 2-(3-butenyl)oxazolines derived from (1R,2S)- and (1S,2R)-norephedrine. Tetrahedron: Asymmetry 2006, 17, 2857–2863. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).