Synthesis and Structure-Activity Relationships of Novel Amino/Nitro Substituted 3-Arylcoumarins as Antibacterial Agents
Abstract
:1. Introduction
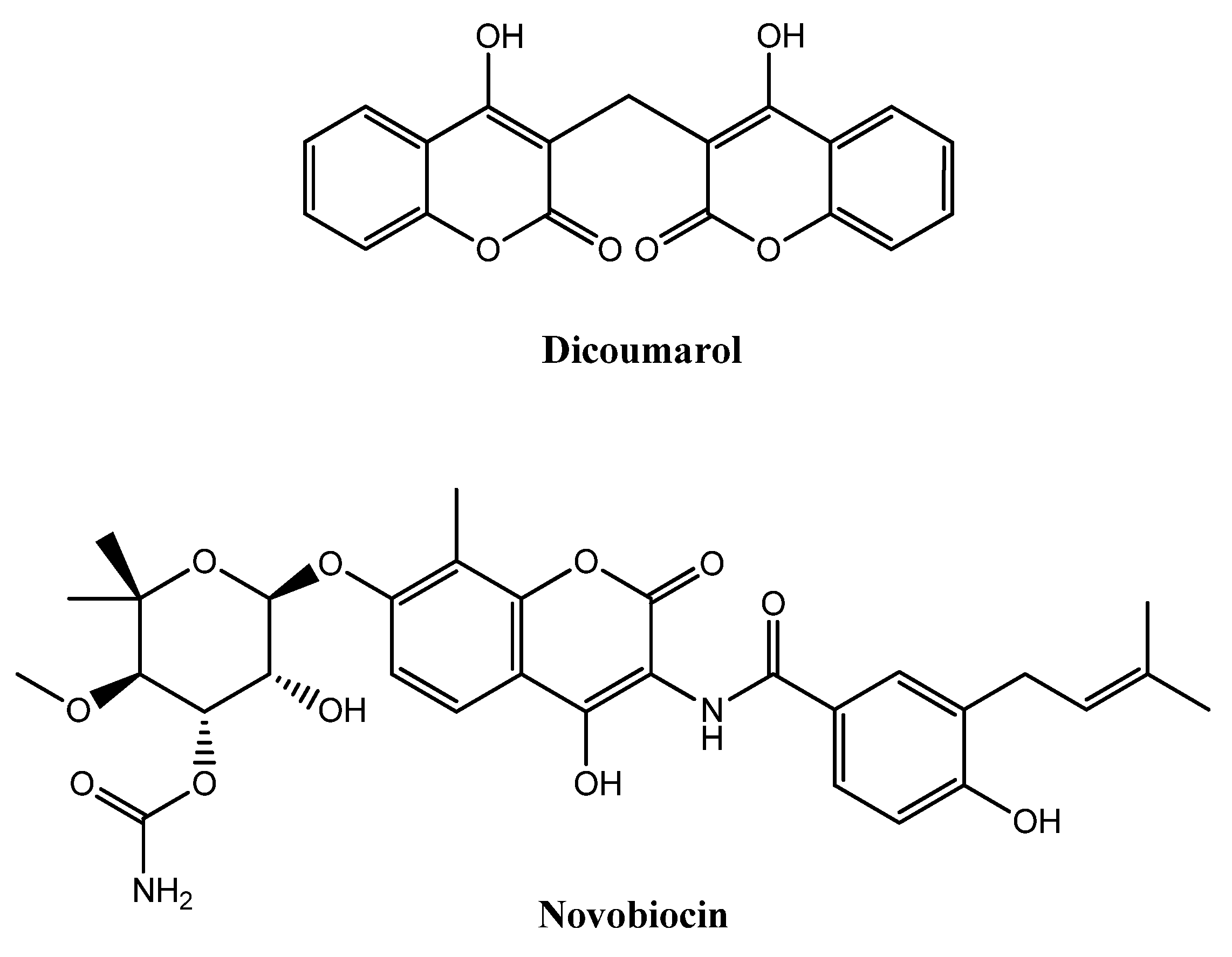
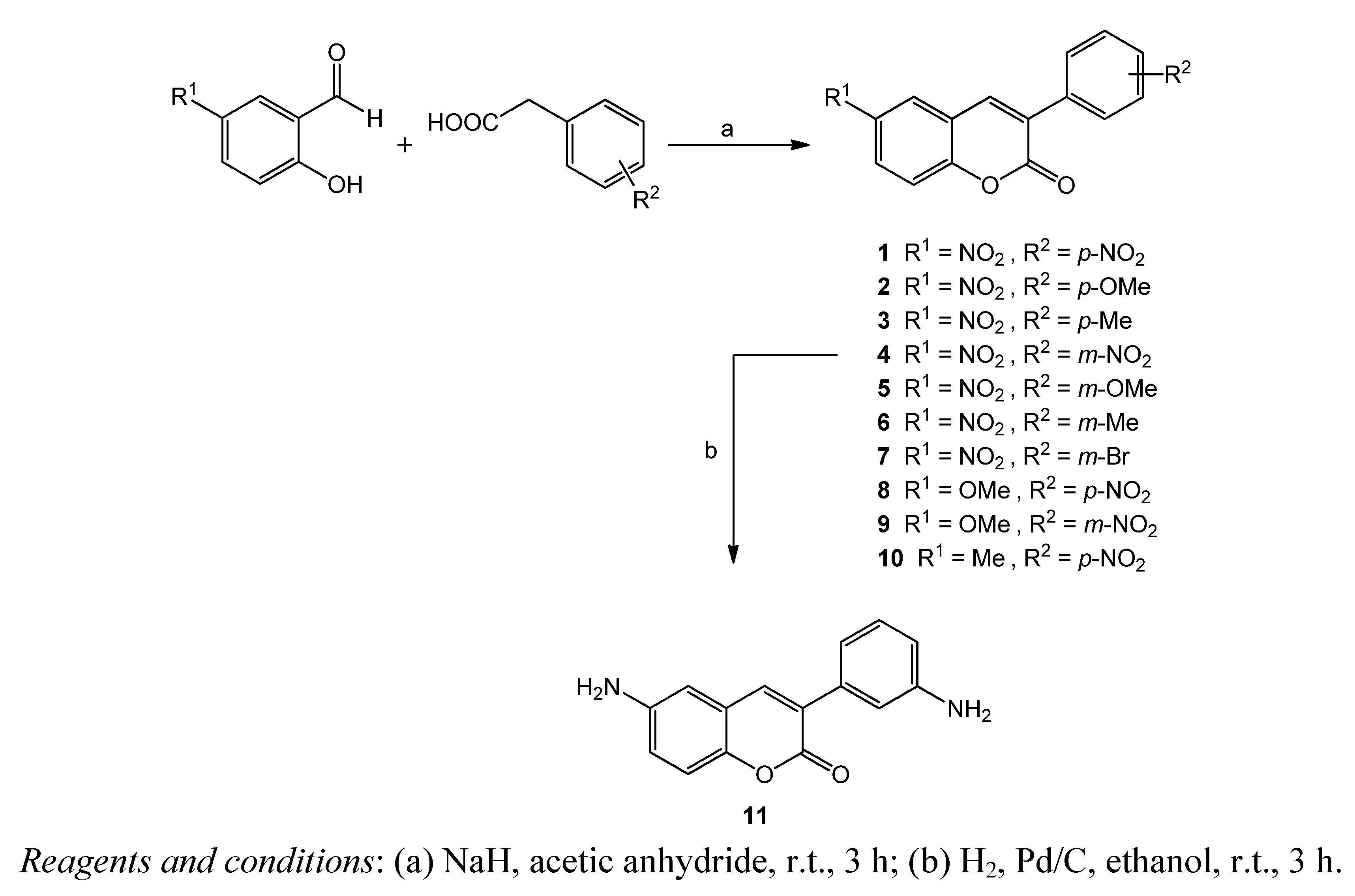
2. Results and Discussion
| Compounds | Method A (mm) | Method B (µg/mL) | ||
|---|---|---|---|---|
| S. aureus | E. coli | S. aureus | E. coli | |
| 1 | 19 | NA | 128 | - |
| 2 | 25 | NA | 64 | - |
| 3 | 22 | NA | 32 | - |
| 4 | 22 | NA | 32 | - |
| 5 | 25 | NA | 64 | - |
| 6 | 32 | NA | 8 | - |
| 7 | 14 | NA | 256 | - |
| 8 | NA | NA | >512 | - |
| 9 | NA | NA | >512 | - |
| 10 | NA | NA | >512 | - |
| 11 | 18 | NA | 128 | - |
| Oxolinic acid | 26 | 31 | 2 | <1 |
| Ampicillin | 32 | 23 | 2 | 8 |
| Compounds | LogP |
|---|---|
| 1 | 3,631 |
| 2 | 3,729 |
| 3 | 4,120 |
| 4 | 3,607 |
| 5 | 3,705 |
| 6 | 4,096 |
| 7 | 4,457 |
| 8 | 3,729 |
| 9 | 3,705 |
| 10 | 4,120 |
| 11 | 1,841 |
3. Experimental
3.1. Chemistry
Procedure for the Preparation of 3-(3′-Aminophenyl)-6-Aminocoumarin (11)
4. Conclusions
Acknowledgments
References
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Uriarte, E.; Santana, L. Simple coumarins: Privileged scaffolds in medicinal chemistry. Front. Med. Chem. 2009, 4, 23–85. [Google Scholar]
- Chilin, A.; Battistutta, R.; Bortolato, A.; Cozza, G.; Zanatta, S.; Poletto, G.; Mazzorana, M.; Zagotto, G.; Uriarte, E.; Guiotto, A.; et al. Coumarin as Attractive Casein Kinase 2 (CK2) Inhibitor scaffold: An integrate approach to elucidate the putative binding motif and explain structure–activity relationships. J. Med. Chem. 2008, 51, 752–759. [Google Scholar]
- Hooper, D.C.; Wilfson, J.S.; McHugh, G.L.; Winters, M.B.; Swartz, M.N. In vitro activity of DNA gyrase inhibitors, singly and in combination, against Mycobacterium avium complex. Antimicrob. Agents Chemother. 1982, 22, 662–671. [Google Scholar] [CrossRef]
- Ostrov, D.A.; Hernández Prada, J.A.; Corsino, P.E.; Finton, K.A.; Le, N.; Rowe, T.C. Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Antimicrob. Agents Chemother. 2007, 51, 3688–3698. [Google Scholar] [CrossRef]
- Chimenti, F.; Bizzarri, B.; Bolasco, A.; Secci, D.; Chimenti, P.; Granese, A.; Carradori, S.; Rivanera, D.; Zicari, A.; Scaltrito, M.M.; et al. Synthesis, Selective anti-Helicobacter pylori activity, And cytotoxicity of novel N-substituted-2-oxo-2H-1-benzopyran-3-carboxamides. Bioorg. Med. Chem. Lett. 2010, 20, 4922–4926. [Google Scholar] [CrossRef]
- Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Fuentes-Edfuf, C.; Santos, Y.; Muñoz-Crego, A. Looking for new targets: Simple coumarins as antibacterial agents. Med. Chem. 2012, 8, 1140–1145. [Google Scholar]
- Vilar, S.; Quezada, E.; Santana, L.; Uriarte, E.; Yanez, M.; Fraiz, N.; Alcaide, C.; Cano, E.; Orallo, F. Design, Synthesis, and Vasorelaxant and platelet antiaggregatory activities of coumarin-resveratrol hybrids. Bioorg. Med. Chem. Lett. 2006, 16, 257–261. [Google Scholar] [CrossRef]
- Riveiro, M.E.; Moglioni, A.; Vazquez, R.; Gomez, N.; Facorro, G.; Piehl, L.; de Celis, E.R.; Shayo, C.; Davio, C. Structural insights into hydroxycoumarin-induced apoptosis in U-937 cells. Bioorg. Med. Chem. 2008, 16, 2665–2675. [Google Scholar]
- Belluti, F.; Fontana, G.; Bo, L.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, Synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg. Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef]
- Roussaki, M.; Kontogiorgis, C.; Hadjipavlou-Litina, D.J.; Hamilakis, S. A novel synthesis of 3-aryl coumarins and evaluation of their antioxidant and lipoxygenase inhibitory activity. Bioorg. Med. Chem. Lett. 2010, 20, 3889–3892. [Google Scholar] [CrossRef]
- Fais, A.; Corda, M.; Era, B.; Fadda, M.B.; Matos, M.J.; Quezada, E.; Santana, L.; Picciau, C.; Podda, G.; Delogu, G. Tyrosinase inhibitor activity of coumarin-resveratrol hybrids. Molecules 2009, 14, 2514–2520. [Google Scholar] [CrossRef]
- Matos, M.J.; Viña, D.; Quezada, E.; Picciau, C.; Delogu, G.; Orallo, F.; Santana, L.; Uriarte, E. A New series of 3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 3268–3270. [Google Scholar] [CrossRef]
- Matos, M.J.; Viña, D.; Janeiro, P.; Borges, F.; Santana, L.; Uriarte, E. New halogenated 3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 5157–5160. [Google Scholar] [CrossRef]
- Matos, M.J.; Terán, C.; Pérez-Castillo, Y.; Uriarte, E.; Santana, L.; Viña, D. Synthesis and study of a series of 3-arylcoumarins as potent and selective monoamine oxidase B inhibitors. J. Med. Chem. 2011, 54, 7127–7137. [Google Scholar] [CrossRef]
- Matos, M.J.; Viña, D.; Picciau, C.; Orallo, F.; Santana, L.; Uriarte, E. Synthesis and evaluation of 6-methyl-3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5053–5055. [Google Scholar] [CrossRef]
- Delogu, G.; Picciau, C.; Ferino, G.; Quezada, E.; Podda, G.; Uriarte, E.; Viña, D. Synthesis, Human monoamine oxidase inhibitory activity and molecular docking studies of 3-heteroarylcoumarin derivatives. Eur. J. Med. Chem. 2011, 49, 1147–1152. [Google Scholar]
- Goth, A. The antibacterial properties of dicumarol. Science 1945, 101, 383. [Google Scholar]
- Hoettecke, N.; Rotzoll, S.; Albrecht, U.; Lalk, M.; Fischer, C.; Langer, P. Synthesis and antimicrobial activity of 2-alkenylchroman-4-ones, 2-alkenylthiochroman-4-ones and 2-alkenylquinol-4-ones. Bioorg. Med. Chem. 2008, 16, 10319–10325. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, C. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 21, 956–960. [Google Scholar] [CrossRef]
- Patil, S.A.; Unki, S.N.; Kulkarni, A.D.; Naik, V.H.; Badami, P.S. Synthesis, Characterization, In vitro antimicrobial and DNA cleavage studies of Co(II), Ni(II) and Cu(II) complexes with ONOO donor coumarin Schiff bases. J. Mol. Struct. 2011, 985, 330–338. [Google Scholar] [CrossRef]
- Sabry, N.M.; Mohamed, H.M.; Khattab, E.S.A.E.H.; Motlaq, S.S.; El-Agrody, A.M. Synthesis of 4H-chromene, Coumarin, 12H-chromeno[2,3-d]pyrimidine derivatives and some of their antimicrobial and cytotoxicity activities. Eur. J. Med. Chem. 2011, 46, 765–772. [Google Scholar] [CrossRef]
- Kawase, M.; Varu, B.; Shah, A.; Motohashi, M.; Tani, S.; Saito, S. Antimicrobial activity of new coumarin derivatives. Arzneimittelforschung 2001, 51, 67–71. [Google Scholar]
- Patel, D.; Kumari, P.; Patel, N. Synthesis, characterization and biological evaluation of some thiazolidinone derivatives as antimicrobial agents. J. Chem. Pharm. Res. 2010, 2, 84–91. [Google Scholar]
- Patel, D.; Kumari, P.; Patel, N. Synthesis and characterization of some new thiazolidinones containing coumarin moiety and their antimicrobial study. Arch. Appl. Sci. Res. 2010, 2, 68–75. [Google Scholar]
- Laurin, P.; Ferroud, D.; Klich, M.; Dupuis-Hamelin, C.; Mauvais, P.; Lassaigne, P.; Bonnefoy, A.; Musicki, B. Synthesis and in vitro evaluation of novel highly potent coumarin inhibitors of gyrase B. Bioorg. Med. Chem. Lett. 1999, 9, 2079–2084. [Google Scholar] [CrossRef]
- Nathan, C. Antibiotics at the crossroads. Nature 2004, 431, 899–902. [Google Scholar] [CrossRef]
- Rachakonda, S.; Cartee, L. Challenges in antimicrobial drug discovery and the potential of nucleoside antibiotics. Curr. Med. Chem. 2004, 11, 775–793. [Google Scholar] [CrossRef]
- Walsh, C. Antibiotics, Actions, Origins, Resistance; ASM: Washington, DC, USA, 2003. [Google Scholar]
- Von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Häbich, D. Antibacterial natural products in medicinal chemistry - exodus or revival? Angew. Chem. Int. Ed. 2006, 45, 5072–5129. [Google Scholar] [CrossRef]
- Matos, M.J.; Delogu, G.; Podda, G.; Santana, L.; Uriarte, E. Regioselective synthesis of bromo-substituted 3-arylcoumarins. Synthesis 2010, 16, 2763–2766. [Google Scholar]
- Matos, M.J.; Vazquez-Rodriguez, S.; Borges, F.; Santana, L.; Uriarte, E. Synthesis of 3-arylcoumarins via Suzuki-cross-coupling reactions of 3-chlorocoumarin. Tetrahedron Lett. 2011, 72, 1225–1227. [Google Scholar]
- Krishnaswamy, N.R.; Seshadri, T.R.; Sharma, B.R. Nitro derivatives of 3-phenylcoumarins. Ind. J. Chem. 1969, 7, 49–55. [Google Scholar]
- Rao, D.V.; Sayigh, A.R.; Ulrich, H. Aminophenyl alkoxy coumarins. US 3676436 A, 11 July 1972. [Google Scholar]
- Kremky, E. Chromatographic method for the separation and identification of some coumarin derivatives. Organika 1979, 159–163. [Google Scholar]
- Piazzi, L.; Cavalli, A.; Belluti, F.; Bisi, A.; Gobbi, S.; Rizzo, S.; Bartolini, M.; Andrisano, V.; Recanatini, M.; Rampa, A. Extensive SAR and computational studies of 3-{4-[(benzylmethylamino)methyl]phenyl}-6,7-dimethoxy-2H-2-chromenone (AP2238) Derivatives. J. Med. Chem. 2007, 50, 4250–4254. [Google Scholar] [CrossRef]
- M cheminformatics, Bratislava, Slovak Republic. Available online: http://www.molinspiration.com/services/properties.html (accessed on 14 December 2009).
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Fuentes-Edfuf, C.; Santos, Y.; Muñoz-Crego, A. Synthesis and Structure-Activity Relationships of Novel Amino/Nitro Substituted 3-Arylcoumarins as Antibacterial Agents. Molecules 2013, 18, 1394-1404. https://doi.org/10.3390/molecules18021394
Matos MJ, Vazquez-Rodriguez S, Santana L, Uriarte E, Fuentes-Edfuf C, Santos Y, Muñoz-Crego A. Synthesis and Structure-Activity Relationships of Novel Amino/Nitro Substituted 3-Arylcoumarins as Antibacterial Agents. Molecules. 2013; 18(2):1394-1404. https://doi.org/10.3390/molecules18021394
Chicago/Turabian StyleMatos, Maria J., Saleta Vazquez-Rodriguez, Lourdes Santana, Eugenio Uriarte, Cristina Fuentes-Edfuf, Ysabel Santos, and Angeles Muñoz-Crego. 2013. "Synthesis and Structure-Activity Relationships of Novel Amino/Nitro Substituted 3-Arylcoumarins as Antibacterial Agents" Molecules 18, no. 2: 1394-1404. https://doi.org/10.3390/molecules18021394
APA StyleMatos, M. J., Vazquez-Rodriguez, S., Santana, L., Uriarte, E., Fuentes-Edfuf, C., Santos, Y., & Muñoz-Crego, A. (2013). Synthesis and Structure-Activity Relationships of Novel Amino/Nitro Substituted 3-Arylcoumarins as Antibacterial Agents. Molecules, 18(2), 1394-1404. https://doi.org/10.3390/molecules18021394






