Synthesis and Conformation of Substituted Chiral Binaphthyl-Azobenzene Cyclic Dyads with Chiroptical Switching Capabilities
Abstract
:1. Introduction

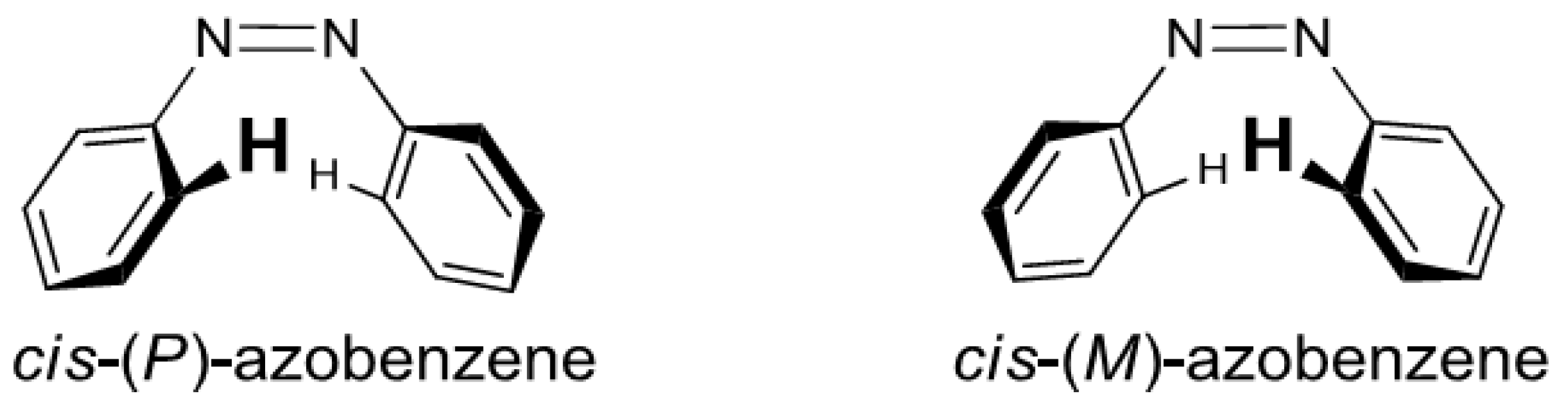
2. Results and Discussion
2.1. Binaphthyl-Azobenzene Cyclic Dyads
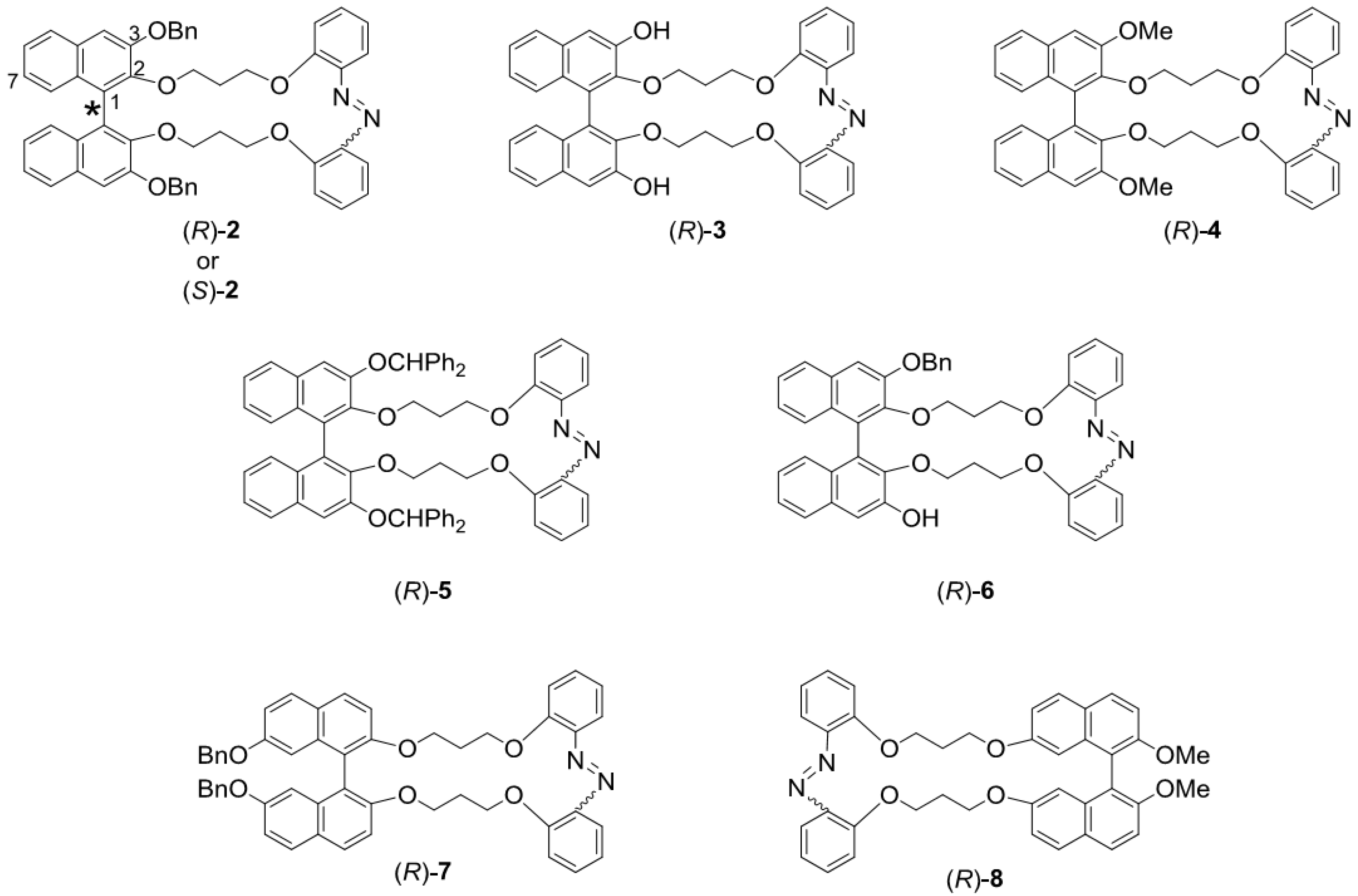
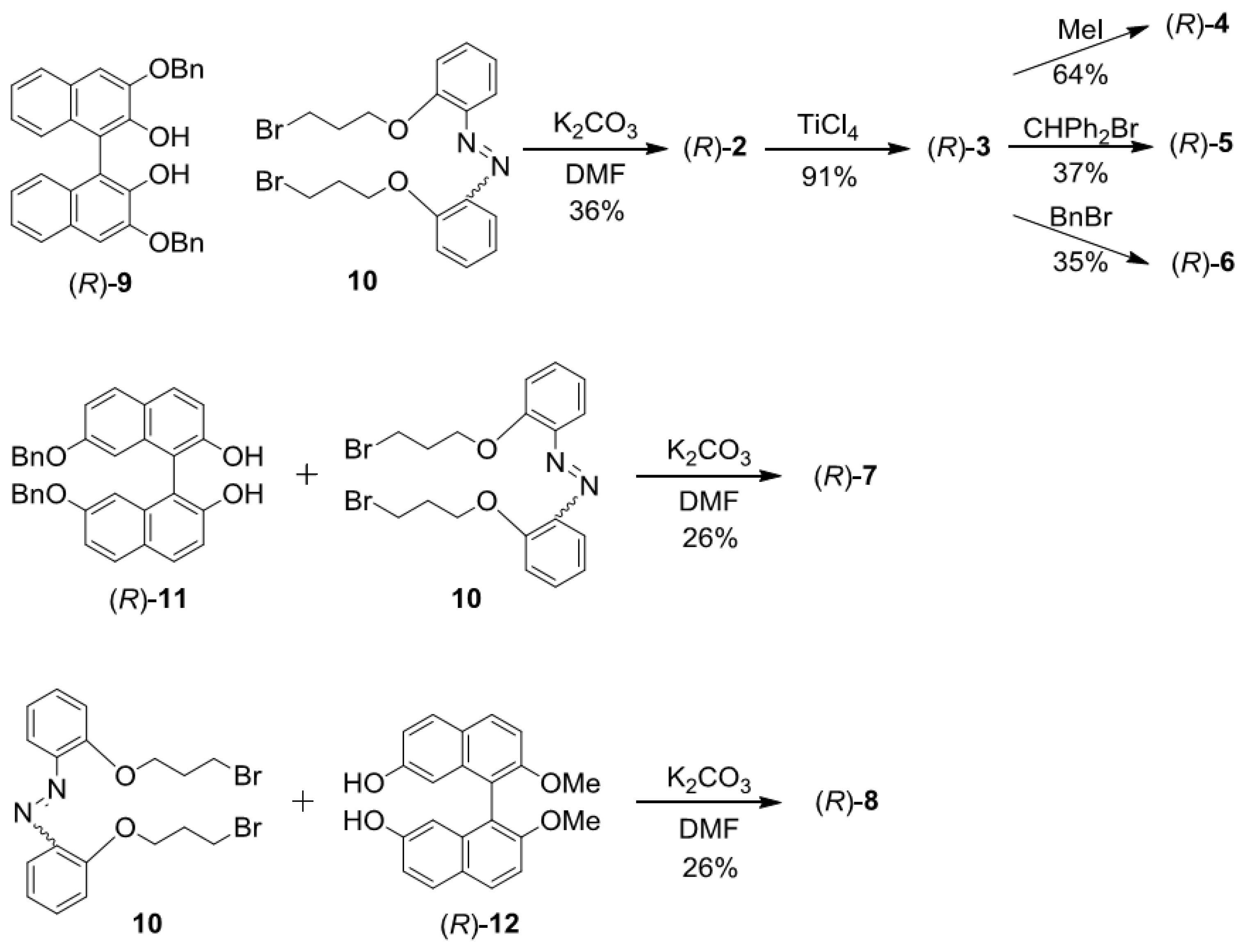
2.2. Synthesis of 2–8
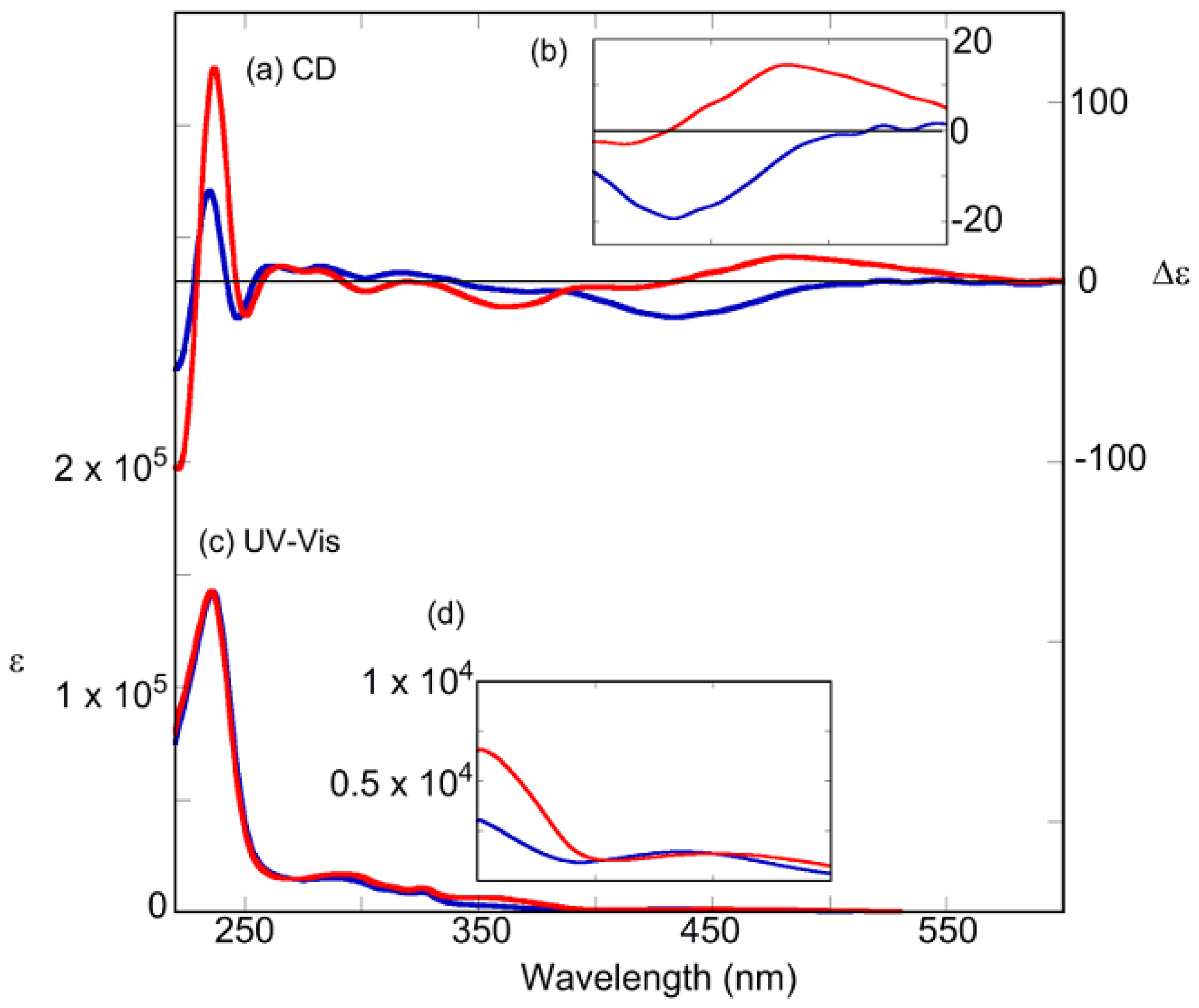
2.3. Photoswitching of Absorption, CD, and NMR Spectra
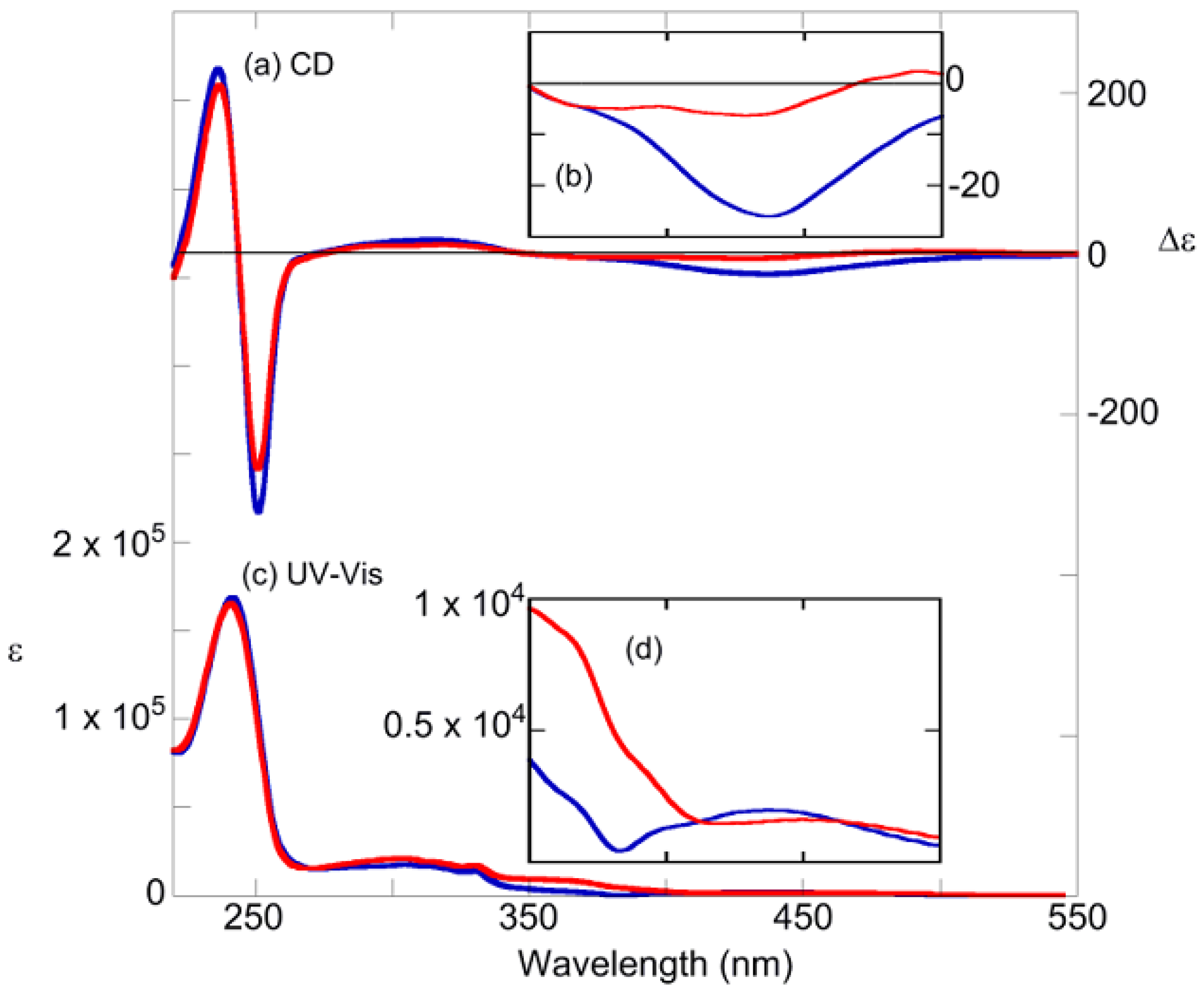
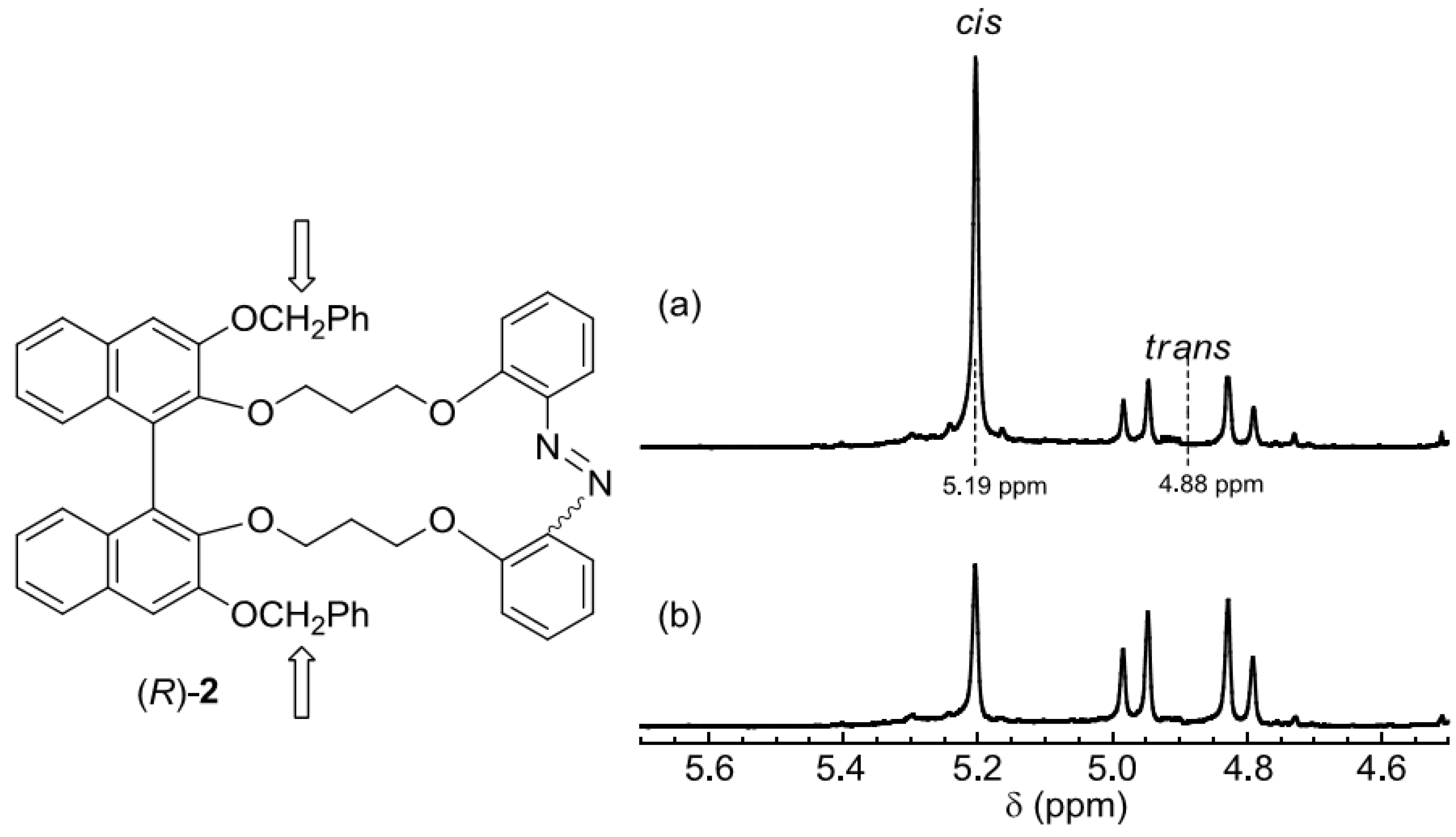
2.4. Photoswiching of Optical Rotation
| Compound | [α]D after 365 nm irradiation (deg) | [α]D after 436 nm irradiation (deg) | cis:trans after 365 nm irradiation | cis:trans after 436 nm irradiation |
|---|---|---|---|---|
| (R)-2 | −314 | +642 | 71:29 | 31:69 |
| (R)-3 | −427 | −490 | 65:35 | 31:69 |
| (R)-4 | −370 | +248 | 72:28 | 22:78 |
| (R)-5 | −252 | +278 | 69:31 | 35:65 |
| (R)-6 | −415 | −285 | 68:32 | 29:71 |
| (R)-7 | −544 | −7 | 80:20 | 20:80 |
| (R)-8 | +358 | +282 | 80:20 | 26:74 |
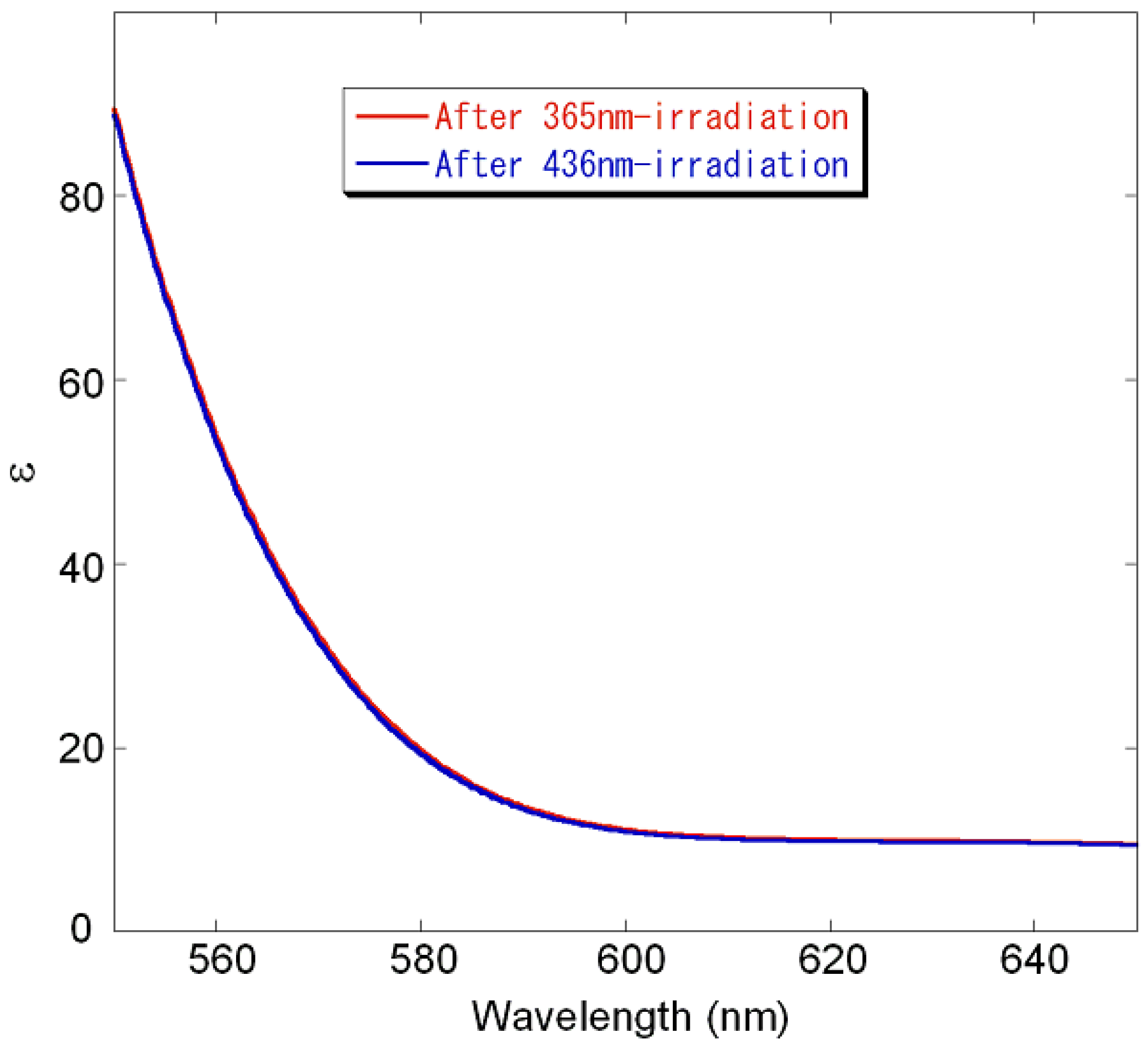
2.5. Thermodynamic Parameters of Trans to cis Isomerization Process
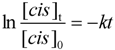
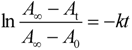
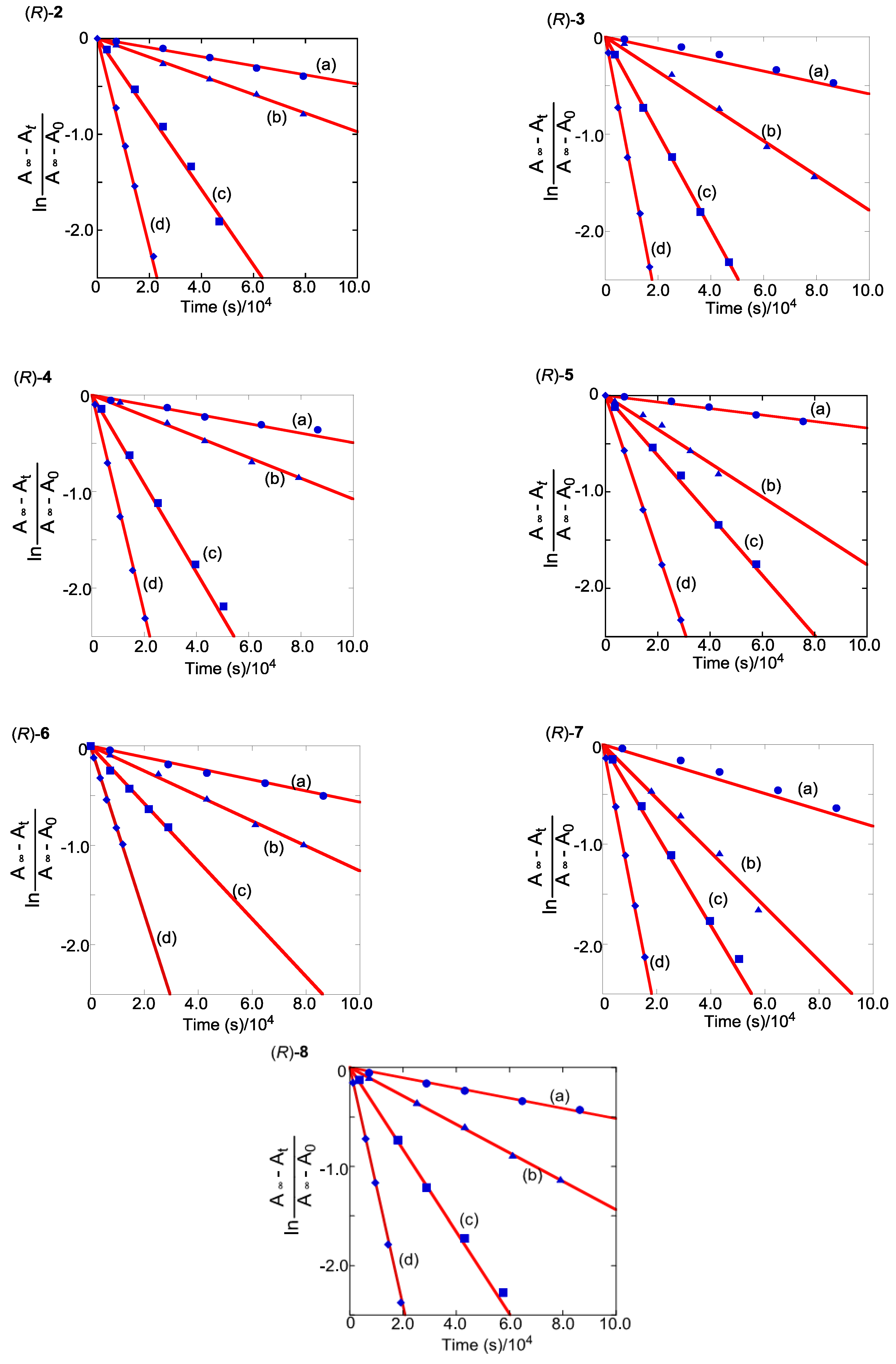
| Compound | k at 35 °C | k at 45 °C | k at 55 °C | k at 65 °C |
|---|---|---|---|---|
| (R)-2 | 4.7 × 10−6 | 9.8 × 10−6 | 4.0 × 10−5 | 1.1 × 10−4 |
| (R)-3 | 5.9 × 10−6 | 1.8 × 10−5 | 5.0 × 10−5 | 1.4 × 10−4 |
| (R)-4 | 5.0 × 10−6 | 1.1 × 10−5 | 4.6 × 10−5 | 1.1 × 10−4 |
| (R)-5 | 3.4 × 10−6 | 1.8 × 10−5 | 3.1 × 10−5 | 8.1 × 10−5 |
| (R)-6 | 5.7 × 10−6 | 1.3 × 10−5 | 2.9 × 10−5 | 8.5 × 10−5 |
| (R)-7 | 8.2 × 10−6 | 2.7 × 10−5 | 4.5 × 10−5 | 1.4 × 10−4 |
| (R)-8 | 5.2 × 10−6 | 1.4 × 10−5 | 4.2 × 10−5 | 1.2 × 10−4 |

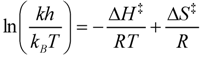
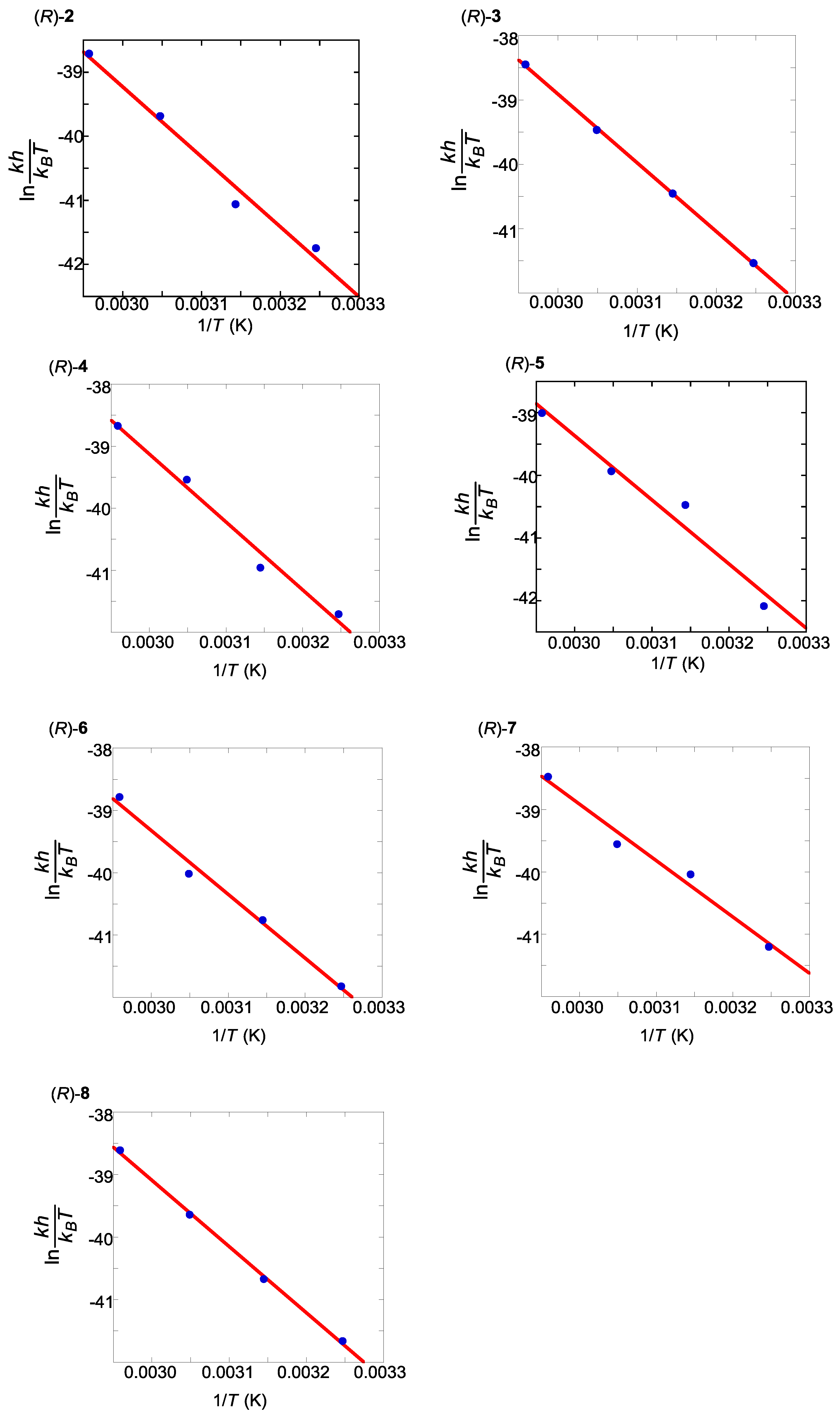
| Compound | ΔH‡ (kcal/mol) | ΔS‡ (cal/mol·K) | t1/2 at 298 K (h) |
|---|---|---|---|
| (R)-2 | 22 | −13 | 157 |
| (R)-3 | 21 | −14 | 120 |
| (R)-4 | 22 | −13 | 163 |
| (R)-5 | 20 | −17 | 148 |
| (R)-6 | 18 | −25 | 101 |
| (R)-7 | 18 | −23 | 61 |
| (R)-8 | 21 | −15 | 128 |
2.6. Helical Chirality of cis-Azobenzenes
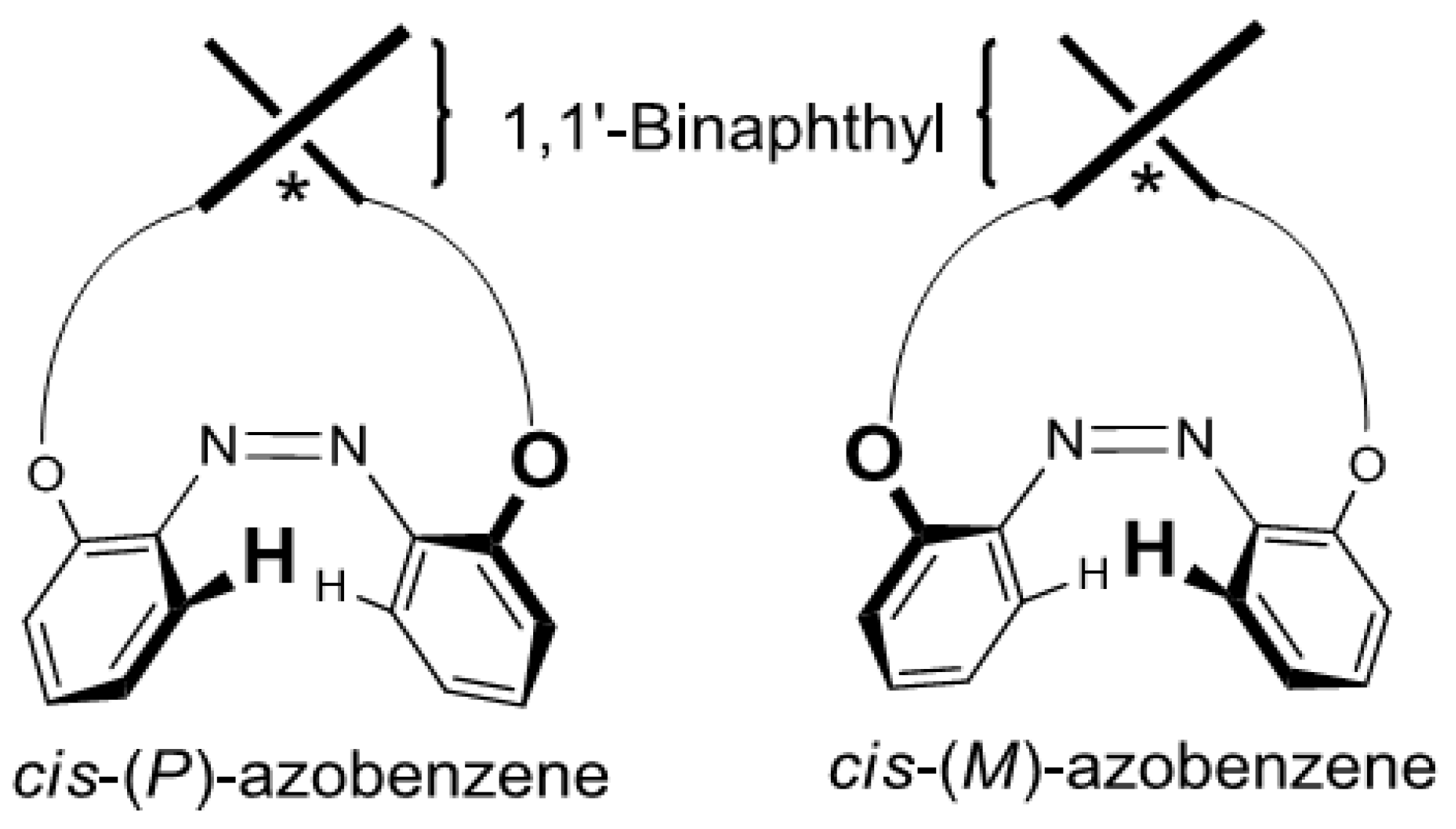
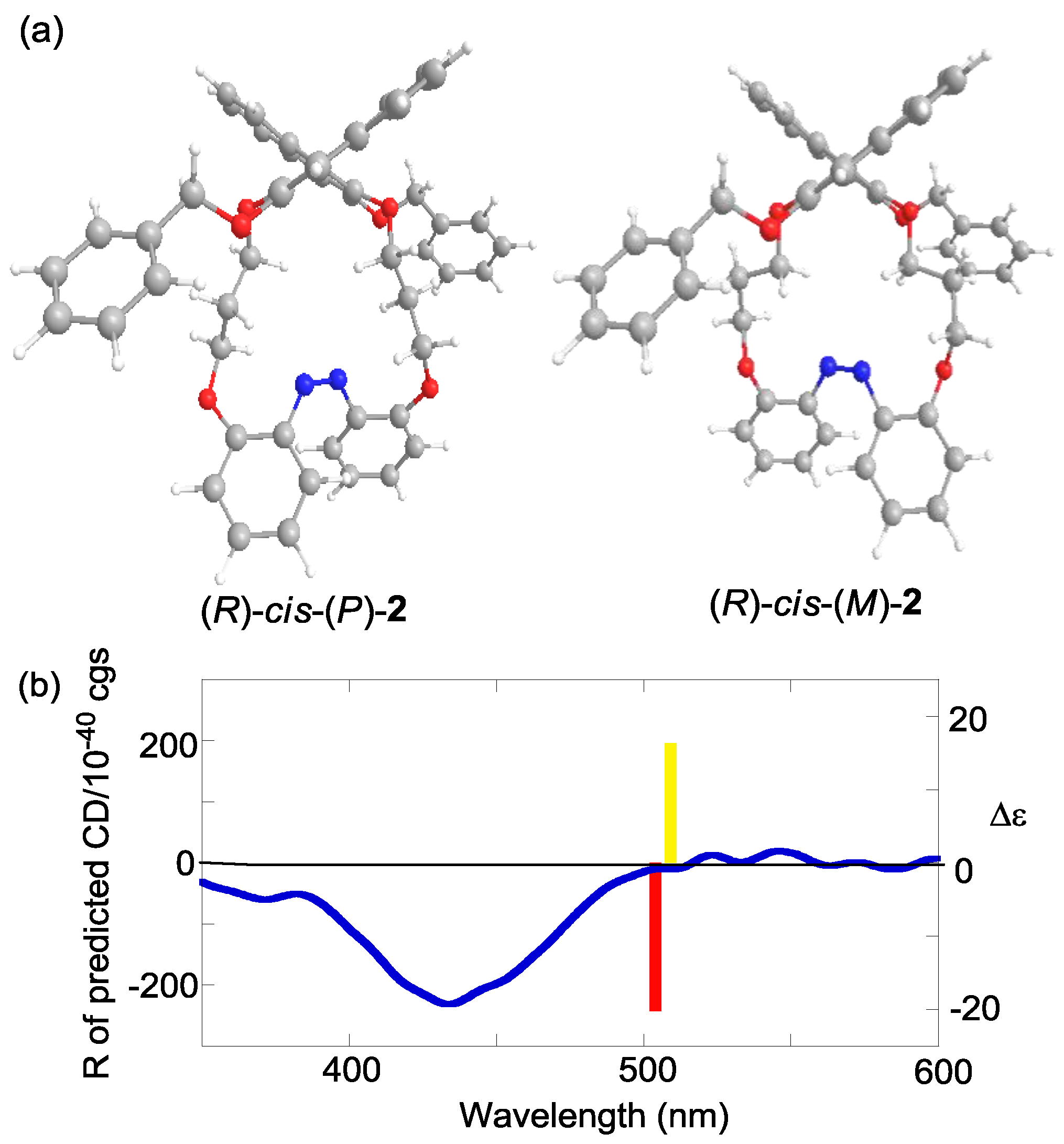
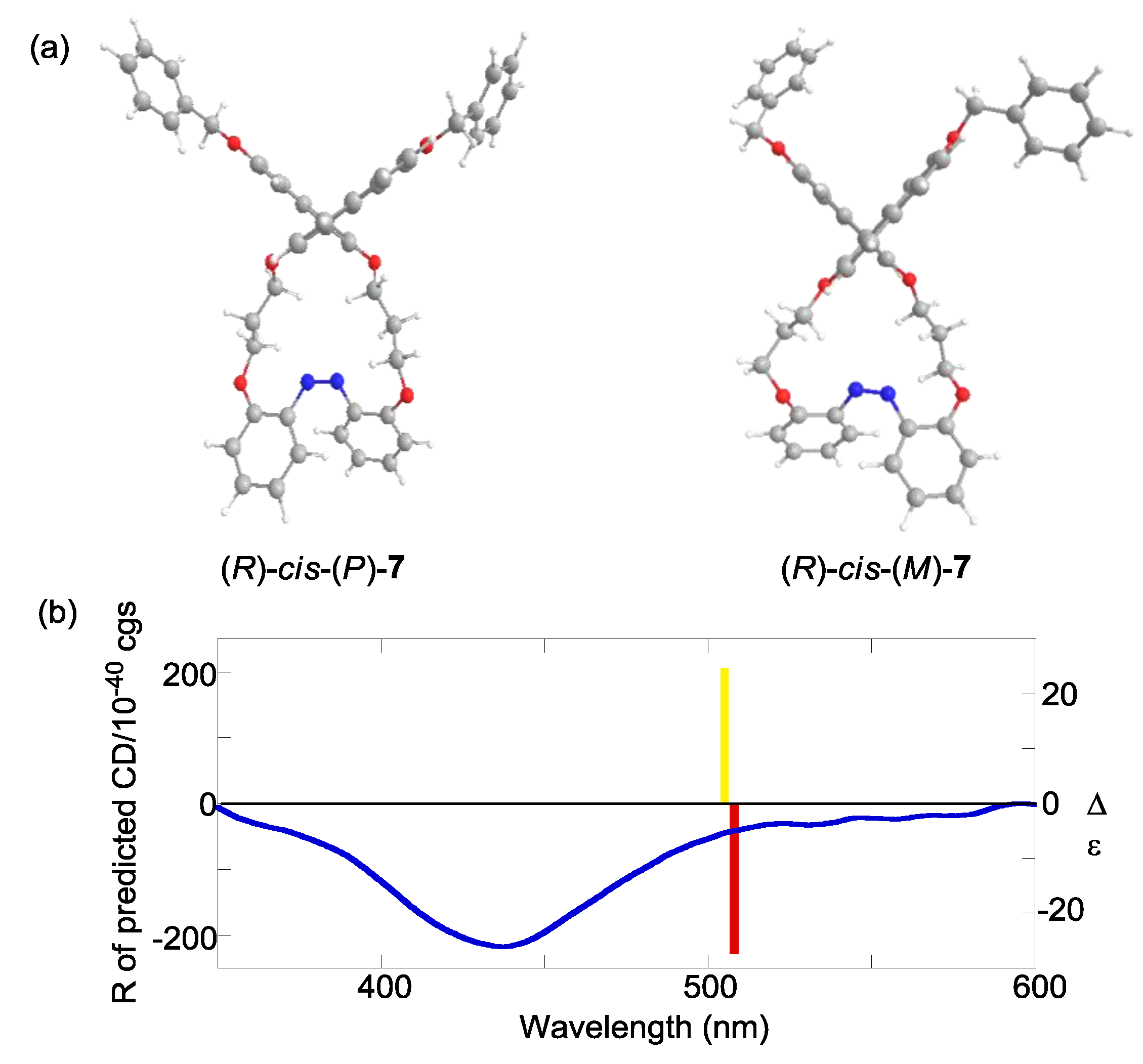
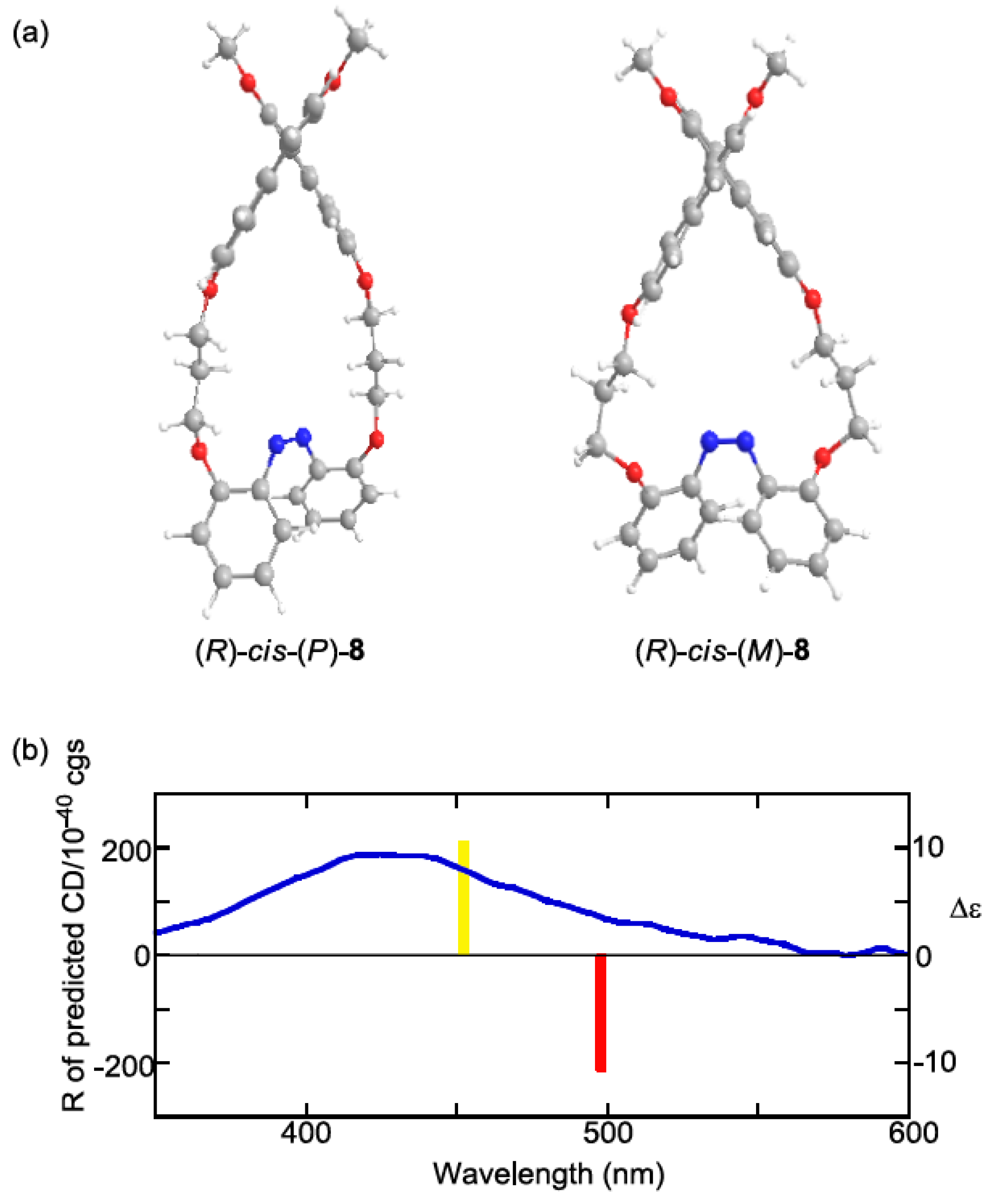
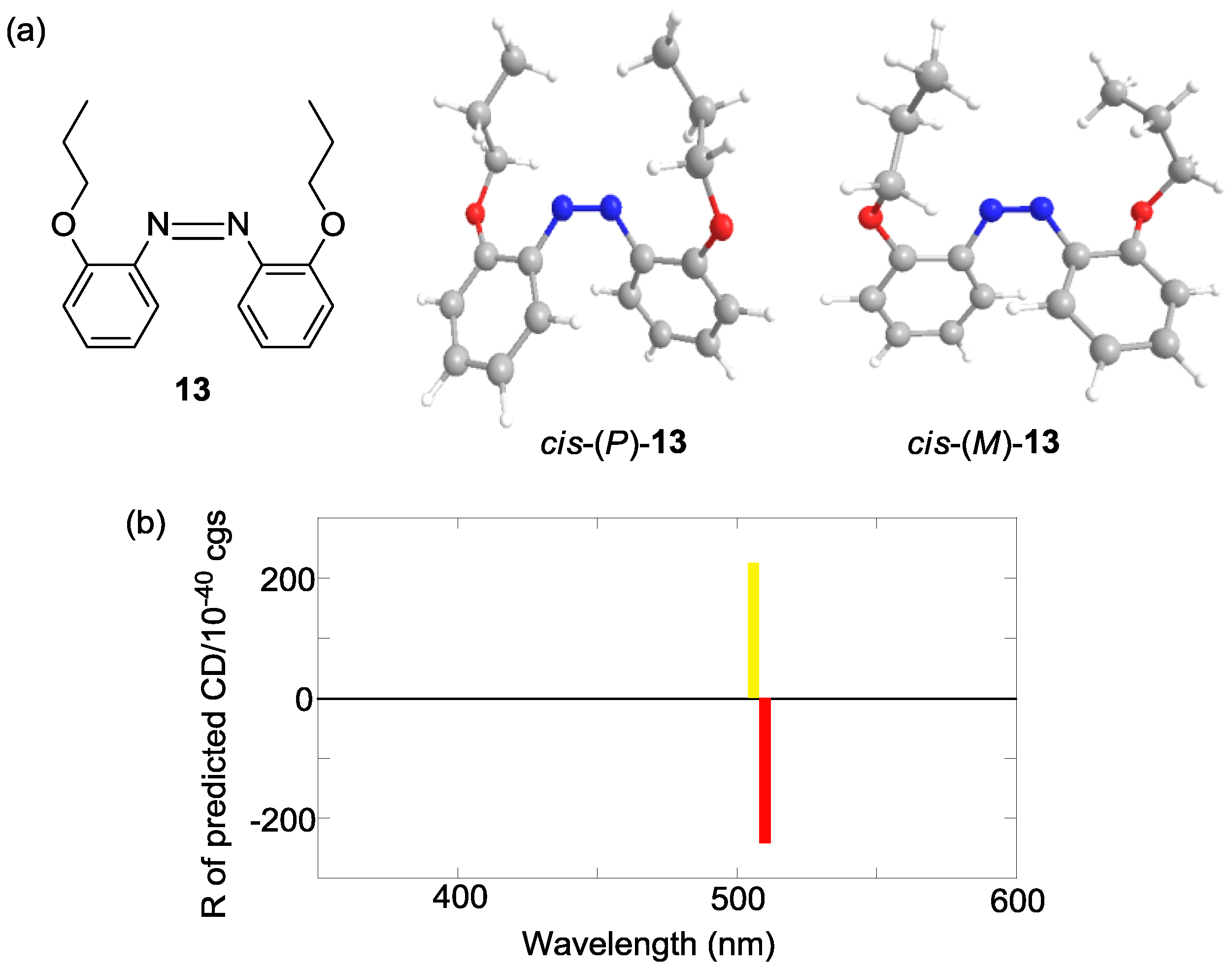
| Compound | Sign of Δε[a][b] | |||
|---|---|---|---|---|
| Experimental | Calculated cis-(P)-form | Calculated cis-(M)-form | Chirality of cis-azobenzene[c] | |
| (R)-2 | – | – | + | (P) |
| (R)-3 | – | – | + | (P) |
| (R)-4 | – | – | + | (P) |
| (R)-5 | – | – | + | (P) |
| (R)-6 | – | – | + | (P) |
| (R)-7 | – | – | + | (P) |
| (R)-8 | + | – | + | (M) |
| (S)-2 | + | – | + | (M) |
| 13 | – | + | ||

3. Conclusions
Acknowledgements
References
- Ogoshi, T.; Shiga, R.; Yamagishi, T.; Nakamoto, Y. Planar-chiral pillar[5]arene: Chiral switches induced by multiexternal stimulus of temperature, solvents, and addition of achiral guest molecule. J. Org. Chem. 2011, 76, 618–622. [Google Scholar] [CrossRef]
- Liu, W.; Cao, D.; Peng, J.; Zhang, H.; Meier, H. A dendrimer chiroptical switch based on the reversible intramolecular photoreaction of anthracene and benzene rings. Chem. Asian J. 2010, 5, 1896–1901. [Google Scholar] [CrossRef]
- Zheng, Y.; Cui, J.; Zheng, J.; Wan, X. Near-infrared electrochromic and chiroptical switching polymers: Synthesis and characterization of helical poly(N-propargylamides) carrying anthraquinone imide moieties in side chains. J. Mater. Chem. 2010, 20, 5915–5922. [Google Scholar] [CrossRef]
- Li, D.; Wang, Z.Y.; Ma, D. Electrically-controlled near-infrared chiroptical switching of enantiomeric dinuclear ruthenium complexes. Chem. Commun. 2009, 1529–1531. [Google Scholar]
- Deng, J.; Zhou, C.; Chen, C.; Song, N.; Su, Z. Synthesis and redox-driven chiroptically switching properties of viologen-containing optically active polymer with main-chain axial chirality. Macromolecules 2008, 41, 7805–7811. [Google Scholar] [CrossRef]
- Deng, J.; Song, N.; Zhou, Q.; Su, Z. Electrically-driven chiroptical switches based on axially dissymmetric 1,1’-binaphthyl and electrochromic viologens: Synthesis and optical properties. Org. Lett. 2007, 9, 5393–5396. [Google Scholar] [CrossRef]
- Rajakumar, P.; Selvam, S. Synthesis, complexation, and photoisomerization studies on some chiral monocyclic stilbenophanes and bis-cyclophanes. Tetrahedron 2007, 63, 8891–8901. [Google Scholar] [CrossRef]
- van Delden, R.A.; Mecca, T.; Rosini, C.; Feringa, B.L. A chiroptical molecular switch with distinct chiral and photochromic entities and its application in optical switching of a cholesteric liquid crystal. Chem. Eur. J. 2004, 10, 61–70. [Google Scholar] [CrossRef]
- Feringa, B.L. In control of motion: From molecular switches to molecular motors. Acc. Chem. Res. 2001, 34, 504–513. [Google Scholar] [CrossRef]
- Cornelissen, J.J.L.M.; Rowan, A.E.; Nolte, R.J.M.; Sommerdijk, N.A.J.M. Chiral architectures from macromolecular building blocks. Chem. Rev. 2001, 101, 4039–4070. [Google Scholar] [CrossRef]
- Feringa, B.L.; van Delden, R.A.; Koumura, N.; Geertsema, E.M. Chiroptical molecular switches. Chem. Rev. 2000, 100, 1789–1816. [Google Scholar] [CrossRef]
- Brunel, J.M. BINOL: A versatile chiral reagent. Chem. Rev. 2005, 105, 857–897. [Google Scholar] [CrossRef]
- Chen, Y.; Yekta, S.; Yudin, A. K. Modified BINOL ligands in asymmetric catalysis. Chem. Rev. 2003, 103, 3155–3211. [Google Scholar] [CrossRef]
- Berthod, M.; Mignani, G.; Woodward, G.; Lemaire, M. Modified BINAP: The How and the Why. Chem. Rev. 2005, 105, 1801–1836. [Google Scholar] [CrossRef]
- Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. Synthesis of 2,2’-bis(dipheny1phosphino)-1,l’-binaphthyl (BINAP), an atropisomeric chiral bis(triaryl)-phosphine, and its use in the rhodium(I)-catalyzed asymmetric hydrogenation of a (acylamino)acrylic acids. J. Am. Chem. Soc. 1980, 102, 7932–7934. [Google Scholar] [CrossRef]
- Yukawa, T.; Seelig, B.; Xu, Y.; Morimoto, H.; Matsunaga, S.; Berkessel, A.; Shibasaki, M. Catalytic asymmetric aza-Morita−Baylis−Hillman reaction of methyl acrylate: Role of a bifunctional La(O-iPr)3/linked-BINOL complex. J. Am. Chem. Soc. 2010, 132, 11988–11992. [Google Scholar]
- Mirri, G.; Bull, S.D.; Horton, P.N.; James, T.D.; Male, L.; Tucker, J.H.R. Electrochemical method for the determination of enantiomeric excess of binol using redox-active boronic acids as chiral sensors. J. Am. Chem. Soc. 2010, 132, 8903–8905. [Google Scholar]
- Pignataro, L.; Carboni, S.; Civera, M.; Colombo, R.; Piarulli, U.; Gennari, C. PhthalaPhos: Chiral supramolecular ligands for enantioselective rhodium-catalyzed hydrogenation reactions. Angew. Chem. Int. Ed. 2010, 49, 6633–6637. [Google Scholar]
- Li, N.; Chen, X.-H.; Zhou, S.-M.; Luo, S.-W.; Song, J.; Ren, L.; Gong, L.-Z. Asymmetric Amplification in Phosphoric Acid Catalyzed Reactions. Angew. Chem. Int. Ed. 2010, 49, 6378–6381. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, J.; He, P.; Wang, J.; Qin, B.; Liu, X.; Lin, L.; Feng, X. Highly enantioselective insertion of carbenoids into N-H bonds catalyzed by copper(I) complexes of binol derivatives. Angew. Chem. Int. Ed. 2010, 49, 4763–4766. [Google Scholar]
- Shirakura, M.; Suginome, M. Nickel-catalyzed asymmetric addition of alkyne C-H bonds across1,3-dienes using taddol-based chiral phosphoramidite ligands. Angew. Chem. Int. Ed. 2010, 49, 3827–3829. [Google Scholar] [CrossRef]
- Hatano, M.; Moriyama, K.; Maki, T.; Ishihara, K. Which is the actual catalyst: Chiral phosphoric acid or chiral calcium phosphate? Angew. Chem. Int. Ed. 2010, 49, 3823–3826. [Google Scholar] [CrossRef]
- Teichert, J.F.; Feringa, B.L. Phosphoramidites: Privileged ligands in asymmetric catalysis. Angew. Chem. Int. Ed. 2010, 49, 2486–2528. [Google Scholar] [CrossRef]
- Aikawa, K.; Hioki, Y.; Mikami, K. Highly enantioselective alkynylation of trifluoropyruvate with alkynylsilanes catalyzed by the BINAP-Pd complex: Access to α-trifluoromethyl-substituted tertiary alcohols. Org. Lett. 2010, 12, 5716–5719. [Google Scholar] [CrossRef]
- Arai, N.; Akashi, M.; Sugizaki, S.; Ooka, H.; Inoue, T.; Ohkuma, T. Asymmetric hydrogenation of bicyclic ketones catalyzed by BINAP/IPHAN-Ru(II) complex. Org. Lett. 2010, 12, 3380–3383. [Google Scholar] [CrossRef]
- Fraile, J.M.; Garcia, J.I.; Mayoral, J.A. Noncovalent Immobilization of Enantioselective Catalysts. Chem. Rev. 2009, 109, 360–417. [Google Scholar] [CrossRef]
- Yamamoto, H.; Abel, J.P. Catalytic enantioselective pudovik reaction of aldehydes and aldimines with tethered bis(8-quinolinato) (TBOx) aluminum complex. J. Am. Chem. Soc. 2008, 130, 10521–10523. [Google Scholar] [CrossRef]
- Rabalakos, C.; Wulff, W.D. Enantioselective organocatalytic direct Michael addition of nitroalkanes to nitroalkenes promoted by a unique bifunctional DMAP-thiourea. J. Am. Chem. Soc. 2008, 130, 13524–13525. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Chen, S.-Y.; Li, K.; Yu, X.-Q.; Pu, L. Enantioselective gel collapsing: A new means of visual chiral sensing. J. Am. Chem. Soc. 2010, 130, 7297–7299. [Google Scholar]
- Liu, H.-L.; Zhu, H.-P.; Hou, X.-L.; Lin Pu, L. Highly enantioselective fluorescent recognition of serine and other amino acid derivatives. Org. Lett. 2010, 12, 4172–4175. [Google Scholar] [CrossRef]
- Liu, H.-L.; Peng, Q.; Wu, Y.-D.; Chen, D.; Hou, X.-L.; Sabat, M.; Pu, L. Highly enantioselective recognition of structurally diverse α-hydroxycarboxylic acids using a fluorescent sensor. Angew. Chem. Int. Ed. 2010, 49, 602–606. [Google Scholar] [CrossRef]
- Sambasivan, S.; Kim, D.; Ahn, K.H. Chiral discrimination of α-amino acids with a C2-symmetric homoditopic receptor. Chem. Commun. 2010, 46, 541–543. [Google Scholar] [CrossRef]
- Yang, L.; Qin, S.; Su, X.; Yang, F.; You, J.; Hu, C.; Xie, R.; Lan, J. 1,1’-Binaphthyl-based imidazolium chemosensors for highly selective recognition of tryptophan in aqueous solutions. Org. Biomol. Chem. 2010, 8, 339–348. [Google Scholar] [CrossRef]
- Ema, T.; Hamada, K.; Sugita, K.; Nagata, Y.; Sakai, T.; Ohnishi, A. Synthesis and evaluation of chiral selectors with multiple hydrogen-bonding sites in the macrocyclic cavities. J. Org. Chem. 2010, 75, 4492–4500. [Google Scholar] [CrossRef]
- Nandhakumar, R.; Ryu, J.; Park, H.; Tang, L.; Choi, S.; Kim, K.M. Effects of ring substituents on enantioselective recognition of amino alcohols and acids in uryl-based binol receptors. Tetrahedron 2008, 64, 7704–7708. [Google Scholar] [CrossRef]
- Ema, T.; Tanida, D.; Sugita, K.; Sakai, T.; Miyazawa, K.; Ohnishi, A. Chiral selector with multiple hydrogen-bonding sites in a macrocyclic cavity. Org. Lett. 2008, 10, 2365–2368. [Google Scholar]
- Ema, T.; Tanida, D.; Hamada, K.; Sakai, T. Tuning the chiral cavity of macrocyclic receptor for chiralrecognition and discrimination. J. Org. Chem. 2008, 73, 9129–9132. [Google Scholar] [CrossRef]
- Park, H.; Nandhakumar, R.; Hong, J.; Ham, S.; Chin, J.; Kim, K. M. Stereoconversion of amino acids and peptides in uryl-pendant binol schiff bases. Chem. Eur. J. 2008, 14, 9935–9942. [Google Scholar]
- Wang, Q.; Chen, X.; Tao, L.; Wang, L.; Xiao, D.; Yu, X.-Q.; Pu, L. Enantioselective fluorescent recognition of amino alcohols by a chiral tetrahydroxyl 1,1’-binaphthyl compound. J. Org. Chem. 2007, 72, 97–101. [Google Scholar] [CrossRef]
- Bříza, T.; Kejík, Z.; Vašek, P.; Králová, J.; Martásek, P.; Císařová, I.; Král, V. Chromophoric binaphthylderivatives. Org. Lett. 2005, 7, 3661–3664. [Google Scholar] [CrossRef]
- Tsubaki, K.; Tanaka, H.; Morikawa, H.; Fuji, K. Synthesis and recognition of amino acids by binaphthyl-crown receptors. Tetrahedron 2003, 59, 3195–3199. [Google Scholar] [CrossRef]
- Mathews, M.; Zola, R.S.; Hurley, S.; Yang, D.-K.; White, T.J.; Bunning, T.J.; Li, Q. Light-driven reversible handedness inversion in self-organized helical superstructures. J. Am. Chem. Soc. 2010, 132, 18361–18366. [Google Scholar]
- Han, Y.; Pacheco, K.; Bastiaansen, C.W.M.; Broer, D.J.; Sijbesma, R.P. Optical monitoring of gases with cholesteric liquid crystals. J. Am. Chem. Soc. 2010, 132, 2961–2967. [Google Scholar]
- Akagi, K. Helical polyacetylene: Asymmetric polymerization in a chiral liquid-crystal field. Chem. Rev. 2009, 109, 5354–5361. [Google Scholar] [CrossRef]
- Yoshizawa, A.; Kogawa, Y.; Kobayashi, K.; Takanishi, Y.; Yamamoto, J. A binaphthyl derivative with a wide temperature range of a blue phase. J. Mater. Chem. 2009, 19, 5759–5764. [Google Scholar] [CrossRef]
- Goh, M.; Kyotani, M.; Akagi, K. Highly twisted helical polyacetylene with morphology free from the bundle of fibrils synthesized in chiral nematic liquid crystal reaction field. J. Am. Chem. Soc. 2007, 129, 8519–8527. [Google Scholar] [CrossRef]
- Yoshizawa, A.; Kobayashi, K.; Sato, M. Host-guest effect on chirality transfer from a binaphthyl derivative to a host nematic liquid crystal. Chem. Commun. 2007, 257–259. [Google Scholar]
- Li, Q.; Green, L.; Venkataraman, N.; Shiyanovskaya, I.; Khan, A.; Urbas, A.; Doane, J.W. Reversible photoswitchable axially chiral dopants with high helical twisting power. J. Am. Chem. Soc. 2007, 129, 12908–12909. [Google Scholar]
- Eelkema, R.; Feringa, B.L. Amplification of chirality in liquid crystals. Org. Biomol. Chem. 2006, 4, 3729–3745. [Google Scholar] [CrossRef]
- Pieraccini, S.; Ferrarini, A.; Fuji, K.; Gottarelli, G.; Lena, S.; Tsubaki, K.; Spada, G.P. Homochiral helices of oligonaphthalenes inducing opposite-handed cholesteric phases. Chem. Eur. J. 2006, 12, 1121–1126. [Google Scholar] [CrossRef]
- Akagi, K.; Guo, S.; Mori, T.; Goh, M.; Piao, G.; Kyotan, M. Synthesis of helical polyacetylene in chiral nematic liquid crystals using crown ether type binaphthyl derivatives as chiral dopants. J. Am. Chem. Soc. 2005, 127, 14647–14654. [Google Scholar] [CrossRef]
- Holzwarth, R.; Bartsch, R.; Cherkaoui, Z.; Solladié, G. New 2,2'-substituted 4,4'-dimethoxy-6,6'-dimethyl[1,1'-biphenyls], inducing a strong helical twisting power in liquid crystals. Chem. Eur. J. 2004, 10, 3931–3935. [Google Scholar] [CrossRef]
- Carlo, R.; Piero, S.G.; Gloria, P.; Stefano, M.; Simone, S. Conformational analysis of some trans-4,5-Diaryl-1,3-dioxolanes by CD spectroscopy and induction of cholesteric mesophases in nematic solvents: A correlation between twisting power and structure of the dopant. J. Am. Chem. Soc. 1997, 119, 506–512. [Google Scholar]
- Gottarelli, G.; Spada, G.P. Induction of the cholesteric mesophase in nematic liquid crystals: Correlation between the conformation of open-chain chiral 1,l’-binaphthyls and their twisting powers. J. Org. Chem. 1986, 51, 589–592. [Google Scholar] [CrossRef]
- Gottarelli, G.; Hibert, M.; Samori, B.; Solladie, G.; Spada, G.P.; Zimmermann, R. Induction of the cholesteric mesophase in nematic liquid crystals: Mechanism and application to the determination of bridged biaryl configurations. J. Am. Chem. Soc. 1983, 105, 7318–7321. [Google Scholar] [CrossRef]
- Reimann, S.; Urakawa, A.; Baiker, A. BINAP adsorption on palladium: A combined infrared spectroscopy and theoretical study. J. Phys. Chem. C 2010, 114, 17836–17844. [Google Scholar] [CrossRef]
- Nishizaka, M.; Mori, T.; Inoue, Y. Experimental and theoretical studies on the chiroptical properties of donor-acceptor binaphthyls. Effects of dynamic conformer population on circular dichroism. J. Phys. Chem. Lett. 2010, 1, 1809–1812. [Google Scholar] [CrossRef]
- Bunzen, J.; Bruhn, T.; Bringmann, G.; Lűtzen, A. Synthesis and helicate formation of a new family of BINOL-based bis(bipyridine) ligands. J. Am. Chem. Soc. 2009, 131, 3621–3630. [Google Scholar]
- Sahnoun, R.; Koseki, S.; Fujimura, Y. Density functional theoretical study on enantiomerization of 2,2'-biphenol. J. Phys. Chem. A 2006, 110, 2440–2447. [Google Scholar] [CrossRef]
- Albrow, V.; Biswas, K.; Crane, A.; Chaplin, N.; Easun, T.; Gladiali, S.; Lygo, B.; Woodward, S. Synthesis of an octahydro-1,1’-binaphthyl thioether ligand and comparison with unhydrogenated binaphthyl analogues. Tetrahedron Asymmetry 2003, 14, 2813–2819. [Google Scholar] [CrossRef]
- Setnička, V.; Urbanová, M.; Bouř, P.; Král, V.; Volka, K. Vibrational circular dichroism of 1,1’-binaphthyl derivatives: Experimental and theoretical study. J. Phys. Chem. A 2001, 105, 8931–8938. [Google Scholar]
- Kranz, M.; Clark, T.; Schleyer, P.v.R. Rotational barriers of 1,1'-binaphthyls: A computational study. J. Org. Chem. 1993, 58, 3317–3325. [Google Scholar] [CrossRef]
- Dube, H.; Ams, M.R.; Rebek, J., Jr. Supramolecular control of fluorescence through reversible encapsulation. J. Am. Chem. Soc. 2010, 132, 9984–9985. [Google Scholar] [CrossRef]
- Han, J.; Yan, D.; Shi, W.; Ma, J.; Yan, H.; Wei, M.; Evans, D.G.; Duan, X. Layer-by-layer ultrathin films of azobenzene-containing polymer/layered double hydroxides with reversible photoresponsive behavior. J. Phys. Chem. B 2010, 114, 5678–5685. [Google Scholar] [CrossRef]
- Liu, M.; Yan, X.; Hu, M.; Chen, X.; Zhang, M.; Zheng, B.; Hu, X.; Shao, S.; Huang, F. Photoresponsive host-guest systems based on a new azobenzene-containing crytpand. Org. Lett. 2010, 12, 2558–2561. [Google Scholar]
- Oka, Y.; Tamaoki, N. Structure of silver(I) complex prepared from azobenzenonaphthalenophane, photochemical coordination change of silver(I) and silver(I)-induced acceleration of Z-E thermal isomerization of azobenzene unit. Inorg. Chem. 2010, 49, 4765–4767. [Google Scholar] [CrossRef]
- Kawamoto, M.; Sassa, T.; Wada, T. Photoinduced control over the self-organized orientation of amorphous molecular materials using polarized light. J. Phys. Chem. B 2010, 114, 1227–1232. [Google Scholar]
- Chen, W.-C.; Lee, Y.-W.; Chen, C.-T. Diastereoselective, synergistic dual-mode optical switch with integrated chirochromic helicene and photochromic bis-azobenzene moieties. Org. Lett. 2010, 12, 1472–1475. [Google Scholar] [CrossRef]
- Basheer, M.C.; Oka, Y.; Mathews, M.; Tamaoki, N. A light-controlled molecular brake with complete ON-OFF rotation. Chem. Eur. J. 2010, 16, 3489–3496. [Google Scholar] [CrossRef]
- Kawamoto, M.; Shiga, N.; Takaishi, K.; Yamashita, T. Non-destructive erasable molecular switches and memory using light-driven twisting motions. Chem. Commun. 2010, 46, 8344–8346. [Google Scholar]
- Sadovski, O.; Beharry, A.A.; Zhang, F.; Woolley, G.A. Spectral tuning of azobenzene photoswitches for biological applications. Angew. Chem. Int. Ed. 2009, 48, 1484–1486. [Google Scholar] [CrossRef]
- Zou, G.; Jiang, H.; Kohn, H.; Manaka, T.; Iwamoto, M. Control and modulation of chirality for azobenzene-substituted polydiacetylene LB films with circularly polarized light. Chem. Commun. 2009, 5627–5629. [Google Scholar]
- Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. Highly efficient reversible Z-E photoisomerization of a bridged azobenzene with visible light through resolved S1(nπ*) absorption bands. J. Am. Chem. Soc. 2009, 131, 15594–15595. [Google Scholar]
- Uno, S.; Dohno, C.; Bittermann, H.; Malinovskii, V.L.; Häner, R.; Nakatani, K. A light-driven supramolecular optical switch. Angew. Chem. Int. Ed. 2009, 48, 7362–7365. [Google Scholar] [CrossRef]
- Chen, J.; Serizawa, T.; Komiyama, M. Peptides recognize photoresponsive targets. Angew. Chem. Int. Ed. 2009, 48, 2917–2920. [Google Scholar] [CrossRef]
- Puntoriero, F.; Bergamini, G.; Ceroni, P.; Balzania, V.; Vögtle, F. A fluorescent guest encapsulated by a photoreactive azobenzene dendrimer. New J. Chem. 2008, 32, 401–406. [Google Scholar] [CrossRef]
- Tamaoki, N.; Mathews, M. Planar chiral azobenzenophanes as chiroptic switches for photon mode reversible reflection color control in induced chiral nematic liquid crystals. J. Am. Chem. Soc. 2008, 130, 11409–11416. [Google Scholar] [CrossRef]
- King, E.D.; Tao, P.; Sanan, T.T.; Hadad, C.M.; Parquetted, J.R. Photomodulated chiral induction in helical azobenzene oligomers. Org. Lett. 2008, 10, 1671–1674. [Google Scholar] [CrossRef]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 2007, 46, 72–191. [Google Scholar] [CrossRef]
- Vera, F.; Tejedor, R.M.; Romero, P.; Barberá, J.; Ros, M.B.; Serrano, J.L.; Sierra, T. Light-driven supramolecular chirality in propeller-like hydrogen-bonded complexes that show columnar mesomorphism. Angew. Chem. Int. Ed. 2007, 46, 1873–1877. [Google Scholar] [CrossRef]
- Alam, M.Z.; Yoshioka, T.; Ogata, T.; Nonaka, T.; Kurihara, S. Influence of helical twisting poweron the photoswitching behavior of chiral azobenzene compounds: Applications to high-performance switching devices. Chem. Eur. J. 2007, 13, 2641–2647. [Google Scholar] [CrossRef]
- Choi, S.-W.; Kawauchi, S.; Ha, N.Y.; Takezoe, H. Photoinduced chirality in azobenzene-containing polymer systems. Phys. Chem. Chem. Phys. 2007, 9, 3671–3681. [Google Scholar]
- Muraoka, T.; Kinbara, K.; Aida, T. Mechanical twisting of a guest by a photoresponsive host. Nature 2006, 440, 512–515. [Google Scholar] [CrossRef]
- Jousselme, B.; Blanchard, P.; Allain, M.; Levillain, E.; Dias, M.; Roncali, J. Structural control of the electronic properties of photodynamic azobenzene-derivatized π-conjugated oligothiophenes. J. Phys. Chem. A 2006, 110, 3488–3494. [Google Scholar] [CrossRef]
- Balzani, V.; Venturi, M.; Credi, A. Molecular Devices and Machines; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Feringa, B.L. Molecular Switches; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Kawamoto, M.; Aoki, T.; Wada, T. Light-driven twisting behaviour of chiral cyclic compounds. Chem. Commun. 2007, 930–932. [Google Scholar] [CrossRef]
- Takaishi, K.; Kawamoto, M.; Tsubaki, K.; Wada, T. Photoswitching of dextro/levo rotation with axially chiral binaphthyls linked to an azobenzene. J. Org. Chem. 2009, 74, 5723–5726. [Google Scholar] [CrossRef]
- Takaishi, K.; Kawamoto, M.; Tsubaki, K.; Furuyama, T.; Muranaka, A.; Uchiyama, M. Helical chirality of azobenzenes induced by an intramolecular chiral axis and potential as chiroptical switches. Chem. Eur. J. 2011, 17, 1778–1782. [Google Scholar] [CrossRef]
- Haberhauer, G.; Kallweit, C. A bridged azobenzene derivative as a reversible, light-induced chirality switch. Angew. Chem. Int. Ed. 2010, 49, 2418–2421. [Google Scholar] [CrossRef]
- Tsubaki, K.; Morikawa, H.; Tanaka, H.; Fuji, K. Convenient synthesis and efficient resolution of 3,3'-bis(benzyloxy)-1,1’-binaphthalene-2,2’-diol. Tetrahedron Asymmetry 2003, 14, 1393–1396. [Google Scholar] [CrossRef]
- Chang, C.-F.; Yang, L.-Y.; Chang, S.-W.; Fang, Y.-T.; Lee, Y.-J. Total synthesis of demethylwedelolactone and wedelolactone by Cu-mediated/Pd(0)-catalysis and oxidative-cyclization. Tetrahedron 2008, 68, 3661–3666. [Google Scholar]
- Surivet, J.-P.; Vatèle, J.-M. First total synthesis of (-)-8-epi-9-deoxygoniopypyrone. Tetrahedron Lett. 1998, 39, 9681–9682. [Google Scholar] [CrossRef]
- Hori, H.; Nishida, Y.; Ohrui, H.; Meguro, H. Regioselective de-O-benzylation with lewis acids. J. Org. Chem. 1989, 54, 1346–1353. [Google Scholar] [CrossRef]
- Bandin, M.; Casolari, S.; Cozzi, P.G.; Proni, G.; Schmohel, E.; Spada, G.P.; Tagliavini, E.; Umani-Ronchi, A. Synthesis and characterization of new enantiopure 7,7’-disubstituted 2,2’-dihydroxy-1,1’-binaphthyls: Useful ligands for the asymmetric allylation reaction of aldehydes. Eur. J. Org. Chem. 2000, 491–497. [Google Scholar]
- Diederich, F.; Hester, M.R.; Uyeki, M.A. Auf 2,2’,7,7’-Tetrahydroxy-1,1’-binaphthyl basierende neuartige chirale mono- und ditope cyclophanartige wirtverbindungen mit einer unpolaren bindungsstelle. Angew. Chem. 1988, 100, 1775–1777. [Google Scholar] [CrossRef]
- Horiuchi, T.; Ohta, T.; Stephan, M.; Takaya, H. Synthesis of (R)- and (S)-7,7’-bis(diphenylphosphino)-2,2’-dimethoxy-l,l’-binaphthyl, a new axially dissymmetric bis(triarylphosphine). Tetrahedron Asymmetry 1994, 5, 325–328. [Google Scholar] [CrossRef]
- Reeder, J.; Castro, P.P.; Knobler, C.B. Chiral recognition of cinchona alkaloids at the minor and major grooves of 1,l’-binaphthyl receptors. J. Org. Chem. 1994, 59, 3151–3160. [Google Scholar] [CrossRef]
- Takaishi, K.; Sue, D.; Kuwahara, S.; Harada, N.; Kawabata, T.; Tsubaki, K. Synthesis and properties of S,R-alternating octinaphthalenes. Tetrahedron 2009, 65, 6135–6140. [Google Scholar] [CrossRef]
- Sue, D.; Takaishi, K.; Harada, T.; Kuroda, R.; Kawabata, T.; Tsubaki, K. Synthesis of chiral dotriacontanaphthalenes: How many naphthalene units are we able to elaborately connect? J. Org. Chem. 2009, 74, 3940–3943. [Google Scholar]
- Bari, L.D.; Pescitelli, G.; Marchetti, F.; Salvadori, P. Anomalous CD/UV exciton splitting of a binaphthyl derivative: The case of 2,2’-diiodo-1,1’-binaphthalene. J. Am. Chem. Soc. 2000, 122, 6395–6398. [Google Scholar] [CrossRef]
- Harada, N.; Nakanishi, K. The exciton chirality method and its application to configurational and conformational studies of natural products. Acc. Chem. Res. 1972, 5, 257–263. [Google Scholar] [CrossRef]
- Jaffé, H.H.; Orchin, M. Theory and Applications of Ultraviolet Spectroscopy; Wiley: New York, NY, USA, 1962. [Google Scholar]
- Graule, S.; Rudolph, M.; Vanthuyne, N.; Autschbach, J.; Roussel, C.; Crassous, J.; Réau, R. Metal-bis(helicene) assemblies incorporating π-conjugated phosphole-azahelicene ligands: Impacting chiroptical properties by metal variation. J. Am. Chem. Soc. 2009, 131, 3183–3185. [Google Scholar]
- Rzepa, H.S. The chiro-optical properties of a lemniscular octaphyrin. Org. Lett. 2009, 11, 3088–3091. [Google Scholar]
- Katzenelson, O.; Edelstein, J.; Avnir, D. Quantitative chirality of helicenes. Tetrahedron Asymmetry 2000, 11, 2695–2704. [Google Scholar] [CrossRef]
- Okumura, T.; Tani, Y.; Miyake, K.; Yokoyama, Y. Chiral helicenoid diarylethene with large change in specific optical rotation by photochromism. J. Org. Chem. 2007, 72, 1634–1638. [Google Scholar] [CrossRef]
- Wigglesworth, T.J.; Sud, D.; Norsten, T.B.; Lekhi, V.S.; Branda, N.R. Chiral discrimination in photochromic helicenes. J. Am. Chem. Soc. 2005, 127, 7272–7273. [Google Scholar]
- Wang, Z.Y.; Todd, E.K.; Meng, X.S.; Gao, J.P. Dual modulation of a molecular switch with exceptional chiroptical properties. J. Am. Chem. Soc. 2005, 127, 11552–11553. [Google Scholar]
- Eyring, H. The activated complex and the absolute rate of chemical reactions. Chem. Rev. 1935, 17, 65–77. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H.P.; Izmaylov, A.F.; Bloino, J.; Zheng, G.; Sonnenberg, J.L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J.A., Jr.; Peralta, J.E.; Ogliaro, F.; M. Bearpark, M.; Heyd, J.J.; Brothers, E.; Kudin, K.N.; Staroverov, V.N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J.C.; Iyengar, S.S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J.M.; Klene, M.; Knox, J.E.; Cross, J.B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski, J.W.; Martin, R.L.; Morokuma, K.; Zakrzewski, V.G.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Dapprich, S.; Daniels, A.D.; Farkas, O.; Foresman, J.B.; Ortiz, J.V.; Cioslowski, J.; Fox, D.J. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Ma, X.; Wang, Q.; Qu, D.; Xu, Y.; Ji, F.; Tian, H. A light-driven pseudo[4]rotaxane encoded by induced circular dichroism in a hydrogel. Adv. Funct. Mater. 2007, 17, 829–837. [Google Scholar] [CrossRef]
- Kobayashi, N.; Higashi, R.; Titeca, B.C.; Lamote, F.; Ceulemans, A. Substituent-induced circular dichroism in phthalocyanines. J. Am. Chem. Soc. 1999, 121, 12018–12028. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functional. Theor. Chem. Account. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2–8 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Takaishi, K.; Kawamoto, M. Synthesis and Conformation of Substituted Chiral Binaphthyl-Azobenzene Cyclic Dyads with Chiroptical Switching Capabilities. Molecules 2011, 16, 1603-1624. https://doi.org/10.3390/molecules16021603
Takaishi K, Kawamoto M. Synthesis and Conformation of Substituted Chiral Binaphthyl-Azobenzene Cyclic Dyads with Chiroptical Switching Capabilities. Molecules. 2011; 16(2):1603-1624. https://doi.org/10.3390/molecules16021603
Chicago/Turabian StyleTakaishi, Kazuto, and Masuki Kawamoto. 2011. "Synthesis and Conformation of Substituted Chiral Binaphthyl-Azobenzene Cyclic Dyads with Chiroptical Switching Capabilities" Molecules 16, no. 2: 1603-1624. https://doi.org/10.3390/molecules16021603
APA StyleTakaishi, K., & Kawamoto, M. (2011). Synthesis and Conformation of Substituted Chiral Binaphthyl-Azobenzene Cyclic Dyads with Chiroptical Switching Capabilities. Molecules, 16(2), 1603-1624. https://doi.org/10.3390/molecules16021603




