Synthesis and Anti-Fungal Activity of Seven Oleanolic Acid Glycosides
Abstract
:1. Introduction
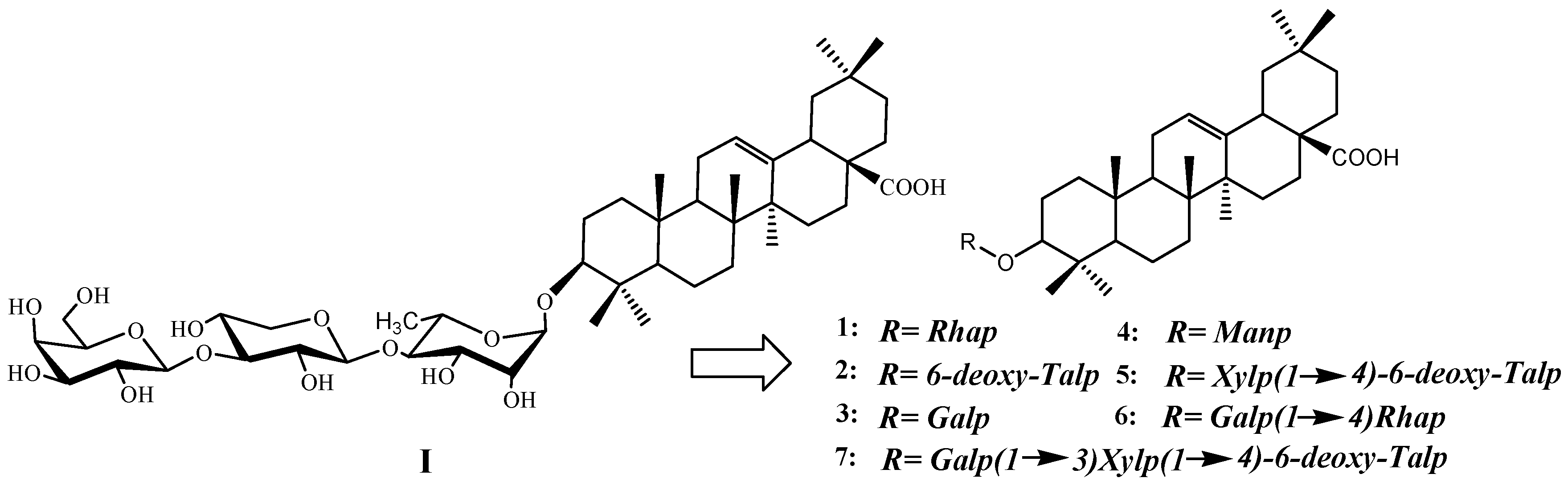
2. Results and Discussion
2.1. Chemistry
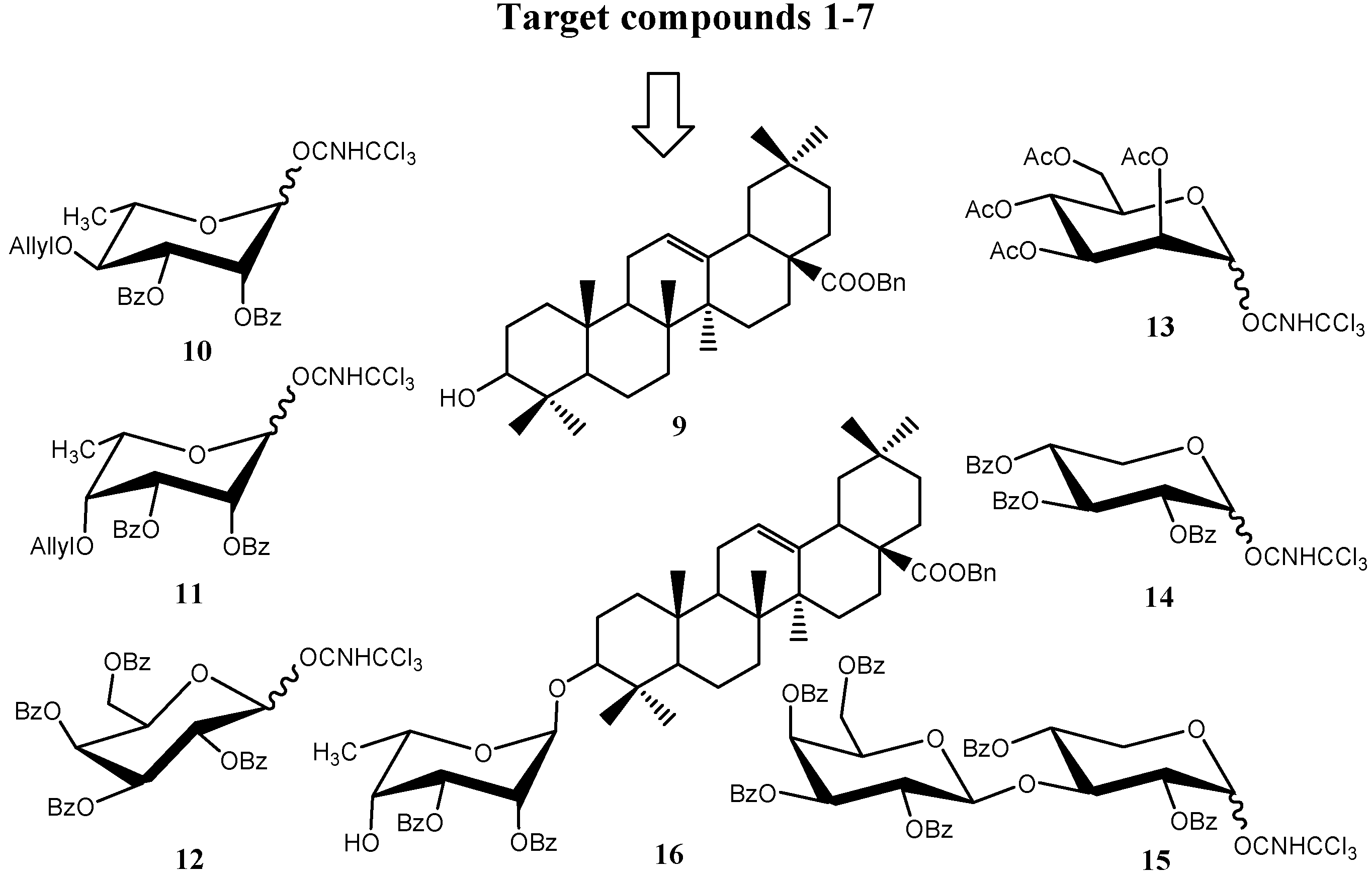
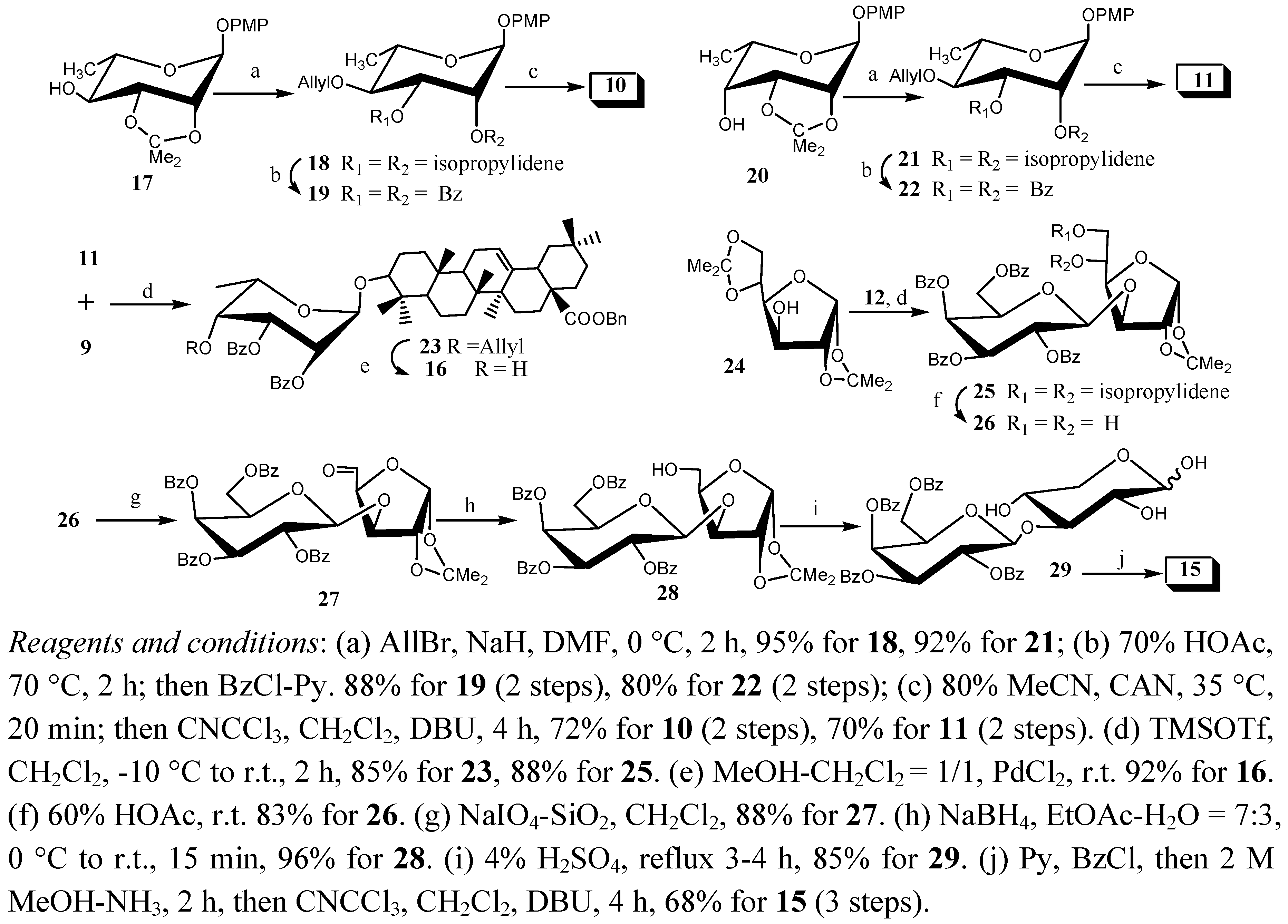

2.2. Bioassay of Fungicidal Activities
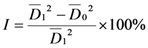
 is the average diameter of mycelia in the blank test, and
is the average diameter of mycelia in the blank test, and 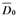 is the average diameter of mycelia in the presence of compounds 1-7: The inhibition rates of compounds 1-7 against the four fungi at 50 µg/mL are given in Table 1. Compounds 1-7 exhibited more fungicidal activity against R. solani than the other fungi, compounds 1 and 2 are more active against B. cinerea and Phytophthora parasitica Dast than the other compounds.
is the average diameter of mycelia in the presence of compounds 1-7: The inhibition rates of compounds 1-7 against the four fungi at 50 µg/mL are given in Table 1. Compounds 1-7 exhibited more fungicidal activity against R. solani than the other fungi, compounds 1 and 2 are more active against B. cinerea and Phytophthora parasitica Dast than the other compounds. | Compd no. | Inhibition rate (%) | ||||
|---|---|---|---|---|---|
| S. sclerotiorum | R. solani | B. cinerea | Phytophthora parasitica Dast | ||
| 1 | 71.90 | 96.05 | 75.41 | 79.21 | |
| 2 | 67.35 | 93.24 | 77.29 | 83.54 | |
| 3 | 78.27 | 95.29 | 68.42 | 67.24 | |
| 4 | 65.16 | 96.17 | 74.59 | 63.55 | |
| 5 | 73.47 | 93.86 | 71.73 | 52.57 | |
| 6 | 71.90 | 95.93 | 71.44 | 69.72 | |
| 7 | 71.10 | 88.48 | 67.17 | 70.06 | |
3. Experimental
3.1. General methods
3.2. Chemical synthesis
 -61.4 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 7.00-6.81 (m, 4 H, Bz-H), 5.93 (m, 1 H, CH2=CH-CH2O), 5.59 (s, 1 H, H-1), 5.32-5.16 (m, 2 H), 4.40-4.32 (m, 3 H), 4.14 (m, 1 H), 3.83-3.77 (m, 4 H, H-5, OCH3), 3.21 (m, 1 H), 1.56 (s, 3 H, CH3), 1.40 (s, 3 H, CH3), 1.23 (d, 3 H, J = 6.3 Hz, H-6). Anal. Calcd. for C19H26O6: C, 65.13; H, 7.48; found: C, 65.29; H, 7.63.
-61.4 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 7.00-6.81 (m, 4 H, Bz-H), 5.93 (m, 1 H, CH2=CH-CH2O), 5.59 (s, 1 H, H-1), 5.32-5.16 (m, 2 H), 4.40-4.32 (m, 3 H), 4.14 (m, 1 H), 3.83-3.77 (m, 4 H, H-5, OCH3), 3.21 (m, 1 H), 1.56 (s, 3 H, CH3), 1.40 (s, 3 H, CH3), 1.23 (d, 3 H, J = 6.3 Hz, H-6). Anal. Calcd. for C19H26O6: C, 65.13; H, 7.48; found: C, 65.29; H, 7.63. +21.1 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.07-7.34 (m, 10 H, Bz-H), 7.08-6.83 (m, 4 H, MeOC6H4), 5.87-5.75 (m, 3 H), 5.52 (d, 1 H, J = 1.8 Hz, H-1), 5.17 (m, 1 H), 5.08 (m, 1 H), 4.20-4.08 (m, 3 H), 3.78-3.71 (m, 4 H, H-5, OCH3), 1.41 (d, J = 6.2 Hz, 3 H, H-6); Anal. Calcd. for C30H30O8: C, 69.49; H, 5.83; found: C, 69.55; H, 5.58;
+21.1 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.07-7.34 (m, 10 H, Bz-H), 7.08-6.83 (m, 4 H, MeOC6H4), 5.87-5.75 (m, 3 H), 5.52 (d, 1 H, J = 1.8 Hz, H-1), 5.17 (m, 1 H), 5.08 (m, 1 H), 4.20-4.08 (m, 3 H), 3.78-3.71 (m, 4 H, H-5, OCH3), 1.41 (d, J = 6.2 Hz, 3 H, H-6); Anal. Calcd. for C30H30O8: C, 69.49; H, 5.83; found: C, 69.55; H, 5.58; +39.3 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.74 (s, 1 H, C=NH), 8.06-7.34 (m, 10 H, Bz-H ), 6.39 (d, 1 H, J = 1.9 Hz, H-1), 5.85-5.71 (m, 3 H), 5.21-5.07 (m, 2 H), 4.22-4.15 (m, 3 H), 3.77 (dd, 1 H, J = 9.6, 9.6 Hz, H-4), 1.48 (d, J = 6.2 Hz, 3 H, H-6). Anal. Calcd. for C25H24Cl3NO7: C, 53.93; H, 4.34; N, 2.52; found: C, 53.79; H, 4.23; N, 2.29.
+39.3 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.74 (s, 1 H, C=NH), 8.06-7.34 (m, 10 H, Bz-H ), 6.39 (d, 1 H, J = 1.9 Hz, H-1), 5.85-5.71 (m, 3 H), 5.21-5.07 (m, 2 H), 4.22-4.15 (m, 3 H), 3.77 (dd, 1 H, J = 9.6, 9.6 Hz, H-4), 1.48 (d, J = 6.2 Hz, 3 H, H-6). Anal. Calcd. for C25H24Cl3NO7: C, 53.93; H, 4.34; N, 2.52; found: C, 53.79; H, 4.23; N, 2.29. -49.1 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 7.01-6.80 (m, 4 H, Bz-H), 5.93 (m, 1 H, CH2=CH-CH2O), 5.56 (d, 1 H, J = 1.5 Hz, H-1), 5.29-5.17 (m, 2 H), 4.49 (m, 1 H), 4.34-4.25 (m, 2 H), 4.09-4.00 (m, 2 H), 3.77 (s, 3 H, OCH3), 3.60 (m, 1 H), 1.59 (s, 3 H, CH3), 1.40 (s, 3 H, CH3), 1.31 (d, 3 H, J = 6.6 Hz, H-6). Anal. Calcd. for C19H26O6: C, 65.13; H, 7.48; found: C, 65.25; H, 7.29.
-49.1 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 7.01-6.80 (m, 4 H, Bz-H), 5.93 (m, 1 H, CH2=CH-CH2O), 5.56 (d, 1 H, J = 1.5 Hz, H-1), 5.29-5.17 (m, 2 H), 4.49 (m, 1 H), 4.34-4.25 (m, 2 H), 4.09-4.00 (m, 2 H), 3.77 (s, 3 H, OCH3), 3.60 (m, 1 H), 1.59 (s, 3 H, CH3), 1.40 (s, 3 H, CH3), 1.31 (d, 3 H, J = 6.6 Hz, H-6). Anal. Calcd. for C19H26O6: C, 65.13; H, 7.48; found: C, 65.25; H, 7.29. -7.7 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.25-7.25 (m, 10 H, Bz-H), 7.07-6.82 (m, 4 H, MeOC6H4), 5.93 (m, 1 H, CH2=CH-CH2O), 5.77 (dd, 1 H, J = 3.49, 3.34 Hz, H-3), 5.68-5.67 (m, 2 H), 5.29-5.12 (m, 2 H), 4.33-4.27 (m, 2 H), 4.07 (m, 1 H), 3.83-3.76 (m, 4 H, CH2=CH-CH2O, OCH3), 1.37 (d, J = 6.5 Hz, 3 H, H-6); Anal. Calcd. for C30H30O8: C, 69.49; H, 5.83; found: C, 69.63; H, 5.66.
-7.7 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.25-7.25 (m, 10 H, Bz-H), 7.07-6.82 (m, 4 H, MeOC6H4), 5.93 (m, 1 H, CH2=CH-CH2O), 5.77 (dd, 1 H, J = 3.49, 3.34 Hz, H-3), 5.68-5.67 (m, 2 H), 5.29-5.12 (m, 2 H), 4.33-4.27 (m, 2 H), 4.07 (m, 1 H), 3.83-3.76 (m, 4 H, CH2=CH-CH2O, OCH3), 1.37 (d, J = 6.5 Hz, 3 H, H-6); Anal. Calcd. for C30H30O8: C, 69.49; H, 5.83; found: C, 69.63; H, 5.66. +6.14 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.74 (s, 1 H, C=NH), 8.24-7.32 (m, 10 H, Bz-H), 6.48 (d, J = 1.4 Hz, 1 H, H-1), 5.90 (m, 1 H), 5.67-5.62 (m, 2 H), 5.18-5.09 (m, 2 H), 4.31-4.07 (m, 2 H), 3.87 (m, 1 H), 3.70 (m, 1 H), 1.44 (d, J = 6.5 Hz, 3 H, H-6). Anal. Calcd. for C25H24Cl3NO7: C, 53.93; H, 4.34; N, 2.52; found: C, 53.87; H, 4.15; N, 2.78.
+6.14 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.74 (s, 1 H, C=NH), 8.24-7.32 (m, 10 H, Bz-H), 6.48 (d, J = 1.4 Hz, 1 H, H-1), 5.90 (m, 1 H), 5.67-5.62 (m, 2 H), 5.18-5.09 (m, 2 H), 4.31-4.07 (m, 2 H), 3.87 (m, 1 H), 3.70 (m, 1 H), 1.44 (d, J = 6.5 Hz, 3 H, H-6). Anal. Calcd. for C25H24Cl3NO7: C, 53.93; H, 4.34; N, 2.52; found: C, 53.87; H, 4.15; N, 2.78. +39.3 (c 0.5, CHCl3), 1H-NMR (CDCl3): δ 8.23-7.30 (m, 15 H, Ar-H), 5.88 (m, 1 H, CH2=CH-CH2O), 5.53 (dd, 1 H, J = 3.4, 3.5 Hz, H-3'), 5.42 (m, 1 H), 5.29 (br s, 1 H, H-12), 5.23-5.02 (m, 5 H), 4.32-4.23 (m, 2 H), 4.03 (m, 1 H), 3.76 (s, 1 H, CH2=CH-CH2O), 3.17 (dd, 1 H, J = 5.1, 10.7 Hz, H-3), 2.90 (dd, 1 H, J = 3.8, 13.7 Hz, H-18), 1.35 (d, 3 H, J = 6.5 Hz, H-6'), 1.12, 1.01, 0.92, 0.91, 0.89, 0.83, 0.60 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3): δ 177.4, 166.3, 165.6 (3 C=O), 143.7, 136.4, 135.0, 133.1, 133.0, 130.3, 130.1, 129.7, 129.7, 128.4, 128.4, 128.4, 128.4, 128.4, 128.2, 128.2, 128.0, 128.0, 128.0, 127.9, 122.5, 116.7, 100.6 (C-1'), 89.3, 76.4, 74.3, 70.1, 69.0, 66.5, 65.9, 55.4, 47.6, 46.7, 45.9, 41.7, 41.4, 39.3, 39.0, 38.4, 36.7, 33.9, 33.1, 32.7, 32.4, 30.7, 28.3, 27.6, 25.8, 25.2, 23.6, 23.4, 23.1, 18.3, 16.9, 16.5, 16.5, 15.3; Anal. Calcd. for C60H76O9: C, 76.56; H, 8.14; found: C, 76.65; H, 8.31.
+39.3 (c 0.5, CHCl3), 1H-NMR (CDCl3): δ 8.23-7.30 (m, 15 H, Ar-H), 5.88 (m, 1 H, CH2=CH-CH2O), 5.53 (dd, 1 H, J = 3.4, 3.5 Hz, H-3'), 5.42 (m, 1 H), 5.29 (br s, 1 H, H-12), 5.23-5.02 (m, 5 H), 4.32-4.23 (m, 2 H), 4.03 (m, 1 H), 3.76 (s, 1 H, CH2=CH-CH2O), 3.17 (dd, 1 H, J = 5.1, 10.7 Hz, H-3), 2.90 (dd, 1 H, J = 3.8, 13.7 Hz, H-18), 1.35 (d, 3 H, J = 6.5 Hz, H-6'), 1.12, 1.01, 0.92, 0.91, 0.89, 0.83, 0.60 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3): δ 177.4, 166.3, 165.6 (3 C=O), 143.7, 136.4, 135.0, 133.1, 133.0, 130.3, 130.1, 129.7, 129.7, 128.4, 128.4, 128.4, 128.4, 128.4, 128.2, 128.2, 128.0, 128.0, 128.0, 127.9, 122.5, 116.7, 100.6 (C-1'), 89.3, 76.4, 74.3, 70.1, 69.0, 66.5, 65.9, 55.4, 47.6, 46.7, 45.9, 41.7, 41.4, 39.3, 39.0, 38.4, 36.7, 33.9, 33.1, 32.7, 32.4, 30.7, 28.3, 27.6, 25.8, 25.2, 23.6, 23.4, 23.1, 18.3, 16.9, 16.5, 16.5, 15.3; Anal. Calcd. for C60H76O9: C, 76.56; H, 8.14; found: C, 76.65; H, 8.31. +45.0 (c 0.5, CHCl3), 1H-NMR (CDCl3): δ 8.07-7.26 (m, 15 H, Ar-H), 5.49-5.47 (m, 2 H), 5.29 (br s, 1 H, H-12), 5.07 (m, 3 H), 4.32-3.96 (m, 2 H, H-4', H-5'), 3.20 (dd, 1 H, J = 5.8, 9.8 Hz, H-3), 2.90 (dd, 1 H, J = 4.4, 13.9 Hz, H-18), 2.55 (d, 1 H, J = 11.1 Hz, OH), 1.34 (d, 3 H, J = 6.5 Hz, H-6'), 1.12, 1.02, 0.92, 0.92, 0.89, 0.85, 0.61 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3): δ 177.4, 165.5, 165.5 (3 C=O), 143.7, 136.5, 133.6, 133.2, 129.8, 129.8, 129.7, 129.7, 129.5, 128.7, 128.7, 128.4, 128.4, 128.3, 128.0, 128.0, 127.9, 127.9, 126.8, 122.5, 100.4 (C-1'), 89.8, 70.6, 70.2, 68.9, 66.7, 65.9, 55.4, 47.6, 46.8, 45.9, 41.7, 41.4, 39.3, 39.0, 38.4, 36.7, 33.8, 33.1, 32.7, 32.4, 30.7, 28.3, 27.6, 25.9, 25.3, 23.6, 23.4, 23.1, 18.3, 16.9, 16.5, 16.2, 15.3; Anal. Calcd. for C57H72O9: C, 75.97; H, 8.05; found: C, 75.83; H, 8.19.
+45.0 (c 0.5, CHCl3), 1H-NMR (CDCl3): δ 8.07-7.26 (m, 15 H, Ar-H), 5.49-5.47 (m, 2 H), 5.29 (br s, 1 H, H-12), 5.07 (m, 3 H), 4.32-3.96 (m, 2 H, H-4', H-5'), 3.20 (dd, 1 H, J = 5.8, 9.8 Hz, H-3), 2.90 (dd, 1 H, J = 4.4, 13.9 Hz, H-18), 2.55 (d, 1 H, J = 11.1 Hz, OH), 1.34 (d, 3 H, J = 6.5 Hz, H-6'), 1.12, 1.02, 0.92, 0.92, 0.89, 0.85, 0.61 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3): δ 177.4, 165.5, 165.5 (3 C=O), 143.7, 136.5, 133.6, 133.2, 129.8, 129.8, 129.7, 129.7, 129.5, 128.7, 128.7, 128.4, 128.4, 128.3, 128.0, 128.0, 127.9, 127.9, 126.8, 122.5, 100.4 (C-1'), 89.8, 70.6, 70.2, 68.9, 66.7, 65.9, 55.4, 47.6, 46.8, 45.9, 41.7, 41.4, 39.3, 39.0, 38.4, 36.7, 33.8, 33.1, 32.7, 32.4, 30.7, 28.3, 27.6, 25.9, 25.3, 23.6, 23.4, 23.1, 18.3, 16.9, 16.5, 16.2, 15.3; Anal. Calcd. for C57H72O9: C, 75.97; H, 8.05; found: C, 75.83; H, 8.19. -61.4 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.09-7.25 (m, 20 H, Bz-H), 5.99 (dd, 1 H, J = 0.8, 3.3 Hz), 5.76 (dd, 1 H, J = 7.9, 10.5 Hz, H-2'), 5.62 (dd, 1 H, J = 3.4, 10.4 Hz, H-3'), 5.50 (d, 1 H, J = 3.6 Hz), 4.95 (d, 1 H, J = 7.9 Hz, H-1'), 4.67 (dd, 1 H, J = 6.3, 11.1 Hz), 4.50-4.25 (m, 6 H), 4.16-4.03 (m, 2 H), 1.43, 1.42, 1.34, 1.12 (s, 4 × 3 H, CH3); 13C-NMR (CDCl3): δ 166.0, 165.5, 165.5, 164.9 (4 C=O), 133.6, 133.5, 133.3, 133.3, 129.9, 129.9, 129.9, 129.9, 129.9, 129.8, 129.6, 129.6, 129.4, 129.1, 129.0, 128.7, 128.7, 128.6, 128.6, 128.5, 128.5, 128.3, 128.3, 111.9, 108.6, 104.9, 100.6 (2 × C-1), 82.9, 81.8, 80.5, 77.2, 73.1, 71.8, 71.5, 69.9, 68.0, 66.3, 61.9, 26.7, 26.6, 25.9, 25.3; Anal. Calcd. for C46H46O15: C, 65.86; H, 5.53; found: C, 65.72; H, 5.75.
-61.4 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.09-7.25 (m, 20 H, Bz-H), 5.99 (dd, 1 H, J = 0.8, 3.3 Hz), 5.76 (dd, 1 H, J = 7.9, 10.5 Hz, H-2'), 5.62 (dd, 1 H, J = 3.4, 10.4 Hz, H-3'), 5.50 (d, 1 H, J = 3.6 Hz), 4.95 (d, 1 H, J = 7.9 Hz, H-1'), 4.67 (dd, 1 H, J = 6.3, 11.1 Hz), 4.50-4.25 (m, 6 H), 4.16-4.03 (m, 2 H), 1.43, 1.42, 1.34, 1.12 (s, 4 × 3 H, CH3); 13C-NMR (CDCl3): δ 166.0, 165.5, 165.5, 164.9 (4 C=O), 133.6, 133.5, 133.3, 133.3, 129.9, 129.9, 129.9, 129.9, 129.9, 129.8, 129.6, 129.6, 129.4, 129.1, 129.0, 128.7, 128.7, 128.6, 128.6, 128.5, 128.5, 128.3, 128.3, 111.9, 108.6, 104.9, 100.6 (2 × C-1), 82.9, 81.8, 80.5, 77.2, 73.1, 71.8, 71.5, 69.9, 68.0, 66.3, 61.9, 26.7, 26.6, 25.9, 25.3; Anal. Calcd. for C46H46O15: C, 65.86; H, 5.53; found: C, 65.72; H, 5.75. +98.2 (c 1.0, CHCl3); 1H-NMR (CDCl3): δ 8.08-7.23 (m, 20 H, Bz-H), 6.01 (d, 1 H, J = 2.5 Hz), 5.79 (dd, 1 H, J = 8.0, 10.5 Hz, H-2'), 5.61 (dd, 1 H, J = 3.4, 10.5 Hz, H-3'), 5.53 (d, 1 H, J = 3.7 Hz), 4.98 (d, 1 H, J = 7.9 Hz, H-1'), 4.57 (d, 2 H, J = 6.1 Hz), 4.50-4.29 (m, 3 H), 4.23 (d, 1 H, J = 3.7 Hz), 4.19-4.07 (m, 3 H), 3.92-3.85 (m, 1 H), 3.69 (dd, 1 H, J = 5.7, 11.5 Hz, H-18), 1.42, 1.06 (s, 2 × 3 H, CH3); 13C- NMR (CDCl3): δ 166.1, 165.5, 165.5, 164.8 (4 C=O), 133.8, 133.6, 133.4, 133.4, 133.3, 130.0, 129.9, 129.8, 129.8, 129.8, 129.6, 129.1, 129.0, 129.0, 128.8, 128.8, 128.7, 128.7, 128.6, 128.6, 128.5, 128.3, 128.3, 112.2, 105.2, 101.9 (2 × C-1), 83.6, 83.2, 80.0, 77.2, 72.4, 71.3, 69.5, 68.7, 68.0, 64.4, 62.2, 26.7, 26.2; Anal. Calcd. for C43H42O15: C, 64.66; H, 5.30; found: C, 64.49; H, 5.38.
+98.2 (c 1.0, CHCl3); 1H-NMR (CDCl3): δ 8.08-7.23 (m, 20 H, Bz-H), 6.01 (d, 1 H, J = 2.5 Hz), 5.79 (dd, 1 H, J = 8.0, 10.5 Hz, H-2'), 5.61 (dd, 1 H, J = 3.4, 10.5 Hz, H-3'), 5.53 (d, 1 H, J = 3.7 Hz), 4.98 (d, 1 H, J = 7.9 Hz, H-1'), 4.57 (d, 2 H, J = 6.1 Hz), 4.50-4.29 (m, 3 H), 4.23 (d, 1 H, J = 3.7 Hz), 4.19-4.07 (m, 3 H), 3.92-3.85 (m, 1 H), 3.69 (dd, 1 H, J = 5.7, 11.5 Hz, H-18), 1.42, 1.06 (s, 2 × 3 H, CH3); 13C- NMR (CDCl3): δ 166.1, 165.5, 165.5, 164.8 (4 C=O), 133.8, 133.6, 133.4, 133.4, 133.3, 130.0, 129.9, 129.8, 129.8, 129.8, 129.6, 129.1, 129.0, 129.0, 128.8, 128.8, 128.7, 128.7, 128.6, 128.6, 128.5, 128.3, 128.3, 112.2, 105.2, 101.9 (2 × C-1), 83.6, 83.2, 80.0, 77.2, 72.4, 71.3, 69.5, 68.7, 68.0, 64.4, 62.2, 26.7, 26.2; Anal. Calcd. for C43H42O15: C, 64.66; H, 5.30; found: C, 64.49; H, 5.38. +70.2 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 9.68 (d, 1 H, J = 1.5 Hz, CHO), 8.08-7.25 (m, 20 H, Bz-H), 5.97 (dd, 1 H, J = 0.9, 3.4 Hz), 5.74-5.59 (m, 3 H), 4.89 (d, 1 H, J = 7.8 Hz, H-1'), 4.70-4.54 (m, 3 H), 4.48-4.30 (m, 3 H), 1.44, 1.18 (s, 2 × 3 H, CH3); 13C-NMR (CDCl3): δ 197.9, 166.0, 165.7, 165.5, 164.8 (5 C=O), 133.7, 133.6, 133.3, 133.3, 130.3, 130.0, 130.0, 129.9, 129.8, 129.8, 129.8, 129.6, 129.6, 129.4, 129.2, 129.1, 129.0, 128.9, 128.8, 128.7, 128.7, 128.6, 128.5, 128.3, 112.8, 105.7, 100.6 (2 × C-1), 83.9, 83.0, 82.9, 77.2, 71.8, 71.5, 69.6, 67.9, 61.9, 26.6, 26.1; Anal. Calcd. for C43H40O14: C, 66.15; H, 5.16; found: C, 66.34; H, 5.25.
+70.2 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 9.68 (d, 1 H, J = 1.5 Hz, CHO), 8.08-7.25 (m, 20 H, Bz-H), 5.97 (dd, 1 H, J = 0.9, 3.4 Hz), 5.74-5.59 (m, 3 H), 4.89 (d, 1 H, J = 7.8 Hz, H-1'), 4.70-4.54 (m, 3 H), 4.48-4.30 (m, 3 H), 1.44, 1.18 (s, 2 × 3 H, CH3); 13C-NMR (CDCl3): δ 197.9, 166.0, 165.7, 165.5, 164.8 (5 C=O), 133.7, 133.6, 133.3, 133.3, 130.3, 130.0, 130.0, 129.9, 129.8, 129.8, 129.8, 129.6, 129.6, 129.4, 129.2, 129.1, 129.0, 128.9, 128.8, 128.7, 128.7, 128.6, 128.5, 128.3, 112.8, 105.7, 100.6 (2 × C-1), 83.9, 83.0, 82.9, 77.2, 71.8, 71.5, 69.6, 67.9, 61.9, 26.6, 26.1; Anal. Calcd. for C43H40O14: C, 66.15; H, 5.16; found: C, 66.34; H, 5.25. +184.2 (c 1.0, CHCl3); 1H-NMR (CDCl3): δ 8.08-7.26 (m, 20 H, Bz-H), 6.00 (dd, 1 H, J = 0.8, 3.3 Hz), 5.80-5.55 (m, 3 H), 4.96 (d, 1 H, J = 7.9 Hz, H-1'), 4.70-4.30 (m, 6 H), 4.16-3.91 (m, 2 H), 2.56 (dd, 1 H, J = 6.7, 6.7 Hz), 1.44, 1.12 (s, 2 × 3 H, CH3); 13C-NMR (CDCl3): δ 166.1, 165.5, 165.5, 164.9 (4 C=O), 133.8, 133.6, 133.4, , 133.4, 130.1, 130.1, 130.0, 130.0, 129.8, 129.8, 129.7, 129.6, 129.2, 129.0, 128.9, 128.8, 128.7, 128.7, 128.7, 128.6, 128.5, 128.5, 128.3, 112.1, 104.9, 101.3 (2 × C-1), 83.6, 82.8, 79.8, 77.2, 72.1, 71.4, 69.6, 68.0, 62.2, 59.9, 26.9, 26.1; Anal. Calcd. for C42H40O14: C, 65.62; H, 5.24; found: C, 65.35; H, 5.37.
+184.2 (c 1.0, CHCl3); 1H-NMR (CDCl3): δ 8.08-7.26 (m, 20 H, Bz-H), 6.00 (dd, 1 H, J = 0.8, 3.3 Hz), 5.80-5.55 (m, 3 H), 4.96 (d, 1 H, J = 7.9 Hz, H-1'), 4.70-4.30 (m, 6 H), 4.16-3.91 (m, 2 H), 2.56 (dd, 1 H, J = 6.7, 6.7 Hz), 1.44, 1.12 (s, 2 × 3 H, CH3); 13C-NMR (CDCl3): δ 166.1, 165.5, 165.5, 164.9 (4 C=O), 133.8, 133.6, 133.4, , 133.4, 130.1, 130.1, 130.0, 130.0, 129.8, 129.8, 129.7, 129.6, 129.2, 129.0, 128.9, 128.8, 128.7, 128.7, 128.7, 128.6, 128.5, 128.5, 128.3, 112.1, 104.9, 101.3 (2 × C-1), 83.6, 82.8, 79.8, 77.2, 72.1, 71.4, 69.6, 68.0, 62.2, 59.9, 26.9, 26.1; Anal. Calcd. for C42H40O14: C, 65.62; H, 5.24; found: C, 65.35; H, 5.37. +331.6 (c 1.0, CHCl3); 1H-NMR (CDCl3): δ 8.10-7.25 (m, 20 H, Bz-H), 6.01 (d, 1 H, J = 3.3 Hz), 5.85 (m, 1 H), 5.65 (m, 1 H), 5.07-5.02 (m, 2 H), 4.58-4.40 (m, 3 H), 3.79-3.74 (m, 3 H), 3.50 (m, 1 H), 3.27 (m, 1 H); 13C-NMR (CDCl3): δ 166.1, 165.6, 165.5, 165.5 (4 C=O), 133.7, 133.5, 133.4, 133.4, 130.0, 130.0, 129.8, 129.7, 129.1, 129.0, 128.8, 128.8, 128.7, 128.6, 128.5, 128.5, 128.4, 128.3, 102.8, 102.6 (2 × C-1), 97.3, 92.4, 88.2, 85.7, 77.2, 73.4, 72.0, 71.5, 70.7, 70.0, 69.9, 68.2, 68.1, 62.4, 62.0; Anal. Calcd. for C39H36O14: C, 64.28; H, 4.98; found: C, 64.39; H, 4.83.
+331.6 (c 1.0, CHCl3); 1H-NMR (CDCl3): δ 8.10-7.25 (m, 20 H, Bz-H), 6.01 (d, 1 H, J = 3.3 Hz), 5.85 (m, 1 H), 5.65 (m, 1 H), 5.07-5.02 (m, 2 H), 4.58-4.40 (m, 3 H), 3.79-3.74 (m, 3 H), 3.50 (m, 1 H), 3.27 (m, 1 H); 13C-NMR (CDCl3): δ 166.1, 165.6, 165.5, 165.5 (4 C=O), 133.7, 133.5, 133.4, 133.4, 130.0, 130.0, 129.8, 129.7, 129.1, 129.0, 128.8, 128.8, 128.7, 128.6, 128.5, 128.5, 128.4, 128.3, 102.8, 102.6 (2 × C-1), 97.3, 92.4, 88.2, 85.7, 77.2, 73.4, 72.0, 71.5, 70.7, 70.0, 69.9, 68.2, 68.1, 62.4, 62.0; Anal. Calcd. for C39H36O14: C, 64.28; H, 4.98; found: C, 64.39; H, 4.83. +18.4 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.52 (s, 1 H, C=NH), 8.18-7.08 (m, 30 H, Bz-H ), 6.54 (d, 1 H, J = 3.5 Hz), 5.87 (d, 1 H, J = 3.3 Hz), 5.66 (dd, 1 H, J = 7.9, 10.4 Hz), 5.48-5.37 (m, 2 H), 5.27 (dd, 1 H, J = 3.5, 9.7 Hz), 5.14 (d, 1 H, J = 7.9 Hz, H-1'), 4.67 (dd, 1 H, J = 9.4, 9.4 Hz), 4.41-4.18 (m, 4 H), 3.95 (dd, 1 H, J = 10.9, 11.0 Hz). Anal. Calcd. for C55H44Cl3NO16: C, 61.09; H, 4.10; N, 1.30; found: C, 61.34; H, 4.27; N, 1.49.
+18.4 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.52 (s, 1 H, C=NH), 8.18-7.08 (m, 30 H, Bz-H ), 6.54 (d, 1 H, J = 3.5 Hz), 5.87 (d, 1 H, J = 3.3 Hz), 5.66 (dd, 1 H, J = 7.9, 10.4 Hz), 5.48-5.37 (m, 2 H), 5.27 (dd, 1 H, J = 3.5, 9.7 Hz), 5.14 (d, 1 H, J = 7.9 Hz, H-1'), 4.67 (dd, 1 H, J = 9.4, 9.4 Hz), 4.41-4.18 (m, 4 H), 3.95 (dd, 1 H, J = 10.9, 11.0 Hz). Anal. Calcd. for C55H44Cl3NO16: C, 61.09; H, 4.10; N, 1.30; found: C, 61.34; H, 4.27; N, 1.49. +73.7 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.06-7.31 (m, 15 H, Ar-H), 5.81 (m, 1 H, CH2=CH-CH2O), 5.66 (dd, 1 H, J = 3.2, 9.5 Hz, H-3'), 5.57 (dd, 1 H, J = 1.7, 3.2 Hz, H-2'), 5.29 (br s, 1 H, H-12), 5.20-5.03 (m, 4 H, PhCH2, CH2=CH-CH2O), 4.97 (d, 1 H, J1,2 = 1.7 Hz, H-1'), 4.22-4.04 (m, 3 H), 3.67 (dd, 1 H, J = 9.5, 9.5 Hz, H-4'), 3.15 (dd, 1 H, J = 6.3, 9.8 Hz, H-3), 2.91 (dd, 1 H, J = 4.1, 13.4 Hz, H-18), 1.40 (d, 3 H, J = 6.2 Hz, H-6'), 1.12, 1.02, 0.92, 0.92, 0.89, 0.88, 0.61 (s, 7 × 3H, CH3); 13C-NMR (CDCl3): δ 177.4, 165.5, 165.3 (3 C=O), 143.7, 136.4, 134.5, 133.2, 132.9, 130.0, 130.0, 129.9, 129.7, 129.5, 129.5, 128.4, 128.4, 128.4, 128.4, 128.3, 128.0, 128.0, 127.9, 122.5, 117.2, 99.6 (C-1'), 89.7, 79.1, 77.2, 74.0, 72.5, 71.5, 67.8, 65.9, 55.4, 47.6, 45.9, 41.7, 41.4, 39.3, 39.0, 38.4, 36.7, 33.9, 33.1, 32.7, 32.4, 30.7, 28.3, 27.6, 25.9, 25.3, 23.6, 23.4, 23.1, 18.3, 18.0, 16.9, 16.5, 15.3; Anal. Calcd. for C60H76O9: C, 76.56; H, 8.14; found: C, 76.73; H, 8.43.
+73.7 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.06-7.31 (m, 15 H, Ar-H), 5.81 (m, 1 H, CH2=CH-CH2O), 5.66 (dd, 1 H, J = 3.2, 9.5 Hz, H-3'), 5.57 (dd, 1 H, J = 1.7, 3.2 Hz, H-2'), 5.29 (br s, 1 H, H-12), 5.20-5.03 (m, 4 H, PhCH2, CH2=CH-CH2O), 4.97 (d, 1 H, J1,2 = 1.7 Hz, H-1'), 4.22-4.04 (m, 3 H), 3.67 (dd, 1 H, J = 9.5, 9.5 Hz, H-4'), 3.15 (dd, 1 H, J = 6.3, 9.8 Hz, H-3), 2.91 (dd, 1 H, J = 4.1, 13.4 Hz, H-18), 1.40 (d, 3 H, J = 6.2 Hz, H-6'), 1.12, 1.02, 0.92, 0.92, 0.89, 0.88, 0.61 (s, 7 × 3H, CH3); 13C-NMR (CDCl3): δ 177.4, 165.5, 165.3 (3 C=O), 143.7, 136.4, 134.5, 133.2, 132.9, 130.0, 130.0, 129.9, 129.7, 129.5, 129.5, 128.4, 128.4, 128.4, 128.4, 128.3, 128.0, 128.0, 127.9, 122.5, 117.2, 99.6 (C-1'), 89.7, 79.1, 77.2, 74.0, 72.5, 71.5, 67.8, 65.9, 55.4, 47.6, 45.9, 41.7, 41.4, 39.3, 39.0, 38.4, 36.7, 33.9, 33.1, 32.7, 32.4, 30.7, 28.3, 27.6, 25.9, 25.3, 23.6, 23.4, 23.1, 18.3, 18.0, 16.9, 16.5, 15.3; Anal. Calcd. for C60H76O9: C, 76.56; H, 8.14; found: C, 76.73; H, 8.43. +29.5 (c 1.0, CHCl3); 1H- NMR (CDCl3): δ 8.09-7.26 (m, 15 H, Ar-H), 5.57-5.48 (m, 2 H), 5.29 (br s, 1 H, H-12), 5.07 (dd, 2 H, J = 12.6, 17.1 Hz, PhCH2), 5.00 (d, 1 H, J = 1.6 Hz, H-1'), 4.06-3.87 (m, 2 H), 3.18 (dd, 1 H, J = 2.9, 13.0 Hz, H-3), 2.90 (dd, 1 H, J = 4.4, 14.2 Hz, H-18), 2.49 (d, 1 H, J = 5.1 Hz, OH), 1.41 (d, 3 H, J = 6.1 Hz, H-6'), 1.12, 1.01, 0.92, 0.92, 0.89, 0.87, 0.61 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3): δ 177.4, 166.8, 165.6 (3 C=O), 143.6, 136.4, 133.3, 133.2, 129.7, 129.7, 129.7, 129.7, 129.5, 129.4, 128.4, 128.4, 128.3, 128.3, 127.9, 127.9, 127.9, 127.8, 126.8, 122.4, 99.7 (C-1'), 89.7, 73.4, 72.1, 71.3, 68.7, 65.9, 55.4, 47.5, 46.7, 45.8, 41.6, 41.4, 39.3, 38.9, 38.4, 36.7, 33.8, 33.0, 32.7, 32.3, 30.6, 28.3, 27.6, 25.8, 25.3, 23.6, 23.4, 23.0, 18.2, 17.5, 16.8, 16.5, 15.3; Anal. Calcd. for C57H72O9: C, 75.97; H, 8.05; found: C, 75.81; H, 8.29.
+29.5 (c 1.0, CHCl3); 1H- NMR (CDCl3): δ 8.09-7.26 (m, 15 H, Ar-H), 5.57-5.48 (m, 2 H), 5.29 (br s, 1 H, H-12), 5.07 (dd, 2 H, J = 12.6, 17.1 Hz, PhCH2), 5.00 (d, 1 H, J = 1.6 Hz, H-1'), 4.06-3.87 (m, 2 H), 3.18 (dd, 1 H, J = 2.9, 13.0 Hz, H-3), 2.90 (dd, 1 H, J = 4.4, 14.2 Hz, H-18), 2.49 (d, 1 H, J = 5.1 Hz, OH), 1.41 (d, 3 H, J = 6.1 Hz, H-6'), 1.12, 1.01, 0.92, 0.92, 0.89, 0.87, 0.61 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3): δ 177.4, 166.8, 165.6 (3 C=O), 143.6, 136.4, 133.3, 133.2, 129.7, 129.7, 129.7, 129.7, 129.5, 129.4, 128.4, 128.4, 128.3, 128.3, 127.9, 127.9, 127.9, 127.8, 126.8, 122.4, 99.7 (C-1'), 89.7, 73.4, 72.1, 71.3, 68.7, 65.9, 55.4, 47.5, 46.7, 45.8, 41.6, 41.4, 39.3, 38.9, 38.4, 36.7, 33.8, 33.0, 32.7, 32.3, 30.6, 28.3, 27.6, 25.8, 25.3, 23.6, 23.4, 23.0, 18.2, 17.5, 16.8, 16.5, 15.3; Anal. Calcd. for C57H72O9: C, 75.97; H, 8.05; found: C, 75.81; H, 8.29. +6.1 (c 0.5, MeOH); 1H-NMR (pyridine-d5): δ 5.47 (br s, 1 H, H-12), 5.31 (d, 1 H, J = 1.3 Hz, H-1'), 4.85 (dd, 1 H, J = 1.5, 3.0 Hz, H-2'), 4.25-4.21 (m, 2 H), 4.06 (d, 1 H, J = 1.4 Hz), 3.29 (dd, 1 H, J = 4.0, 13.7 Hz, H-3), 3.13 (dd, 1 H, J = 4.3, 11.5 Hz, H-18), 1.54 (d, 3 H, J = 6.5 Hz, H-6'), 1.28, 1.00, 0.99, 0.95, 0.90, 0.85, 0.80 (s, 7 × 3 H, CH3); 13C-NMR (pyridine-d5): δ 180.2, 144.9, 122.6, 104.9 (C-1'), 88.6, 74.3, 72.4, 67.8, 67.5, 55.7, 48.1, 46.7, 46.6, 42.2, 42.1, 39.8, 39.2, 38.6, 37.1, 34.3, 33.4, 33.3, 33.2, 31.0, 28.4, 28.3, 26.2, 25.8, 23.9, 23.9, 23.8, 18.7, 17.5, 17.4, 16.8, 15.5; HRESIMS: m/z calcd. for C36H58O7Na[M+Na+]: 625.4080; found: m/z 625.4059.
+6.1 (c 0.5, MeOH); 1H-NMR (pyridine-d5): δ 5.47 (br s, 1 H, H-12), 5.31 (d, 1 H, J = 1.3 Hz, H-1'), 4.85 (dd, 1 H, J = 1.5, 3.0 Hz, H-2'), 4.25-4.21 (m, 2 H), 4.06 (d, 1 H, J = 1.4 Hz), 3.29 (dd, 1 H, J = 4.0, 13.7 Hz, H-3), 3.13 (dd, 1 H, J = 4.3, 11.5 Hz, H-18), 1.54 (d, 3 H, J = 6.5 Hz, H-6'), 1.28, 1.00, 0.99, 0.95, 0.90, 0.85, 0.80 (s, 7 × 3 H, CH3); 13C-NMR (pyridine-d5): δ 180.2, 144.9, 122.6, 104.9 (C-1'), 88.6, 74.3, 72.4, 67.8, 67.5, 55.7, 48.1, 46.7, 46.6, 42.2, 42.1, 39.8, 39.2, 38.6, 37.1, 34.3, 33.4, 33.3, 33.2, 31.0, 28.4, 28.3, 26.2, 25.8, 23.9, 23.9, 23.8, 18.7, 17.5, 17.4, 16.8, 15.5; HRESIMS: m/z calcd. for C36H58O7Na[M+Na+]: 625.4080; found: m/z 625.4059. +70.6 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 7.47-7.27 (m, 5 H, Bn-H), 5.35-5.24 (m, 3 H), 5.16-5.06 (m, 3 H), 4.97 (d, 1 H, J = 1.7 Hz, H-1'), 4.25 (dd, 1 H, J = 5.7, 12.5 Hz, H-3), 4.15-4.10 (m, 2 H), 3.21 (dd, 1 H, J = 4.0, 11.3 Hz, H-3), 2.90 (dd, 1 H, J = 4.0, 13.6 Hz, H-18), 2.16, 2.09, 2.05, 2.00 (s, 4 × 3 H, CH3CO), 1.11, 1.00, 0.92, 0.89, 0.89, 0.82, 0.60 (s, 7 × 3 H, CH3); 13C- NMR (CDCl3): δ 177.4, 170.6, 170.2, 169.9, 169.8 (5 C=O), 143.6, 136.3, 128.3, 128.3, 127.9, 127.9, 122.4, 94.6 (C-1'), 84.7, 77.2, 70.7, 69.2, 69.0, 66.4, 66.3, 65.9, 62.6, 55.6, 47.6, 46.7, 45.8, 41.6, 41.3, 39.3, 38.3, 38.0, 36.8, 33.8, 33.0, 32.7, 32.3, 30.6, 28.7, 27.6, 25.8, 23.6, 23.4, 23.0, 22.1, 20.8, 20.6, 20.6, 18.2, 16.8, 16.4, 15.2; HRESIMS: m/z calcd. for C51H72O8Na[M+Na+]: 835.5125; found: m/z 835.5118.
+70.6 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 7.47-7.27 (m, 5 H, Bn-H), 5.35-5.24 (m, 3 H), 5.16-5.06 (m, 3 H), 4.97 (d, 1 H, J = 1.7 Hz, H-1'), 4.25 (dd, 1 H, J = 5.7, 12.5 Hz, H-3), 4.15-4.10 (m, 2 H), 3.21 (dd, 1 H, J = 4.0, 11.3 Hz, H-3), 2.90 (dd, 1 H, J = 4.0, 13.6 Hz, H-18), 2.16, 2.09, 2.05, 2.00 (s, 4 × 3 H, CH3CO), 1.11, 1.00, 0.92, 0.89, 0.89, 0.82, 0.60 (s, 7 × 3 H, CH3); 13C- NMR (CDCl3): δ 177.4, 170.6, 170.2, 169.9, 169.8 (5 C=O), 143.6, 136.3, 128.3, 128.3, 127.9, 127.9, 122.4, 94.6 (C-1'), 84.7, 77.2, 70.7, 69.2, 69.0, 66.4, 66.3, 65.9, 62.6, 55.6, 47.6, 46.7, 45.8, 41.6, 41.3, 39.3, 38.3, 38.0, 36.8, 33.8, 33.0, 32.7, 32.3, 30.6, 28.7, 27.6, 25.8, 23.6, 23.4, 23.0, 22.1, 20.8, 20.6, 20.6, 18.2, 16.8, 16.4, 15.2; HRESIMS: m/z calcd. for C51H72O8Na[M+Na+]: 835.5125; found: m/z 835.5118. +79.8 (c 0.5, MeOH); 1H-NMR (pyridine-d5): δ 5.54 (d, 1 H, J = 1.0 Hz, H-1'), 5.46 (br s, 1 H, H-12), 4.69 (m, 1 H), 4.59-4.50 (m, 3 H), 4.46-4.38 (m, 2 H), 3.47 (dd, 1 H, J = 4.2, 11.4 Hz, H-3), 3.28 (dd, 1 H, J = 4.0, 13.5 Hz, H-18), 1.24, 1.15, 1.00, 0.97, 0.94, 0.81, 0.79 (s, 7 × 3 H, CH3); 13C-NMR (pyridine-d5): δ 180.1 (C=O), 144.8, 124.1, 122.4, 97.7 (C-1'), 81.8, 75.8, 73.2, 72.9, 69.2, 63.4, 55.7, 47.9, 46.6, 46.4, 42.1, 41.9, 39.7, 38.5, 38.1, 37.1, 34.2, 33.2, 33.1, 33.1, 30.9, 29.0, 28.2, 26.1, 23.7, 23.6, 22.0, 18.5, 17.3, 16.9, 15.3; HRESIMS: m/z calcd. for C36H58O8Na[M+Na+]: 641.4029; found: m/z 641.4037.
+79.8 (c 0.5, MeOH); 1H-NMR (pyridine-d5): δ 5.54 (d, 1 H, J = 1.0 Hz, H-1'), 5.46 (br s, 1 H, H-12), 4.69 (m, 1 H), 4.59-4.50 (m, 3 H), 4.46-4.38 (m, 2 H), 3.47 (dd, 1 H, J = 4.2, 11.4 Hz, H-3), 3.28 (dd, 1 H, J = 4.0, 13.5 Hz, H-18), 1.24, 1.15, 1.00, 0.97, 0.94, 0.81, 0.79 (s, 7 × 3 H, CH3); 13C-NMR (pyridine-d5): δ 180.1 (C=O), 144.8, 124.1, 122.4, 97.7 (C-1'), 81.8, 75.8, 73.2, 72.9, 69.2, 63.4, 55.7, 47.9, 46.6, 46.4, 42.1, 41.9, 39.7, 38.5, 38.1, 37.1, 34.2, 33.2, 33.1, 33.1, 30.9, 29.0, 28.2, 26.1, 23.7, 23.6, 22.0, 18.5, 17.3, 16.9, 15.3; HRESIMS: m/z calcd. for C36H58O8Na[M+Na+]: 641.4029; found: m/z 641.4037. -24.6 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.33-7.26 (m, 30 H, Ar-H), 5.67 (dd, 1 H, J = 8.2, 9.1 Hz, H-2''), 5.58 (dd, 1 H, J = 6.7, 9.1 Hz, H-3''), 5.48 (d, 1 H, J = 2.2 Hz, H-1'), 5.40 (dd, 1 H, J = 3.6, 3.6 Hz, H-3'), 5.28 (br s, 1 H, H-12), 5.07 (dd, 2 H, J = 12.5, 17.6 Hz, PhCH2), 4.98-4.92 (m, 2 H), 4.70 (d, 1 H, J = 6.7 Hz, H-1''), 4.29-4.19 (m, 2 H), 3.50 (m, 1 H), 3.14-3.04 (m, 2 H), 2.89 (dd, 1 H, J = 4.1, 9.6 Hz, H-18), 1.19 (d, 3 H, J = 6.5 Hz, H-6'), 1.10, 0.97, 0.91, 0.89, 0.89, 0.81, 0.59 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3): δ 177.4, 166.3, 166.2, 165.6, 165.2, 164.9 (6 C=O), 143.7, 140.9, 138.8, 136.4, 133.3, 133.2, 133.1, 133.0, 130.4, 130.0, 130.0, 129.9, 129.8, 129.7, 129.2, 129.1, 128.6, 128.5, 128.4, 128.4, 128.3, 128.3, 128.3, 128.0, 127.9, 127.8, 127.6, 127.5, 126.9, 122.4, 103.1, 100.7 (2 × C-1), 99.4, 89.5, 78.1, 77.3, 76.6, 76.4, 74.2, 73.7, 72.8, 72.3, 71.8, 71.6, 69.9, 68.6, 68.3, 65.9, 65.6, 65.3, 62.1, 60.2, 55.5, 55.4, 47.5, 46.7, 45.9, 41.7, 41.4, 39.3, 38.9, 38.4, 36.7, 33.1, 32.4, 30.7, 28.3, 27.6, 25.8, 23.6, 23.4, 23.1, 18.2, 16.9, 16.5, 16.1, 15.3; HRESIMS: m/z calcd. for C83H92O16Na[M+Na+]: 1367.6283; found: m/z 1367.6290.
-24.6 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.33-7.26 (m, 30 H, Ar-H), 5.67 (dd, 1 H, J = 8.2, 9.1 Hz, H-2''), 5.58 (dd, 1 H, J = 6.7, 9.1 Hz, H-3''), 5.48 (d, 1 H, J = 2.2 Hz, H-1'), 5.40 (dd, 1 H, J = 3.6, 3.6 Hz, H-3'), 5.28 (br s, 1 H, H-12), 5.07 (dd, 2 H, J = 12.5, 17.6 Hz, PhCH2), 4.98-4.92 (m, 2 H), 4.70 (d, 1 H, J = 6.7 Hz, H-1''), 4.29-4.19 (m, 2 H), 3.50 (m, 1 H), 3.14-3.04 (m, 2 H), 2.89 (dd, 1 H, J = 4.1, 9.6 Hz, H-18), 1.19 (d, 3 H, J = 6.5 Hz, H-6'), 1.10, 0.97, 0.91, 0.89, 0.89, 0.81, 0.59 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3): δ 177.4, 166.3, 166.2, 165.6, 165.2, 164.9 (6 C=O), 143.7, 140.9, 138.8, 136.4, 133.3, 133.2, 133.1, 133.0, 130.4, 130.0, 130.0, 129.9, 129.8, 129.7, 129.2, 129.1, 128.6, 128.5, 128.4, 128.4, 128.3, 128.3, 128.3, 128.0, 127.9, 127.8, 127.6, 127.5, 126.9, 122.4, 103.1, 100.7 (2 × C-1), 99.4, 89.5, 78.1, 77.3, 76.6, 76.4, 74.2, 73.7, 72.8, 72.3, 71.8, 71.6, 69.9, 68.6, 68.3, 65.9, 65.6, 65.3, 62.1, 60.2, 55.5, 55.4, 47.5, 46.7, 45.9, 41.7, 41.4, 39.3, 38.9, 38.4, 36.7, 33.1, 32.4, 30.7, 28.3, 27.6, 25.8, 23.6, 23.4, 23.1, 18.2, 16.9, 16.5, 16.1, 15.3; HRESIMS: m/z calcd. for C83H92O16Na[M+Na+]: 1367.6283; found: m/z 1367.6290. -30.7 (c 0.5, MeOH); 1H-NMR (pyridine-d5): δ 5.47 (br s, 1 H, H-12), 5.27 (s, 1 H, H-1'), 4.80 (d, 1 H, J = 7.4 Hz, H-1''), 4.34-4.30 (m, 2 H), 4.25-4.20 (m, 3 H), 4.13-3.93 (m, 3 H), 3.69 (dd, 1 H, J = 9.6, 10.9 Hz), 3.29 (dd, 1 H, J = 3.9, 13.6 Hz, H-3), 3.11 (dd, 1 H, J = 4.3, 11.6 Hz, H-18), 1.70 (d, 3 H, J = 6.6 Hz, H-6'), 1.29, 1.00, 1.00, 0.95, 0.92, 0.84, 0.79 (s, 7 × 3 H, CH3); 13C-NMR (pyridine-d5): δ 180.1 (C=O), 144.8, 122.4, 106.3, 104.9 (2 × C-1), 88.6, 83.3, 77.8, 74.7, 71.9, 70.5, 67.2, 67.1, 66.7, 55.5, 47.9, 46.6, 46.4, 42.1, 42.0, 39.7, 39.1, 38.4, 36.9, 34.2, 33.2, 33.2, 33.1, 30.9, 28.2, 26.1, 25.6, 23.7, 23.7, 23.7, 23.6, 18.5, 17.3, 17.0, 16.6, 15.4; HRESIMS: m/z calcd. for C41H66O11Na[M+Na+]: 757.4503; found: m/z 757.4515.
-30.7 (c 0.5, MeOH); 1H-NMR (pyridine-d5): δ 5.47 (br s, 1 H, H-12), 5.27 (s, 1 H, H-1'), 4.80 (d, 1 H, J = 7.4 Hz, H-1''), 4.34-4.30 (m, 2 H), 4.25-4.20 (m, 3 H), 4.13-3.93 (m, 3 H), 3.69 (dd, 1 H, J = 9.6, 10.9 Hz), 3.29 (dd, 1 H, J = 3.9, 13.6 Hz, H-3), 3.11 (dd, 1 H, J = 4.3, 11.6 Hz, H-18), 1.70 (d, 3 H, J = 6.6 Hz, H-6'), 1.29, 1.00, 1.00, 0.95, 0.92, 0.84, 0.79 (s, 7 × 3 H, CH3); 13C-NMR (pyridine-d5): δ 180.1 (C=O), 144.8, 122.4, 106.3, 104.9 (2 × C-1), 88.6, 83.3, 77.8, 74.7, 71.9, 70.5, 67.2, 67.1, 66.7, 55.5, 47.9, 46.6, 46.4, 42.1, 42.0, 39.7, 39.1, 38.4, 36.9, 34.2, 33.2, 33.2, 33.1, 30.9, 28.2, 26.1, 25.6, 23.7, 23.7, 23.7, 23.6, 18.5, 17.3, 17.0, 16.6, 15.4; HRESIMS: m/z calcd. for C41H66O11Na[M+Na+]: 757.4503; found: m/z 757.4515. +64.5 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.07-7.05 (m, 35 H, Ar-H), 5.97 (d, 1 H, J = 3.1 Hz, H-4''), 5.75 (dd, 1 H, J = 7.9, 10.4 Hz, H-2''), 5.55-5.47 (m, 2 H, H-2', H-3'), 5.41 (dd, 1 H, J = 3.3, 10.4 Hz, H-3''), 5.30 (br s, 1 H, H-12), 5.13-5.07 (m, 3 H, H-1'', PhCH2), 4.94 (d, 1 H, J = 1.4 Hz, H-1'), 4.71-4.37 (m, 3 H), 4.21-4.05 (m, 2 H), 3.14 (dd, 1 H, J = 7.5, 8.6 Hz, H-3), 2.91 (dd, 1 H, J = 3.9, 13.7 Hz, H-18), 1.50 (d, 1 H, J = 6.0 Hz, H-6'), 1.12, 0.96, 0.92, 0.92, 0.89, 0.85, 0.61 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3): δ 177.4, 166.1, 165.5, 165.4, 165.4, 165.3, 164.7 (7 C=O), 143.7, 136.4, 133.5, 133.3, 133.2, 133.1, 132.8, 129.9, 129.7, 129.7, 129.7, 129.6, 129.6, 129.6, 129.6, 129.6, 129.5, 129.5, 129.4, 129.3, 129.0, 128.8, 128.6, 128.6, 128.5, 128.5, 128.4, 128.4, 128.4, 128.4, 128.4, 128.4, 128.4, 128.4, 128.3, 128.2, 128.1, 128.0, 128.0, 128.0, 128.0, 127.9, 122.5, 101.5, 99.8 (2 × C-1), 89.9, 77.6, 77.2, 72.5, 71.9, 71.1, 70.9, 69.7, 68.0, 67.1, 65.9, 62.0, 55.4, 47.6, 46.7, 45.9, 41.7, 41.4, 39.3, 38.9, 38.5, 36.7, 33.8, 33.0, 32.7, 32.4, 30.6, 28.3, 27.6, 25.8, 25.4, 23.6, 23.4, 23.1, 18.2, 18.1, 16.8, 16.5, 15.3; HRESIMS: m/z calcd. for C91H98O18Na[M+Na+]: 1501.6651; found: m/z 1501.6629.
+64.5 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.07-7.05 (m, 35 H, Ar-H), 5.97 (d, 1 H, J = 3.1 Hz, H-4''), 5.75 (dd, 1 H, J = 7.9, 10.4 Hz, H-2''), 5.55-5.47 (m, 2 H, H-2', H-3'), 5.41 (dd, 1 H, J = 3.3, 10.4 Hz, H-3''), 5.30 (br s, 1 H, H-12), 5.13-5.07 (m, 3 H, H-1'', PhCH2), 4.94 (d, 1 H, J = 1.4 Hz, H-1'), 4.71-4.37 (m, 3 H), 4.21-4.05 (m, 2 H), 3.14 (dd, 1 H, J = 7.5, 8.6 Hz, H-3), 2.91 (dd, 1 H, J = 3.9, 13.7 Hz, H-18), 1.50 (d, 1 H, J = 6.0 Hz, H-6'), 1.12, 0.96, 0.92, 0.92, 0.89, 0.85, 0.61 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3): δ 177.4, 166.1, 165.5, 165.4, 165.4, 165.3, 164.7 (7 C=O), 143.7, 136.4, 133.5, 133.3, 133.2, 133.1, 132.8, 129.9, 129.7, 129.7, 129.7, 129.6, 129.6, 129.6, 129.6, 129.6, 129.5, 129.5, 129.4, 129.3, 129.0, 128.8, 128.6, 128.6, 128.5, 128.5, 128.4, 128.4, 128.4, 128.4, 128.4, 128.4, 128.4, 128.4, 128.3, 128.2, 128.1, 128.0, 128.0, 128.0, 128.0, 127.9, 122.5, 101.5, 99.8 (2 × C-1), 89.9, 77.6, 77.2, 72.5, 71.9, 71.1, 70.9, 69.7, 68.0, 67.1, 65.9, 62.0, 55.4, 47.6, 46.7, 45.9, 41.7, 41.4, 39.3, 38.9, 38.5, 36.7, 33.8, 33.0, 32.7, 32.4, 30.6, 28.3, 27.6, 25.8, 25.4, 23.6, 23.4, 23.1, 18.2, 18.1, 16.8, 16.5, 15.3; HRESIMS: m/z calcd. for C91H98O18Na[M+Na+]: 1501.6651; found: m/z 1501.6629. +24.6 (c 0.5, MeOH); 1H-NMR (pyridine-d5): δ 5.46 (br s, 1 H, H-12), 5.27 (s, 1 H, H-1'), 5.18 (d, 1 H, J = 7.8 Hz, H-1''), 4.60-4.24 (m, 8 H), 4.14 (dd, 1 H, J = 3.4, 9.5 Hz), 3.95 (dd, 1 H, J = 6.1, 6.4 Hz, H-2''), 3.29 (dd, 1 H, J = 4.2, 13.7 Hz, H-3), 3.11 (dd, 1 H, J = 4.5, 11.6 Hz, H-18),1.74 (d, 3 H, J = 6.2 Hz, H-6'), 1.27, 1.00, 0.99, 0.95, 0.89, 0.83, 0.76 (s, 7 × 3 H, CH3); 13C-NMR (pyridine-d5): δ 180.1 (C=O), 144.8, 122.7, 122.5, 107.3, 103.9 (2 × C-1), 88.5, 85.2, 76.9, 75.6, 74.1, 72.9, 71.9, 70.0, 68.1, 62.0, 55.5, 47.9, 46.6, 46.4, 42.1, 42.0, 39.7, 39.1, 38.4, 36.9, 34.2, 33.2, 33.2, 33.1, 30.9, 28.3, 28.2, 26.1, 25.7, 23.7, 23.7, 18.5, 18.3, 17.3, 16.7, 15.4; HRESIMS: m/z calcd. for C42H68O12Na[M+Na+]: 787.4608; found: m/z 787.4579.
+24.6 (c 0.5, MeOH); 1H-NMR (pyridine-d5): δ 5.46 (br s, 1 H, H-12), 5.27 (s, 1 H, H-1'), 5.18 (d, 1 H, J = 7.8 Hz, H-1''), 4.60-4.24 (m, 8 H), 4.14 (dd, 1 H, J = 3.4, 9.5 Hz), 3.95 (dd, 1 H, J = 6.1, 6.4 Hz, H-2''), 3.29 (dd, 1 H, J = 4.2, 13.7 Hz, H-3), 3.11 (dd, 1 H, J = 4.5, 11.6 Hz, H-18),1.74 (d, 3 H, J = 6.2 Hz, H-6'), 1.27, 1.00, 0.99, 0.95, 0.89, 0.83, 0.76 (s, 7 × 3 H, CH3); 13C-NMR (pyridine-d5): δ 180.1 (C=O), 144.8, 122.7, 122.5, 107.3, 103.9 (2 × C-1), 88.5, 85.2, 76.9, 75.6, 74.1, 72.9, 71.9, 70.0, 68.1, 62.0, 55.5, 47.9, 46.6, 46.4, 42.1, 42.0, 39.7, 39.1, 38.4, 36.9, 34.2, 33.2, 33.2, 33.1, 30.9, 28.3, 28.2, 26.1, 25.7, 23.7, 23.7, 18.5, 18.3, 17.3, 16.7, 15.4; HRESIMS: m/z calcd. for C42H68O12Na[M+Na+]: 787.4608; found: m/z 787.4579.  -26.6 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.28-7.16 (m, 45 H, Ar-H), 5.78 (d, 1 H, J = 3.5 Hz), 5.59 (dd, 1 H, J = 7.9, 10.4 Hz), 5.47-5.41 (m, 2 H), 5.26-5.20 (m, 2 H), 5.06 (dd, 2 H, J = 12.5, 17.3 Hz, PhCH2), 4.93-4.86 (m, 3 H), 4.44 (d, 1 H, J = 1.4 Hz), 4.39-3.92 (m, 6 H), 3.30 (dd, 1 H, J = 5.6, 12.1 Hz, H-3), 3.06-2.87 (m, 3 H), 0.99 (d, 1 H, J = 6.5 Hz, H-6'), 1.09, 0.93, 0.91, 0.89, 0.86, 0.77, 0.58 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3) δ 177.4, 166.3, 166.2, 165.8, 165.4, 165.3, 165.0, 164.9, 163.9 (9 C=O), 143.7, 136.4, 133.3, 133.3, 133.3, 133.2, 133.2, 133.1, 133.0, 132.9, 130.5, 130.0, 129.9, 129.9, 129.8, 129.8, 129.7, 129.7, 129.7, 129.7, 129.7, 129.7, 129.6, 129.6, 129.6, 129.5, 129.5, 129.4, 129.4, 129.4, 129.4, 129.2, 129.0, 128.7, 128.6, 128.5, 128.5, 128.5, 128.5, 128.5, 128.4, 128.4, 128.4, 128.4, 128.4, 128.4, 128.3, 128.3, 128.2, 128.2, 128.1, 128.0, 127.9, 127.9, 127.8, 127.8, 122.4, 103.3, 101.0, 100.6 (3 × C-1), 89.3, 77.3, 73.2, 71.7, 70.9, 70.2, 69.7, 68.5, 68.0, 67.5, 65.9, 65.4, 62.4, 61.2, 55.3, 47.5, 46.7, 45.9, 41.7, 41.4, 39.3, 38.9, 38.3, 36.6, 33.8, 33.0, 32.6, 32.3, 30.6, 28.2, 27.6, 25.8, 25.1, 23.6, 23.3, 23.0, 18.2, 16.8, 16.4, 15.9, 15.3; HRESIMS: m/z calcd. for C110H114O24Na[M+Na+]: 1841.7598; found: m/z 1841.7579.
-26.6 (c 0.5, CHCl3); 1H-NMR (CDCl3): δ 8.28-7.16 (m, 45 H, Ar-H), 5.78 (d, 1 H, J = 3.5 Hz), 5.59 (dd, 1 H, J = 7.9, 10.4 Hz), 5.47-5.41 (m, 2 H), 5.26-5.20 (m, 2 H), 5.06 (dd, 2 H, J = 12.5, 17.3 Hz, PhCH2), 4.93-4.86 (m, 3 H), 4.44 (d, 1 H, J = 1.4 Hz), 4.39-3.92 (m, 6 H), 3.30 (dd, 1 H, J = 5.6, 12.1 Hz, H-3), 3.06-2.87 (m, 3 H), 0.99 (d, 1 H, J = 6.5 Hz, H-6'), 1.09, 0.93, 0.91, 0.89, 0.86, 0.77, 0.58 (s, 7 × 3 H, CH3); 13C-NMR (CDCl3) δ 177.4, 166.3, 166.2, 165.8, 165.4, 165.3, 165.0, 164.9, 163.9 (9 C=O), 143.7, 136.4, 133.3, 133.3, 133.3, 133.2, 133.2, 133.1, 133.0, 132.9, 130.5, 130.0, 129.9, 129.9, 129.8, 129.8, 129.7, 129.7, 129.7, 129.7, 129.7, 129.7, 129.6, 129.6, 129.6, 129.5, 129.5, 129.4, 129.4, 129.4, 129.4, 129.2, 129.0, 128.7, 128.6, 128.5, 128.5, 128.5, 128.5, 128.5, 128.4, 128.4, 128.4, 128.4, 128.4, 128.4, 128.3, 128.3, 128.2, 128.2, 128.1, 128.0, 127.9, 127.9, 127.8, 127.8, 122.4, 103.3, 101.0, 100.6 (3 × C-1), 89.3, 77.3, 73.2, 71.7, 70.9, 70.2, 69.7, 68.5, 68.0, 67.5, 65.9, 65.4, 62.4, 61.2, 55.3, 47.5, 46.7, 45.9, 41.7, 41.4, 39.3, 38.9, 38.3, 36.6, 33.8, 33.0, 32.6, 32.3, 30.6, 28.2, 27.6, 25.8, 25.1, 23.6, 23.3, 23.0, 18.2, 16.8, 16.4, 15.9, 15.3; HRESIMS: m/z calcd. for C110H114O24Na[M+Na+]: 1841.7598; found: m/z 1841.7579. -36.8 (c 0.5, MeOH); 1H- NMR (pyridine-d5): δ 5.47 (br s, 1 H, H-12), 5.27-5.25 (m, 2 H), 4.72 (d, 1 H, J = 7.8 Hz), 4.54-4.50 (m, 2 H), 4.40-3.91 (m, 12 H), 3.85 (dd, 1 H, J = 7.0, 7.0 Hz), 3.59 (dd, 1 H, J = 10.2, 11.2 Hz), 3.28 (dd, 1 H, J = 4.2, 10.0 Hz, H-3), 3.09 (dd, 1 H, J = 4.4, 11.4 Hz, H-18), 1.67 (d, 3 H, J = 6.5 Hz, H-6'), 1.28, 1.00, 0.99, 0.95, 0.90, 0.83, 0.78 (s, 7 × 3 H, CH3); 13C-NMR (pyridine-d5): δ 180.2, 144.9, 122.5, 106.3, 105.9, 104.9 (3 × C-1), 88.8, 86.8, 83.6, 77.3, 75.2, 73.5, 73.1, 72.0, 70.2, 69.0, 67.1, 66.7, 66.5, 62.1, 57.4, 55.6, 48.0, 46.7, 46.5, 42.2, 42.1, 39.8, 39.2, 38.5, 37.0, 34.3, 33.3, 33.2, 31.0, 28.4, 28.3, 26.2, 25.7, 23.8, 23.7, 19.2, 18.6, 17.4, 17.0, 16.8, 15.5; HRESIMS: m/z calcd. for C47H76O16Na[M+Na+]: 919.5031; found: m/z 919.5018.
-36.8 (c 0.5, MeOH); 1H- NMR (pyridine-d5): δ 5.47 (br s, 1 H, H-12), 5.27-5.25 (m, 2 H), 4.72 (d, 1 H, J = 7.8 Hz), 4.54-4.50 (m, 2 H), 4.40-3.91 (m, 12 H), 3.85 (dd, 1 H, J = 7.0, 7.0 Hz), 3.59 (dd, 1 H, J = 10.2, 11.2 Hz), 3.28 (dd, 1 H, J = 4.2, 10.0 Hz, H-3), 3.09 (dd, 1 H, J = 4.4, 11.4 Hz, H-18), 1.67 (d, 3 H, J = 6.5 Hz, H-6'), 1.28, 1.00, 0.99, 0.95, 0.90, 0.83, 0.78 (s, 7 × 3 H, CH3); 13C-NMR (pyridine-d5): δ 180.2, 144.9, 122.5, 106.3, 105.9, 104.9 (3 × C-1), 88.8, 86.8, 83.6, 77.3, 75.2, 73.5, 73.1, 72.0, 70.2, 69.0, 67.1, 66.7, 66.5, 62.1, 57.4, 55.6, 48.0, 46.7, 46.5, 42.2, 42.1, 39.8, 39.2, 38.5, 37.0, 34.3, 33.3, 33.2, 31.0, 28.4, 28.3, 26.2, 25.7, 23.8, 23.7, 19.2, 18.6, 17.4, 17.0, 16.8, 15.5; HRESIMS: m/z calcd. for C47H76O16Na[M+Na+]: 919.5031; found: m/z 919.5018.3.3. Fungicidal activity bioassay
4. Conclusions
Acknowledgements
References and Notes
- Papadopoulou, K.; Melton, R.E.; Legget, M.; Daniels, M.J.; Osbourn, A.E. Compromised disease resistance in saponin-deficient plants. Proc. Natl. Acad. Sci. USA 1999, 96, 12923–12928. [Google Scholar]
- Mahato, S.B.; Garai, S. Triterpenoid saponins. Progr. Chem. Org. Nat. Prod. 1998, 74, 1–196. [Google Scholar]
- Gauthier, C.; Legault, J.; Pichette, A. Recent progress in the synthesis of naturally occurring triterpenoid saponins. Mini-Rev. Org. Chem. 2009, 6, 321–344. [Google Scholar] [CrossRef]
- Yu, B.; Sun, J. Current synthesis of triterpene saponins. Chem. Asian J. 2009, 4, 642–654. [Google Scholar] [CrossRef]
- Paczkowski, C.; Wojciechowski, Z.A. Glucosylation and galactosylation of diosgenin and solasodine by soluble glycosyltransferase(s) from Solanum melongena leaves. Phytochemistry 1994, 35, 1429–1434. [Google Scholar]
- Wojciechowski, Z.A. Biosynthesis of oleanolic acid glycosides by subcellular fractions of Calendula officinalis seedlings. Phytochemistry 1975, 14, 1749–1753. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marton, A. Saponins. Chemistry and Pharmacology of Natural Products; Cambridge University Press: Cambridge, UK, 1995; p. 560. [Google Scholar]
- Yadava, R.N.; Jharbade, J. A new bioactive triterpenoid saponin from the seeds of Lactuca scariola Linn. Nat. Prod. Res. Part A: Struct. Synth. 2007, 21, 500–506. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Kinzy, W. Anomeric-oxygen activation for glycoside synthesis: the trichloroacetimidate method. Adv. Carbohydr. Chem. Biochem. 1994, 50, 21–123. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.H.; Kong, X.G.; Lan, E.; Huang, Z.J.; Peng, S.X.; Kaufman, D.L.; Tian, J.D. Design, synthesis, and antihepatocellular carcinoma activity of nitric oxide releasing derivatives of oleanolic aci. J. Med. Chem. 2008, 51, 4834–4838. [Google Scholar] [CrossRef]
- Lafont, D.; Bouchu, M.N.; Girard-Egrot, A.; Boullanger, P. Syntheses and interfacial behavior of neoglycolipid analogues of glycosyl ceramides. Carbohydr. Res. 2001, 336, 181–194. [Google Scholar]
- Kimmel, R.; Kafka, S.; Kosmrlj, J. Selective formation of glycosidic linkages of N-unsubstituted 4-hydroxyquinolin-2-(1H)-ones. Carbohydr. Res. 2010, 345, 768–779. [Google Scholar] [CrossRef]
- Sha, Y.; Yan, M.C.; Liu, J.; Liu, Y.; Cheng, M.S. Facile synthesis of oleanolic acid monoglycosides and diglycosides. Molecules 2008, 13, 1472–1486. [Google Scholar] [CrossRef]
- Sarkar, K.; Mukherjee, I.; Roy, N. Synthesis of the trisaccharide repeating unit of the O-antigen related to the enterohemorrhagic Escherichia coli type O26:H. J. Carbohydr. Chem. 2003, 22, 95–107. [Google Scholar]
- Yan, S.Q.; Wu, X.M.; Liang, X.M.; Zhang, J.J.; Wang, D.Q. Synthesis of di- and tetrasaccharide containing 6-deoxy-L-talose from the O-antigenic polysaccharide of Burkholderia pseudomallei strain 304b. Chin. Chem. Lett. 2009, 20, 582–585. [Google Scholar]
- Agarwal, A.; Vankar, Y.D. Selective deprotection of terminal isopropylidene acetals and trityl ethers using HClO4 supported on silica gel. Carbohydr. Res. 2005, 340, 1661–1667. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Shing, T.K.M. Efficient and Facile Glycol Cleavage Oxidation Using Improved Silica Gel-Supported Sodium Metaperiodate. J. Org. Chem. 1997, 62, 2622–2624. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liang, X.M.; Wang, D.Q.; Kong, F.Z. Regio- and stereoselective anomeric esterification of glucopyranose 1,2-diols and a facile preparation of 2-O-acetylated glucopyranosyl trichloroacetimidates from the corresponding 1,2-diols. Carbohydr. Res. 2007, 342, 797–805. [Google Scholar]
- Guo, T.; Liu, Q.; Wang, P.; Zhang, L.; Zhang, W.; Li, Y. Facile synthesis of three bidesmosidic oleanolic acid saponins with strong inhibitory activity on pancreatic lipase. Carbohydr. Res. 2009, 344, 1167–1174. [Google Scholar] [CrossRef]
- Chen, N.C. Bioassay of Pesticides; Beijing Agricultural University Press: Beijing, China, 1991; pp. 161–162. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, H.; Zong, G.; Zhang, J.; Wang, D.; Liang, X. Synthesis and Anti-Fungal Activity of Seven Oleanolic Acid Glycosides. Molecules 2011, 16, 1113-1128. https://doi.org/10.3390/molecules16021113
Zhao H, Zong G, Zhang J, Wang D, Liang X. Synthesis and Anti-Fungal Activity of Seven Oleanolic Acid Glycosides. Molecules. 2011; 16(2):1113-1128. https://doi.org/10.3390/molecules16021113
Chicago/Turabian StyleZhao, Hanqing, Guanghui Zong, Jianjun Zhang, Daoquan Wang, and Xiaomei Liang. 2011. "Synthesis and Anti-Fungal Activity of Seven Oleanolic Acid Glycosides" Molecules 16, no. 2: 1113-1128. https://doi.org/10.3390/molecules16021113
APA StyleZhao, H., Zong, G., Zhang, J., Wang, D., & Liang, X. (2011). Synthesis and Anti-Fungal Activity of Seven Oleanolic Acid Glycosides. Molecules, 16(2), 1113-1128. https://doi.org/10.3390/molecules16021113




