Abstract
Phytohemical investigation on the heartwood of Dalbergia odorifera resulted in the isolation of nine flavonoids. Their structures were elucidated as sativanone (1), (3R)-vestitone (2), (3R)-2',3',7-trihydroxy-4'-methoxyisoflavanone (3), (3R)-4'-methoxy-2',3,7-trihydroxyisoflavanone (4), carthamidin (5), liquiritigenin (6), isoliquiritigenin (7), (3R)-vestitol (8), and sulfuretin (9) based on their spectral data. All compounds were evaluated for their inhibitory activity against Ralstonia solanacearum. This is the first report about anti-R. solanacearum activity of the compounds from D. odorifera.
1. Introduction
Ralstonia solanacearum, the pathogen that is the causal agent of bacterial wilt, is one of the best-known bacterial diseases, and is found in tropical, subtropical, and some temperate regions of the World. This soilborne pathogen attacks more than 200 plant species, including many agriculturally important crops [1]. This bacterium can also be free-living as a saprophyte in water or in the soil in the absence of host plants [2]. Streptomycin is widely used in agriculture, but the overuse of it can lead to bacterial resistance [3]. Thus, it is very necessary to search for more potent anti-R. solanacearum compounds.
The heartwood of Dalbergia odorifera T. Chen, named “Jiangxiang” in Chinese traditional medicine, was used in China and Korea for the treatment of blood stagnation syndrome, ischemia, swelling, necrosis and rheumatic pain [4,5]. Previous chemical investigations on this plant have led to the isolation of flavonoids and phenolic compounds [6,7,8]. Some flavonoids have been reported to possess various pharmacological effects such as anti-inflammatory, antibacterial, antiplasmodial, antinephritic, neuroprotective and antioxidant activities [9,10,11,12,13,14]. During the course of our screening for anti-R. solanacearum agents from tropical medicinal plants, the crude ethanol extract of the heartwood of D.odorifera showed anti-R. solanacearum activity. In this paper, we described the isolation, identification and anti-R. solanacearum activity of compounds 1−9.
2. Results and Discussion
The compounds (Figure 1) were identified as: sativanone (1), (3R)-vestitone (2), (3R)-2',3',7-trihydroxy-4'-methoxyisoflavanone (3), (3R)-4'-methoxy-2',3,7-trihydroxyisoflavanone (4), carthamidin (5), liquiritigenin (6), isoliquiritigenin (7), (3R)-vestitol (8), and sulfuretin (9) by comparison of their spectral data with the literature.
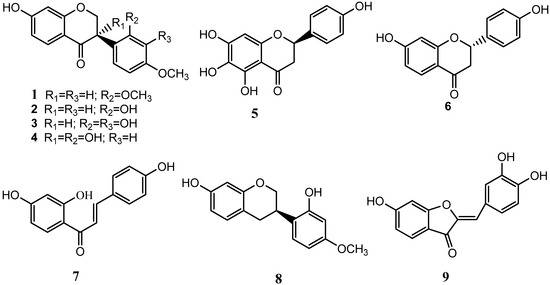
Figure 1.
Structures of compounds 1−9.
Compounds 1−9 were next evaluated for their inhibitory activity against R. solanacearum (Table 1). Among the nine flavonoids, compound 8 exhibited the strongest antibacterial activity, with an inhibition zone diameter of 16.62 mm, which was close to that of streptomycin sulfate (the positive control). Compounds 2, 6 and 7 also showed stronger antibacterial activities than the rest of compounds, with inhibition zone diameters of 11.19, 12.23, and 14.15 mm, respectively.

Table 1.
Antibacterial activity of compounds 1−9from Dalbergia odorifera (mm).
| Compound | Ralstonia solanacearum | Compound | Ralstonia solanacearum |
|---|---|---|---|
| 1 | 6.53 ± 0.05 | 6 | 12.23 ± 0.45 |
| 2 | 11.19 ± 0.15 | 7 | 14.15 ± 0.95 |
| 3 | 8.11 ± 0.14 | 8 | 16.62 ± 1.07 |
| 4 | 9.99 ± 1.25 | 9 | 9.10 ± 1.22 |
| 5 | 8.34 ± 0.16 | Streptomycin sulfate a | 16.80 ± 0.33 |
The results of diffusion method are presented as diameters of inhibition zones in mm. Each value represents mean ± SD (n = 3). a Streptomycin sulfate was used as positive control.
Compounds 1−4 belong to the isoflavanone class. Compound 1 showed lower activity than the other compounds, and this may be due to the absence of the 2'-OH group, suggesting that this 2'-OH is a favorable group for activity. Compounds 2−4 had a B-ring OH group (2' position), and 3 had a B-ring OH group (3' position), while 4 had a C-ring OH group (3 position). Lower activity of 3compared to that of 2 seemed to be because the 3'-OH and 2'-OH formed a stable five-membered ring, which reduced the inhibition of the 2'-OH group. Compound 4 had slightly reduced inhibition compared with 2, which leads us to speculate that the 3-OH and 2'-OH formed an unstable six-membered ring. Compound 8 belong to the isoflavane class which lack the C(4)=O in the C-ring compared with 2, and its activity was higher than that of 2. The result suggests that the presence of C(4)=O will reduced the inhibitory effect.
3. Experimental
3.1. General
The NMR spectra were recorded on a Bruker AV-400 spectrometer, using TMS as an internal standard. Column chromatography was performed with silica gel (Marine Chemical Industry Factory, Qingdao, China) and Sephadex LH-20 (Merck). TLC was preformed with silica gel GF254 (Marine Chemical Industry Factory, Qingdao, China) plates.
3.2. Plant Materials
The dried heartwood of D. odorifera was purchased from the Haikou Free Market of Agricultural Products, Hainan Province, China, in October, 2010. The specimen was identified by Professor Zheng-fu Dai of the Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, where a voucher specimen (No. 20101009) has been deposited.
3.3. Extraction, Fractionation and Identification of the Flavonoids
The dried and crushed heartwood of D. odorifera (8.4 kg) was extracted three times with 95% ethanol (50 L) at room temperature for three weeks totally. The ethanol extract was then filtered through absorbent gauze, and the filtrate was concentrated on a rotary evaporator under reduced pressure at 50 °C to remove ethanol, resulting in a crude ethanolic extract. This was partitioned with petroleum ether, ethyl acetate and n-butanol. The ethyl acetate phase (477.0 g) was submitted to column chromatography (CC) over silica gel eluted with a mixture of chloroform and methanol (100:1–0:100, v/v) of increasing polarity resulting in eighteen fractions (Fr.1–Fr.18). Compound 1 (100.0 mg) obtained by recrystallization from Fr.6 (52.0 g). Repeated CC on silica gel CC eluted with CHCl3-MeOH (100:1–0:1, v/v) and Sephadex LH-20 (CHCl3-MeOH, 1:1, v/v), led to the isolation of compounds 2 (34.0 mg), 3 (4.0 mg), 4 (64.4 mg), 5 (5.0 mg), 6 (10.4 mg), 7 (70.0 mg) and 8 (5.0 mg) from Fr.10 (45.0 g). Fr.12 (51.3 g) was submitted to column chromatography over silica gel eluted with CHCl3-MeOH (50:1–0:1, v/v) and further purification with Sephadex LH-20 (95% EtOH) to afford compound 9 (7.6 mg). The physicochemical and spectrometric data of nine flavonoids were as follows:
Sativanone (1). White powder; C17H16O5; 1H-NMR (CD3OD), δ: 4.40 (1H, dd, J = 11.0, 5.5 Hz, H-2a), 4.54 (1H, d, J = 11.0 Hz, H-2b), 4.16 (1H, dd, J = 11.0, 5.5 Hz, H-3), 7.76 (1H, d, J = 8.7 Hz, H-5), 6.49 (1H, d, J = 8.7 Hz, H-6), 6.51 (1H, s, H-8), 6.33 (1H, d, J = 2.3 Hz, H-3′), 6.46 (1H, dd, J = 8.4, 2.3 Hz, H-5′), 6.97 (1H, d, J = 8.4 Hz, H-6′), 3.77 (3H, s, 2′-OCH3), 3.74 (3H, s, 4′-OCH3); 13C-NMR (CD3OD), δ: 70.1 (C-2), 47.5 (C-3), 192.4 (C-4), 129.9 (C-5), 101.8 (C-6), 164.2 (C-7), 98.0 (C-8), 163.6 (C-9), 113.7 (C-10), 115.3 (C-1′), 157.7 (C-2′), 109.8 (C-3′), 160.0 (C-4′), 104.0 (C-5′), 128.5 (C-6′), 54.0 (2′-OCH3), 54.2 (4′-OCH3). These data were equal to those of literature [15].
(3R)-Vestitone (2). Yellow crystals; C16H14O5; 1H-NMR (CD3OD), δ: 4.40 (1H, dd, J = 11.0, 5.4 Hz, H-2a), 4.56 (1H, d, J = 11.0 Hz, H-2b), 4.12 (1H, dd, J = 11.0, 5.4 Hz, H-3), 7.74 (1H, d, J = 8.8 Hz, H-5), 6.48 (1H, dd, J = 8.8, 2.2 Hz, H-6), 6.31 (1H, d, J = 2.2 Hz, H-8), 6.38 (1H, d, J = 2.4 Hz, H-3′), 6.34 (1H, d, J = 8.4 Hz, H-5′), 6.88 (1H, d, J = 8.4 Hz, H-6′), 3.70 (3H, s, 4′-OCH3); 13C-NMR (CD3OD), δ: 72.0 (C-2), 48.7 (C-3), 194.7 (C-4), 130.4 (C-5), 111.7 (C-6), 166.4 (C-7), 103.6 (C-8), 165.8 (C-9), 115.7 (C-10), 115.8 (C-1′), 157.6 (C-2′), 102.7 (C-3′), 161.8 (C-4′), 106.0 (C-5′), 131.8 (C-6′), 55.7 (4′-OCH3). These data were identical to those reported [16,17,18].
(3R)-2',3',7-Trihydroxy-4'-methoxyisoflavanone (3). White powder; C16H14O6; 1H-NMR (CD3OD), δ: 4.49 (1H, d, J = 5.4 Hz, H-2a), 4.63 (1H, dd, J = 10.8, 5.4 Hz, H-2b), 4.17 (1H, dd, J = 10.8, 5.4 Hz, H-3), 7.78 (1H, d, J = 8.7 Hz, H-5), 6.53 (1H, dd, J = 8.7, 2.0 Hz, H-6), 6.35 (1H, d, J = 2.0 Hz, H-8), 6.45 (1H, d, J = 8.5 Hz, H-5′), 6.51 (1H, d, J = 8.5 Hz, H-6′), 3.83 (3H, s, 4′-OCH3); 13C-NMR (CD3OD, 100 MHz), δ: 71.9 (C-2), 48.7 (C-3), 194.5 (C-4), 130.4 (C-5), 111.7 (C-6), 149.1 (C-7), 104.2 (C-8), 165.6 (C-9), 116.8 (C-10), 115.5 (C-1′), 145.1 (C-2′), 135.2 (C-3′), 166.2 (C-4′), 103.6 (C-5′), 120.6 (C-6′), 56.6 (4′-OCH3). These data were consistent with those reported in [19].
(3R)-4'-Methoxy-2',3,7-trihydroxyisoflavanone (4). White crystals; C16H14O6; 1H-NMR (acetone-d6), δ: 4.30 (1H, d, J = 11.8 Hz, H-2a), 4.88 (1H, d, J = 11.8 Hz, H-2b), 7.74 (1H, d, J = 8.7 Hz, H-5), 6.56 (1H, d, J = 8.7 Hz, H-6), 6.36 (3H, overlapped, H-3′, 5′, 8), 7.32 (1H, d, J = 9.3 Hz, H-6′), 3.67 (3H, s, 4′-OCH3); 13C-NMR (acetone-d6), δ: 75.5 (C-2), 76.0 (C-3), 191.6 (C-4), 131.6 (C-5), 112.8 (C-6), 166.5 (C-7), 104.4 (C-8), 164.9 (C-9), 114.5 (C-10), 118.9 (C-1′), 158.5 (C-2′), 104.1 (C-3′), 162.9 (C-4′), 106.7 (C-5′), 129.7 (C-6′), 56.4 (4′-OCH3). These data were in accordance with those reported in [9].
Carthamidin (5). White crystals; C15H12O6; 1H-NMR (CD3OD), δ: 5.44 (1H, dd, J = 13.0, 2.8 Hz, H-2), 2.83 (1H, dd, J = 17.1, 2.8 Hz, H-3a), 3.20 (1H, dd, J = 17.1, 13.0 Hz, H-3b), 6.05 (1H, s, H-8), 7.42 (2H, d, J = 8.5 Hz, H-2′, 6′), 6.96 (2H, d, J = 8.5 Hz, H-3′, 5′). These data were identical to those in the literature [20].
Liquiritigenin (6). White crystals; C15H12O4; 1H-NMR (CD3OD), δ: 5.58 (1H, dd, J = 13.2, 2.8 Hz, H-2), 2.95 (1H, dd, J = 16.9, 2.8 Hz, H-3a ), 3.26 (1H, dd, J = 16.9, 13.2 Hz, H-3b), 7.97 (1H, d, J = 8.7 Hz, H-5), 6.74 (1H, dd, J = 8.7, 2.2 Hz, H-6), 6.62 (1H, d, J = 2.2 Hz, H-8), 7.53 (2H, d, J = 8.6 Hz, H-2′, 6′), 7.08 (2H, d, J = 8.6 Hz, H-3′, 5′); 13C-NMR (CD3OD), δ: 80.3 (C-2), 44.5(C-3), 193.0 (C-4), 129.5 (C-5), 111.5 (C-6), 166.0 (C-7), 103.6 (C-8), 164.8 (C-9), 114.4 (C-10), 130.5 (C-1′), 128.4 (C-2′, 6′), 116.1 (C-3′, 5′), 158.1 (C-4′). These data were in accordance with those reported previously [19].
Isoliquiritigenin (7). Yellow crystals; C15H12O4; 1H-NMR (CD3OD), δ: 7.54 (3H, dd, J = 15.4, 6.0 Hz, H-2, 6, α), 6.88 (2H, d, J = 8.6 Hz, H-3, 5), 6.25 (1H, d, J = 2.4 Hz, H-3′), 6.37 (1H, dd, J = 8.8, 2.4 Hz, H-5′), 7.89 (1H, d, J = 8.8 Hz, H-6′), 7.73 (1H, d, J = 15.4 Hz, H-β); 13C-NMR (CD3OD) δ: 127.9 (C-1), 131.8 (C-2), 116.9 (C-3), 161.5 (C-4), 116.9 (C-5), 131.8 (C-6), 114.7 (C-1′), 166.4 (C-2′), 103.9 (C-3′), 167.5 (C-4′), 109.2 (C-5′), 133.4 (C-6′), 118.4 (C-α), 145.7 (C-β), 193.6 (C=O). These data were identical to those in the literature [15,19].
(3R)-Vestitol (8). White crystals; C16H16O4; 1H-NMR (CD3OD), δ: 3.93 (1H, t, J = 10.1 Hz, H-2a), 4.21 (1H, dd, J = 10.1, 4.1 Hz, H-2b), 3.42 (1H, m, H-3), 2.77 (1H, dd, J = 15.5, 4.1 Hz, H-4a), 2.93 (1H, dd, J = 15.5, 10.9 Hz, H-4b), 6.86 (1H, d, J = 8.2 Hz, H-5), 6.22 (1H, d, J = 2.4 Hz, H-8), 6.31 (1H, dd, J = 8.2, 2.4 Hz, H-3′), 6.37 (2H, m, H-6, 5′), 6.96 (1H, d, J = 8.2 Hz, H-6′), 3.71 (3H, s, 4′-OCH3); 13C-NMR (CD3OD), δ: 71.2 (C-2), 33.2 (C-3), 31.4 (C-4), 131.2 (C-5), 109.1 (C-6), 156.4 (C-7), 103.9 (C-8), 157.3 (C-9), 115.0 (C-10), 121.5 (C-1′), 157.5 (C-2′), 102.5 (C-3′), 160.9 (C-4′), 105.8 (C-5′), 128.8 (C-6′), 55.6 (4′-OCH3). These data were identical to those in the literature [19].
Sulfuretin (9). White crystals; C15H10O5; 1H-NMR (CD3OD), δ: 6.85 (1H, d, J = 8.2 Hz, H-4), 7.24 (1H, d, J = 8.2 Hz, H-5), 6.70 (3H, overlapped, H-7, 10, 6′), 7.54 (1H, s, H-2′), 7.61 (1H, d, J = 8.3 Hz, H-5′); 13C-NMR (CD3OD), δ: 147.7 (C-2), 184.4 (C-3), 126.9 (C-4), 116.8 (C-5), 169.9 (C-6), 99.4 (C-7), 168.7 (C-8), 114.8 (C-9), 114.8 (C-10), 125.4 (C-1′), 114.3 (C-2′), 146.8 (C-3′), 149.7 (C-4′), 119.0 (C-5′), 126.5 (C-6′). These data were consistent with those previously reported [21].
3.4. Bacterial Strains
The R. solanacearum strain was obtained from Professor Ming-he Mo of the Key Laboratory of Protection and Utilization of Biological Resources, Yunnan University, and maintained on a nutrient agar (NA) slant at 4 °C.
3.5. Antibacterial Activity
These compounds were individually tested for in vitro antibacterial activity against R. solanacearum strain by the filter paper disc agar diffusion method [22]. The NA medium was mixed with suspension (2 mL) containing 107 CFU/mL of R. solanacearum, and then poured into Petri-plates to a uniform depth of 5 mm and was allowed to solidify. The isolated compounds dissolved in dimethyl sulfoxide (DMSO) (1.6 µL, 50 mg/mL) were impregnated on sterile filter paper discs (6 mm diameter) and then applied aseptically to the surface of the agar plates. Streptomycin sulfate (1.6 µL, 50 mg/mL) was used as positive control. The plates were incubated at 37 °C for 24 h. Then the diameters of the inhibition zones including the 6 mm disc diameter were measured. Experiments were done in triplicate, and the results were mean values.
4. Conclusions
In conclusion, a total of nine compounds including four isoflavanones 1−4, two flavanones 5 and 6, one chalcone 7, one isoflavane 8 and one aurone 9 were isolated from D. odorifera and identified by comparison of their NMR data with data reported in the literature. In addition, all compounds were evaluated for their inhibitory activity against R. solanacearum. Among the nine flavonoids, compound 8 exhibited the strongest antibacterial activity and compounds 2, 6, and 7 showed strong antibacterial activity. This is the first report of the anti-R. solanacearum activity of the compounds from D.odorifera.
Acknowledgements
This research was financially supported by International Cooperation Projects of Hainan Province (GJXM20100005).
References
- Vailleau, F.; Sartorel, E.; Jardinaud, M.F.; Chardon, F.; Genin, S.; Huguet, T.; Gentzbittel, L.; Petitprez, M. Characterization of the interaction between the bacterial wilt pathogen Ralstonia solanacearum and the model legume plant Medicago truncatula. Mol. Plant Microbe Interact. 2007, 20, 159–167. [Google Scholar] [CrossRef]
- Genin, S.; Boucher, C. Lessons learned from the genome analysis of Ralstonia solanacearum. Annu. Rev. Phytopathol. 2004, 42, 107–134. [Google Scholar] [CrossRef]
- Lai, R.Q.; Zeng, W.L.; Jiang, G.H.; Li, L.Y.; Xie, X.H. Preliminary study on control effects of ethanol extracts from garlic plant against Ralstonia solanacearum and TMV. J. Yunnan Agric. Univ. 2011, 26, 284–287. [Google Scholar]
- The State Pharmacopoeia Commission of PR China, Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2000; 1, p. 184.
- Kang, T.H.; Tian, Y.H.; Kim, Y.C. Isoliquiritigenin: A competitive tyrosinase inhibitor from the heartwood of Dalbergia odorifera. Biomol. Ther. 2005, 13, 32–34. [Google Scholar]
- Goda, Y.; Katayama, M.; Ichikawa, K.; Shibuya, M.; Kiuchi, F.; Sankawa, U. Inhibitors of prostaglandin biosynthesis from Dalbergia odorifera. Chem. Pharm. Bull. 1985, 33, 5606–5609. [Google Scholar] [CrossRef]
- Yahara, S.; Saijo, R.; Nohara, T.; Konishi, R.; Yamahara, J.; Kawasaki, T.; Miyahara, K. Novel Bi-Isoflavonoids from Dalbergia odorifera. Chem. Pharm. Bull. 1985, 33, 5130–5133. [Google Scholar]
- Ogata, T.; Yahara, S.; Hisatsune, R.; Konishi, R.; Nohara, T. Isoflavan and related compounds from Dalbergia odorifera. II. Chem. Pharm. Bull. 1990, 38, 2750–2755. [Google Scholar] [CrossRef]
- Chan, S.C.; Chang, Y.S.; Wang, J.P.; Chen, S.C.; Kuo, S.C. Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood of Dalbergia odorifera. Planta Med. 1998, 64, 153–158. [Google Scholar]
- Yu, X.; Wang, W.; Yang, M. Antioxidant activities of compounds isolated from Dalbergia odorifera T. Chen and their inhibition effects on the decrease of glutathione level of rat lens induced by UV irradiation. Food Chem. 2007, 104, 715–720. [Google Scholar] [CrossRef]
- Liu, R.X.; Wang, W.; Wang, Q.; Bi, K.S.; Guo, D. Identification and determination of major flavonoids in rat urine by HPLC-UV and HPLC-MS methods following oral administration of Dalbergia odorifera extract. Biomed. Chromatogr. 2006, 20, 101–108. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, H.; Xu, X.R.; Mei, W.L.; Dai, H.F. Antioxidant phenolic compounds of Dracaena cambodiana. Molecules 2010, 15, 8904–8914. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Woelfle, U.; Schempp, C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar] [CrossRef]
- An, R.B.; Jeong, G.S.; Kim, Y.C. Flavonoids from the heartwood of Dalbergia odorifera and their protective effect on glutamate-induced oxidative injury in HT22 cells. Chem. Pharm. Bull. 2008, 56, 1722–1724. [Google Scholar] [CrossRef]
- Guo, L.B.; Wang, L. Studies on the flavonoids from lignum Dalbergia odorifera. Chin. Tradit. Herb. Drugs 2008, 39, 1147–1149. [Google Scholar]
- Dewick, P.M. Biosynthesis of pterocarpan phytoalexins in Trifolium pretense. Phytochemistry 1977, 16, 93–97. [Google Scholar] [CrossRef]
- Ingham, J.L. A new isoflavonoid phytoalexin from Medicago rugosa. Planta Med. 1982, 45, 46–47. [Google Scholar]
- Macias, F.A.; Simonet, A.M.; Galindo, J.C.; Castellano, D. Bioactive phenolics and polar compounds from Melilotus messanensis. Phytochemistry 1999, 50, 35–46. [Google Scholar]
- Yahara, S.; Ogata, T.; Saijo, R.; Konishi, R.; Yamahara, J.; Miyahara, K.; Nohara, T. Isoflavan and related compounds from Dalbergia odorifera. I. Chem. Pharm. Bull. 1989, 37, 979–987. [Google Scholar] [CrossRef]
- Obara, H.; Onodera, J.I.; Kurihara, Y.J.; Yamamoto, F. Synthesis of 2′,3′,4,4′,6′- pentahydroxychalcone, an aglycone of carheamin, and its isomerization into 4′,5,6,7- and 4′,5,7,8-tetrahydroxyflavanone, carthamidin and isocarthamidin. Bull. Chem. Soc. Jpn. 1978, 51, 3627–3630. [Google Scholar] [CrossRef]
- Zhao, A.H.; Zhao, Q.S.; Peng, L.Y.; Zhang, J.X.; Lin, Z.W.; Sun, H.D. A new chalcone glycoside from Bidens pilosa. Acta Bot. Yunnanica 2004, 26, 121–126. [Google Scholar]
- Xu, S.Y.; Bian, R.L.; Chen, X. Methods of Pharmacology Experiment; People’s Sanitation Press: Beijing, China, 2002; pp. 1651–1653. [Google Scholar]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).