Abstract
A new oleanane-type triterpenoid saponin, named platycoside N (1), together with six known saponins, was isolated from the roots of Platycodon grandiflorum. On the basis of acid hydrolysis, comprehensive spectroscopic data analyses and comparison with the spectral data of the known compounds, its structure was elucidated as 3-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl-2β,3β,16α,23-tetrahydroxyolean-12-en-28-oic acid 28-O-β-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside. The six known compounds were platycodin D (2), deapioplatycodin D (3), platycodin D3 (4), deapio- platycodin D3 (5), platycoside E (6) and deapioplatycoside E (7).
1. Introduction
The roots of Platycodon grandiflorum A. DC (Campanulaceae), Platycodi Radix, are traditionally used as food and a herbal medicine in the treatment of a wide range of diseases, including bronchial asthma, hepatic fibrosis, bone disorders [1,2,3,4], hypercholesterolemia and hyperlipidemia [5]. The principal bioactive constituents of this herb are triterpenoid saponins (platycosides), which exhibit a variety of pharmacological activities, such as anti-inflammatory [6,7], anti-obesity [8,9,10,11], anti-cancer [12,13,14,15] and hypoglycemic effects [16,17]. To date, more than 30 saponins have been isolated from this plant [18,19,20,21,22,23,24,25]. In order to find more bioactive compounds, we have now studied the chemical constituents of P. platycodiflorum, and in this paper, we report the presence in this species of a new oleanane-type triterpenoid saponin, named platycoside N, together with six known compounds, from the roots of Platycodon grandiflorum. (Figure 1)
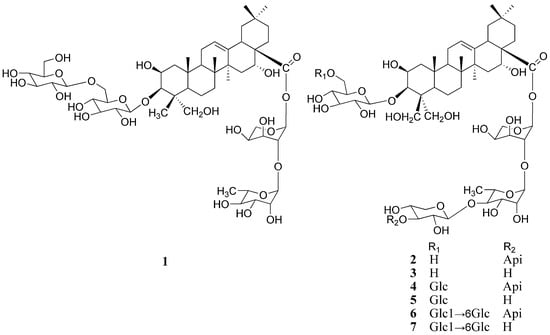
Figure 1.
Chemical structures of compounds 1-7.
2. Results and Discussion
Platycoside N (1) was a white amorphous powder, and its molecular formula C53H86O24 was determined based on the HR-ESI-MS spectra. The oleanane-type triterpenoid saponin nature of compound 1 was revealed through analysis of its spectral features. The IR spectrum exhibited absorptions at 3,425 cm−1 (OH), 1,647 cm−1 (ester carbonyl), and 1,616 cm−1 (double bond). Six methyl groups (δ 0.89 × 2, 0.98, 1.17, and 1.58 × 2) and one olefinic proton (δ 5.46, br s) of the aglycon were observed in the 1H-NMR spectrum. The 13C-NMR spectrum showed that the aglycon had six methyl carbons at δ 16.0, 18.6, 17.7, 27.2, 33.3, and 24.8, two olefinic carbons at δ 123.1 (CH) and 144.5 (C), one oxymethylene and three oxymethine carbons at δ 66.6, and 70.1, 74.2 and 83.1, respectively, and one carbonyl carbon at δ 176.0 (Table 1). The information of the 1H-NMR spectrum coupled with the 13C-NMR spectrum indicated that 1 had 2β,3β,16α,23-tetrahydroxyolean-12-en-28-oic acid (polygalacic acid) as an aglycon [20]. The 13C-NMR spectrum showed 53 signals, of which 30 were assigned to a triterpenoid moiety and 23 to the saccharide portion. The downfield shift of C-3 (δ 83.1) and for the upfield shift of C-28 (δ 176.0), revealed that the sugar moieties were attached to the aglycon at these two positions. The 1H and 13C-NMR spectra of 1 exhibited four anomeric protons at δ 5.10 × 2 (2H, d, J = 7.5 Hz), 6.27(1H, d, J = 2.5 Hz), 5.68 (1H, br s) ppm and carbons at δ 106.7 × 2, 93.9, 101.5 (Table 1). In the 1H-NMR spectrum, one methyl signal at δ 1.59 (3H, d, J = 5.5 Hz) belonging to rhamnose was observed. In addition, the monosaccharides were identified as glucose, rhamnose and arabinose by TLC and a combination of DEPT, HMQC and HMBC experiments. Acid hydrolysis of 1 also gave glucose, arabinose and rhamnose in a ratio of 2:1:1 respectively, as confirmed by GC analysis of the respective trimethylsilyl derivatives [20]. The 1H- and 13C-NMR and 2D-NMR analysis indicated that all the monosaccharides of 1 were in pyranose forms. The β-anomeric configurations of the D-gulucose units were determined by its 3JH1,H2 coupling constants (7.5 Hz). The α-anomeric configurations of the L-arabinose and L-rhamnose were determined by the broad singlet of their anomeric protons [24]. The linkages between sugar moieties and C-3 of the aglycon were corroborated through HMBC experiments, i.e., H-1 (δ 5.10) of the terminal glucose correlated with C-6 (δ 70.8) of the inner glucose, and H-1 (δ 5.10) of the inner glucose correlated with C-3 (δ 83.1) of the sapogenin. The linkages of sugar moieties at C-28 were established based on HMBC correlations between H-1 (δ 5.68) of rhamnose and C-2 (δ 75.3) of arabinose, and H-1 (δ 6.27) of arabinose and C-28 (δ 176.0) of aglycone (Figure 2). On the basis of all the above evidence, platycoside N (1) was identified as 3-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl-2β,3β,16α,23-tertahydroxyolean-12-en-28-oic acid 28-O-β-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside.

Table 1.
13C-NMR data of compound 1 in pyridine–d5 (δ ppm).
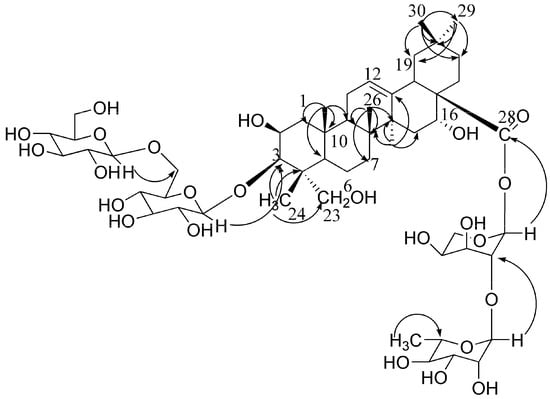
Figure 2.
The key HMBC correlations of compound 1 (from H to C).
The six known saponins were identified as platycodin D (2), deapio platycodin D (3), platycodin D3 (4), deapio platycodin D3 (5), platycoside E (6) and deapio platycoside E (7) through comparison of their UV, IR, NMR and MS data with literature values [25,26].
3. Experimental
3.1. General
ESI-MS (negative mode) measurements were carried out on an Agilent 1100 series LC/MSD Trap SL mass spectrometer. HR-ESI-MS (positive and negative modes) was analyzed on a Bruker FT-ICRMS spectrometer. IR spectra were recorded on an IR-47 spectrometer. NMR spectra were recorded on a Bruker Avance DRX 400 NMR spectrometer using TMS as internal standard, and chemical shifts δ were given in ppm. Silica gel (200–300 mesh) for column chromatography and silica gel G for TLC were purchased from Qingdao Marine Chemical Factory, Qingdao, China. AB-8 macroporous resin was purchased from Tianjin Nankai factory. Preparative HPLC was performed on a Waters 600 liquid chromatography instrument with a UV detector, monitored at 210 nm using a C18 column (Zorbax Eclipse XDB, 250 mm × 9 mm; 10 μm)
3.2. Plant material
The roots of P. grandiflorum were purchased at Changchun Guangfulu market in Changchun-city of Jilin province, China and identified by Prof. Yi-Nan Zheng, College of Chinese Material Medicine, Jilin Agricultural University. A voucher specimen (No.20050116) has been deposited in the herbarium of the same college.
3.3. Extraction and isolation
Dry and powdered roots of P. grandiflorum (2.0 Kg) were refluxed three times with 30 L of 70% methanol, 3 h each time. Extracts were concentrated, suspended in water and sequentially partitioned with ethyl acetate and n-butanol. The n-butanol fraction was subjected to macroporous resin AB-8 column and eluted sequentially with water, 30% ethanol and 70% ethanol. The 30% ethanol elution was repeatedly chromatographed on a reverse-phase column, and eluted with aqueous methanol, affording three fractions A-C. Fraction A was purified by HPLC to afford compounds 1 (23 mg), 2 (15 mg) and 3 (40 mg). Fraction B and C gave 4 (32 mg), 5 (22 mg), 6 (16 mg) and 7 (15 mg). Platycoside N (1): White amorphous powder; IR (KBr) cm−1: 3425, 2947, 1647, 1616, 1114; ESI-MS m/z: 1105 [M-H]-, HR-ESI-MS m/z 1105.5408 [M-H]- (Calcd for C53H85O24, 1105.5431). 1H-NMR (400 MHz, pyridine-d5) δ: 0.89 × 2, 0.98, 1.17, 1.58 × 2 (each 3H, s, CH3 of C-26, C-29, C-30, C-24, C-25, C-27), 1.59 (3H, d, CH3 of rhamnose), 4.36 (1H, d, J = 3.0Hz, H-3), 3.82, 4.60 (each 1H, d, H-23), 4.72(1H, m, H-2), 5.10 × 2 (each 1H, d, J = 7.5 Hz, H-1′ and H-1of glucose), 5.68 (1H, br s, H-1 of rhamnose), 6.27 (1H, br s, H-1 of arabinose), 5.46 (1H, br s, H-12) 5.03 (1H, br s, H-16). 13C-NMR (100 MHz, pyridine-d5) data: see Table 1.
3.4. Acid hydrolysis of 1
Compound 1 (2.0 mg) was refluxed with 4.0 M HCl (5.0 mL) for 1 h at 95 °C, and the reaction mixture was extracted with ethyl acetate. The aqueous layer was then adjusted to pH 7.0 with NaHCO3. After evaporating to dryness, the sugar mixture was dissolved in pyridine and developed on silica gel TLC [CHCl3-MeOH-H2O (7:3:0.5, lower phase), n-BuOH-AcOH-H2O (4:1:5, upper phase). Three spots were seen on the TLC after spraying with 4% α-naphthol-EtOH-5% H2SO4. Through comparison with authentic sugar standards (purchased from Sigma), it was found that compound 1 possessed D-glucose, L-rhamnose and L-arabinose units.
4. Conclusions
In summary, we have isolated a new oleanane-type triterpenoid saponin, named platycoside N (1), together with six known saponins from the roots of Platycodon grandiflorum.
Acknowledgements
This work was supported by the grant from Key Project in the National Science & Technology Pillar Program during the Eleventh Five-Year Plan Period (2007BAI38B03); Jilin Science & Technology Development Plan (20100938).
References and Notes
- Lee, K.J.; Kim, J.Y.; Jung, K.S.; Choi, C.Y.; Chung, Y.C.; Kim, D.H.; Jeong, H.G. Suppressive effects of Platycodon grandiflorum on the progress of carbon tetrachloride-induced hepatic fibrosis. Arch. Pharm. Res. 2004, 27, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Hwang, Y.P.; Lee, H.S.; Jeong, H.G. Inhibitory effect of Platycodi Radix on ovalbumin-induced airway inflammation in a murine model of asthma. Food Chem. Toxicol. 2009, 47, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Han, E.H.; Jin, Y.H.; Hwang, Y.P.; Kim, H.G.; Park, B.H.; Kim, J.Y.; Chung, Y.C.; Lee, K.Y.; Jeong, H.G. Saponins from the roots of Platycodon grandiflorum stimulate osteoblast differentiation via p38 MAPK- and ERK-dependent RUNX2 activation. Food Chem. Toxicol. 2010, 48, 3362–3368. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B. Pharmacological studies on Platycodon grandiflorum A. DC. IV. A comparison of experimental pharmacological effects of crude platycodin with clinical indications of platycodi radix. Yakugaku Zasshi 1973, 93, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Ezaki, O.; Ikemoto, S.; Itakura, H. Effects of Platycodon grandiflorum feeding on serum and liver lipid concentrations in rats with diet-induced hyperlipidemia. J. Nutr. Sci. Vitaminol. 1995, 41, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.Y.; Lee, W.J.; Lee, E.B.; Choi, E.Y.; Ko, K.H. Platycodin D and D3 increase airway mucin release in vivo and in vitro in rats and hamsters. Planta Med. 2002, 68, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.P.; Lee, E.B.; Kim, S.Y.; Li, D.; Ban, H.S.; Lim, S.S.; Shin, K.H.; Ohuchi, K. Inhibition of prostaglandin E2 production by platycodin D isolated from the root of Platycodon grandiflorum. Planta Med. 2001, 67, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.; Xu, B.J.; Kimura, Y.; Zheng, Y.N.; Okuda, H. Platycodi radix affects lipid metabolism in mice with high fat diet-induced obesity. J. Nutr. 2000, 130, 2760–2764. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.; Zheng, Y.N.; Xu, B.J.; Okuda, H.; Kimura, Y. Saponins from platycodi radix ameliorate high fat diet-induced obesity in mice. J. Nutr. 2002, 132, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Han, L.K.; Zheng, Y.N.; Lee, J.H.; Sung, C.K. In vitro inhibitory effect of triterpenoidal saponins from Platycodi Radix on pancreatic lipase. Arch. Pharm. Res. 2005, 28, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Kim, Y.S. Determination of the kinetic properties of platycodin D for the inhibition of pancreatic lipase using a 1,2-diglyceride-based colorimetric assay. Arch. Pharm. Res. 2004, 27, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.O.; Moon, D.O.; Choi, Y.H.; Shin, D.Y.; Kang, H.S.; Choi, B.T.; Lee, J.D.; Li, W.; Kim, G.Y. Platycodin D induces apoptosis and decreases telomerase activity in human leukemia cells. Cancer Lett. 2008, 261, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, K.W.; Moon, K.D.; Lee, M.K.; Choi, J.; Yee, S.T.; Shim, K.H.; Seo, K.I. Induction of apoptosis in HT-29 colon cancer cells by crude saponin from Platycodi Radix. Food Chem. Toxicol. 2008, 46, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Kim, G.Y.; Li, W.; Choi, B.T.; Kim, N.D.; Kang, H.S.; Choi, Y.H. Implication of intracellular ROS formation, caspase-3 activation and Egr-1 induction in platycodon D-induced apoptosis of U937 human leukemia cells. Biomed. Pharmacother. 2009, 63, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.H.; Tian, J.K. Cytotoxic triterpenoid saponins from the roots of Platycodon grandiflorum. Molecules 2007, 12, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, R.; Kim, Y.S.; Chung, S.I.; Yoon, Y. Platycodin D inhibits adipogenesis of 3T3-L1 cells by modulating kruppel-like factor 2 and peroxisome proliferator-activated receptor gamma. Phytother. Res. 2009, 24, S161–S167. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, J.; Ji, B.; Li, Y.; Zhang, X. Antihyperglycemic effects of Platycodon grandiflorum (Jacq.) A. DC. extract on streptozotocin-induced diabetic mice. Plant Foods Hum. Nutr. 2007, 62, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yoo, D.S.; Choi, C.W.; Cha, M.R.; Kim, Y.S.; Lee, H.S.; Lee, K.R.; Ryu, S.Y. Platyconic acid A, a genuine triterpenoid saponin from the roots of Platycodon grandiflorum. Molecules 2008, 13, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Dou, D.Q.; Zhao, C.J.; Shimizu, N.; Pei, Y.P.; Pei, Y.H.; Chen, Y.J.; Takeda, T. Triterpenoid saponins from Platycodon grandiflorum. J. Asian Nat. Prod. Res. 2007, 9, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Shimizu, N.; Dou, D.Q.; Takeda, T.; Fu, R.; Pei, Y.H.; Chen, Y.J. Five new triterpenoid saponins from the roots of Platycodon grandiflorum. Chem. Pharm. Bull 2006, 54, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Choi, S.U.; Kim, J.S.; Lee, H.S.; Roh, S.H.; Jeong, Y.C.; Kim, Y.K.; Ryu, S.Y. Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta Med. 2005, 71, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Shimizu, N.; Takeda, T.; Dou, D.Q.; Chen, B.; Pei, Y.H.; Chen, Y.J. New A-ring lactone triterpenoid saponins from the roots of Platycodon grandiflorum. Chem. Pharm. Bull. 2006, 54, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Hou, W.B.; Dou, D.Q.; Hua, H.M.; Gui, M.H.; Fu, R.; Chen, Y.J.; Pei, Y.H. Saponins of polygalacic acid type from Platycodon grandiflorum (in Chinese). Yao Xue Xue Bao 2006, 41, 358–360. [Google Scholar] [PubMed]
- Nikaido, T.; Koike, K.; Mitsunaga, K.; Saeki, T. Two new triterpenoid saponins from Platycodon grandiflorum. Chem. Pharm. Bull. 1999, 47, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiang, L.; Zhang, J.; Zheng, Y.N.; Han, L.K.; Saito, M. A New Triterpenoid Saponin from the Roots of Platycodon grandiflorum. Chin. Chem. Lett. 2007, 18, 306–308. [Google Scholar] [CrossRef]
- He, Z.; Qiao, C.; Han, Q.; Wang, Y.; Ye, W.; Xu, H. New triterpenoid saponins from the roots of Platycodon grandiflorum. Tetrahedron 2005, 61, 2211–2215. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors (liwei7727@126.com). |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).