Abstract
Flash vacuum pyrolysis (FVP) of 4-aryl-3-buten-2-ols [ArCH=CH-CH(CH3)OH, where Ar is phenyl, p-MeO, p-Me, p-Cl, p-NO2] gave the corresponding buta-1,3-dien-1-ylbenzene (ArCH=CH-CH=CH2, where Ar is Ph, p-MeO, p-Me, p-Cl, p-NO2) and 7-X-1,2-dihydronaphthalene derivatives (where X is H, MeO); FVP of 1-aryl-3-benzyloxy1-1-butenes and benzyl cinnamyl ethers [ArCH=CHCH(X)OCH2Ph, where Ar is phenyl, p-MeO, p-Me, p-Cl, X is H, Me, Ph] gave the corresponding but-2-en-1-ylbenzene derivatives (ArCH2CH=CH-X, where X is H, Me, Ph) together with benzaldehyde. The proposed mechanism of these pyrolytic transformations was supported by kinetic and product analysis.
Introduction
Recently we have shown that gas-phase pyrolysis of α-substituted propionic acids (Scheme 1, where the substituents G = PhO, PhS, PhNH) proceeds by elimination of GH and formation of acetaldehyde and carbon monoxide by the mechanistic pathway presented in Equation 1 [1]. On the other hand, the analogous β-substituted propionic acids undergo retro-Michael elimination to give GH and acrylic acid, as shown in Equation 2 [2]. Moreover, various 3-anilino-1-propanol derivatives have been shown to undergo gas-phase pyrolysis via retro-ene reactions to give anilines, ethylene and carbonyl compounds, as shown in Equation 3 [3,4].
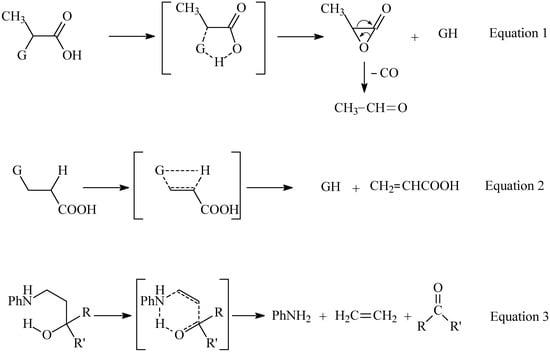
Scheme 1.
Pyrolysis product and mechanism of gas-phase of α-substituted propanoic acids.
This study reports the gas-phase pyrolytic reactions of 4-aryl-3-buten-2-ols 1a-e and allyl benzyl ethers 5a-f. Pyrolysis of 1a-e is expected to proceed via 1,2-elimination similar to those shown in Equation 2 to produce 1-aryl-1,3-butadienes. On the other hand, pyrolysis of 5a-f is expected to proceed via a retro-ene mechanism similar to that shown in Equation 3 and which has widely been used in many useful synthetic applications [3,4].
Results and Discussion
Synthesis
The required substrates 1a-e, 5a-f was prepared and fully characterized by NMR and MS, as described in the Experimental section.
Pyrolysates
Flash vacuum pyrolysis of the 4-aryl-3-buten-2-ols 1a-e at 500 °C and 10-2 Torr afforded the corresponding 1-aryl-1,3-butadienes 3a-e and 1,2-dihydronphthalenes 4a,b (Scheme 2). The pyrolysates were qualitatively and quantitatively analyzed by HPLC, the conversion yields were also determined from 1H-NMR and the results are recorded in Table 1. The possible mechanistic route for the formation of 3 obtained from the FVP of substrates 1a-e involves the 4-membered transition state 2. A similar transition state has been shown to account for the pyrolysis products of β-substituted propionic acids (Equation 2, Scheme 1) [2]. The formation of dihydronaphthalenes 4a,b involves 6π-electrocyclization of 3a,b followed by 1,5-H-shift (Scheme 2). The thermal conversion of 3a to 1,2-dihydronaphthalene 4a has been previously reported by Badwa [6].
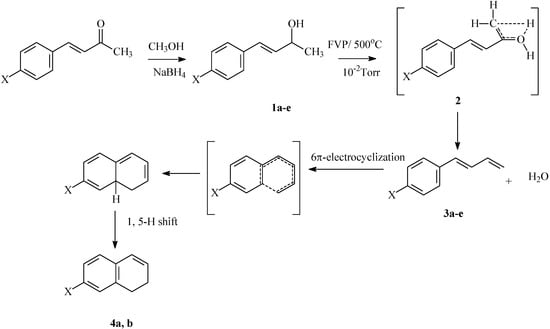
Scheme 2.
Synthesis and FVP products of 4-aryl-3-buten-2-ols 1a-e.

Table 1.
Pyrolysis products of 1a-e at 500 °C, 10-2 Torr and% yielda from FVP.
| Cpd | Substrate (X) | Pyrolysis products (% yield)b | Recovered substrate | |
|---|---|---|---|---|
| 1a | H | 3a (40) | 4a (50) | 1a (0) |
| b | OCH3 | 3b (38) | 4b (55) | 2a (0) |
| c | CH3 | 3c (45)b | - | 1c (40) |
| d | Cl | 3d (68)b | - | 1d (25) |
| e | NO2 | 3e (85)b | - | 1e (15) |
a) Yields measured by 1H-NMR spectroscopy in CDCl3 based on the 1H doublets at δ 5.23, 5.13, 5.18, 5.23, 5.37 for compounds 3a-e and 2H multiplets at δ 2.37, 2.30 for 4a,b; b) This yield refers to conversion yield based on the substrate consumed.
Flash vacuum pyrolysis of the allyl benzyl ethers 5a-d at 600 °C and 10-2 Torr afforded but-2-en-1-ylbenzene derivatives 8a-d in good yield, together with benzaldehyde 7 (Scheme 3). The pyrolysates were qualitatively and quantitatively analyzed by HPLC, yields were also determined from 1H-NMR and the results are recorded in Table 2. Similarly, FVP of benzyl cinnamyl ether 5e and 3-benzyloxy-1,3-diphenylpropene 5f at 600 °C and 10-2 Torr afforded allylbenzene 8e and 1,3-diphenylpropene 8f, respectively, together with benzaldehyde (Scheme 3); yields are recorded in Table 2. The possible mechanistic route to the formation of 8a-f and 7 obtained from the FVP of substrates 5a-f involves the hetero-retro-ene six-membered transition state 6 (Scheme 3).
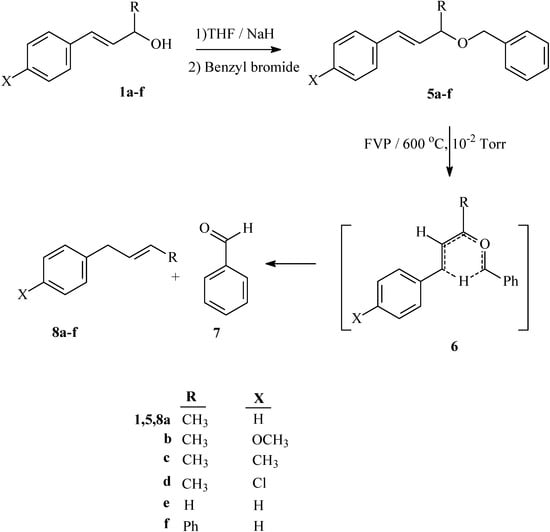
Scheme 3.
Synthesis and FVP products of allyl benzyl ethers 5a-d.

Table 2.
Pyrolysis products of 5a-f at 600 °C, 10-2 Torr and % yielda from FVP.
| Cpd | R | X | Pyrolysis products (% yield)b | Recovered substrate | |
|---|---|---|---|---|---|
| 5a | CH3 | H | 8a (78) | 7 (86) | 5a (0) |
| 5b | CH3 | OCH3 | 8b (91) | 7 (93) | 5b (0) |
| 5c | CH3 | CH3 | 8c (85) | 7 (64) | 5c (14) |
| 5d | CH3 | Cl | 8d (68) | 7 (56) | 5d (20) |
| 5e | H | H | 8e (70) | 7 (69) | 5e (0) |
| 5f | Ph | H | 8f (60) | 7 (59) | 5f (0) |
a) Yields measured by 1H-NMR spectroscopy in CDCl3; b) This yield refers to conversion yield based on the substrate consumed.
Kinetic Analysis
Analysis of the kinetic data reported in Table 3 using the proposed reaction mechanism in Scheme 3 reflects the influence of the substituent group (X) on the reactivity of allyl benzyl ethers 5a-d. The rate enhancement in going from OCH3 > CH3 > H > Cl, is systematic and consistent with the electron donating effects of the OCH3 on the protophilicity of the π-bond involved in the transition state 6.
Experimental
General
All melting points are uncorrected. IR spectra were recorded in KBr disks on a Perkin Elmer System 2000 FT-IR spectrophotometer. 1H- and 13C-NMR spectra were recorded on a Bruker DPX 400 MHz super-conducting NMR spectrometer. Mass spectra were measured on VG Autospec-Q (high resolution, high performance, tri-sector GC/MS/MS) and with LCMS using Agilent 1100 series LC/MSD with an API-ES/APCI ionization mode. Microanalyses were performed on a LECO CH NS-932 Elemental Analyzer. The HPLC analysis was performed using a Metrohm HPLC (pump model 7091C and Shimadzu SPD-10 UV/VIS detector), and Supelco LC-8 chromatography column (2.5 cm length × 4.6 mm ID, 5 μm particle size). The reactor used for kinetic and product analysis is a Chemical Data System (CDS) custom-made pyrolyzer consisting of an insulated aluminium-alloy block fitted with a platinum resistance thermocouple connected to a Comark microprocessor thermometer for reactor temperature read-out, accurate to < 0.5 °C (Figure 1). The alloy was chosen for its high thermal conductivity and low temperature gradient, and may be heated for up to 526 °C. The temperature of the reactor is controlled by means of a Eurotherm 093 precision temperature regulator to provide 0.1 °C incremental changes. The reaction tubes were Pyrex: 8 cm long for kinetic runs, and 12 cm for product analysis; with internal and outer diameters 1.5 cm and 1.7 cm, respectively, for both tubes.
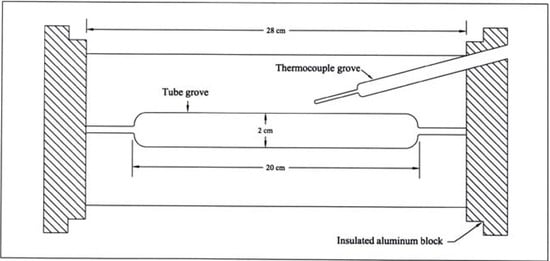
Figure 1.
Schematic drawing of the reactor used for kinetic studies by static pyrolysis.
General Procedure of Synthesis of Starting Compounds 1a-e
To a cooled solution of the appropriate 4-aryl-3-buten-2-one [7] (10 mmol) in methanol (20 mL) was added portionwise with stirring NaBH4 (0.5 g, 15 mmol) [7]. The reaction mixture was then stirred at room temperature for 1 h and the solvent was then removed in vacuo. The product was extracted with dichloromethane (DCM, 3 × 50 mL), washed with water and dried over anhydrous sodium sulfate. The solvent was then removed in vacuo and the remaining products collected and purified to give 1a-e [6,7].
4-Pheny-3-buten-2-ol (1a). Colorless crystals from hexane, mp 40-43 °C, yield 90%; MS: m/z = 148 (M+, 20%), 130 (100%), 105 (50%); 1H-NMR (CDCl3): δ 1.40 (d, 3H, J = 6.4 Hz), 2.42 (br, 1H, OH), 4.50 (quin, 1H, J = 6.4 Hz), 6.29 (dd, 1H, J = 15.9, 6.4 Hz), 6.58 (d, 1H, J = 15.9 Hz), 7.29 (t, 1H, J = 7.2 Hz), 7.36 (t, 2H, J = 7.0 Hz), 7.44 (d, 2H, J = 7.4 Hz) [6,7].
4-(4-Methoxphenyl)-3-buten-2-ol (1b). Colorless crystals from pet. ether (60-80), mp 70-73 °C, yield 86%; MS: m/z = 178 (M+, 30%), 163 (10%), 121 (100%); 1H-NMR (CDCl3): δ 1.38 (d, 3H, CH3 J = 6.4 Hz), 1.61 (br, 1H, OH), 3.83 (s, 3H, OCH3), 4.48 (quin, 1H, J = 6.4 Hz), 6.15 (dd, 1H, J = 15.7, 6.4 Hz), 6.53 (d, 1H, J = 15.7), 6.87 (d, 2H, J = 8.6 Hz), 7.34 (d, 2H, J = 8.6 Hz ) [7].
4-(4-Methylphenyl)-3-buten-2-ol (1c). Colorless crystals from pet. ether (60-80), mp 40-42 °C, yield 94%; MS: m/z = 162 (M+, 10%), 145 (100%), 119 (60%), 105 (60%); 1H-NMR (CDCl3): δ 1.40 (d, 3H, J = 6.4 Hz), 1.58 (br, 1H, OH), 2.36 (s, 3H, CH3), 4.50 (quin, 1H, J = 6.4 Hz), 6.24 (dd, 1H, J = 15.9, 6.4 Hz), 6.56 (d, 1H, J = 15.9 Hz), 7.16 (d, 2H, J = 7.8 Hz), 7.31 (d, 2H, J = 7.8 Hz ); 13C-NMR (CDCl3): δ 137.6 (C), 133.9 (C), 132.6 (CH), 129.5 (CH), 129.4 (2CH), 126.5 (2CH), 69.1 (C), 23.4 (CH3), 21.2 (CH3) [7].
4-(4-Chlorophyl)-2-buten-2-ol (1d). Colorless oil, yield 88%; MS: m/z = 182 (M+, 90%), 165 (100%), 125 (80%); 1H-NMR (CDCl3): δ 1.39 (d, 3H, J = 6.2 Hz), 1.68 (br, 1H, OH), 4.40 (quin, 1H, J = 6.2 Hz), 6.25 (dd, 1H, J = 15.9, 6.2 Hz), 6.54 (d, 1H, J = 15.9 Hz), 7.29-7.35 (m, 4H) [7].
4-(4-Nitrophenyl)-3-buten-2-ol (1e). Yellow crystals from hexane, mp 40-42 °C (lit. [9] mp 40 °C), yield 68%; MS: m/z = 193 (M+, 10%), 175 (100%), 146 (60%); 1H-NMR (CDCl3): δ 1.42 (d, 3H, J = 6.4 Hz), 1.68 (br, 1H, OH), 4.57 (quin,1H, J = 6.4 Hz), 6.47 (dd, 1H, J = 15.9, 6.4 Hz), 6.68 (d, 1H, J = 15.9 Hz), 7.53 (d, 2H, J = 8.6 Hz), 8.20 (d, 2H, J = 8.6 Hz); 13C-NMR (CDCl3): δ 23.4 (CH3), 68.4 (C), 124.0 (2CH), 126.9 (2CH), 127.0 (CH), 129.4 (C), 138.3 (CH), 143.6 (C).
Synthesis of starting compounds 5a-f: General procedure
To a mixture of the appropriate 1a-f [6,7] (10 mmol) and NaH (60% w/w in mineral oil, 15 mmol) in dry THF (20 mL) was added benzyl bromide (10 mmol). The reaction mixture was heated under reflux for 3 h, the solvent was then removed in vacuo and the residue was extracted with ether (100 mL). The ethereal solution was washed with water (100 mL) and brine (100 mL) and dried over anhydrous Na2SO4. After removal of the solvent the product was purified by column chromatography using the appropriate solvent to give 5a-f [9].
(3-Benzyloxybut-1-enyl)benzene (5a). Colorless oil, yield 61%; [Rf = 0.84 EtOAc/pet.ether (60-80) 1:7]; MS: m/z = 238 (M+, 10%), 180 (30%), 146 (10%), 131 (100%); 1H-NMR (CDCl3): δ 1.60 (d, 3H, J = 6.4 Hz), 4.30 (quin,1H, J = 6.4 Hz), 4.64 (d, 1H, J = 12.0 Hz), 4.82 (d, 1H, J = 12.0 Hz), 6.38 (dd, 1H, J = 15.9, 6.4 Hz), 6.74 (d, 1H, J = 15.9 Hz), 7.38- 7.69 (m, 10H); 13C-NMR (CDCl3): δ 22.6 (CH3), 70.8 (CH2), 76.6 (C), 127.3 (2CH), 128.2 (CH), 128.4 (2CH), 128.5 (CH), 129.0 (2CH), 129.4 (2CH), 132.2 (CH), 132.5 (CH), 137.4 (C), 139.6 (C) [9].
1-(3-Benzyloxybut-1-enyl)-4-methoxylbenzene (5b). Yellow oil, yield 68%; [Rf = 0.68, EtOAc/pet.ether (60-80) 1:9]; MS: m/z = 268 (M+, 20%), 252 (30%), 163 (60%). IR: 3063, 2925, 1720, 1453, 1272, 1046, 1070, 751, 699; 1H-NMR (CDCl3): δ 1.42 (d, 3H, J = 6.4 Hz), 3.86 (s, 3H, OCH3), 4.13 (quin,1H, J = 6.4 Hz), 4.47 (d, 1H, J = 12.0 Hz), 4.66 (d, 1H, J = 12.0 Hz), 6.07 (dd, 1H, J = 15.9, 6.4 Hz), 6.53 (d, 1H, J = 15.9 Hz), 6.92 (d, 2H, J = 8.4 Hz), 7.30-7.40 (m, 7H); 13C-NMR (CDCl3): δ 21.9 (CH3), 55.2 (CH2), 69.9 (OCH3), 76.0 (C), 113.9 (2CH), 127.4 (CH), 127.6 (2CH), 127.7 (2CH), 128.3 (2CH), 129.3 (C), 129.4 (CH), 131.0 (CH), 138.8 (C), 159.2 (C); Anal. calc. for C18H20O2 (268.4): C 80.56; H 7.51. Found: C 80.48; H 7.48.
1-(3-Benzyloxybut-1-enyl)-4-methylbenzene (5c). Yellow oil, yield 72%; [Rf = 0.8 EtOAc/pet.ether (60-80) 1:9]; MS: m/z = 252 (M+, 10%), 146 (40%), 131 (100%), 105 (50%).IR: 3028, 2925, 1704, 1514, 1454, 1145, 1071, 970, 801, 735, 697 ; 1H-NMR (CDCl3): δ 1.46 (d, 3H, J = 6.4 Hz), 2.43 (s, 3H, CH3), 4.17 (quin,1H, J = 6.4 Hz), 4.52 (d, 1H, J = 12.0 Hz), 4.67 (d, 1H, J = 12.0 Hz), 6.19 (dd, 1H, J = 15.9, 6.4 Hz), 6.58 (d, 1H, J = 15.9 Hz), 7.21 (d, 2H, J = 8.0 Hz), 7.33- 7.46 (m, 7H); 13C-NMR (CDCl3): δ 21.2 (CH3), 21.9 (CH3), 70.0 (CH2), 76.0 (C), 126.4 (2CH), 127.4 (CH), 127.8 (2CH), 128.4 (2CH), 129.3 (2CH), 130.7 (CH), 131.4 (CH), 133.9 (C), 137.6 (C), 138.9 (C); Anal. calc. for C18H20O (252.4): C 85.67; H 7.99. Found: C 85.48; H 7.88.
1-(3-Benzyloxybut-1-enyl)-4-chlorobenzene (5d). Yellow oil, yield 64%; [Rf = 0.8 ether/pet. ether (60-80) 1:1]; MS: m/z = 272 (M+, 10%), 214 (25%), 165 (100%), 91 (80%). IR: 3030, 2975, 1703, 1492, 1271, 1096, 1013, 970, 809, 737, 698 ; 1H-NMR (CDCl3): δ 1.41 (d, 3H, J = 6.4 Hz), 4.19 (quin, 1H, J = 6.4 Hz), 4.54 (d, 1H, J = 12.0 Hz), 4.70 (d, 1H, J = 12.0 Hz), 6.24 (dd, 1H, J = 15.9, 6.4 Hz), 6.59 (d, 1H, J = 15.9 Hz), 7.39 (m, 5H), 7.46 (m, 4H); 13C-NMR (CDCl3): δ 21.6 (CH3), 70.1 (CH2), 75.7 (C), 127.5 (CH), 127.6 (2CH), 127.7 (2CH), 128.3 (2CH), 128.7 (2CH), 130.0 (CH), 132.4 (CH), 133.2 (C), 135.1 (C), 138.7 (C); Anal. calc. for C17H17O (272.8): C 74.86; H 6.28. Found: C 74.68; H 6.14.
3-Benzyl cinnamyl ether (5e). Colorless oil, yield 70%; [Rf = 0.8 ether/hexane 5:95]; MS: m/z = 225 (M+, 20%), 118 (80%); 1H-NMR (CDCl3): δ 4.30 (d, 2H, J = 5.4 Hz), 4.68 (s, 2H), 6.45 (m, 1H), 6.73 (d, 1H, J = 15.9 Hz), 7.33-7.52 (m, 10H) [9].
(3-Benzyloxy-3-phenylprop-1-enyl)benzene (5f). Colorless oil, yield 56%; [Rf = 0.76 ether/hexane 1:9]; MS: m/z = 300 (M+, 20%), 194 (20%), 106 (90%), 104 (80%); IR: 3030, 2925, 1721, 1452, 1270, 1097, 1026, 750,700; 1H-NMR (CDCl3): δ 4.62 (s, 2H ), 5.06 (d, 1H, J = 7.2 Hz), 6.38 (dd, 1H, J = 16.0, 7.2 Hz), 6.67 (d, 1H, J = 16.0 Hz), 7.18 (d, 2H, J = 7.2 Hz), 7.23 (m, 2H), 7.25-7.44 (m, 9H), 7.8 (d, 2H, J = 7.6 Hz) [11].
Flash vacuum pyrolysis
The apparatus used was similar to the one which has been described in our recent publication [1]. The sample was volatilized from a tube in a Buchi Kugelrohr oven through a 30 × 2.5 cm horizontal fused quartz tube. This was heated externally by a Carbolite Eurotherm tube furnace MTF-12/38A to a temperature of 500 °C, the temperature being monitored by Pt/Pt-13%Rh thermocouple situated at the center of the furnace. The products were collected in a U-shaped trap cooled in liquid nitrogen. The whole system was maintained at a pressure of 10-2 Torr by an Edwards Model E2M5 high capacity rotary oil pump, the pressure being measured by a Pirani gauge situated between the cold trap and pump. Under these condition the contact time in the hot zone was estimated to be =10 ms. The different zones of the product collected in the U-shaped trap were analyzed by 1H, 13C-NMR, IR and GC-MS. Relative and percent yields were determined from NMR.
Pyrolysis products
Buta-1,3-dien-1-ylbenzene (3a). LCMS: m/z = 131 (M + 1); 1H-NMR (CDCl3): δ 5.23 (d, 1H, J = 10.0 Hz), 5.39 (d, 1H, J = 16.7 Hz), 6.60 (m, 2H), 6.84 (m, 1H), 7.29 (t, 1H, J = 7.2 Hz), 7.38 (t, 2H, J = 7.4 Hz), 7.41 (d, 2H, J = 7.6 Hz) [12].
1-(Buta-1,3-dien-1-yl)-4-methoxybenzene (3b). LCMS: m/z = 161 (M + 1); 1H-NMR (CDCl3): δ 3.84 (s, 3H, OCH3), 5.13 (d, 1H, J = 9.0 Hz), 5.30 (d, 1H, J = 16.0 Hz), 6.48 (m, 2H), 6.69 (m, 1H), 6.87 (d, 2H, J = 8.4 Hz), 7.37 (d, 2H, J = 8.4 Hz) [13].
1-(Buta-1,3-dien-1-yl)-4-methylbenzene (3c). LCMS: m/z = 145 (M+1); 1H-NMR (CDCl3): δ 2.37 (s, 3H, CH3), 5.18 (d, 1H, J = 10.6 Hz), 5.34 (d, 1H, J = 15.4 Hz), 6.56 (m, 2H), 6.78 (m, 1H), 7.19 (d, 2H, J = 8.2 Hz), 7.34 (d, 2H, J = 8.2 Hz) [14].
1-(Buta-1,3-dien-1-yl)-4-chlorobenzene (3d). LCMS: m/z = 165 (M + 1); 1H-NMR (CDCl3): δ 5.23 (d, 1H, J = 9.6 Hz), 5.43 (d, 1H, J = 16.4 Hz), 6.53 (m, 2H), 6.78 (m, 1H), 7.26-7.36 (m, 4H) [15].
1-(Buta-1,3-dien-1-yl)-4-nitrorobenzene (3e). LCMS: m/z = 176 (M + 1); 1H-NMR (CDCl3): δ 5.37 (d, 1H, J = 9.6 Hz), 5.49 (d, 1H, J = 15.9 Hz), 6.54 (m, 2H), 6.94 (dd, 1H, J = 15.9, 9.6 Hz), 7.53 (d, 2H, J = 8.8 Hz), 8.20 (d, 2H, J = 8.8 Hz); 13C NMR (CDCl3): δ 121.0, 124.1, 126.8, 130.4, 134.0, 136.4, 143.6, 146.8 [16].
1,2-Dihydronaphthalene (4a). LCMS m/z = 131 (M + 1); 1H-NMR (CDCl3): δ 2.37 (m, 2H), 2.86 (t, 2H, J = 8.2 Hz), 6.08 (m, 1H), 6.54 (d, 1H, J = 8.6 Hz ), 7.07 (d, 1H, J = 6.8 Hz), 7.14-7.22 (m, 3H) [17a].
7-Methoxy-1,2-dihydronaphthalene (4b). LCMS: m/z = 161 (M + 1); 1H-NMR (CDCl3): δ 2.30 (m, 2H), 2.79 (t, 2H, J = 8.2 Hz), 3.81 (s, 3H, OCH3) 5.91 (m, 1H), 6.55 (d, 1H, J = 8.6 Hz ), 6.69 (m, 2H), 6.96 (d, 1H, J = 8.6 Hz).
Benzaldehyde (7). 1H-NMR (CDCl3): δ 7.58 (t, 2H, J = 8.0 Hz), 7.69 (t, 1H, J = 7.8 Hz ), 7.94 (d, 2H, J = 7.9 Hz), 10.06 (s, 1H) [17b].
But-2-enylbenzene (8a). LCMS: m/z = 133 (M + 1); 1H-NMR (CDCl3): δ 1.75 (d, 3H, J = 6.2 Hz), 3.37 (d, 2H, J = 6.2 Hz), 5.61 (m, 2H), 7.22 (t, 2H, J = 7.6 Hz), 7.33 (m, 3H) [18].
1-(But-2-enyl)-4-methoxybenzene (8b). LCMS: m/z = 163 (M + 1); 1H-NMR (CDCl3): δ 1.74 (d, 3H, J = 6.2 Hz), 3.31 (d, 2H, J = 6.2 Hz), 3.83 (s, 3H, OCH3) 5.58 (m, 2H), 6.89 (d, 2H, J = 8.4 Hz), 7.15 (d, 2H, J = 8.4 Hz) [19].
1-(But-2-enyl)-4-methylbenzene (8c).LCMS: m/z = 147 (M+1); 1H-NMR (CDCl3): δ 1.75 (d, 3H, J = 6.2 Hz), 2.41 (s, 3H, CH3), 3.36 (d, 2H, J = 6.2 Hz), 5.62 (m, 2H), 7.16 (d, 2H, J = 8.2 Hz), 7.43 (d, 2H, J = 8.2 Hz) [20].
1-(But-2-enyl)-4-chlorobenzene (8d. LCMS: m/z = 167 (M + 1); 1H-NMR (CDCl3): δ 1.73 (d, 3H, J = 6.2 Hz), 3.31 (d, 2H, J = 6.2 Hz), 5.56 (m, 2H), 7.14 (d, 2H, J = 8.2 Hz), 7.36 (d, 2H, J = 8.2 Hz) [21].
Allylbenzene (8e. LCMS: m/z = 119 (M + 1); 1H-NMR (CDCl3): δ 3.46 (d, 2H, J = 6.2 Hz), 5.17 (m, 2H), 6.05 (m, 1H), 7.27 (d, 2H, J = 7.8 Hz), 7.39 (t, 2H, J = 7.8 Hz), 7.45(t, 1H, J = 7.8 Hz) [22].
1,3-Diphenylpropene (8f). LCMS: m/z = 195 (M + 1); 1H-NMR (CDCl3): δ 3.68 (d, 2H, J = 6.4 Hz), 6.53 (m, 1H), 6.59 (d, 1H, J = 15.8 Hz), 7.28- 7.53 (m, 10H).
Kinetic runs
Stock solution (7 mL) was prepared by dissolving 5–10 mg of the substrate in acetonitrile to give a concentration of 1 × 103 – 2 × 103 ppm. An internal standard was then added, the amount of which was adjusted to give the desired peak area ratio of substrate to standard (2.5:1). The solvent and standard were selected to be stable under the conditions of pyrolysis, and because they do not interact or react with either substrate or product. The internal standard used in the present study was 1,3-dichlorobenzene and 1,2,4-trichlorobenzene. Each mixture was filtered before use to ensure a homogeneous solution.
The ratio of the amount of substrate with respect to the internal standard was calculated from the ratio of the substrate peak area to the peak area of the internal standard. The kinetic rate was obtained by tracing the rate of disappearance of the substrate with respect to the internal standard as follows: An aliquot part (0.2 mL) of each solution containing the substrate and the internal standard was pipetted into the pyrolysis tube, which was then cooled in liquid nitrogen and sealed at reduced pressure (0.20 mbar). The tube was then placed in the pyrolyzer for 6 minutes under non-thermal conditions (ambient temperature). A sample was then analyzed using HPLC with uv/vis detector at wavelength, λ = 256 nm to calculate the standardization value (Ao). Several HPLC measurements were obtained with accuracy ≥2%. The temperature of the pyrolysis block was then raised until ca 10% pyrolysis of the substrate was deemed to occur over 600 s interval. This process was repeated after each ca 10 °C rise in the reaction temperature until >95% reaction was achieved. The relative ratios of the integration values of the sample and the internal standard (A) at the pyrolysis temperature were then calculated. A minimum of two kinetic runs were carried out at each reaction temperature following each 10 °C rise in the pyrolyzer temperature to ensure reproducible values of (A). Treatment of the kinetic data and calculation of Arrhenius parameters and reaction rate constants have been detailed elsewhere [22]. Figure 2 shows Arrhenius plot for pyrolysis of compounds 5b as a selected example.

Table 3.
Rate coefficient (k/s-1), Arrhenius parameters of compounds 5a-d. 

| Cpd | X | T /K | 104k /s-1 | log A /s-1 | Ea / kJ mol-1 | k450K/s-1 |
|---|---|---|---|---|---|---|
| 5a | H | 427.05 | 1.133 | 13.48 ± 0.26 | 258.2 ± 4.88 | 8.47 × 10-4 |
| 440.05 | 3.477 | |||||
| 437.15 | 2.175 | |||||
| 456.95 | 14.611 | |||||
| 466.85 | 34.889 | |||||
| 5b | OCH3 | 396.70 | 1.059 | 14.62 ± 0.32 | 141.4 ± 2.37 | 16.30 × 10-3 |
| 406.55 | 2.764 | |||||
| 413.55 | 5.604 | |||||
| 427.25 | 23.126 | |||||
| 434.05 | 39.985 | |||||
| 5c | CH3 | 412.75 | 1.617 | 20.25 ± 0.57 | 190.1 ± 4.63 | 15.39 × 10-3 |
| 417.50 | 2.939 | |||||
| 422.45 | 5.222 | |||||
| 427.45 | 10.91 | |||||
| 432.45 | 19.81 | |||||
| 5d | Cl | 467.15 | 1.583 | 7.21 ± 0.34 | 138.1 ± 6.49 | 6.60 × 10-5 |
| 477.35 | 3.162 | |||||
| 487.05 | 4.737 | |||||
| 497.05 | 9.068 | |||||
| 506.85 | 12.128 | |||||
| 516.80 | 18.945 | |||||
| 526.45 | 21.774 |
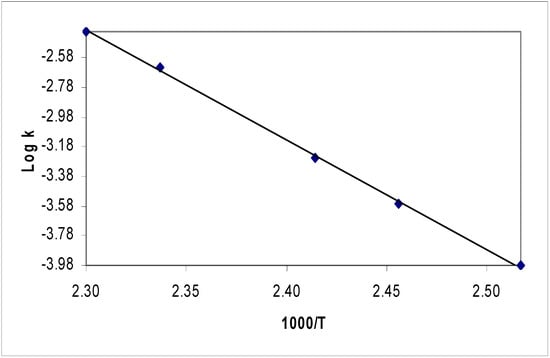
Figure 2.
Arrhenius plot for pyrolysis of compounds 5b.
Conclusions
This work describes pyrolytic synthesis of 1-aryl-1,3-butadienes and 3-arylalkenes. The proposed mechanism is supported by kinetic studies. This represents a general method for the synthesis of such important olefinic derivatives.
Acknowledgements
The support of the University of Kuwait received through research grant # SC04/08 and the General Facilities Projects grant # GS01/01 and GS03/01 are gratefully acknowledged.
- Sample Availability: Samples of the compounds 1a-f, 3a-e, 5a-f and 8a-f are available from the authors.
References and Notes
- Al-Awadi, N.A.; Kaul, K.; El-Dusouqui, O.M.E. Kinetics and mechanism of thermal gas-phase elimination of α-substituted carboxylic acids: Role of relative basicity of α-substituents and acidity of incipient proton. J. Phys. Org. Chem. 2000, 13, 499–505. [Google Scholar]
- Al-Awadi, S.; Abdallah, M.; Hasan, M.; Al-Awadi, N.A. Kinetics and mechanism of thermal gas-phase elimination of α- and β- (N-arylamino)propanoic acid: experimental and theoretical analysis. Tetrahedron 2004, 60, 3045–3049. [Google Scholar]
- Ibrahim, M. Gas-phase pyrolytic reaction of 3-anilino-1-propanol derivatives: Kinetic and mechanistic study. Tetrahedron 2007, 63, 4768–4772. [Google Scholar]
- Dib, H.; Ibrahim, M.R.; Al-Awadi, N.A.; Ibrahim, Y.A. as-phase pyroltic reaction of 3-phenoxy and 3-phenylsulfanyl-1-propanol derivatives. Kinetic and mechanistic study. Int. Chem. Kint. 2008, 40, 51–58. [Google Scholar]
- Padwa, A.; Caruso, T.; Naham, S. Flash vacuum pyrolysis of N-allyl-substituted 1,3,4-oxadiazolin-5-ones. J. Org. Chem. 1980, 45, 4065–4067. [Google Scholar]
- Gergory, B.; Hinz, W.; Jones, R.A.; Sepulveda, A.J. Utilizing of carbon-13-NMR spectrscopy for the identification of E and Z-α,β-unsaturated esters. J. Chem. Res. 1984, 10, 11–12. [Google Scholar]
- Onaran, M.B.; Seto, T.C. Using a lipase as a high-throughput screening method for measuring the enantiomeric excess of allylic acetates. J. Org. Chem. 2003, 68, 8136–8141. [Google Scholar]
- Wang, D.; Chen, D.; Haberman, J.; Li, C. Ruthenium-catalyzed isomerization of homoallylic alcohols in water. Tetrahedron 1998, 54, 5129–5142. [Google Scholar]
- Elisabetta, B.; Nicola, C.; Claudio, F.; Daniela, F.; Piero, G. Enantioselective synthesis of β-substituted butyric acid derivatives via orthoester Claisen rearrangement of enzymatically resolved allylic alcohols: application to the synthesis of (R)-(−)-baclofen. Tetrahedron Asymmetry 1997, 8, 3801–3805. [Google Scholar]
- Saijiko, H.; Hirota, K. A novel type of PdM/C-catalyzed hydrogenation using a catalyst poison: Chemoselective inhibition of the hydrogenolysis for O-benzyl protective group by the addition of a nitrogen-containing base. Tetrahedron 1998, 54, 13981–13996. [Google Scholar]
- Anthony, H.; Eric, J.S.; Matthew, J.P.; Vandana, K.G. General method for the palladium-catalyzed allylation of aliphatic alcohols. J. Org. Chem. 2003, 68, 8092–8096. [Google Scholar]
- Chinkov, N.; Majumdar, S.; Marek, I. Stereoselective synthesis and reactivity of dienyl zirconocene derivatives. Synthesis 2004, 14, 2411–2417. [Google Scholar]
- 12. Shi, M.; Wang, B.Y.; Huang, J.W. Palladium-catalyzed isomerization of methylenecyclopropanes in acetic acid. J. Org. Chem. 2005, 70, 5606-5610 .
- Murakami, M.; Ubukata, M.; Ito, Y. Ruthenium-catalyzed coupling of unactivated olefins with unactivated alkynes. Tetrahedron Lett. 1998, 39, 7361–7364. [Google Scholar]
- Luton, N.D.; Tylor, K.D. New mechanistic aspects on the catalytic transformation of vinylthiiranes to mono and disubstituted 3,6-dihydro-1,2-dithiins by tungsten pentacarbonyl monoacetonitrile. Tetrahedron 2002, 58, 4517–4527. [Google Scholar]
- Hattori, T.; Suzuki, Y.; Ito, Y.; Hotta, D.; Miyano, S. Synthesis of 3,6-dihydro-2H-pyran-2-ones via cationic palladium(II) complex-catalyzed tandem [2+2] cycloaddition-allylic rearrangement of ketene with α,β-unsaturated aldehydes and ketones. Tetrahedron 2002, 58, 5215–5223. [Google Scholar] [CrossRef]
- FT NMR, Aldrich Catalog (a) II, 25C, (b) II, 932B.
- Simmons, H.E.; Park, C.H. Macrobicyclic amines. II. out-out in-in Prototropy in 1, (k + 2)-diazabicyclo [k.l.m] alkaneammonium ions. J. Am. Chem. Soc. 1968, 90, 2429–2431. [Google Scholar] [CrossRef]
- Lajis, N.H.; Khan, M.N. Solvolytic stereoselective coupling reaction of p-methoxyphenylmagnesium bromide with substituted allylic chlorides. Tetrahedron 1992, 48, 1109–1114. [Google Scholar] [CrossRef]
- Ichihara, J. Functional selectivity in Friedel–Crafts alkylations with allylic halides promoted by solid composite lead fluoride reagent. Chem. Comm. 1997, 19, 1921–1922. [Google Scholar] [CrossRef]
- Ibrahim, Y.A.; Al-Awadi, N.A.; Ibrahim, M.R. Gase-phase thermolysis of thieno[3,2-e][1,2,4]triazines. Interesting route towards hetercyclic ring system. Tetrahedron 2004, 60, 9121–9130. [Google Scholar] [CrossRef]
- FT NMR, Aldrich Catalog II, 24B.
- Al-Awadi, N.A.; Ibrahim, Y.A.; Mehul, P.; Bobby, J.; and Al-Etaibi, A.M. Comparative studies on the pyrolysis of N-arylideneaminoamides: Kinetic and mechanistic studies. Int. J. Chem. Kinet. 2007, 39, 59–66. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).