Abstract
It was found that cesium carbonate has a unique catalytic ability on the reaction of carbonyl compounds with diphenyl diselenide to give the corresponding α-phenylseleno carbonyl compounds in moderate to good yields. Similarly, the α-phenylthiolation of carbonyl compounds with diphenyl disulfide was promoted by the cesium carbonate catalyst.
Introduction
α-Phenylselenocarbonyl compounds are useful synthetic intermediates in organic synthesis [1,2,3,4,5] and much effort is being devoted to accomplish the synthesis of these compounds. There are many reports on the preparation of α-phenylselenocarbonyl compounds: (i) electrophilic reaction with PhSeX, PhSeX3, PhSeO2CCF3 or phenylselenium cation (PhSe+), which was generated in situ from the treatment of diphenyl diselenide with SeO2, benzeneseleninic anhydride, chloramine-T, or Et4NBr or MgBr2 under electrolysis [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]; (ii) nucleophilic displacement of α-halocarbonyl compounds with sodium and lithium phenylselenolate [22]; (iii) insertion of carbene into carbon-selenium bond of selenol esters [23]; (iv) palladium-catalyzed coupling of phenyl tributylstannyl selenide and α-halocarbonyl compounds [24] and (v) organocatalyst-catalyzed reaction of carbonyl compounds with N-(phenylseleno)phthalimide [25,26,27,28]. However, many of these synthetic methods suffer from the the problems of handling the selenium reagents used as selenium sources because of their instability towards air and moisture, acidic or basic reaction conditions, and the use of stoichiometric amounts of acid or base. Thus, the development of new synthetic methods using stable selenium reagents under mild conditions would have significant synthetic value. We now discovered that a cesium salt acts as a catalyst on the α-phenylselenation of carbonyl compounds with diphenyl diselenide giving the corresponding α-phenylselenocarbonyl compounds in moderate to good yields (Scheme 1).
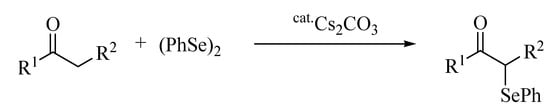
Scheme 1.
Cesium carbonate-catalyzed α-phenylselenation of carbonyl compounds with diphenyl diselenide.
Results and Discussion
When 5-nonanone (1a; 0.30 mmol) was allowed to react with an equivalent amount of diphenyl diselenide (0.30 mmol) in the presence of a catalytic amount of cesium carbonate (0.05 mmol, 17 mol%) in DMA solvent at 70 °C for 5 h, the α-phenylselenation of 1a smoothly proceeded to give 4-phenylseleno-5-nonanone (2a) in 0.03 mmol yield (entry 2 in Table 1). In the absence of the cesium salt, 2a was not formed and the starting materials were recovered (entry 1).

Table 1.
Reaction of 5-nonanone with diphenyl diselenide. a
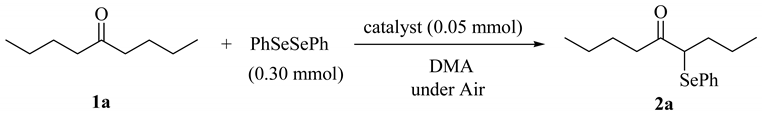 | |||||
| entry | 1a/mmol | temp/ °C | time/h | catalyst | yield/mmol b |
| 1 | 0.30 | 70 | 5 | none | trace |
| 2 | 0.30 | 70 | 5 | Cs2CO3 | 0.03 |
| 3 | 0.90 | 70 | 5 | Cs2CO3 | 0.03 |
| 4 | 1.50 | 70 | 5 | Cs2CO3 | 0.05 |
| 5 | 3.00 | 70 | 5 | Cs2CO3 | 0.07 |
| 6 | 3.00 | 70 | 10 | Cs2CO3 | 0.23 |
| 7 | 3.00 | 100 | 5 | Cs2CO3 | 0.59 |
| 8 c | 3.00 | 100 | 5 | Cs2CO3 | 0.27 |
| 9 | 3.00 | 100 | 5 | CsF | 0.42 |
| 10 | 3.00 | 100 | 5 | CsCl | 0.06 |
| 11 | 3.00 | 100 | 5 | CsBr | 0.08 |
| 12 | 3.00 | 100 | 5 | CsI | 0.02 |
| 13 | 3.00 | 100 | 5 | K2CO3 | 0.48 |
| 14 | 3.00 | 100 | 5 | Na2CO3 | 0.17 |
a Reaction conditions: PhSeSePh (0.30 mmol), catalyst (0.05 mmol), and DMA (2.5 mL); b GC yield. c Under a nitrogen atmosphere.
Increasing the amount of 1a, extending the reaction time, and elevating the reaction temperature led to the increase the yield of 2a (entries 2-7). When diphenyl diselenide was treated with ten equivalent amounts of 1a (3.00 mmol) under air at 100 °C for 5 h, 2a was obtained in 0.59 mmol yield, along with a formation of a small amount of 4,4-bisphenylseleno-5-nonanone (entry 7). It is interested to note that both phenylseleno groups on diphenyl diselenide are efficiently utilized on the reaction. When the reaction was carried out under a nitrogen atmosphere, the yield of 2a was decreased (entry 8). Similarly, in the presence of a catalytic amount of cesium fluoride, the α-phenylselenation of 1a occurred to give 2a in 0.42 mmol yield (entry 9). In contrast to cesium carbonate and fluoride, in the case of the other cesium salts, such as cesium chloride, bromide and iodide, the α-phenylselenation did not proceed (entries 10-12). In the presence of another alkaline metal carbonates, the yields of 2a decreased slightly (entries 13 and 14) [29].
To determine the scope and limitation of the cesium salt-catalyzed α-phenylselenation of carbonyl compounds with diphenyl diselenide, various carbonyl compounds were allowed to react with diphenyl diselenide in the presence of a catalytic amount of cesium carbonate (17 mol%) and these results are shown in Table 2. The treatment of cyclohexanone with diphenyl diselenide under the same reaction conditions as that of 5-nonanone afforded 2-phenylselenocyclohexanone in 0.43 mmol yield (entry 2). For the reaction of the acetophenone and propiophenone, which have an aromatic ring adjacent to the carbonyl group, the α-phenylselenation of these compounds took place smoothly to give the corresponding α-phenylselenoacetophenone and -propiophenone in 0.51 and 0.59 mmol yields, respectively (entries 4 and 8).
4’-Methyl-, 4’-methoxy-, and 4’-chloro-α-phenylselenoacetophenone were prepared by the cesium carbonate-catalyzed reaction of the corresponding acetophenone derivatives with diphenyl diselenide in moderate to good yields (entries 5-7). When an unsymmetrical dialkyl ketone such as 2-octanone was treated with diphenyl diselenide in the presence of cesium carbonate, the phenylselenation of methylene carbon predominantly proceeded to give 3-phenylseleno-2-octanone in 0.41 mmol yield with the formation of 1-phenylseleno-2-octanone (0.17 mmol) (entry 3). In the case of hexanal, the yield of α-phenylselenoaldehyde was low (0.13 mmol) under the same reaction conditions as that of 5-nonanone, owing to the formation of a complex reaction mixture that included the aldol type products; however, the α-phenylselenated product yield was improved, when the reaction was carried out under the lower reaction temperature (65 °C) (entry 9).
Next, the cesium carbonate-catalyzed reaction of carbonyl compounds with diphenyl disulfide was examined (Table 3). When 5-nonanone (3.0 mmol) was allowed to react with diphenyl disulfide (1.0 mmol) in the presence amount of cesium carbonate at 100 °C for 5 h, the α-phenylthiolation of 5-nonanone was successfully occurred and 4-phenylthio-5-nonanone was obtained in 0.44 mmol yield (entry 1). The treatment of cyclohexanone or acetophenone with diphenyl disulfide also afforded the α-phenylthioketone in 0.60 or 0.51 mmol yields (entries 2 and 3).

Table 2.
Cesium carbonate-catalyzed α-phenylselenation of carbonyl compounds with diphenyl diselenides. a
| entry | carbonyl compound | product | yield/mmol b |
|---|---|---|---|
| 1 | 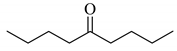 | 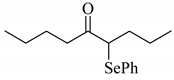 | 0.59 |
| 2a | |||
| 2 | 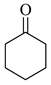 | 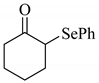 | 0.43 |
| 2b | |||
| 3 | 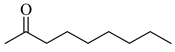 | 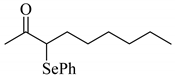 | 0.41 |
| 2c | |||
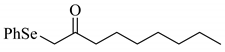 | 0.17 | ||
| 2c’ | |||
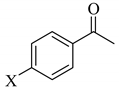 | 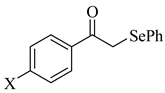 | ||
| 4 | X = H | 2d; X = H | 0.51 |
| 5 | X = CH3 | 2e; X = CH3 | 0.54 |
| 6 | X = OCH3 | 2f; X = OCH3 | 0.48 |
| 7 | X = Cl | 2g; X = Cl | 0.56 |
| 8 | 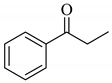 | 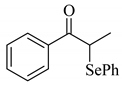 | 0.59 |
| 2h | |||
| 9 | 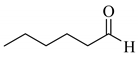 | 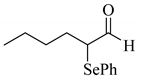 | 0.43 |
| 2i |
a Reaction conditions: PhSeSePh (0.30 mmol), carbonyl compound (3.00 mmol), Cs2CO3 (0.05 mmol), and DMA (2.5 mL) under air at 100 °C for 5 h. b GC yield.

Table 3.
Cesium carbonate-catalyzed α-phenylthiolation of carbonyl compounds with diphenyl disulfide. a
| entry | carbonyl compound | product | yield/mmol b |
|---|---|---|---|
| 1 | 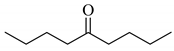 | 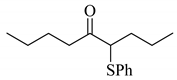 | 0.44 |
| 3a | |||
| 2 | 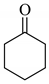 | 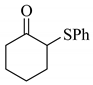 | 0.60 |
| 3b | |||
| 3 | 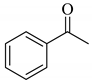 | 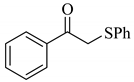 | 0.51 |
| 3c |
a Reaction conditions: PhSSPh (1.0 mmol), carbonyl compound (3.0 mmol), Cs2CO3 (0.2 mmol), and DMA (2.5 mL) under air at 100 °C for 5 h. b GC yield.
Although we cannot explain the reaction pathway of the carbonyl compounds with diphenyl diselenide in the presence of cesium carbonate catalyst in detail, one possible reaction pathway is shown in Scheme 2. The first step of the reaction is the generation of the enolate anion by the deprotonation of carbonyl compound with the cesium salt [30]. The enolate anion was trapped with diselenide to form α-phenylselenocarbonyl compounds, and the phenylselenolate [31]. When the reaction was carried out under air, the product yield was higher than when performed under a nitrogen atmosphere (entries 7 and 8 in Table 1). From the result, it was suggested that phenylselenolate was oxidized by oxygen to form phenylseleno radical and subsequently dimerized giving the diphenyl diselenide.
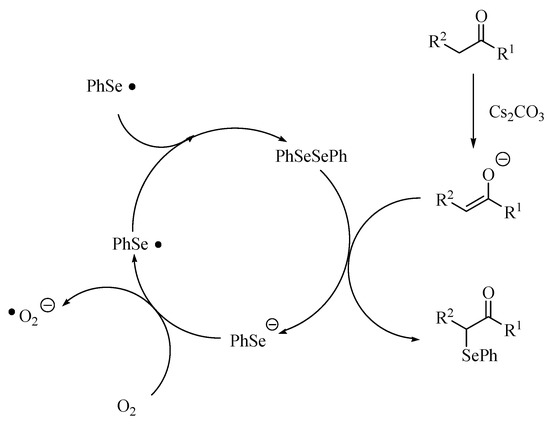
Scheme 2.
A plausible reaction path.
In summary, we found a unique catalytic ability of cesium carbonate in the reaction of carbonyl compounds with diphenyl dichalogenides. The α-phenylchalcogenation of carbonyl compounds with diphenyl dichalcogenides, such as the diselenide or disulfide in the presence of cesium carbonate catalyst efficiently proceeded to give the α-phenylchalcogenocarbonyl compounds in moderate to good yields.
Experimental
General
The 1H- and 13C-NMR spectra were recorded on a JEOL AL400 spectrometer using CDCl3 as the solvent with tetramethylsilane as the internal standard. The IR spectra were recorded with a PerkinElmer FT-IR2000 spectrometer. Gas chromatography (GC) was performed on a Shimadzu GC-14B gas chromatograph using a flame-ionizing detector-equipped instrument and a CBP1 capillary column (0.25 mm × 1200 mm). Preparative HPLC separation was undertaken with a JAI LC-908 chromatograph using 600-mm × 20-mm JAIGEL-1H and 2H GPC columns with CHCl3 as an eluent.
Reagents
Diphenyl disulfide, carbonyl compounds, cesium salts, and alkaline metal salts were purchased as high grade products, and used without purfication. Diphenyl diselenide was synthesized as described in the literature [22]. The solvents were purified before use by the usual methods.
General procedure for cesium salt-catalyzed reaction of carbonyl componds with diphenyl diselenide
A DMA (2.5 mL) solution of diphenyl diselenide (94 mg, 0.30 mmol), carbonyl compound (3.00 mmol), and cesium carbonate (16 mg, 0.05 mmol) was stirred at 100 °C for 5 h under air. After the reaction was complete, H2O was added to the reaction mixture and extracted with diisopropyl ether. The organic layer was dried over MgSO4. The resulting mixture was filtered, and the filtrate was concentrated. Purification of the residue by HPLC afforded the corresponding α-phenylselenated product. The product was characterized by comparing its spectral date with those of authentic samples (2a [32], 2b [27], 2d [24], 2e [24], 2f [24], 2g [24], 2h [24], and 2i [27]). The structures of the product were assigned by their 1H- and 13C-NMR, IR, and Mass spectra.
Mixture 2c and 2c’ (2c:2c’ = 41:17): 1H-NMR (CDCl3) δ 7.53-7.46 (m, 2H), 7.33-7.20 (m, 3H), 3.63 (t, J = 7.4 Hz, 0.70H), 3.56 (s, 0.61H), 2.53 (t. J = 7.4 Hz, 0.61H), 2.26 (s, 2.1H), 1.87-1.76 (m, 0.70H), 1.73-1.62 (m, 0.70H), 1.58-1.48 (m, 0.70H), 1.47-1.38 (m, 0.70H), 1.37-1.14 (m, 7.2H), 0.87 (t, J = 6.8 Hz, 3H); 13C-NMR (CDCl3) δ 205.56, 204.04, 135.36, 135.31, 133.01, 129.06, 128.95, 128.41, 127.60, 127.18, 52.29, 40.51, 35.80, 31.46, 31.40, 30.14, 28.86, 28.81, 28.74, 27.85, 27.19, 23.80, 22.41, 22.35, 13.90, 13.86; IR (NaCl) 3388, 3058, 2925, 2855, 1703, 1578, 1477, 1438, 1355, 1066, 1022, 1000, 739, 691, 670 cm-1; MS m/z 298.
General procedure for cesium salt-catalyzed reaction of carbonyl compounds with diphenyl disulfide
A DMA (2.5 mL) solution of diphenyl disulfide (218 mg, 1.0 mmol), carbonyl compound (3.0 mmol), and cesium carbonate (64 mg, 0.2 mmol) was stirred at 100 °C for 5 h under air. After the reaction was complete, H2O was added to the reaction mixture and extracted with diisopropyl ether. The organic layer was dried over MgSO4. The resulting mixture was filtered, and the filtrate was concentrated. Purification of the residue by HPLC afforded the corresponding α-phenylselenated product. The product was characterized by comparing its spectral date with those of authentic samples (3a [32], 3b [33], and 3c [34]). The structures of the product were assigned by their 1H- and 13C-NMR, IR, and Mass spectra.
Acknowledgments
This research was supported by a Grant-in-Aid for Science Research, and Strategic Project to Support the Formation of Research bases at Private Universities from the Ministry of Education, Culture Sports, Science and Technology of Japan.
- Samples Availability: Samples of the compounds 2a-2i and 3a-3c are available from the authors.
References and Notes
- In Organoselenium Chemistry: A Practical Approach; Back, T.G. (Ed.) Oxford University Press: New York, NY, USA, 1999.
- Krief, A. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon Press: Oxford, UK, 1991; Volume 3, pp. 85–192. [Google Scholar]
- Krief, A.; Hevesi, L. Organoselenium Chemistry; Springer-Verlag: Berlin, Germany, 1988; Volume 1. [Google Scholar]
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis; Pergamon Press: Oxford, UK, 1986. [Google Scholar]
- The Chemistry of Organic Selenium and Tellurium Compounds; Patai, S.; Rappoport, Z. (Eds.) Wiley: New York, NY, USA, 1986; Vols. 1 and 2 and references therein.
- Sharpless, K.B.; Lauer, R.F.; Teranishi, A.Y. Electrophilic and nucleophilic organoselenium reagents. New routes to α,β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 1973, 95, 6137–6139. [Google Scholar]
- Clive, D.L. Fragmentation of selenoxides: A new method for dehydrogenation of ketones. J. Chem. Soc. Chem. Commun. 1973, 695–696. [Google Scholar] [CrossRef]
- Reich, H.J. Organoselenium chemistry. Benzeneselenenyl trifluoroacetate additions to olefins and acetylenes. J. Org. Chem. 1974, 39, 428–429. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Gordon, K.M.; Lauer, R.F.; Singers, S.P.; Young, M.W. The utility of selenium reagents in organic synthesis. Chem. Scr. 1975, 8A, 9–13. [Google Scholar]
- Reich, H.J.; Renga, J.M.; Reich, I.L. Organoselenium chemistry. Conversion of ketones to enones by selenoxide syn elimination. J. Am. Chem. Soc. 1975, 97, 5434–5447. [Google Scholar] [CrossRef]
- Ryu, I.; Murai, S.; Niwa, I.; Sonoda, N. A convenient synthesis of α-phenylseleno ketones and aldehydes from enol silyl ethers and phenylselenenyl bromide. Synthesis 1977, 874–875. [Google Scholar]
- Raucher, S. The regiospecific synthesis of α-phenylseleno ketones from monosubstituted alkenes. Tetrahedron Lett. 1978, 19, 2261–2262. [Google Scholar] [CrossRef]
- Clive, D.L. Modern organoselenium chemistry. Tetrahedron 1978, 34, 1049–1132. [Google Scholar] [CrossRef]
- Torii, S.; Uneyama, K. Handa, K. A facile access to α-phenylselenenyl carbonyl compounds by electrochemical oxidation. Tetrahedron Lett. 1980, 21, 1863–1866. [Google Scholar] [CrossRef]
- Liotta, D.; Zima, G.; Barnum, C.; Saindane, M. Reactions involving selenium metal as an electrophile. The enolate - selenolate transformation. Tetrahedron Lett. 1980, 21, 3643–3646. [Google Scholar] [CrossRef]
- Williams, D.R. Nishitani, T. A mild oxidation of aldehydes to α,β-unsaturated aldehydes. Tetrahedron Lett. 1980, 21, 4417–4420. [Google Scholar] [CrossRef]
- Miyoshi, N.; Yamamoto, T.; Kambe, N.; Murai, S.; Sonoda, N. A convenient method for the synthesis of α-phenylselenenyl carbonyl compounds. Tetrahedron Lett. 1982, 23, 4813–4816. [Google Scholar] [CrossRef]
- Magnus, P.; Rigollier, P. New mild methodology for the synthesis of α-phenylthio and α-phenylseleno ketones. Tetrahedron Lett. 1992, 33, 6111–6114. [Google Scholar] [CrossRef]
- Houllemare, D.; Ponthieux, S.; Outurquin, F.; Paulmier, C. Use of phenylselenium trichloride for simple and rapid preparation of α-phenylselanyl aldehydes and ketones. Synthesis 1997, 101–106. [Google Scholar]
- Yang, M.H.; Wang, H.S.; Zheng, Y.F.; Zhu, C.J. Synthesis of α-phenylseleno ketone directly catalyzed by amine. Youji Huaxue 2006, 26, 1268–1271. [Google Scholar]
- Nazari, M.; Movassagh, B. α-Phenylselenenylation of aldehydes and ketones with diphenyl diselenide mediated by KF/Al2O3. Tetrahedron Lett. 2009, 50, 1453–1455. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Lauer, R.F. Mild procedure for the conversion of epoxides to allylic alcohols. First organoselenium reagent. J. Am. Chem. Soc. 1973, 95, 2697–2699. [Google Scholar] [CrossRef]
- Back, T.G.; Kerr, R.G. Homologation of selenoesters to (phenylseleno)- or (methylseleno)methyl ketones with diazomethane. Tetrahedron Lett. 1982, 23, 3241–3244. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Kawamatsu, H.; Funato, S.; Tokunaga, K.; Sonoda, N. Phenyl tributylstannyl selenide as a promising reagent for introducion of the phenylseleno group. J. Org. Chem. 2003, 68, 3599–3602. [Google Scholar] [CrossRef]
- Cossy, J.; Furet, N. N-(Phenylseleno)phthalimide: A useful reagent for the α-selenylation of ketones and aldehydes. Tetrahedron Lett. 1993, 34, 7755–7756. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Li, H. A Simple and efficient l-prolinamide-catalyzed α-selenenylation reaction of aldehydes. Org. Lett. 2004, 6, 2817–2820. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Mei, Y.; Lou, B.; Xu, D.; Xie, D.; Guo, H.; Wang, W. Direct, facile aldehyde and ketone α-selenenylation reactions promoted by l-prolinamide and pyrrolidine sulfonamide organocatalysts. J. Org. Chem. 2005, 70, 5678–5687. [Google Scholar] [CrossRef]
- Giacalone, F.; Gruttadauria, M.; Marculescu, A. Noto, R. Polystyrene-supported proline and prolinamide. Versatile heterogeneous organocatalysts both for asymmetric aldol reaction in water and α-selenenylation of aldehydes. Tetrahedron Lett. 2006, 48, 255–259. [Google Scholar]
- When diphenyl diselenide was treated with 1a in the presence of cesium oxide, no α-phenylselenation was observed.
- The generation of carbon dioxide was not ascertained by the bubbling of the resulting gas into the aq. Ba(OH)2.
- Reich et al. have described that diphenyl diselenide does not react with lithium enolates of ketones but react with ester enolates and more reactive carbanions [10]. In this manuscript, they suggested that the failure is more the result of an unfavorable equilibrium than of a kinetic barrier.
- Paterson, I.; Osbone, S. Enol borinates in organic synthesis: Regioselective α-sulphenylation and α-selenenylation of ketones. Synlett 1991, 145–146. [Google Scholar] [CrossRef]
- Leriverend, P.; Leriverend, M. Chemoselektive methode zur herstellung von phenyl-sulfoxiden durch oxidation von phenyl-sulfiden mit 2-hydroperoxy-2-methoxypropan. Synthesis 1987, 587–588. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Ogawa, T.; Suzuki, H. Sodium telluride-mediated sulfenylation of α-halo carbonyl compounds with diphenyl disulfide. Bull. Chem. Soc. Jpn. 1989, 62, 1358–1360. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).