Abstract
A solvent-free approach for the regioselective synthesis of β-amino alcohols in shorter reaction times and higher yields, compared to conventional heating is described. It involves microwave (MW) exposure of undiluted reactants in the presence of sulphated zirconia (SZ) or sulphated zirconia over MCM-41 (SZM) as catalyst. Both acid materials can be easily recovered and reused.
Introduction
There is a noticeable growing interest in obtaining solid catalysts that should be able to replace those commonly used, such as concentrated H2SO4, HCl and triflates, among others, for chemical transformations because they can be recovered, reused, and are generally innocuous to the environment. Sulphated zirconia, right from its first reported use in 1979 by Hino and co-workers [1], has commanded increasing attention due to its significantly large acidity, which is associated to its relevant properties such as efficient catalysis of organic reactions. In recent literature, descriptions can be found of coumarin synthesis through the Pechmann reaction [2], alcohol and amine acylation reactions [3], Mannich-type reactions between silylated ketones and aldimines [4], xylose to furfural conversion [5], indole synthesis [6] and the synthesis of β-acetamidocarbonyl compounds [7]. Vicinal amino alcohols are present in various natural and synthetic biologically active products used for preparing diabetes treatments, bronco-dilators, anti-inflammatory, anti-hypotensive, anti-depressants, anti-HIV and anti-malarials [8], in addition to compounds used as catalysts in asymmetric synthesis [9].
Epoxide aminolysis reactions, normally catalyzed by acids or bases, are an efficient route for obtaining β-amino alcohols. However, homogeneous catalysis of these reactions is inconvenient, as it entails the impossibility of reusing the catalyst after the reactions, and in addition, the workup processes often can be laborious. Recently, we found a description of the reaction performed under mild conditions in aqueous media [10]. Among the solid materials used to catalyze aminolysis reactions, one may mention the use of silica gel [11], zeolite NaY [12], alumina [13], copper sulphate-supported polymer [14], montmorillonite [15], alumina-supported phosphomolybdic acid [16] and acid resins [17].
Microwave (MW) irradiation, an unconventional energy source, has been used for a variety of applications including organic synthesis, wherein chemical reactions are accelerated because of selective absorption of MW energy by polar molecules, non-polar molecules being inert to the MW dielectric loss [18]. Recently, it has been found that the use of microwave irradiation to assist organic reactions has considerable advantages over thermal reactions. Reactions that typically require high temperatures and extended reaction times have been considerably accelerated using microwave irradiation. The ring-opening of epoxides [19] can be performed in a very short time under microwave irradiation, compared to the conventional thermal epoxide-opening conditions. The use of microwave irradiation also eliminated the problems of decomposition of the substrates or products during the reaction due to prolonged thermal treatment.
Results and Discussion
Our work group has recently shown [20] that MCM-41-supported sulphated zirconia is an efficient catalyst for the regioselective nucleophilic opening of oxiranes with aniline and benzylamine, under thermal and solvent-free conditions, which motivated us to study the corresponding microwave assisted reaction (Scheme 1).
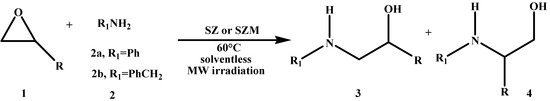
Scheme 1.
Aminolysis of oxiranes.
Scheme 1.
Aminolysis of oxiranes.
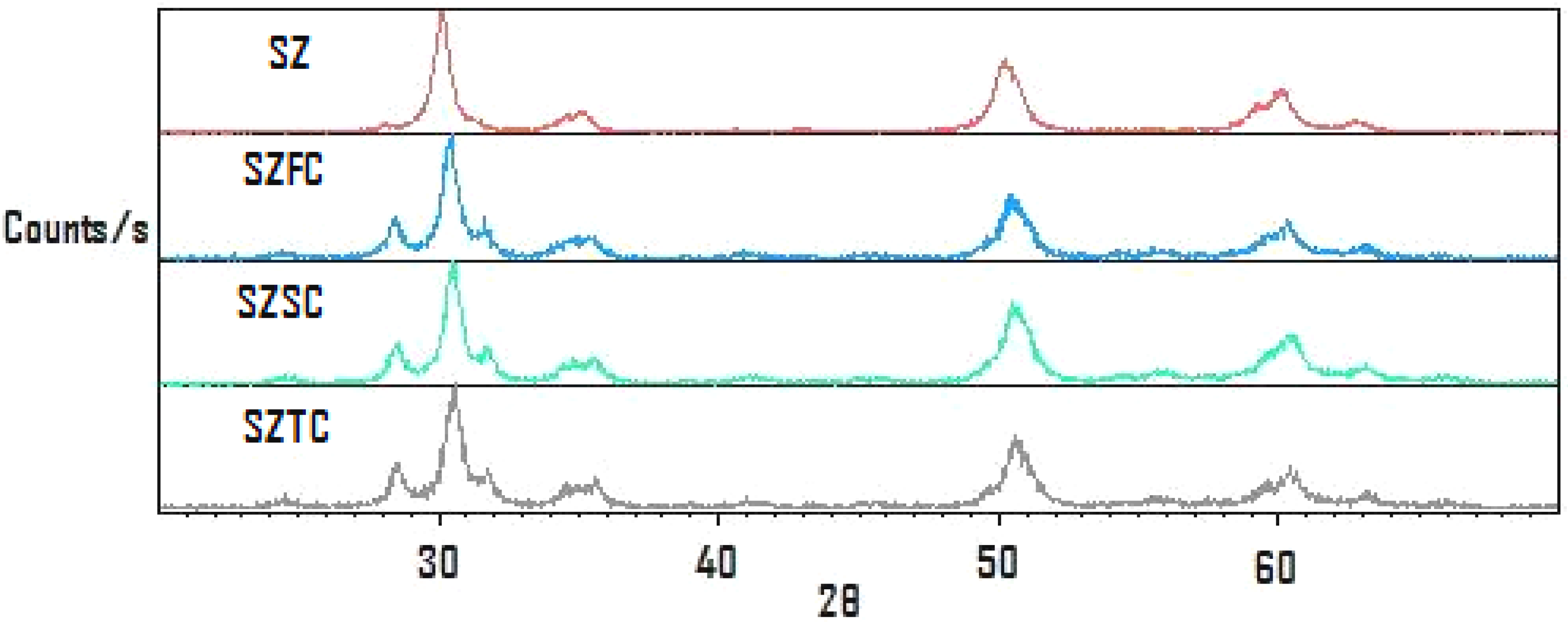
Once the catalyst has been spent, it can be recovered by filtration reactivated by regeneration at 550 °C for one hour. The reactivated samples treated this way displayed the XRD traces shown in Figure 1, where the initial pattern corresponds to as-received sulphated zirconia (SZ); the recovered samples that were used again are labelled SZFC for one cycle, SZSC for a second cycle and SZTC for a third cycle. It can be observed that formation of the monoclinic phase takes place after the first reactivation, while for the other reactivation cycles, the monoclinic: tetragonal phase proportion was maintained.
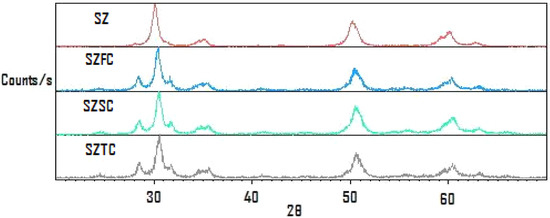
Figure 1.
XRD traces of sulphated zirconia after reactivation cycles.
Figure 1.
XRD traces of sulphated zirconia after reactivation cycles.
The diffraction patterns corresponding to the as-prepared SZ/MCM-41 (SZM), and those of the SZ/MCM-41 recovered after a first cycle (SZMFC), a second cycle (SZMSC) and a third cycle (SZMTC) are shown in Figure 2, which shows that reactivation destroys the MCM-41 phase, because its corresponding peak did appear at 2θ = 2.5.
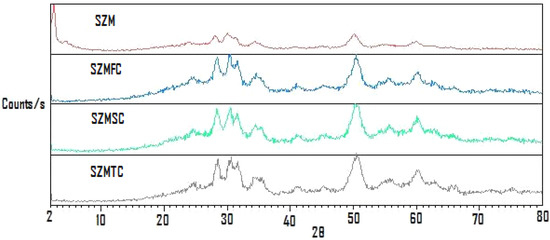
Figure 2.
XRD traces of sulphated zirconia over MCM-41 after reactivation cycles.
Figure 2.
XRD traces of sulphated zirconia over MCM-41 after reactivation cycles.
The textural properties of the sulphated zirconia and the SZ/MCM-41, and those of the reactivated catalysts, shown in Table 1 indicate that the texture of the catalytic material recovered after heating for 1 h at 550 °C, did not undergo significant changes.

Table 1.
Textural properties of the catalysts.
| Sample | Specific area /(m2∙g-1) | Pore volume / (cc∙g-1) | Pore size /(Å) |
|---|---|---|---|
| SZ | 105.73 | 0.12 | 42.79 |
| SZFC | 107.84 | 0.15 | 55.90 |
| SZSC | 86.82 | 0.13 | 63.04 |
| SZTC | 96.83 | 0.13 | 54.59 |
| SZM | 554.21 | 0.40 | 27.19 |
| SZMFC | 580.88 | 0.39 | 24.95 |
| SZMSC | 415.72 | 0.33 | 29.00 |
| SZMTC | 431.29 | 0.29 | 27.03 |
The experimental results related to Scheme 1 are presented and compared in Table 2 (3/4 yield, refers to Scheme 1). They show that the use of the microwave radiation at 60°C (initial power 50W) did not catalyze the reaction. However, if the latter is carried out in the presence of sulphated zirconia or sulphated zirconia supported on MCM-41 the reaction is catalyzed and a significant decrease of the reaction times becomes obvious, as compared to those measured during thermal treatment. Two cases can be mentioned as examples: in entry 1 in Table 2, using only microwaves and no catalyst, no product formation is observed. Using microwaves, 75% of product 3 is obtained with SZ and 70% of product 3 is obtained with SZM, and no product 4 is formed in both cases. In entry 2, using only microwaves and no catalyst, no product formation is observed. Using microwaves and SZ 68% of product 3 is obtained and no product 4, and with SZM 64% of product 3 and 19% of product 4 are obtained, for an 84% overall yield. Reaction times for both entries are shorter than thermal treatment ones.

Table 2.
Yields (%) (3/4 refers to Scheme 1), calculated using GC-FID and compared with published data [20]
| Entry | Epoxide | Amine | MW Time (min) | 3/4 yielda No cat. (%) | 3/4 yieldb SZ (%) | 3/4 yieldc SZM (%) | 60 o Time (min) | 3/4 yieldd SZ (%) | 3/4 yielde SZM (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 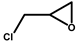 | 2a | 10 | 0 | 75/0 | 70/0 | 30 | 74/0 | 23/0 |
| 2 | 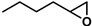 | 2a | 20 | 0 | 68/0 | 64/19 | 300 | 61/20 | 63/0 |
| 3 | 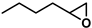 | 2b | 120 | 1 | 78/0 | 40/0 | 360 | 82/0 | 35/0 |
| Entry | Epoxide | Aminea | MW Time (min) | 3/4 yieldb No cat. (%) | 3/4 yieldc SZ (%) | 3/4 yieldd SZM (%) | 60 o Time (min) | 3/4 yielde SZ (%) | 3/4 yieldf SZM (%) |
| 4 |  | 2a | 30 | 0 | 65/15 | 64/12 | 300 | 63/13 | 55/0 |
| 5 | 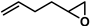 | 2b | 150 | 4 | 72/0 | 46/0 | 300 | 81/0 | 54/0 |
| 6 | 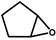 | 2a | 20 | 0 | 82/0 | 83/0 | 300 | 97/0 | 84/0 |
| 7 |  | 2b | 170 | 0 | 66/0 | 11/0 | 300 | 71/0 | 14/0 |
| 8 | 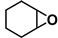 | 2a | 10 | 0 | 95/0 | 86/0 | 300 | 93/0 | 99/0 |
| 9 |  | 2b | 120 | 0 | 95/0 | 47/0 | 300 | 83/0 | 45/0 |
| 10 | 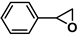 | 2a | 10 | 0 | 4/92 | 5/85 | 60 | 0/96 | 5/91 |
| 11 | 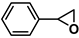 | 2b | 180 | 11 | 61/23 | 23/9 | 360 | 53/31 | 55/25 |
| 12 | 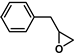 | 2a | 110 | 2 | 85/0 | 77/4 | 300 | 81/0 | 83/0 |
| 13 | 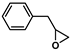 | 2b | 120 | 12 | 77/0 | 44/0 | 300 | 87/0 | 81/0 |
| 14 | 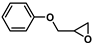 | 2a | 10 | 0 | 95/0 | 80/0 | 30 | 86/0 | 81/0 |
| 15 |  | 2b | 20 | 11 | 83/0 | 70/0 | 30 | 87/0 | 52/0 |
| 16 | 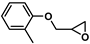 | 2a | 30 | 2 | 86/0 | 77/0 | 60 | 76/0 | 57/0 |
| 17 | 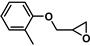 | 2b | 50 | 36 | 85/0 | 55/0 | 60 | 77/0 | 51/0 |
| 18 | 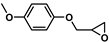 | 2a | 10 | 0 | 85/0 | 80/0 | 60 | 76/0 | 59/0 |
| 19 | 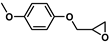 | 2b | 20 | 9 | 89/0 | 70/0 | 60 | 85/0 | 82/0 |
a 2a = aniline; 2b = benzylamine; b MW radiation-assisted reaction without catalyst; c MW assisted reaction with SZ; d MW assisted reaction with SZM; e Solvent-free reaction at 60 °C with SZ [20]; f Solvent-free reaction at 60 °C with SZM [20].
In order to assess the catalytic activity of the reactivated materials, these were used in the test reaction given in entry 15 in Table 2, using 2,3-epoxy-3-phenoxypropane as substrate and benzylamine (2b) as nucleophile, under the same conditions previously described. It was noted that their activity was maintained, as shown in Table 3: regardless of the reactivation process of the SZ/MCM-41, a destruction of the MCM-41 occurs.

Table 3.
Catalytic activity of reactivated catalysts. (3/4 yield refers to Scheme 1).
| Calcination | MW Time (min) | 3/4 Yield (%) SZ | 3/4 Yield (%) SZM |
|---|---|---|---|
| 1st | 20 | 77/0 | 76/0 |
| 2nd | 20 | 79/0 | 68/0 |
| 3rd | 20 | 65/0 | 70/0 |
Conclusions
When the aminolysis reaction takes place mainly through microwave assistance, the yields are typically lower than 5 %, although entries 11, 13, 15, 17 and 19 gave yields of 11, 12, 11, 36 and 9 %, respectively. Entries 1, 2, 4, 6, 7, 8, 9, 10, 14 and 18 did not afford any reaction products. The application of microwave irradiation in conjunction with the use of sulphated zirconia and SZ/MCM-41, under solvent-free conditions, enables the regioselective synthesis of β-amino alcohols, thus providing a unique chemical processes with special attributes such as enhanced reaction rates, higher yields, and the associated ease of manipulation. The catalyst can be easily recovered and reused for at least three cycles without any significant decreases in yield and regioselectivity. It is worthwhile to mention that the reactions where the SZ/MCM-41 was used, microwave irradiation destroyed the characteristic structure of MCM-41 though its activity was maintained even after three reaction cycles, which indicates that catalytic activity depends on the presence of the sulphated zirconia, be it tetragonal or monoclinic.
Experimental
General
The sulphated zirconia was characterized using a Phillips X´Pert Instrument diffractometer with Cu Kα, and nitrogen physisorption was measured at -196 °C with a Micromeritics ASAP 2020. The reaction products were analyzed using an HP-5 column on a Hewlett Packard model 6890 Gas Chromatograph equipped with a model 5973 mass detector system, with the oven programmed from 70-200°C (10°C/min) for 4 min then 200-280°C (10°C/min) for 3 min, inj. 250ºC, det. 280 °C; the detector was set in the Chemical Ionization mode using methane as reactive gas.
Synthesis of sulphated zirconia supported on MCM-41
Preparation of the sulphated zirconia supported on MCM-41 (SZM) has been already described. [20] Siliceous MCM-41 (300 mg) were mixed with zirconium sulphate (15 mL) in methanol (1 wt % S), the heterogeneous mixture was stirred 14 h. Then, the impregnated solid was dried for 6 days at 50 °C and finally calcined at 660 °C for 3 h in air flow.
General experimental procedure for β-amino alcohol synthesis
A mixture of oxirane 1 (186 mg, 1.0 mmol), aniline (2a) or benzylamine (2b) (93 mg or 107 mg, 1.1 mmol), and sulphated zirconia or sulphated zirconia on MCM-41 (50 mg) were introduced into a pressurised reaction tube (10 mL) equipped with a magnetic stirrer, which was irradiated at 60 °C (initial power 50 W) for 10 min in a self tuning single mode CEM Labmate® microwave synthesizer. After completion of the reaction, the mixture was cooled rapidly to room temperature, passing compressed nitrogen through the microwave cavity for 5 min. The catalyst was then recovered by filtration and the organic product was dried under reduced pressure. All products were identified by comparison with the previously described physical and spectroscopic data of the corresponding β-amino alcohols [20].
Acknowledgements
The authors would like to thank Consejo Nacional de Ciencia y Tecnología, CONACYT (project 59417) for financial support of this work. GNS, LLR and EGZ thank the SNI for the distinction of their membership and the stipend received.
References and notes
- Hino, M.; Kobayashi, S.; Arata, K. Reactions of butane and isobutane catalyzed by zirconium oxide treated with sulfate ion.Solid superacid catalyst. J. Am. Chem. Soc. 1979, 101, 6439–6441. [Google Scholar]
- Tyagi, B.; Mishra, M. K.; Jasra, R. V. Synthesis of 7-substituted 4-methyl coumarins by Pechmann reaction using nano-crystalline sulfated-zirconia. J. Mol. Catal. A: Chem. 2007, 276, 47–56. [Google Scholar]
- Ratnama, K. J.; Reddy, R. S.; Sekhar, N. S.; Kantam, M. L.; Figueras, F. Sulphated zirconia catalyzed acylation of phenols, alcohols and amines under solvent free conditions. J. Mol. Catal. A: Chem. 2007, 276, 230–234. [Google Scholar]
- Wang, S.; Matsumura, S.; Toshima, K. Sulfated zirconia (SO4/ZrO2) as a reusable solid acid catalyst for the Mannich-type reaction between ketene silyl acetals and aldimines. Tetrahedron Lett. 2007, 48, 6449–6452. [Google Scholar]
- Dias, A. S.; Lima, S.; Pillinger, M.; Valente, A. A. Modified versions of sulfated zirconia as catalysts for the conversion of xylose to furfural. Catal. Lett. 2007, 114, 151–160. [Google Scholar]
- Reddy, B. M.; Sreekanth, P. M.; Lakshmanan, P. Sulfated zirconia as an efficient catalyst for organic synthesis and transformation. J. Mol. Catal. A: Chem. 2005, 237, 93–100. [Google Scholar]
- Das, B.; Krishnaiah, M.; Laxminarayana, K.; Reddy, K. R. A simple and efficient one-pot synthesis of β-acetamido carbonyl compounds using sulfated zirconia as a heterogeneous recyclable catalyst. J. Mol. Catal. A: Chem. 2007, 270, 284–288. [Google Scholar]
- Smith, D. J.; Jalkanen, M.; Lazar, L.; Szakonky, Z.; Bernarth, G. Inhibitors of copper-containing amine oxidases. WO Pat. 0202090, 2002. [Google Scholar] Kato, H.; Kurata, S. α-(Alkylamino-methyl)-o-chlorobenzyl alcohols. DE Pat. 2244737, 1973. [Google Scholar] Bogan, J. A. Tulobuterol hydro-chloride. Drugs Today 1982, 18, 238–243. [Google Scholar] Lyrenas, S.; Grahnen, A.; Lindberg, B.; Lindstrom, B.; Lonnerholm, G. Pharmacokinetics of terbutaline during pregnancy. Eur. J. Clin. Pharmacol. 1986, 29, 619–623. [Google Scholar] Litosch, I.; Hudson, T. H.; Mills, I.; Li, S. Y.; Fain, J. N. Forskolin as an activator of cyclic AMP accumulation and lipolysis in rat adipocytes. Mol. Pharmacol. 1982, 22, 109–115. [Google Scholar] Farr, R. A.; Cregge, R. J.; Janowick, D. A.; Kohlman, D. T.; Van Dorsselaer, V.; Schirlin, D. G.; Tarnus, C. Difluorostatone antiviral agents. WO Pat. 9602499, 1996. [Google Scholar] Kato, S.; Harada, H.; Hirokawa, Y.; Yoshida, N.; Kawashima, H. Preparation and formulation of indole derivatives as β3 adrenergic receptor stimulants. US Pat. 5817689, 1996. [Google Scholar] De Amici, M.; De Micheli, C.; Kassi, L.; Carrea, G.; Ottolina, G.; Colombo, G. β3-Adrenergic receptor ligands: insight into structure–activity relationships using Monte-Carlo conformational analysis in water. Tetrahedron 2001, 57, 1849–1855. [Google Scholar] Cecchi, R.; Boigegrain, R.; Bianchetti, A.; Poggesi, E.; Croci, T. Phenylethanolaminotetralins, a process for their preparation, and pharmaceutical compositions containing them. US Pat. 4707497, 1987. [Google Scholar]
- Steiner, D.; Sethofer, S. G.; Goralskib, C. T.; Singaram, B. Asymmetric addition of diethylzinc to aldehydes catalyzed by β-amino alcohols derived from limonene oxide. Tetrahedron Asymmetry 2002, 13, 1477–1483. [Google Scholar] Scarpi, D.; Galbo, F. L.; Occhiato, E. G.; Guarna, A. Enantioselective addition of diethylzinc to aldehydes using 1,4-aminoalcohols as chiral ligands. Tetrahedron Asymmetry 2004, 15, 1319–1324. [Google Scholar]
- Azizi, N.; Said, M. R. Highly chemoselective addition of amines to epoxides in water. Org. Lett 2005, 7, 3649–3651. [Google Scholar] Stuart, J. K.; Howard, S. W.; Cydnie, E. B. Aqueous-phase aminolysis: Approach for the analysis of epoxides in water. Anal. Chem. 2006, 78, 2608–2616. [Google Scholar]
- Chakraborti, A. K.; Rudrawar, S.; Kondaskar, A. An efficient synthesis of 2-amino alcohols by silica gel catalysed opening of epoxide rings by amines. Org. Biomol. Chem 2004, 2, 1277–1280. [Google Scholar] Sreedhar, B.; Radhika, B.; Neelima, B.; Hebalkar, N. Regioselective ring opening of epoxides with amines using monodispersed silica nanoparticles in water. J. Mol. Catal. A: Chem. 2007, 272, 159–163. [Google Scholar]
- Kureshy, R. I.; Singh, S.; Khan, N. H.; Razi Abdi, S. H.; Suresh, E.; Jasra, R. V. Efficient method for ring opening of epoxides with amines by NaY zeolite under solvent-free conditions. J. Mol. Catal. A: Chem 2007, 264, 162–169. [Google Scholar]
- Xue, W. M.; Kung, M. C.; Kozlov, A. I.; Popp, K. E.; Kung, H. H. Catalytic aminolysis of epoxide by alumina prepared from amine-protected Al precursor. Catal. Today 2003, 85, 219–224. [Google Scholar]
- Yarapathy, V. R.; Mekala, S.; Rao, B. V.; Shekharam, T. Polymer supported copper sulphate promoted aminolysis of epoxides with aromatic amines. Catal. Commun. 2006, 7, 466–471. [Google Scholar]
- Chakraborti, A. K.; Kondaskar, A.; Rudrawar, S. Scope and limitations of montmorillonite K10 catalyzed opening of epoxide rings by amines. Tetrahedron 2004, 60, 9085–9091. [Google Scholar]
- Kumar, S. R.; Leelavathi, P. Phosphomolybdic acid-Al2O3: A mild, efficient, heterogeneous and reusable catalyst for regioselective opening of oxiranes with amines to β-amino alcohols. J. Mol. Catal. A: Chem. 2007, 266, 65–68. [Google Scholar]
- Vijender, M.; Kishore, P.; Narender, P.; Satyanarayana, B. Amberlist-15 as heterogeneous reusable catalyst for regioselective ring opening of epoxides with amines under mild conditions. J. Mol. Catal. A: Chem. 2007, 266, 290–293. [Google Scholar]
- Desai, H.; D’Souza, B. R; Foether, D.; Johnson, B. F.; Lindsay, H.A. Regioselectivity in a highly efficient, microwave-assisted epoxide aminolysis. Synthesis 2007, 902–910. [Google Scholar] Tan, W.; Zhao, B. X.; Sha, L.; Jiao, P. F.; Wan, M. S. Microwave-assisted ring opening of epoxides in solvent-free conditions. Synth. Commun. 2006, 36, 1353–1359. [Google Scholar]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—a review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] Hayes, B L. Microwave Synthesis; CEM Publishing: Matthews, NC, 2002; p. 292. [Google Scholar] Ollevier, T.; Nadeau, E. Microwave-enhanced bismuth triflate-catalyzed epoxide opening with aliphatic amines. Tetrahedron Lett. 2008, 49, 1546–1550. [Google Scholar] Yang, S. M.; Murray, W. V. Microwave assisted ring-opening of epoxides with N-biaryl sulfonamides in the synthesis of matrix metalloproteinase-9 inhibitors. Tetrahedron Lett. 2008, 49, 835–839. [Google Scholar] Robin, A.; Brown, F.; Bahamontes-Rosa, N.; Wu, B.; Beitz, E.; Kun, J. F.; Flitsch, S. L. Microwave-assisted ring opening of epoxides: A general route to the synthesis of 1-Aminopropan-2-ols with anti malaria parasite activities. J. Med. Chem. 2007, 50, 4243–4249. [Google Scholar]
- Negrón, G.; Hernández, C. X.; Angeles, D.; Lomas, L.; González, E.; Méndez, J. Comparative study of the regioselective synthesis of β-Aminoalcohols under solventless conditions catalyzed by sulfated zirconia and SZ/MCM-41. Molecules 2007, 12, 2515–2532. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
