Abstract
Condensation of aromatic primary bis-amines with isatin (1H-indole-2,3-dione) and 5-flouroisatin occurred cleanly and efficiently in a water suspension medium without using any organic solvent or acid catalyst. The corresponding bis-Schiff bases were obtained in good yields and were easily isolated by filtration. Their structures were confirmed by 1H-NMR, 13C-NMR, IR and mass spectra.
Introduction
A big challenge facing academia and industry is the relationship of modern societies to the environment that requires reinventing the manufacture and use of materials. Synthetic methodologies nowadays should be designed to use and generate substances that possess little or no toxicity to human health and the environment. Schiff bases belong to a widely used group of organic intermediates important for production of specialty chemicals, e.g. pharmaceuticals, or rubber additives [1] and as amino protective groups in organic synthesis [2,3,4,5]. They also have uses as liquid crystals, [6] and in analytical, [7] medicinal [8] and polymer chemistry [9]. Conventionally Schiff bases have been prepared by refluxing mixtures of the amine and the carbonyl compound in an organic solvent, for example, ethanol or methanol [10], but variations are known, such as treatment of the same mixture at room temperature, refluxing the mixture in heptane in the presence of acetic acid [12], or azeotroping the mixture with benzene in a Dean-Stark apparatus in the presence of acid [13]. In general, ketones react more slowly than aldehydes and higher temperatures and longer reaction times are often required as a result. In addition, the equilibrium must often be shifted, usually by removal of the water, either azeotropically by distillation or with suitable drying agents [14,15]. In recent years, environmentally benign synthetic methods have received considerable attention and some solvent-free protocols have been developed [16]. Catalyzed synthesis of imines under solvent-free conditions using microwave irradiation has been reported [17]. Grinding together solid anilines and solid benzaldehydes yielded various kinds of benzylideneanilines [18]. Bergman et al. reported the synthesis of primary imines by condensation of 2-hydroxylaryl ketones with ammonium iodide and piperidine under solvent free conditions [19]. Based on these facts, we decided to synthesize some new bis-Schiff bases of isatin and 5-fluoroisatin in water. We have found that the condensation of isatin and 5-fluoroisatin 1 with diamines 2 to give the corresponding Schiff bases 3 occurred cleanly and efficiently in a water suspension medium without using any acid catalyst or organic solvent and the products were isolated simply by filtration.
Results and Discussion
Isatin and 5-fluoroistin were chosen as the starting materials. Treatment of different aromatic bis- amines 2 with isatin or its derivative, 1, in a small amount of water gave the desired bis-Schiff bases 3 (Scheme 1). The results of this study are summarized in Table 1.
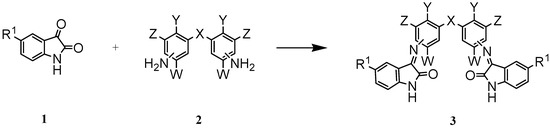
Scheme 1.
Scheme 1.
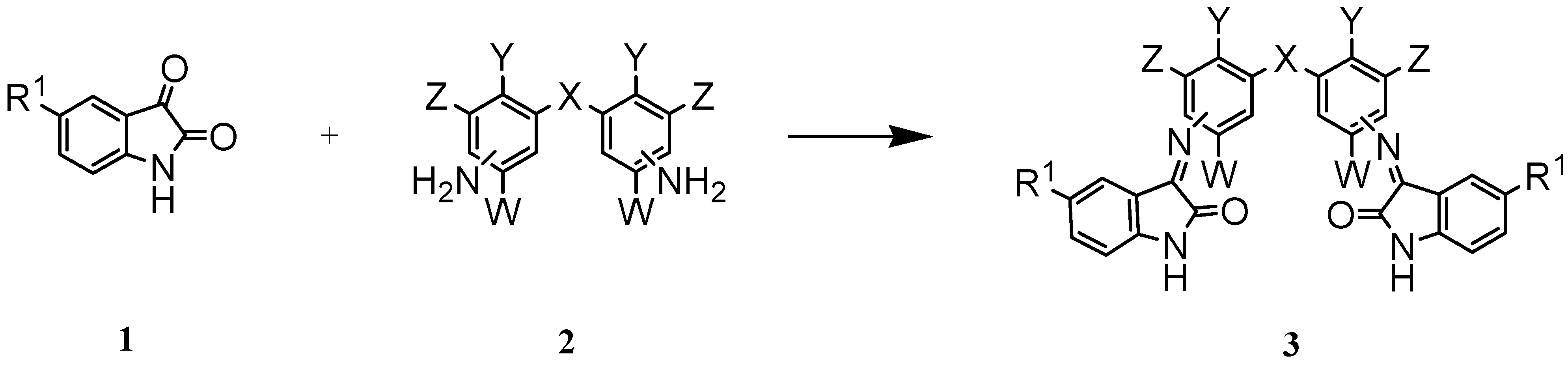
The preparation of 3,3´-[methylenebis-(3,1-phenylenenitrilo)]-bis-[1,3-dihydro]-2H-indol-2-one (3b) is representative. For this, a mixture of isatin and 4,4´-diaminodiphenyl ether was stirred in a small amount of water at room temperature for 30 hours. The yellow crystalline powder of 3b was collected by filtration, washed with water and dried. Among the products, 3h was formed under reflux because of the steric hindrance by the ethyl groups in the starting diamine. The presence of the fluorine atom in 5-fluoroisatin increased the yields of the reactions and reduced the reaction times. Better yields were also obtained when the amino groups were located in the para position with respect to the substituent X.

Table 1.
Synthesis of new bis-Schiff bases of isatin and 5-fluoroisatin in water
| 3 | R1 | X | Y | W | Z | Position of C=N relative to X | Reaction time/h | Yield % |
| a* | H | CH2 | H | H | H | 4,4′ | 22 | 98.0 |
| b | H | CH2 | H | H | H | 3,3′ | 30 | 72.7 |
| c | H | O | H | H | H | 3,4′ | 45 | 80.0 |
| d* | H | O | H | H | H | 4,4′ | 24 | 92.9 |
| e | F | CH2 | H | H | H | 4,4′ | 17 | 82.1 |
| f | F | CH2 | H | H | H | 3,3′ | 22 | 99.0 |
| g | F | O | H | H | H | 4,4′ | 17 | 90.2 |
| h | H | CH2 | Cl | Et | Et | 4,4′ | 48 | 70.0 |
* These compounds have been synthesized previously [20].
Experimental
General:
All melting points were taken in open capillaries on a Büchi 530 apparatus and are uncorrected. FT-IR spectra were recorded on a Shimadzu 8000 instrument using KBr disks. 1H-NMR and 13C-NMR were run on a Bruker Avance DPX-250 (1H-NMR at 250 MHz, 13C-NMR at 62.9 MHz) using DMSO- d6 as solvent and TMS as internal standard. Mass spectra were recorded on a Shimadzu GC MS– QP1000 EX at 70 eV. Column chromatography was carried out on Merck silica gel 60 (30 – 270 mesh). Thin layer chromatography (TLC) was carried out on silicagel plates (Fluka-Kieselgel, 0.2 mm thickness) and the plates were scanned under 254 nm ultraviolet light.
Typical procedure for the preparation of bis-Schiff bases of isatin and 5-fluoroisatin
A mixture of powdered crystalline isatin (0.50 g, 3.40 mmol) and 3,3-diaminodiphenylmethane (0.34 g, 1.70 mmol) was stirred in a small amount of water (9 mL) at room temperature for 30 hours. The crystalline powder formed was collected by filtration, washed with water and dried to give 3b, (0.56 g, 72.70%). The crude crystals thus obtained were recrystallized from EtOH to give pure 3,3´- [methylenebis(3,1-phenylenenitrilo)]bis[1,3-dihydro]-2H-indol-2-one (3b) as yellow crystals; m.p. >260 °C; IR (cm-1): 1652.0 (C=N), 1726.2 (C=O), 3168.8 (N-H); 1H-NMR δ (ppm): 4.07 (2H, s, CH2), 6.29-7.43 (16H, m, ArH), 10.96 (1H, s, N-H); 13C-NMR δ (ppm): 72.54, 115.63, 116.39, 119.11, 120.04, 121.65, 122.25, 124.28, 126.41, 127.20, 130.18, 130.35, 134.66, 139.19, 147.51, 151.73, 155.53, 159.76, 168.29; MS (m/z): 458, 457, 456, 327, 312, 299, 284, 283, 200, 181, 180, 166, 165, 152, 115, 104, 91, 90, 84, 83, 77, 76, 71, 69, 65, 57, 56, 50, 45, 44.
The following compounds were similarly prepared:
3,3´-[Methylenebis(4,1-phenylenenitrilo)]bis[1,3-dihydro]-2H-indol-2-one (3a): m.p. >260 °C; IR (cm-1): 1640.0 (C=N), 1735.0 (C=O), 3222.8 (N-H); 1H-NMR δ (ppm): 4.01 (2H, s, CH2), 6.44-7.56 (16H, m, ArH), 10.88 (1H, s, N-H); 13C-NMR δ (ppm): 111.03, 111.87, 114.44, 116.05, 117.99, 120.22, 122.01, 122.98, 125.58, 128.86, 129.50, 130.19, 134.37, 138.19, 138.52, 147.05, 148.85, 155.27, 158.82, 163.86; MS (m/z): 456, 327, 310, 299, 285, 284, 207, 200, 180, 165, 164, 106, 104, 91, 90, 89, 77, 76, 64, 63, 57, 55, 51, 48, 45, 44.
3,3´-[Oxybis[(3,1)-(4´,1´)phenylenenitrilo]]bis[1,3-dihydro]-2H-indol-2-one (3c): m.p. >260 °C; IR (cm-1): 1620.1 (C=N), 1733.9 (C=O), 3195 (N-H); 1H-NMR δ (ppm): 6.16-7.77 (16H, m, ArH), 10.94 (1H, s, N-H); 13C-NMR δ (ppm): 111.27, 111.97, 112.92, 115.97, 117.35, 118.60, 122.29, 122.76, 123.46, 124.29, 124.46, 125.98, 130.10, 131.25, 132.75, 135.11, 135.60, 147.51, 153.48, 153.86, 154.00, 155.41, 163.66; MS (m/z): 458, 330, 329, 302, 301, 234, 201, 200, 194, 171, 156, 154, 151, 150, 133, 132, 128, 117, 109, 104, 93, 92, 91, 90, 84, 77, 75, 66, 65, 64, 63, 44.
3,3´-[Oxybis(4,1-phenylenenitrilo)]bis[1,4-dihydro]-2H-indol-2-one (3d): m.p. >260 °C; IR (cm-1): 1612.4 (C=N), 1741.6 (C=O), 3193.9 (N-H); 1H-NMR δ (ppm): 6.62-7.58 (16H, m, ArH), 10.89 (16H, s, N-H); 13C-NMR δ (ppm): 111.03, 111.88, 116.05, 118.64, 119.88, 120.02, 122.18, 122.95, 125.64, 134.34, 134.77, 144.44, 145.78, 146.20, 154.51, 155.49, 158.91, 163.88; MS (m/z): 458, 329, 168, 133, 108, 92, 91, 76, 69, 64, 55, 45, 44.
3,3´-[Methylenebis(4,1-phenylenenitrilo)]bis[1,4-dihydro]-5-fluoro-2H-indol-2-one (3e): m.p. >260°C; IR (cm-1): 1618.2 (C=N), 1739.7 (C=O), 3261.4 (N-H); 1H-NMR δ (ppm): 4.03 (2H, s, CH2), 6.09-7.41 (14H, m, ArH), 11.01 (1H, s, N-H); 13C-NMR δ (ppm): 41.61, 113.239, 118.67, 121.18, 139.82, 149.14, 153.68, 159, 164, 1; MS (m/z): 492, 467, 439, 411, 395, 368, 339, 313. 285, 264, 237, 236, 211, 194, 171, 152, 129, 111, 83, 57.
3,3´-[Methylenebis(3,1-phenylenenitrilo)]bis[1,3-dihydro]-5-fluoro-2H-indol-2-one (3f): m.p. >260°C; IR (cm-1): 1622.0 (C=N), 1733.9 (C=O), 3290.3 (N-H); 1H-NMR δ (ppm): 4.06 (2H, s, CH2), 6.78-7.48 (14H, m, ArH), 10.86 (1H, s, N-H); 13C-NMR δ (ppm): 40.50, 112.02, 112.45, 112.88, 115.35, 117.48, 120.83, 121.21, 126.09, 130.21, 143.13, 143.52, 150.67, 163.73; MS (m/z): 492, 466, 449, 423, 393, 368, 339, 313, 285, 264, 236, 206, 178, 164, 147, 146, 119, 91, 73, 43.
3,3´-[Oxybis(4,1-phenylenenitrilo)]bis[1,3-dihydro]-5-fluoro-2H-indol-2-one (3g): m.p. >260 °C; IR (cm-1): 1618.0 (C=N), 1739.7 (C=O), 3261.4 (N-H); 1H-NMR δ (ppm): 6.25-7.43 (14H, m, ArH), 11.07 (1H, s, N-H); 13C-NMR δ (ppm): 111.55, 113.85, 115.41, 119.27, 119.94, 121.00, 125.23, 130.54, 143.61, 15375, 174.10; MS (m/z): 494, 466, 444, 426, 396, 368, 339, 313, 285, 264, 237, 236, 219, 200, 168, 151, 123, 98, 97, 95, 82, 81, 76, 72, 71, 69, 57, 55, 44, 43.
3,3´-[Methylenebis(2-chloro-3,5-diethyl-4,1-phenylenenitrilo)]bis[1,3-dihydro]-5-fluoro-2H-indol-2- one (3h): This compound was also prepared in water but at reflux. m.p. >260 °C; IR (cm-1): 1614.3 (C=N), 1735.8 (C=O), 3247.9 (N-H); 1H-NMR δ (ppm): 0.86-1.05 (12H, t, 4CH3), 4.19 (CH2), 6.66-7.70 (10H, m, ArH), 10.98 (1H, s, N-H); 13C-NMR δ (ppm): 13.21, 13.73, 23.02, 23.77, 37.50, 112.10, 116.51, 122.26, 125.01, 128.41, 128.74, 132.10, 133.37, 135.52, 147.13, 156.83, 163.42; MS (m/z): 637, 603, 577, 555, 523, 507, 479, 456, 423, 368, 339, 313, 285, 264, 236, 211, 194, 171, 147, 129, 113, 97, 96, 95, 82, 81, 76, 72, 69, 44, 43.
Acknowledgments
The authors deeply thank the Shiraz University Research Council for financial support (Grant No. 83-GR-SC-31).
References
- Macho, V.; Kralik, M.; Hudec, J.; Cingelova, J. J. Mol. Catal. A: Chem. 2004, 209, 69. [CrossRef]
- Bey, P.; Vevert, J. P. Tetrahedron Lett. 1977, 18, 1455. [CrossRef]
- Lucas, R. A.; Dickel, D. F.; Dziemian, M. J.; Hensle, B. L.; Mcphillarney, H. B. J. Am. Chem. Soc. 1960, 82, 5688.
- Fleet, G. W. J.; Fleming, I. J. Chem. Soc. 1969, 1758.
- Bezas, B.; Zervas, L. J. Am. Chem. Soc. 1961, 83, 719.
- Adams, J. P. J. Chem. Soc., Perkin Trans. 1 2000, 125. [CrossRef]
- Layer, R. W. Chem. Rev. 1963, 63, 489.Abbaspour, A.; Esmaeilbeig, A. R.; Jarrahpour, A. A.; Khajeh, B.; Kia, R. Talanta 2002, 58, 397.
- Jarrahpour, A. A.; Motamedifar, M.; Pakshir, K.; Hadi, N.; Zarei, M. Molecules 2009, 9, 815.Alexander, V. Chem. Rev. 1995, 95, 273.
- Higuchi, M.; Yamamoto, K. Org. Lett. 1999, 1, 1881.
- Sridhar, S. K.; Saravanan, M.; Ramesh, A. Eur. J. Med. Chem. 2001, 36, 615.
- Deshmukh, A. R. A. S.; Jayanthi, A.; Puranik, V.G.; Bhawal, B. M. Synthesis 2004, 1249.Karupaiyan, K.; Puranik, V. G.; Deshmukh, A. R. A. S.; Bhawal, B. M. Tetrahedron 2000, 56, 8555.
- Kunz, H.; Pfrengle, W.; Ruck, K.; Sager, W. Synthesis 1991, 1039.Kunz, H.; Sager, W. Angew. Chem. Int. Ed. Engl. 1987, 26, 557.
- Vazzana, I.; Terranova, E.; Mattioli, F.; Sparatore, F. Arkivoc 2004, 364.
- Weingarten, H.; Chupp, J. P.; White, W. A. J. Org. Chem. 1967, 32, 3246.
- Bonett, R.; Emerson, T. R. J. Chem. Soc. 1965, 4508. [CrossRef]Roelofsen, D. P.; van Bekkum, H. Recl. Trav. Chim. Pays-Bays 1972, 91, 605.
- Tanaka, T.; Toda, F. Chem. Rev. 2000, 100, 1025.Varma, R. S. Green Chem. 1999, 1, 43.
- Varma, R. S.; Dahiya, R.; Kumar, S. Tetrahedron Lett. 1997, 38, 2039. [CrossRef]
- Schmeyers, J.; Toda, F.; Boy, J.; Kaupp, J. J. Chem. Soc., Perkin Trans.1 1998, 989.
- Bergman, Y.; Perlmutter, P.; Thienthong, N. Green Chem. 2004, 6, 539.
- Bader, B. D.; Vilceanu, R. Rev. Roum. Chim. 1972, 17, 1991.Vilceanu, R.; Bader, B. D.; Radulescu, M.; Marinescu, M. Rev. Roum. Chim. 1973, 18, 1225.
- Sample availability: Contact the authors or MDPI
© 2006 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes
