Abstract
A series of highly functionalized quinones was prepared by an original reaction of 2,3-bis(chloromethyl)-1,4-dimethoxyanthraquinone (6) with various nitronate anions under electron transfer reaction conditions.
Introduction
The efficacy of anthracycline antibiotics such as daunomycin and adriamycin in the treatment of a variety of human malignancies has stimulated a continued interest in the synthesis of this class of antitumor agents [1,2,3]. Unfortunately, their clinical use is limited by a number of problems, including intrinsic and acquired drug resistance and dose-dependent cardiomyopathy. Considerable efforts have been devoted to develop new structurally modified anthracyclines with an improved antineoplastic activity and a low cardiotoxicity.
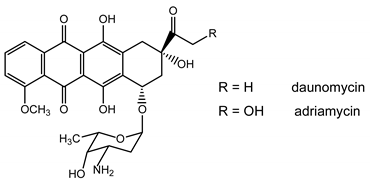
As bioreductible alkylating agents, quinones constitute potential substrates for the radical nucleophilic substitution (SRN1) reaction [4]. In connection with our program directed toward the synthesis of new nitroheterocyclic or quinonic bioreductible alkylating agents using electron transfer methodology [5], we report herein the preparation of 2,3-bis(chloromethyl)-1,4-dimethoxyanthraquinone (6) via a four-step synthesis from phthalic anhydride (1) and 2,3-dimethyl-1,4-hydroquinone (2) and the study of its reactivity with various anions to afford new highly functionalized anthraquinonic derivatives.
Results and Discussion
1,4-Dihydroxy-2,3-dimethylanthraquinone (3) was isolated in 80% yield after condensation of phthalic anhydride (1) and 2,3-dimethyl-1,4-hydroquinone (2) in the presence of aluminium chloride [6]. The methylation of compound 3 with dimethylsulfate (DMS) in acetone followed by radical bromination using N-bromosuccinimide (NBS) and halogen exchange reaction with lithium chloride furnished the target 6 (Scheme 1).
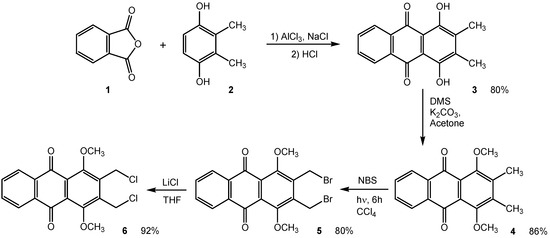
Scheme 1.
Treated under various electron transfer operating conditions with 2-nitropropane anion, the bis-chloride 6 led to the bis-C-alkylation product 7a (Scheme 2). The best yield of bis-C-alkylated product 7a was obtained in phase transfer conditions using dichloromethane as solvent with 5 equivalents of 2-nitropropane anion during 20 minutes under inert atmosphere and light catalysis. The reaction of 6 with 2-nitropropane anion, in the optimal conditions, in presence of classical inhibitors (p-dinitrobenzene as radical-anion scavenger, 2,2,6,6-tetramethyl-1-piperidinyloxy or TEMPO as radical trap and CuCl2) gave effective inhibition, indicating a classical bis-SRN1 mechanism for the formation of 7a.
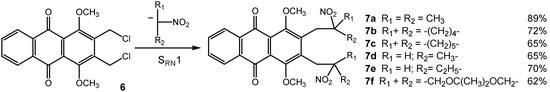
Scheme 2.
This bis-SRN1 reaction was extended with various primary and secondary (cyclic, heterocyclic) nitronate anions (Scheme 2) or S-centered anions (Scheme 3) and allowed us to reach at a series of new highly functionalized anthraquinones. These bis-C-alkylated compounds 7a-f are good candidates for the preparation of anthracycline analogs via an annulation reaction after base promoted nitrous acid elimination [7].
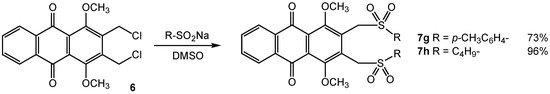
Scheme 3.
The extension of the reactivity to S-centered anions was realized from toluenesulfinic acid sodium salt or butanesulfinic acid sodium salt. This latter anion was formed from butane sulfonyl chloride according to the procedure of Liu [8]. These two reactions treated in refluxed DMSO during 2 hours furnished the corresponding bis-S-alkylated compounds 7g and 7h in 73% and 96% yields, respectively (Scheme 3).
Conclusions
In conclusion, we have demonstrated in this work that the reaction of a new bis(chloromethyl)anthraquinone 6 with nitronate anions proceeded according to an bis-SRN1 mechanism. This procedure permitted us to prepare new highly functionalized anthraquinones in excellent yields. The antiproliferative activities of these compounds are under active investigation.
Experimental
General
Melting points were determined on Büchi B-540 and are uncorrected. Elemental analyses were performed by the Centre de Microanalyses of the University of Aix-Marseille 3. Both 1H- and 13C‑NMR spectra were determined on CDCl3 solutions on a Bruker ARX 200 spectrometer. The 1H chemical shifts are reported as parts per million downfield from tetramethylsilane (Me4Si), and the 13C chemical shifts were referenced to the solvent peak of CDCl3 (76.9 ppm). Silica gel 60 adosrbent (Merck, 230-400 mesh) was used for column chromatography: Thin-layer chromatography was performed with silica gel Merck 60F-254 (0.25 mm layer thickness). Compounds 3-5 were prepared according to Kerdesky method [6]. Nitroalkanes were commercially available or easily prepared by oxidation of the corresponding amine with m-chloroperbenzoic acid in refluxing 1,2-dichloroethane by Gilbert and Borden’s procedure [9]. The nitroheterocyclic nitronate anion was prepared from 2,2-dimethyl-5-hydroxymethyl-5-nitro-1,3-dioxane by the previously described method [10]. Toluene-sulfinic acid sodium salt was commercially available and butanesulfinic acid sodium salt was prepared from butane sulfonyl chloride according to the Liu procedure [8].
2,3-Bis(chloromethyl)-1,4-dimethoxyanthraquinone (6).
In a two-necked flask equipped with a drying tube, a solution of 5 (1.7 g, 3.74 mmol) and of lithium chloride (3.25 g, 76,67 mmol) in anhydrous tetrahydrofuran (50 mL) was stirred at room temperature under an inert atmosphere for 48 h. Then, dichloromethane (100 mL) was added. The organic layer was washed twice with water (50 mL), dried over MgSO4 and removed under reduced pressure. Purification by chromatography on silica gel eluting with dichloromethane and recrystallization from ethanol gave 2,3-bis(chloromethyl)-1,4-dimethoxyanthraquinone (6); mp 140 °C. 1H-NMR δ: 4.04 (s, 6H, 2xOCH3), 4.91 (s, 4H, 2xCH2Cl), 7.75-7.80 (m, 2H, 2xAr-H), 8.18-8.21 (m, 2H, 2xAr-H); 13C-NMR δ: 35.6 (2xCH2Cl), 63.6 (2xOCH3), 126.7 (2xCH), 127.8 (2xC), 133.8 (2xC), 133.9 (2xCH), 140.1 (2xC), 155.7 (2xC), 182.2 (2xC=O); Anal. Calcd for C18H14Cl2O4: C, 59.20; H, 3.86. Found: C, 59.23; H, 3.87.
General procedure for bis-SRN1 reaction with aliphatic and cyclic nitronate anions.
Under a nitrogen atmosphere, a solution of tetrabutylammonium hydroxide (40% in water, 2.1 mL, 2.75 mmol) was treated with nitroalkane (2.75 mmol) for 1 h. A solution of 2,3-bis(chloromethyl)-1,4-dimethoxyanthraquinone (6, 0.20 g, 0.55 mmol) in dichloromethane (20 mL) was then added and the mixture was irradiated with a 300W sun lamp for 20 min at room temperature under an inert atmosphere. The organic layer was separated and the aqueous layer was extracted with dichloro-methane (3 x 10 mL). The combined organic layers were washed twice with water (30 mL), dried over MgSO4 and removed under reduced pressure. Purification by chromatography on silica gel eluting with chloroform and recrystallization from ethanol led to the corresponding products 7a-f.
1,4-Dimethoxy-2,3-bis(2-methyl-2-nitropropyl)anthraquinone (7a): Orange solid, mp 168.4°C (ethanol); 1H-NMR δ: 1.55 (s, 12H, 4xCH3), 3.37 (s, 4H, CH2), 3.83 (s, 6H, 2xOCH3), 7.74-7.77 (m, 2H, 2xAr-H), 8.17-8.20 (m, 2H, 2xAr-H); 13C-NMR δ: 26.1 (4xCH3), 37.0 (2xCH2), 62.0 (2xOCH3), 88.6 (2xCNO2), 125.7 (2xC), 126.7 (2xCH), 133.8 (2xCH), 133.9 (2xC), 139.4 (2xC), 156.2 (2xC), 182.4 (2xC=O); Anal. Calcd for C24H26N2O8: C, 61.27; H, 5.57; N, 5.95. Found: C, 61.42; H, 5.60; N, 5.78.
1,4-Dimethoxy-2,3-bis(1-nitrocyclopentylmethyl)anthraquinone (7b): Orange solid, mp 98°C (ethanol); 1H-NMR δ: 1.58-1.69 (m, 8H, 4xCH2), 1.79-1.90 (m, 4H, 2xCH2), 2.49-2.60 (m, 4H, 2xCH2), 3.47 (s, 4H, 2xCH2), 3.85 (s, 6H, 2xOCH3), 7.74-7.78 (m, 2H, 2xAr-H), 8.18-8.22 (m, 2H, 2xAr-H); 13C-NMR δ: 22.7 (4xCH2), 34.7 (2xCH2), 36.3 (4xCH2), 62.1 (2xOCH3), 101.1 (2xCNO2), 125.9 (2xC), 126.7 (2xCH), 133.8 (2xCH), 134.0 (2xC), 139.9 (2xC), 156.1 (2xC), 182.5 (2xC=O); Anal. Calcd for C28H30N2O8: C, 64.36; H, 5.79; N, 5.36. Found: C, 64.27; H, 5.77; N, 5.17.
1,4-Dimethoxy-2,3-bis(1-nitrocyclohexylmethyl)anthraquinone (7c): Yellow solid, mp 191°C (ethanol); 1H-NMR δ: 1.12-1.27 (m, 4H, 2xCH2), 1.59-1.67 (m, 12H, 6xCH2), 2.31-2.45 (m, 4H, 2xCH2), 3.18 (s, 4H, 2xCH2), 3.84 (s, 6H, 2xOCH3), 7.73-7.77 (m, 2H, 2xAr-H), 8.17-8.21 (m, 2H, 2xAr-H); 13C-NMR δ: 22.2 (6xCH2), 24.3 (2xCH2), 34.2 (2xCH2), 37.5 (2xCH2), 61.9 (2xOCH3), 92.5 (2xCNO2), 125.4 (2xC), 126.6 (2xCH), 133.7 (2xCH), 134.0 (2xC), 139.2 (2xC), 156.0 (2xC), 182.5 (2xC=O); Anal. Calcd for C30H34N2O8: C, 65.44; H, 6.22; N, 5.09. Found: C, 65.48; H, 6.22; N, 4.85.
1,4-Dimethoxy-2,3-bis(2-nitropropyl)anthraquinone (7d): Orange solid, mp 185.9°C (ethanol); 1H‑NMR δ: 1.58 (d, 3H, J = 6.7 Hz), 1.68 (d, 3H, J = 6.7 Hz), 3.19-3.54 (m, 4H, 2xCH2), 3.90 (s, 6H, 2xOCH3), 4.91-5.02 (m, 2H, 2xCHNO2), 7.75-7.79 (m, 2H, 2xAr-H), 8.18-8.22 (m, 2H, 2xAr-H); 13C‑NMR δ: 19.5 (2xCH3), 33.0 (2xCH2), 62.4 (2xOCH3), 82.9 (2xCHNO2), 125.9 (2xC), 126.7 (2xCH), 133.9 (2xCH), 138.8 (2xC), 139.4 (2xC), 156.0 (2xC), 182.3 (2xC=O); Anal. Calcd for C22H22N2O8: C, 59.73; H, 5.01; N, 6.33. Found: C, 59.74; H, 4.84; N, 6.39.
1,4-Dimethoxy-2,3-bis(2-nitrobutyl)anthraquinone (7e): Orange solid, mp 145.8 °C (ethanol); 1H NMR δ: 1.00 (t, 3H, J = 7.4 Hz), 1.05 (t, 3H, J = 7.4 Hz), 1.81-2.18 (m, 4H, 2xCH2), 3.22-3.45 (m, 4H, 2xCH2), 3.90 (s, 6H, 2xOCH3), 4.74-4.83 (m, 2H, 2xCHNO2), 7.75-7.78 (m, 2H, 2xAr-H), 8.18-8.21 (m, 2H, 2xAr-H); 13C NMR δ: 10.2 (2xCH3), 27.9 (2xCH2), 31.4 (2xCH2), 62.3 (2xOCH3), 89.3 (2xCHNO2), 125.7 (2xC), 126.7 (2xCH), 133.8 (2xC), 133.9 (2xCH), 139.5 (2xC), 155.9 (2xC), 182.4 (2xC=O); Anal. Calcd for C24H26N2O8: C, 61.27; H, 5.57; N, 5.95. Found: C, 61.15; H, 5.74; N, 5.95.
2,3-Bis(2,2-dimethyl-5-nitro[1,3]dioxan-5-ylmethyl)-1,4-dimethoxyanthraquinone (7f): Yellow solid, mp 215 °C (ethanol); 1H-NMR δ: 1.35 (s, 6H, 2xCH3), 1.50 (s, 6H, 2xCH3), 3.39 (s, 4H, 2xCH2), 3.85 (s, 6H, 2xOCH3), 4.04 (d, 4H, JAB = 12.8 Hz, 2xCH2O), 4.31 (d, 4H, JAB = 12.8 Hz, 2xCH2O), 7.75-7.80 (m, 2H, 2xAr-H), 8.17-8.22 (m, 2H, 2xAr-H); 13C-NMR δ: 21.9 (2xCH3), 24.7 (2xCH3), 30.5 (2xCH2), 61.4 (2xCH2O), 62.3 (2xOCH3), 63.9 (2xCH2O), 85.2 (2xCNO2), 99.0 (2xC), 126.0 (2xC), 126.7 (2xCH), 133.9 (2xCH), 134.0 (2xC), 137.4 (2xC), 156.1 (2xC), 182.2 (2xC=O); Anal. Calcd for C30H34N2O12: C, 58.63; H, 5.58; N, 4.56. Found: C, 58.76; H, 5.69; N, 4.38.
General procedure for reactions with substituted sulfinate sodium salt
A solution of substituted sulfinate sodium salt (3.3 mmol) in dimethylsulfoxide (10 mL) was added dropwise to a solution of dichloride 6 (0.20 g, 0.55 mmol) in dimethylsulfoxide (6 mL) and stirred under inert atmosphere for 10 min. The reaction mixture was poured into cold water and a precipitate was formed. After filtration, the crude product was recrystallized from the corresponding solvent gave the corresponding bis-S-alkylated product.
1,4-Dimethoxy-2,3-bis-(toluene-4-sulfonylmethyl)anthraquinone (7g): Yellow solid, mp 281°C (ethyl acetate); 1H-NMR δ: 2.42 (s, 6H, 2xCH3), 3.90 (s, 6H, 2xOCH3), 5.07 (s, 4H, 2xCH2SO2), 7.34 (d, 4H, J = 8.2 Hz, 4xAr-H), 7.74 (d, 4H, J = 8.2 Hz, 4xAr-H), 7.76-7.80 (m, 2H, 2xAr-H), 8.17-8.22 (m, 2H, 2xAr-H); 13C-NMR δ: 21.6 (2xCH3), 54.3 (2xCH2SO2), 63.6 (2xOCH3), 126.7 (2xCH), 128.1 (4xCH), 130.0 (4xCH), 132.4 (2xCH), 133.8 (2xC), 134.0 (2xC), 145.3 (2xC), 156.7 (2xC), 182.0 (2xC=O); Anal. Calcd for C32H28O8S2: C, 63.56; H, 4.67. Found: C, 63.65; H, 4.67.
2,3-Bis(butane-1-sulfonylmethyl)-1,4-dimethoxyanthraquinone (7h): Yellow solid, mp 184°C (ethanol); 1H-NMR δ: 0.96 (t, 6H, J = 6.6 Hz, 2xCH3), 1.43-1.55 (m, 4H, 2xCH2), 1.82-1.94 (m, 4H, 2xCH2), 3.04-3.12 (m, 4H, 2xCH2), 3.99 (s, 6H, 2xOCH3), 4.98 (s, 4H, 2xCH2SO2), 7.77-7.81 (m, 2H, 2xAr-H), 8.19-8.23 (m, 2H, 2xAr-H); 13C-NMR δ: 13.5 (2xCH3), 21.7 (2xCH2), 24.0 (2xCH2), 50.6 (2xCH2SO2), 53.6 (2xCH2SO2), 63.6 (2xOCH3), 126.8 (2xCH), 127.3 (2xC), 132.7 (2xC), 133.5 (2xC), 134.1 (2xCH), 156.3 (2xC), 182.1 (2xC=O); Anal. Calcd for C26H32O8S2: C, 58.19; H, 6.01. Found: C, 58.23; H, 5.84.
References and Notes
- Lin, A. J.; Cosby, L. A.; Shansky, C. W.; Sartorelli, A. C. Potential bioreductive alkylating agents. 1. Benzoquinone derivatives. J. Med. Chem. 1972, 15, 1247. [Google Scholar]
- Moore, H. W. Bioactivation as a model for drug design bioreductive alkylation. Science 1977, 197, 527. [Google Scholar] [CrossRef]
- Arcamone, F. Properties of antitumor anthracyclines and new developments in their application: Cain Memorial Award Lecture. Cancer Res. 1985, 45, 5995. [Google Scholar]
- Vanelle, P.; Terme, T.; Giraud, L.; Crozet, M. P. Progress in electron transfer reactions of new quinone bioreductive alkylating agents. Recent Adv. Devel. Org. Chem. 2000, 4, 1. [Google Scholar]
- Terme, T.; Crozet, M. P.; Maldonado, J.; Vanelle, P. Electron Transfer Reactions in Organic Synthesis; Vanelle, P., Ed.; Research Signpost: Trivandrum, 2002; p. 1. [Google Scholar]
- Kerdesky, F. A. J.; Ardecky, R. J.; Lakshmikantham, M. V.; Cava, M. P. Simple o-quino-dimethane route to (±)-4-demethoxydaunomycinone. J. Am. Chem. Soc. 1981, 103, 1992. [Google Scholar] [CrossRef]
- Terme, T.; Crozet, M. P.; Giraud, L.; Vanelle, P. Original annulation in anthraquinone series: synthesis of new 2,3-dialkylnaphthacene-5,12-diones. Tetrahedron 2000, 56, 1097. [Google Scholar] [CrossRef]
- Liu, L. K.; Chi, Y.; Jen, K.-Y. Copper-catalyzed additions of sulfonyl iodides to simple and cyclic alkenes. J. Org. Chem. 1980, 45, 406. [Google Scholar] [CrossRef]
- Gilbert, K. E.; Borden, W. T. Peracid oxidation of aliphatic amines: general synthesis of nitroalkanes. J. Org. Chem. 1979, 44, 659. [Google Scholar] [CrossRef]
- Linden, G. B.; Gold, M. H. Preparation of 2- and 5-substituted 1,3-dioxanes. J. Org. Chem. 1956, 21, 1175. [Google Scholar]
- Samples Availability: Available from the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.