Abstract
We performed a cohort analysis of the entire population of Abruzzo, Italy, to evaluate the real-world effectiveness of SARS-CoV-2 vaccines against infection, COVID-19 hospitalization or death, over time and during the Omicron wave. All resident or domiciled subjects were included, and official vaccination, COVID-19, demographic, hospital and co-pay exemption datasets were extracted up to 18 February 2022. Multivariable analyses were adjusted for age, gender, hypertension, diabetes, major cardio- and cerebrovascular events, COPD, kidney diseases, and cancer. During the follow-up (average 244 days), 252,365 subjects received three vaccine doses (of BNT162b2, ChAdOx1 nCoV-19, mRNA-1273 or JNJ-78436735), 684,860 two doses, 29,401 one dose, and 313,068 no dose. Overall, 13.4% of the individuals were infected with SARS-CoV-2 (n = 170,761); 1.1% of them had severe COVID-19, and 0.6% died. Compared with the unvaccinated, those receiving two or three vaccine doses showed an 80% to 90% lower risk of COVID-19 hospitalization or death. Protection decreased during the Omicron wave and six months after the last dose, but it remained substantial. Lethal disease was uncommon during the Omicron wave and in the young population, even among the unvaccinated. Some of the current policies may need a re-evaluation in light of these findings. The results from the Omicron wave will inevitably require confirmation.
1. Introduction
Vaccination is among the key preventive strategies against SARS-CoV-2 pandemic [,]. Phase 3 trials reported the efficacy of vaccines against symptomatic COVID-19 ranging from 67% to 95%, with acceptable safety profiles [,,,,,,], and these findings were confirmed by several population studies [,,,].
However, several questions remain unanswered. Firstly, the length of follow-up is inevitably short, and vaccine long-term safety and effectiveness is uncertain. Indeed, a growing body of literature suggests that vaccine immunity may decrease over time as a result, among other factors, of the emergence and spread of new virus variants [,,,,]. Secondly, it is unclear whether current vaccines are still effective against the virus variant B.1.1.259, commonly named “Omicron” [], as early investigations found a decrease of 23% in the efficacy against Omicron-related hospitalizations [,]. Third, as a response to the potential waning of vaccine-induced immunity and Omicron spread, many countries introduced a third, booster dose of vaccine, the effectiveness of which remains to be evaluated in large population studies [,].
In this cohort analysis of the entire population of an Italian region, we evaluated the risk of infection, COVID-19 hospitalization and death of the unvaccinated subjects versus those who received different doses of SARS-CoV-2 vaccines, over time and during the Omicron wave.
2. Materials and Methods
This retrospective cohort study follows and expands a previous, preliminary analysis []. Here, we included all the subjects resident or domiciled in the Abruzzo region of Italy on 1 January 2020, with no positive SARS-CoV-2 swab before the start of follow-up, in order to evaluate the effectiveness of SARS-CoV-2 vaccination (one, two or three doses versus none) against infection (detected through RT-PCR-Reverse transcription polymerase chain reaction; tested through nasopharyngeal swabs by the accredited laboratories of the region), virologically confirmed COVID-19 severe disease, diagnosed by a specialist physician and requiring hospital admission, and COVID-19-related death (inside or outside the hospital) [].
According to the Italian National immunization campaign plan, starting from 2 January 2021, Pfizer–Biontech BNT162b2, Oxford–AstraZeneca ChAdOx1 nCoV-19, Moderna mRNA-1273 and Janssen JNJ-78436735 vaccines were gradually administered to the population []. The Italian National Health System (NHS) provides vaccines administration, SARS-CoV-2 testing, and all healthcare services to its residents free of charge. Tests are mandatory for all individuals with suggestive symptoms such as fever or acute respiratory illness, for those that have been in contact with infected persons, and for all individuals returning from travel abroad. On 8 January 2022, a law decree established that, from February 15, vaccination was to become mandatory for all Italian citizens aged 50 years or more []. As the control group for these analyses was the group of unvaccinated persons, which may disappear as a consequence of this obligation, we extracted vaccine data before the mandate ruling (18 December 2021), and COVID data at the first available date after the mandate application: 18 February 2022.
From 2 January 2021 (date of the administration of the first vaccine dose), up to 18 December 2021:
- The subjects who received only one dose of BNT162b2, mRNA-1273 or ChAdOx1 nCoV-19 vaccines, were included in the group “1 dose”;
- The subjects who received only one dose of JNJ-78436735 vaccine, or only two doses of BNT162b2, mRNA-1273 or ChAdOx1 nCoV-19 vaccines, were included in the group “2 doses”;
- The subjects who received two doses of JNJ-78436735 vaccine, or three doses of BNT162b2, mRNA-1273 or ChAdOx1 nCoV-19 vaccines, were included in the group “3 doses”.
For the analyses evaluating the effectiveness of the various vaccines vs. no vaccines, the subjects who received two or three different vaccines were included in a global category named “mixed vaccines”.
The start of follow-up varied across vaccine categories, as all vaccine groups were separately compared versus unvaccinated subjects:
- For the analyses comparing unvaccinated versus those receiving only one vaccine dose (set of analyses “1 dose”), the start of follow-up was set 14 days after the single vaccine dose (to account for the time required for seroconversion) [] for vaccinated subjects and 14 days after the first administration of the first vaccine dose (16 January 2021) for the group “unvaccinated”.
- For the analyses comparing unvaccinated versus those receiving two vaccine doses only (set of analyses “2 doses”), the start of follow-up was set 14 days after the second vaccine dose for vaccinated subjects and 14 days after the first administration of the second vaccine dose (31 January 2021) for the group “unvaccinated”.
- For the analyses comparing unvaccinated versus those receiving three vaccine doses (set of analyses “3 doses”), the start of follow-up was set 14 days after the third vaccine dose for vaccinated subjects and 14 days after the start of the mass administration of the third vaccine dose (17 September 2021) for the group “unvaccinated”.
For the outcome “infection”, the end of follow-up was set:
- −
- On 18 February 2022 for those who had no positive swabs during the follow-up;
- −
- On the day of the first positive swab for those who were not infected before the start of the follow-up (“uninfected” cohort).
For the outcome “COVID-19 hospitalization”, the end of follow-up was set on the day of the hospital admission, or on 18 February 2022, for all the subjects who were not hospitalized with COVID-19 related symptoms. For the outcome “death”, the end of follow-up was set on the day of the death with a positive swab, or on 18 February 2022 for all others. A schematic definition of the study groups, outcomes and follow-up is reported online in Table S1.
To account for some of the main potential confounders of the association between vaccination and COVID-19 hospitalization or death [], we used (a) the COVID-19 database, (b) co-pay exemption database and (c) administrative discharge abstracts from the last ten years to extract the following conditions for each resident: diabetes (ICD-9 cm codes in any diagnosis field—250.xx); hypertension (401.xx–405.xx); major cardiovascular or cerebrovascular diseases (410.xx–412.xx; 414.xx–415.xx; 428.xx or 433.xx–436.xx); chronic obstructive pulmonary diseases—COPD (491.xx–493.xx); kidney diseases (580.xx–589.xx); and cancer (140.xx–172.xx or 174.xx–208.xx).
2.1. Data Collection
All the information about vaccines, laboratory tests, demographic, anamnestic and clinical residents’ data are routinely entered into NHS official datasets, updated daily, and sent to the Italian Institute of Health []. We extracted all data from the official vaccination, COVID-19, demographic, hospital (Italian SDO) and co-pay exemption (Italian “Esenzioni Ticket” file) datasets of the Abruzzo region. Individual data were merged through encrypted fiscal code.
2.2. Statistical Analyses
The main analyses separately compared the risk of the primary infections, COVID-19 hospitalization and COVID-related death of unvaccinated subjects versus individuals who received one, two, or three vaccine doses. Each of these comparisons were stratified by age category (0–29y, 30–59y, 60 + y), vaccine type, and time since the last vaccine dose (≤ or >182 days, or six months, which was the duration of the “green pass” set by the Italian Government for subjects who recovered after a SARS-CoV-2 infection) []. The age ranges were identified to be consistent with the periodical reports of the Italian Institute of Health [], and with the recommendations of the Italian Government [], which identified subjects aged ≥ 60 years as priority targets for vaccination.
As the survival analyses could be biased due to the severe unbalance in the average follow-up duration between the unvaccinated individuals (367 days) and those who received two (181 days) or three (71 days) vaccine doses, and considering that more than 80% of the infections occurred during the restricted time span of the Omicron wave (the last 50 days of follow-up), we opted to use multiple logistic regression instead of the typical Cox proportional hazards analysis to evaluate the independent association between each outcome and vaccination []. All models were adjusted for age, gender, hypertension, diabetes, major cardio- and cerebrovascular events, COPD, kidney diseases, and cancer, all included a priori, and the results of such covariates were reported in the online Table S2. Standard post-estimation tests were used to assess the validity of final models, performing multicollinearity and influential observation analyses (using standardized residuals, change in Pearson and deviance chi-square), and testing for potential interactions between vaccination and other covariates []. The goodness of fit was checked using Hosmer–Lemeshow test, and predictive power was assessed through C-statistics (area under the receiving operator curve).
The analyses were repeated, including only the events that occurred during the Omicron wave (from 27 December 2021 to 18 February 2022, when the proportion of Omicron variant in the available positive swabs was higher than 50%) []. In these analyses, all the subjects with a positive SARS-CoV-2 swab before 27 December 2021 were classified as “infected before the start of follow-up” and excluded.
A two-sided p-value of <0.05 was considered to be significant for all analyses, which were carried out using Stata, version 13.1 (Stata Corp., College Station, TX, USA, 2014).
3. Results
The STROBE flowchart of the study participants is shown in Figure 1. From 2 January 2021 to 18 December 2021, a total of 313,068 unvaccinated (24.5%) and 966,626 vaccinated (who received 2,156,216 doses) residents or domiciled in the Abruzzo region, Italy were included in the analyses. The main demographic characteristics of the population and the proportion of selected comorbidities are reported in Table 1. Overall, 29,401 subjects received only one vaccine dose (2.3% of the population), 684,860 received two doses (53.5%), and 252,365 received three doses (19.7%).
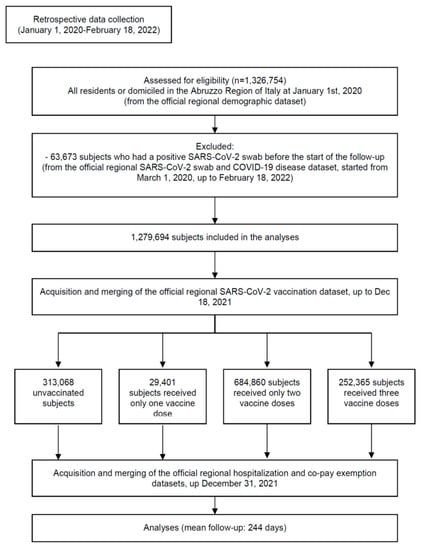
Figure 1.
Study flowchart in line with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement (http://www.strobestatement.org accessed on 16 April 2022).

Table 1.
Main characteristics of the sample, overall and by vaccine status.
The majority of the population (63.9%) received only BNT162b2 doses; 13.0% received only mRNA-1273 doses; 11.5% ChAdOx1 nCoV-19, and 10.4% mixed vaccines.
During the follow-up, 13.4% of the population was infected with SARS-CoV-2 (n = 170,761): 19.1% of the unvaccinated, 13.8% of those receiving one dose, 13.1% of those receiving two doses, and 6.5% of those who received three doses (Table 2). During pre-Omicron waves (344 days of follow-up), we observed a total of 30,726 primary infections (89 per day), 1152 COVID-19 hospitalizations (3.3 per day; 3.8% of the infected), and 933 COVID-19-related deaths (2.7 per day; 3.0% of the infected). During the first 54 days of the Omicron wave, among the 1,245,097 subjects who were not infected before 27 December 2021, we observed 140,035 primary infections (2593 per day), 758 COVID-19 hospitalizations (14 per day; 0.5% of the infected), and 117 COVID-19-related deaths (2.0 per day; 0.1% of the infected).

Table 2.
Main outcomes, overall, by vaccine status, vaccine type, and age category.
The mean follow-up was 244 ± 99 days, but varied widely by vaccine group, being shortest (71 ± 18 days) for the analyses of the effectiveness of the third dose, which was widely administered from September, 2021.
3.1. Risk of Primary Infection
The highest incidence of infection was observed in the young population (0–29 years): 21.8%, as compared to 6.3% among the adults aged ≥ 60 years. In the multivariable analysis (Table 3), when adjusting for age, gender, and comorbidities, the risk of infection was significantly lower for vaccinated subjects. As compared to the unvaccinated, the individuals who received one, two, or three doses showed the following adjusted odds ratios (ORs), respectively: 0.71, 0.75, and 0.74, with 95% confidence intervals (Cis) ranging from 0.68 to 0.76. The results did not substantially vary by vaccine type or age category, but when the analyses were restricted to the Omicron wave, only the subjects who received three doses showed some degree of protection against infection (OR: 0.81; 95% CI: 0.80–0.83), while those who received two doses did not differ from the unvaccinated (OR: 0.98; 0.97–1.00). On the contrary, during the pre-Omicron waves, the risk of infection was significantly lower among the subjects who received any dose of vaccine (online Table S3).

Table 3.
Multivariable analysis ψ of the effectiveness of COVID-19 vaccines.
3.2. Risk of COVID-19 Hospitalization
During the follow-up, 1910 persons had a COVID-19 hospitalization (0.15% of the population; 1.1% of the infected subjects—Table 2). Among the 70,345 infected, young subjects, the incidence of COVID-19 hospitalization was lower than 0.1% (n = 24), rising to 6.2% among older adults. Before the Omicron wave, 5.3% of unvaccinated, infected subjects had severe disease (975/18,489); during the Omicron wave, this value decreased to 0.7% (295/41,281).
Overall, the frequency of COVID-19 hospitalization was considerably higher among the unvaccinated (0.41% of the population; 2.12% of the infected) as compared to those receiving two (0.06% and 0.44%, respectively) or three (0.06% and 1.00%, respectively) vaccine doses. Multivariable analyses confirmed a significantly and substantially lower risk of COVID-19 hospitalization for the subjects who received one (OR: 0.53), two (0.12) or three (0.21) vaccine doses, as compared with the unvaccinated (Table 3; all p < 0.001). The results were similar when the analyses were restricted to the infected persons, and stratified by vaccine type or age category. In contrast, during the Omicron wave (Table 3), and after six months from the last vaccine dose (Table 4), the effectiveness of two doses decreased to approximately 66% and 69%, respectively, although remained highly significant.

Table 4.
Multivariable analysis ψ predicting vaccine effectiveness to prevent COVID-19 hospitalization or death among the infected subjects, according to the duration of follow-up.
3.3. Risk of COVID-19-Related Death
During follow-up, 1050 persons with COVID-19 died (0.08% of the total population; 0.6% of the infected; 54.9% of the hospitalized subjects—Table 2). There were no deaths during follow-up in the population aged 0–29 years. Before the Omicron wave, 4.5% of the unvaccinated, infected subjects died (831/18,489); during the Omicron wave, this value decreased to 0.1% (41/41,281).
Overall, both mortality and lethality were considerably higher among the unvaccinated (0.28% and 1.46%, respectively) as compared to those receiving two (0.02% and 0.12%) or three (0.01% and 0.19%) vaccine doses. Multivariable analyses confirmed a significantly and substantially lower risk of death for the subjects who received one (OR: 0.49), two (0.06) or three (0.24) vaccine doses, as compared with the unvaccinated (Table 3; all p < 0.001). The results were similar when the analyses were restricted to the infected persons, and stratified by vaccine type or age category. A lower, yet considerable protection of two vaccine doses was observed six months after the last dose (OR: 0.25; 95% CI: 0.17–0.35; Table 4) and during the Omicron wave, where the number of deaths was too low to determine a lack of statistical power. Only the group receiving three doses showed a significantly lower risk of death compared to the unvaccinated (OR: 0.42; 95% CI: 0.26–0.68; Table 3).
3.4. Vaccine Safety
The regional surveillance system communicated that, between 2 January 2021 and 31 December 2022, the passive surveillance system received 179 spontaneous reports of possibly vaccine-related adverse events, 64 of which were defined as “severe”, with no deaths from 2,306,000 doses administered during the year 2021 (2.8 severe adverse event ×100,000 doses). The system did not provide any other data, including the proportion of severe adverse events whose correlation with vaccination was assessed and verified [].
4. Discussion
This study evaluated the effectiveness of some of the most commonly used SARS-CoV-2 vaccines in the general population of an Italian region, followed for more than six months on average, in the context of a mass vaccination campaign. The main findings were: (a) compared with the unvaccinated, persons who received two or three vaccine doses showed a 80% to 90% lower risk of COVID-19 hospitalization or death; (b) during the Omicron wave, and six months after the last dose, protection against COVID-19 decreased but remained considerable; (c) the effectiveness against infection was modest at any dose; (d) the risk of a lethal disease was very small during the Omicron wave and in the young population, even among the unvaccinated; and (e) overall, the reported incidence of serious adverse events was very low.
Our results on the effectiveness of current vaccination schedule are consistent with the existing literature on pre-Omicron waves [,]. Since the first months of 2021, two doses of vaccines were able to confer a high real-world protection against severe or lethal COVID-19 in several population studies [,,,,,,]. With regard to the increase in the effectiveness that may derive from a third dose, which has been extensively recommended worldwide, some preliminary reports from Israel, the UK, and USA have recently been published, indicating the very high effectiveness of booster doses against severe or fatal disease [,,]. In this study, we observed a limited or null increase in the protection after a third dose, but the effectiveness was already very high after two doses, and the follow-up was relatively short. Concerning the potential decrease in protection caused by the Omicron variant, our findings were reassuring, in line with those from a few recent studies that also reported a high vaccine effectiveness against severe or lethal COVID-19 during Omicron predominance [,,]. As both present and previous studies could only consider the first weeks of the Omicron wave, further data are inevitably required to confirm whether the current vaccines should still be considered as the pivotal strategy to control the pandemic, or whether new vaccines or policy changes are required.
The first reports of waning vaccine immunity emerged in October 2021, when Tartof et al. hypothesized that vaccine effectiveness against infection decreases with time from immunization, irrespective of the dominant virus variant []. In December 2021, another study reported no residual effectiveness against severe or lethal disease after more than seven months [], and data from Israel suggested that waning immunity may also incur after booster doses []. In our study, we observed a reduction of 30% in effectiveness after six months from the last vaccine dose, but subjects who received two doses still showed a 70% lower risk of COVID-19 hospitalization compared with the unvaccinated, during both pre-Omicron and Omicron waves. Although the biological mechanisms of the waning of vaccine-induced immunity remain to be elucidated [], these findings remain concerning, suggesting that a strategy of periodical immunization should be evaluated if the pandemic does not decrease its morbidity.
Italy, as well as several other countries, has established a different set of restriction measures according to vaccination status, and unvaccinated subjects have been denied access to a number of public or private facilities []. As a consequence, vaccinated subjects may have been exposed to a higher risk of SARS-CoV-2 transmission than unvaccinated individuals []. This clear bias may explain, at least in part, why the effectiveness of current vaccines against infection was modest at any dose.
In line with an ample amount of the literature, the number of COVID-19 hospitalizations was very low, with no deaths among the individuals younger than 30 years [,,,,]. Given that the incidence of COVID-19 hospitalization was as low as 1 out of 2000 infections for unvaccinated individuals, and even though two vaccine doses were able to reduce the risk of hospitalization by 90%, the risk–benefit profile of multiple vaccine doses for this population should be carefully evaluated.
The overall number of serious adverse events following vaccination that was reported by the surveillance system of the Abruzzo region was very low, in agreement with the acceptable safety profile reported by all previous studies [,]. However, we had no access to individual-level data and, as correctly admitted by the operators of the system, the overall number of reports was extremely scarce, suggesting a low efficacy of the current, passive pharmacovigilance system for monitoring vaccine safety []. Furthermore, more detailed data are strongly needed to properly assess the harm–benefit profile of vaccination for specific clusters of the population [].
The strengths of this study are the use of official, routinely collected electronic health databases from the entire, general population of an Italian region, followed for a maximum of 12 months. Importantly, individual-level data were available on several comorbidities that may influence the risk of COVID-19 hospitalization and death, and the analyses were accordingly adjusted for. Finally, although the follow-up was inevitably short, we had the chance to evaluate vaccine effectiveness during the first seven weeks of the Omicron wave, when more than 140,000 new infections were recorded.
This study also has limitations that must be considered when interpreting the results. Some of them have been mentioned: a relatively short follow-up after the booster dose and a higher contagion risk of the vaccinated vs. unvaccinated subjects, resulting from the less stringent restrictions, that may lead to an underestimation of vaccine effectiveness against infection. However, on the other side, under the regulations in force in Italy at the time of this study, unvaccinated individuals were more likely to undergo multiple testing (requested to attend social gatherings) [], which may have enhanced their likelihood of a testing positive for COVID-19, potentially resulting in an overestimation of vaccine effectiveness for this outcome []. Finally, it may also be argued that the negative test case–control design is more appropriate for assessing real-world vaccine effectiveness in the context of a pandemic []; however, a good concordance with findings from cohort studies was observed by previous research, which directly compared the two methodologies []. Likewise, the observational study design was suboptimal for performing head-to-head comparisons of the diverse administered vaccines, all of which showed a similar effectiveness profile.
5. Conclusions
This large population-based cohort study confirmed that two or three doses of all current vaccines were able to confer a strong protection against COVID-19 hospitalization or death. During the Omicron wave, and six months after the last dose, protection decreased but remained high. The effectiveness against infection was modest at any dose, likely as a result of the differences in restriction policies by immunization status. Finally, the rate of reported serious adverse events was very low.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines10050662/s1, Table S1: Schematic definition of the study groups, follow-up, and outcomes. Table S2: Multivariable analysis predicting SARS-CoV-2 infection, COVID-19 hospitalization, and COVID-19-related death (complete results of the logistic models reported in Table 3—Whole period, all subjects). Table S3: Multivariable analysis of the effectiveness of COVID-19 vaccines during pre-Omicron waves.
Author Contributions
Conceptualization, L.M., M.E.F. and G.S.; Methodology, L.M., M.E.F., C.A.M. and G.D.M.; Software, R.C. and G.D.M.; Validation, G.S. and C.A.M.; Formal analysis, L.M. and M.E.F.; Investigation, G.S., C.A.M. and R.C.; Resources, L.M., G.S., G.D.M. and A.C.; Data curation, L.M., G.D.M. and R.C.; Writing—original draft preparation, L.M., M.E.F. and C.A.M.; Writing—review and editing, L.M., G.S. and A.C.; Supervision, L.M. and A.C.; Project administration, G.S., L.M. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this work.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and the research protocol was approved by the Ethics Committee of the Emilia-Romagna Region on 17 June 2021 (protocol code 556/2021). According to the European Union General Data Protection (GDPR) regulation, all datasets were pseudo-anonymized by the Regional Offices before access of the authors using a unique identification code for each subject in each dataset. All data concerning the addresses, phone, email, date of birth, vaccination center, hospital site, swab lab, and municipality of all subjects were not provided to the authors, and the encrypted identification code could not be reversed by the authors or by the regional offices (the encryption was made in two steps, assigning random codes for each fiscal code in the demographic database, and the intermediate codes were deleted). RC was the owner of the data processing and granted LM the permission to release anonymized raw data upon request.
Informed Consent Statement
Patient consent was waived due to the retrospective and pseudo-anonymized nature of the data.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- USA Department of Health & Human Services. Secretarial Directive of Eligibility to Receive COVID-19 Vaccines—17 March 2021; USA Department of Health & Human Services: Washington, DC, USA, 2021.
- European Commission. Communication from the Commission to the European Parliament, the European Council and the Council—A United Front to Beat COVID-19; European Commission: Brussels, Belgium, 2021; Volume 19.1.2021, COM (2021) 35 final.
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.Ş.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Flacco, M.E.; Soldato, G.; Acuti Martellucci, C.; Carota, R.; Di Luzio, R.; Caponetti, A.; Manzoli, L. Interim Estimates of COVID-19 Vaccine Effectiveness in a Mass Vaccination Setting: Data from an Italian Province. Vaccines 2021, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet Lond. Engl. 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions—United States, March–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Fabiani, M.; Puopolo, M.; Morciano, C.; Spuri, M.; Spila Alegiani, S.; Filia, A.; D’Ancona, F.; Del Manso, M.; Riccardo, F.; Tallon, M.; et al. Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe COVID-19 during predominant circulation of the delta variant in Italy: Retrospective cohort study. BMJ 2022, 376, e069052. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 10 January 2022).
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.-G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2021, 386, 494–496. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Dorabawila, V.; Easton, D.; Bauer, U.E.; Kumar, J.; Hoen, R.; Hoefer, D.; Wu, M.; Lutterloh, E.; Conroy, M.B.; et al. COVID-19 Vaccine Effectiveness in New York State. N. Engl. J. Med. 2021, 386, 116–127. [Google Scholar] [CrossRef]
- Fast, H.E.; Zell, E.; Murthy, B.P.; Murthy, N.; Meng, L.; Gibbs Scharf, L.; Black, C.L.; Shaw, L.; Chorba, T.; Harris, L.Q. Booster and Additional Primary Dose COVID-19 Vaccinations Among Adults Aged ≥65 Years—United States, August 13, 2021–November 19, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1735–1739. [Google Scholar] [CrossRef]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to COVID-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef]
- Acuti Martellucci, C.; Flacco, M.E.; Cappadona, R.; Bravi, F.; Mantovani, L.; Manzoli, L. SARS-CoV-2 pandemic: An overview. Adv. Biol. Regul. 2020, 77, 100736. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministry of Health. Piano Vaccini Anti COVID-19 [Italian Mational Immunization Plan against COVID-19]; Italian Ministry of Health: Roma, Italy, 2020.
- Italian Government. Decreto Legge: Misure urgenti per fronteggiare l’emergenza COVID-19. In Particolare Nei Luoghi Di Lavoro, Nelle Scuole E Negli Istituti Della Formazione Superiore; Government, I., Ed.; Gazzetta Ufficiale S.G. n. 4: Roma, Italy, 2022; Volume Decreto Legge n. 1. [Google Scholar]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Tang, P.; Hasan, M.R.; Coyle, P.; Al Kanaani, Z.; et al. Association of Prior SARS-CoV-2 Infection With Risk of Breakthrough Infection Following mRNA Vaccination in Qatar. JAMA 2021, 326, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Flacco, M.E.; Carradori, T.; Volta, C.A.; Cosenza, G.; De Togni, A.; Acuti Martellucci, C.; Parruti, G.; Mantovani, L.; Manzoli, L. Predictors of severe or lethal COVID-19, including Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers, in a sample of infected Italian citizens. PLoS ONE 2020, 15, e0235248. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Andrianou, X.; Bella, A.; Del Manso, M.; Urdiales, A.M.; Fabiani, M.; Bellino, S.; Boros, S.; D’Ancona, F.; Rota, M.C.; et al. COVID-19 Integrated Surveillance System. Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza (accessed on 7 January 2022).
- Italian Government. Proroga Dello Stato Di Emergenza Nazionale E Ulteriori Misure per Il Contenimento Della Diffusione Dell’epidemia da COVID-19; Italian Government, Ed.; Serie Generale, n. 305; Gazzetta Ufficiale: Roma, Italy, 2021.
- Italian Institute of Health. Characteristics of COVID-19 Patients Dying in Italy Report Based on Available Data on 10 January 2022; Italian Institute of Health: Rome, Italy, 2022.
- Italian Government. Raccomandazioni Ad Interim Sui Gruppi Target Della Vaccinazione Anti SARS-CoV-2/COVID-19; Italian Government, Ed.; Serie Generale, n. 72; Gazzetta Ufficiale: Rome, Italy, 2021.
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- Pizzi, C.; Costa, G.M.; Santarella, L.; Flacco, M.E.; Capasso, L.; Bert, F.; Manzoli, L. Depression symptoms and the progression of carotid intima-media thickness: A 5-year follow-up study. Atherosclerosis 2014, 233, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Italian National Institute of Health. Stima Della Prevalenza Delle Varianti VOC (Variants of Concern) in Italia: Beta, Gamma, Delta, Omicron E Altre Varianti Di SARS-CoV-2; Italian National Institute of Health: Roma, Italy, 2021.
- Agenzia Italiana del Farmaco (AIFA). Rapporto Annuale Sulla Sicurezza Dei Vaccini Anti-COVID-19; AIFA: Rome, Italy, 2022.
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef]
- Charmet, T.; Schaeffer, L.; Grant, R.; Galmiche, S.; Chény, O.; Von Platen, C.; Maurizot, A.; Rogoff, A.; Omar, F.; David, C.; et al. Impact of original, B.1.1.7, and B.1.351/P.1 SARS-CoV-2 lineages on vaccine effectiveness of two doses of COVID-19 mRNA vaccines: Results from a nationwide case-control study in France. Lancet Reg. Health—Eur. 2021, 8, 100171. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic COVID-19 infection in Sweden: A nationwide cohort study. Lancet Reg. Health—Eur. 2021, 11, 100249. [Google Scholar] [CrossRef]
- UK Health Security Agency. COVID-19 Vaccine Surveillance Report Week 4; UK Health Security Agency: London, UK, 2022.
- Thompson, M.G.; Natarajan, K.; Irving, S.A.; Rowley, E.A.; Griggs, E.P.; Gaglani, M.; Klein, N.P.; Grannis, S.J.; DeSilva, M.B.; Stenehjem, E.; et al. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 139–145. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by 4th dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 2022. NEJMoa2201570. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Richterman, A.; Cevik, M. COVID-19 vaccination: Evidence of waning immunity is overstated. BMJ 2021, 374, n2320. [Google Scholar] [CrossRef] [PubMed]
- Italian Government. Decreto Legge: Misure Urgenti per La Graduale Ripresa Delle Attivita’ Economiche E Sociali Nel Rispetto Delle Esigenze Di Contenimento Della Diffusione Dell’epidemia Da COVID-19; Italian Government: Roma, Italy, 2021; Gazzetta Ufficiale S.G. 96, Volume D.L. n. 52.
- Bhopal, S.S.; Bagaria, J.; Olabi, B.; Bhopal, R. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc Health 2021, 5, e12–e13. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Italian Agency of Drugs (AIFA). Annual Report on the Safety of Anti-COVID-19 Vaccines; Italian Agency of Drugs (AIFA): Rome, Italy, 2022.
- Doshi, P.; Godlee, F.; Abbasi, K. COVID-19 vaccines and treatments: We must have raw data, now. BMJ 2022, 376, o102. [Google Scholar] [CrossRef]
- Andersson, O.; Campos-Mercade, P.; Meier, A.N.; Wengström, E. Anticipation of COVID-19 vaccines reduces willingness to socially distance. J. Health Econ. 2021, 80, 102530. [Google Scholar] [CrossRef]
- Sullivan, S.G.; Tchetgen Tchetgen, E.J.; Cowling, B.J. Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness. Am. J. Epidemiol. 2016, 184, 345–353. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).