Abstract
Grumixama (Eugenia brasiliensis Lam.) is a native fruit of the Brazilian Atlantic Forest, belonging to the Myrtaceae family, which designatesthe most significant number of species with food potential. It stands out due to its phytochemical characteristics because of the presence of polyphenols and volatile organic compounds. Volatile compounds are substances released by foods that give off an aroma and influence flavor. Solid-phase microextraction is a technique that allows for low-cost, fast, and solvent-free extraction, has an affinity for numerous analytes, and is easily coupled to gas chromatography. The objectives of this work were to evaluate the efficiency of different fibers of SPME (solid-phase microextraction) in the extraction of volatile organic compounds from grumixama pulp; optimize a method for extraction time, temperature, and sample weight; and to determine the characteristic volatile profile of this fruit. For the extraction of volatile compounds, three fibers of different polarities were used: polar polyacrylate (PA) fibers, divinylbenzene/carboxyne/polydimethylsiloxane (DVB/CAR/PDMS) semipolar fibers, and polydimethylsiloxane/divinylbenzene (PDMS/DVB). Fourteen volatile organic compounds (VOCs) were identified by DVB/CAR/PDMS, six by PA, and seven by PDMS/DVB through solid-phase microextraction in the headspace mode (SPME-HS). Considering the total number of compounds identified, regardless of the fiber used, and the optimization of the method, Eugenia brasiliensis presented sesquiterpene fractions (85.7%, 83.3%, and 85.7% of total VOCs) higher than the monoterpene fractions (14.3%, 16.7%, and 14.3%) for DVB/CAR/PDMS, PA, and PDMS/DVB, respectively in its composition. In addition, it was possible to verify that the fiber DVB/CAR/PDMS presented a better efficiency due to the larger chromatographic area observed when the grumixama pulp was subjected to conditions of 75 °C, 2.0 g, and an adsorption time of 20 min.
1. Introduction
Brazil stands out for the immense biological diversity of its flora and is seen as one of the leading centers for the genetic diversity of fruit species worldwide. Although a wide variety of native fruit species is found in the Amazon and the Cerrado, the southern and southeastern regions also present a great richness in wild fruits. The Myrtaceae family stands out for designating the most significant number of species with food potential, which could be marketed in nature for use in the manufacturing of ice cream, juices, yogurts, liqueurs, desserts, cereal bars, jams, and jellies [1,2,3].
The grumixameira is a native tree of the Brazilian Atlantic Forest. The fruit of the grumixameira, Eugenia brasiliensis Lam., is approximately 2.0 cm in diameter. The skin is smooth, shiny, and has different colors [4,5]. In Brazil, grumixama is commercially used for the production of jellies, pies, and liqueurs in the southern and southeastern regions [6], harvested between November and February, and can also be used in public gardens and ecological restoration programs [5,7].
The chemical and physical composition and polyphenol profile of grumixama can vary significantly due to the biome where the fruit is grown [5]. The main bioactive compounds present in fruits are phenolic compounds: mainly flavonoids and ellagitannins [8,9] related to antibiofilm activity [4], the detection of quorum-sensing [10], and anti-inflammatory effects [6,9]. In addition, its flavor characteristics are related to the presence of volatile organic compounds.
Volatile compounds are substances released by foods that give off aromas and influence flavor [11,12,13,14,15], directly affecting consumer purchasing decisions. They arise from a complex mixture of secondary metabolites, including terpenes, esters, aldehydes, ketones, and alcohols. Terpenes, especially sesquiterpenes, have analgesic and antimicrobial properties [12,16]. However, there is not much knowledge regarding these compounds in grumixama.
Many extraction methods are used to analyze these substances, with an emphasis on solid-phase microextraction (SPME) [17,18]. SPME is a technique that allows for low-cost, fast, solvent-free extraction, has an affinity for numerous analytes, and is easily coupled to gas chromatography [19,20,21]. The development of the HS-SPME VOC extraction method involves evaluating many parameters such as fiber type, agitation, extraction time, and temperature [17,19,21].
For the analysis of separation and identification of these compounds, gas chromatography (GC) instruments coupled to mass spectrometry (MS) are used, preceded by the SPME technique, which consists of extracting volatile compounds through the fibers and transferring the material from the fibers through a chromatograph injector [8,17,22]. This extraction can vary according to the polarity of the extracting fiber and sample weight, as well as the time, and temperature of extraction, directly impacting the efficiency of the process. Knowledge of flavors and aromas is still a problem since the set of VOCs is specific for each species and fruit variety [8]. So, the determination of volatile compounds can contribute to the discovery of a new characteristic aroma of the species. The optimization of extraction processes is essential, as the SPME fiber coating material is crucial in obtaining a representative extraction of the volatile compounds profile [23].
Given the above, the objective of this work is to evaluate the efficiency of different fibers of SPME in the extraction of volatile organic compounds from grumixama pulp and to optimize a method to determine the characteristic volatile profile of this fruit.
2. Results and Discussion
In order to determine the ideal conditions for the extraction of VOCs by HS-SPME, the effect of sample weight, temperature, and extraction time for each of the fibers was evaluated. The SPME fibers, PA, DVB/CAR/PDMS, and PDMS/DVB, were analyzed and individually compared according to the sum of the peak areas prepared in the chromatograms of the 19 design tests. The relative areas of the isolated volatile compounds for each of the SPME fibers are shown in Table 1.

Table 1.
Experimental design conditions and the total sum of the chromatographic peaks obtained for each fiber by the HS-SPME-GC-MS method.
In the present study, it was observed that the fiber divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) had a better efficiency due to the larger chromatographic area observed when the grumixama pulp was subjected to the conditions of 75 °C, 2.0 g, and an adsorption time of 20 min. On the other hand, studies on the optimization of extraction conditions in fruits belonging to the Myrtaceae family, such as acerola (Malpighia emarginata D.C.) [17], cagaita (Eugenia dysenterica) [21], and cambuí (Myrciaria floribunda) [14] had a better efficiency for fiber polyacrylate (PA). For the pêra do cerrado, a better performance was observed for the PDMS/DVB fiber [15]. The observed behaviors emphasized that the extracted fiber’s efficiency could vary according to the matrix studied. Figure 1 shows chromatograms obtained from the studied grumixama sample, with the same conditions used with different fibers, differentiated by their colors: orange (DVB/CAR/PDMS fiber), red (PA fiber), and pink (PDMS/DVB fiber).
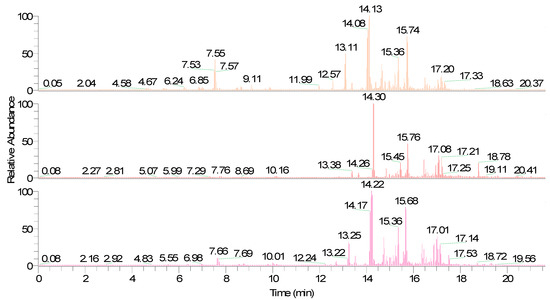
Figure 1.
Chromatogram generated for grumixama fruits.
For the conditions that presented a better efficiency due to the larger chromatographic area, a total of 14 volatile organic compounds (VOCs) were identified by the divinylbenzene/carboxyne/polydimethylsiloxane (DVB/CAR/PDMS), six by polyacrylate fibers (PA), and seven by polydimethylsiloxane/divinylbenzene (PDMS/DVB) through solid-phase microextraction in the headspace mode (SPME-HS). The fruits of grumixama are mainly composed of sesquiterpenes.
Evaluating each individually used fiber in the Eugenia brasiliensis, despite the significant difference in the number of compounds, made it possible to observe that the sum of compounds in sesquiterpene fractions (85.7%, 83.3%, and 85.7% of total VOCs ) was consistently higher than the sum of compounds in monoterpene fractions (14.3%, 16.7%, and 14.3%) for DVB/CAR/PDMS, PA, and PDMS/DVB, respectively (Table 2).

Table 2.
Volatile profile of fruits of Eugenia brasiliensis, isolated by different fibers and SPME-HS/GC–MS.
An aroma is a complex mixture of many volatile compounds, whose composition is specific to each variety and varies depending on the combination of compounds, concentrations, and the perception of each compound [24]. According to Siebert et al., 2015, studies using grumixama essential oil show its composition based on the presence of monoterpenes and sesquiterpenes [25]. Thus, these results were corroborated in the present study, which also detected the presence of these chemical classes of compounds in E. brasiliensis.
Monoterpenes are a class of secondary metabolites found in aromatic plants with diverse food applications [26]. Pinene stands out for being commonly present in the Eugenia genus [27]. This compound confers sensory characteristics, such as a characteristic pine odor, which is also observed in camphene and related to a woody and citrus odor [15].
Sesquiterpenes are also the predominant volatile compounds identified in fruits of different species and are strongly linked to flavor perception and consumer acceptance [28]. In this class, we can highlight longifolene and caryophyllene. These compounds have aromatic characteristics and are important ingredients commonly used in foods [29,30].
Several parameters influence the extraction of compounds, such as the time and temperature of extraction and the weight of the sample to be evaluated. Similarly, the amount of analytes extracted from fruit and vegetable samples depends on the nature of the fiber and the properties of the sample matrix [31]. The present study evaluates the influence of different parameters (time, temperature, and sample weight) on analyte extraction for method optimization (Figure 2).
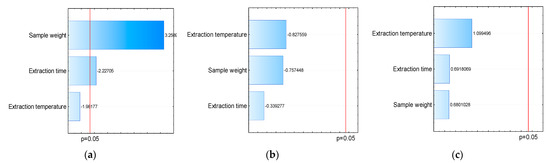
Figure 2.
Effects of parameters: sample weight, extraction time, and extraction temperature on the extraction of volatiles using different fibers for HS-SPME-(a) DVB/CAR/PDMS, (b) PA, and (c) PDMS/DVB.
Through Pareto’s diagram, generated from the total area, it is possible to observe that for all parameters evaluated, there was no significant difference when using PA fibers (Figure 2b) and PDMS/DVB (Figure 2c). However, a significant difference was observed when using DVB/CAR/PDMS fibers and for the variation in sample weight and extraction time (Figure 2a). The graph shows a positive correlation between the increase in weight and the extraction of analytes, so the greater the sample weight, the greater the extraction efficiency. Despite this, when we evaluated the influence of the extraction time, we observed that increasing time decreased the efficiency of analyte extraction.
The total area of the chromatographic peaks corresponding to the grumixama sample for each investigated fiber was used as a response to the presented experimental runs to obtain the response surface graphs, as shown in Figure 3. The graphs show the behavior of the extraction effectiveness of the analytes concerning the parameters evaluated.
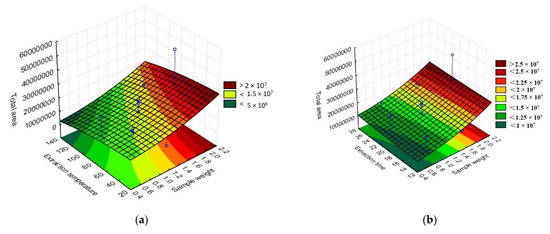
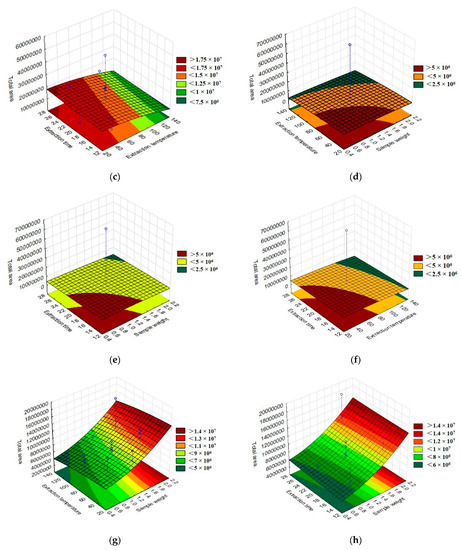
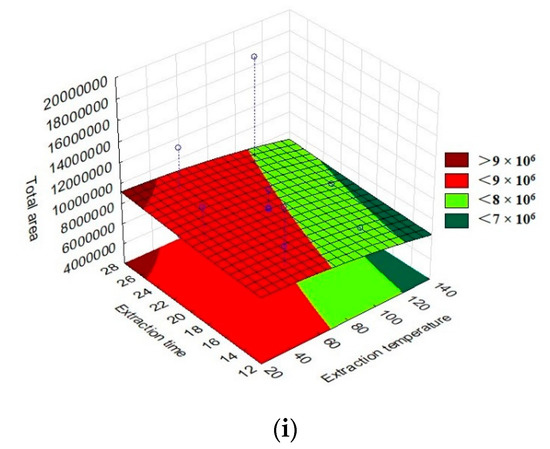
Figure 3.
Three-dimensional response surface (RSM) graphs of the parameters time, extraction temperature, and sample weight in the extraction of volatile compounds using different fibers for HS-SPME: (a) DVB/CAR/PDMS extraction temperature vs. sample weight, (b) DVB/CAR/PDMS extraction time vs. sample weight, (c) DVB/CAR/PDMS extraction time vs. extraction temperature, (d) PA extraction temperature vs. sample weight, (e) PA extraction time vs. sample weight, (f) PA extraction time vs. extraction temperature, (g) DVB/PDMS extraction temperature vs. sample weight, (h) DVB/PDMS extraction time vs. sample weight, and (i) DVB/PDMS extraction time vs. extraction temperature.
By evaluating the response surface graphs obtained from the evaluation of the DVB/CAR/PDMS fiber data, confirmation of the influence of sample weight and time on the efficiency of analyte extraction could be observed. In addition to this, it can also be inferred that there was no significant influence on the parameters evaluated for the PA fiber. For the behavior of the graphs when using the PDMS/DVB fiber, no significant difference was observed, despite the response surface graphic showing a tendency to increase the efficiency of the process with an increase in temperature and sample weight.
3. Experimental Section
3.1. Plant Material
The grumixama pulp was obtained from the “Sítio do Bello” stores located in São Paulo, Brazil (23°27′53.94″ South and 45°42′31.88″ West), in December 2018. The pulp was stored under a temperature of −18 °C and protected from light until analysis.
3.2. Experimental Design
The central composite design (CCD) was used for the experimental design. It consisted of a factorial system of three factors (23), with five central points, and six axial points, totaling 19 runs. The independent variables were the sample weight, adsorption temperature, and extraction time, as shown in Table 3. Statistical analyzes were performed using the Statistica v.10 software (Stat-Soft Inc., Tulsa, OK, USA) (STATSOFT, 2011) [32].

Table 3.
Variables used in factorial planning 23 with a central component for optimizing HS-SPME conditions.
3.3. Extraction of Volatile Compounds
For the extraction of volatile compounds, three fibers of different polarities were used: polar polyacrylate (PA) fibers (85 μm), divinylbenzene/carboxyne/polydimethylsiloxane (DVB/CAR/PDMS) semipolar fibers (50/30 μm), and polydimethylsiloxane/divinilbenzene (PDMS/DVB) fibers (65 μm). In order to optimize the extraction conditions of the volatile organic compounds (VOCs), the samples were subjected to the experimental conditions established in the experimental design. After undergoing the experiment, the samples were refrigerated. According to the experimental method, the samples were weighed in glass vials (capacity of 20 mL) and sealed with an aluminum seal and a rubber septum. Then, they were pre-heated in a heating block, and the fibers to be used for the adsorption of the volatile substances were inserted into each flask [13,14,15,21].
3.4. Identification of Volatile Compounds
The identification of VOCs was performed using a gas chromatograph (Trace GC Ultra) coupled to a mass spectrometer (Polaris Q, Thermo Scientific, San Jose, CA, USA) with an ion trap analyzer using a split/splitless capillary injector.
The instrument conditions adopted were an injector temperature of 250 °C, an ion source temperature of 200 °C, and an interface temperature of 270 °C. Initially, the fiber was exposed to a temperature of 40 °C for 5 min. Then, the temperature was increased at a rate of 2.5 °C min−1 to 125 °C, followed by an increase of 10 °C min−1 at 245 °C and held for 3 min [14,21].
Data acquisition took place in full-scan mode using electronic impact ionization (EI) and a power of 70 eV, with a range from 50 to 300 m/z. The identification of volatile compounds was based on the mass-to-charge ratio (m/z) of the sample ion fragments, using each mass spectrum in the range from 50 to 300 m/z. Using Xcalibur software version 2.1 (Thermo Scientific, San Jose, CA, USA), a comparison was made of the mass spectra corresponding to each peak observed in the chromatogram with data obtained by the NIST library (National Institute of Standards and Technology), considering the level of similarity (reverse lookup index, RSI) greater than 600 [8,14,33].
4. Conclusions
Three experimental parameters were evaluated: sample weight, adsorption temperature, and extraction time (min). No significant differences were observed for PA and PDMS/DVB fibers. However, for the analysis with DVB/CAR/PDMS, there was interference regarding the sample weight and extraction time. Thus, it can be inferred that the method was efficient. Furthermore, it is noteworthy that the best experimental condition for extracting volatile organic compounds in grumixama was 75 °C, 2.0 g of pulp, and 20 min.
The profile of volatile compounds in the grumixama pulp is mainly composed of sesquiterpene compounds, representing 85.7%, 83.3%, and 85.7% for DVB/CAR/PDMS, PA, and PDMS/DVB fiber, respectively, of its composition. Fourteen volatile organic compounds (VOCs) were identified through the DVB/CAR/PDMS, six through the PA, and seven through the PDMS/DVB fiber. By analyzing the relative areas of the chromatographic peaks isolated for each SPME fiber, DVB/CAR/PDMS presented better results.
In this way, the present study presented new information about a fruit species of great importance in the Atlantic Forest, contributing to the deepening of knowledge about the sensory characteristics of grumixama pulp.
Author Contributions
Conceptualization, J.O.F.M., A.L.C.C.R., I.C.A.L., R.L.B.d.A. and R.A.; methodology, A.L.C.C.R., J.O.F.M., R.A. and M.R.S.; software, A.L.C.C.R., M.R.S., A.P.X.M. and T.N.d.A.R.; validation, A.L.C.C.R. and M.R.S.; formal analysis, A.L.C.C.R., A.P.X.M. and T.N.d.A.R.; investigation, A.L.C.C.R., L.A.N. and A.C.d.C.M.; resources, R.A., A.C.C.F.F.d.P., A.C.d.M. and J.O.F.M.; data curation, A.L.C.C.R., L.A.N. and A.C.d.M.; writing—original draft preparation, A.L.C.C.R. and L.A.N.; writing—review and editing, A.L.C.C.R. and M.R.S.; visualization, A.C.C.F.F.d.P., A.C.d.M., R.L.B.d.A. and I.C.A.L.; supervision, R.L.B.d.A., I.C.A.L. and J.O.F.M.; project administration, R.L.B.d.A., I.C.A.L., J.O.F.M. and A.L.C.C.R.; funding acquisition, R.A., J.O.F.M., I.C.A.L., R.L.B.d.A., A.C.C.F.F.d.P. and A.C.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article.
Acknowledgments
The authors would like to thank Universidade Federal de Minas Gerais–UFMG, Pró-Reitoria de Pesquisa–PRPq–UFMG, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–CAPES (88882.380829/2019-01), Instituto Federal de Educação Ciência e Tecnologia de Minas Gerais (IFMG), and Universidade Federal de São João del-Rei (UFSJ) for the equipment loaned to carry out the analyzes and financial support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of grumixama are available from the authors.
References
- Chhikara, N.; Kaur, R.; Jaglan, S.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive Compounds and Pharmacological and Food Applications of Syzygium cumini—A Review. Food Funct. 2018, 9, 6096–6115. [Google Scholar] [CrossRef]
- Medeiros de Aguiar, T.; Ubirajara Oliveira Sabaa-Srur, A.E.; Smith, R. Study of Grumixama (Eugenia brasiliensis, Lam) Fruit Pulp and Development of a Jelly: Rheological, Sensorial and Colorimetric Evaluation. Nat. Prod. J. 2016, 6, 142–151. [Google Scholar] [CrossRef]
- Pereira, M.C.; Steffens, R.S.; Jablonski, A.; Hertz, P.F.; de O. Rios, A.; Vizzotto, M.; Flôres, S.H. Characterization and Antioxidant Potential of Brazilian Fruits from the Myrtaceae Family. J. Agric. Food Chem. 2012, 60, 3061–3067. [Google Scholar] [CrossRef]
- Lazarini, J.G.; de Cássia Orlandi Sardi, J.; Franchin, M.; Nani, B.D.; Freires, I.A.; Infante, J.; Paschoal, J.A.R.; de Alencar, S.M.; Rosalen, P.L. Bioprospection of Eugenia brasiliensis, a Brazilian Native Fruit, as a Source of Anti-Inflammatory and Antibiofilm Compounds. Biomed. Pharmacother. 2018, 102, 132–139. [Google Scholar] [CrossRef]
- Xu, K.; Alves-Santos, A.M.; Dias, T.; Naves, M.M.V. Grumixama (Eugenia brasiliensis Lam.) Cultivated in the Cerrado Has High Content of Bioactive Compounds and Great Antioxidant Potential. Ciênc. Rural 2020, 50, 16. [Google Scholar] [CrossRef]
- Flores, G.; Dastmalchi, K.; Paulino, S.; Whalen, K.; Dabo, A.J.; Reynertson, K.A.; Foronjy, R.F.; D’Armiento, J.M.; Kennelly, E.J. Anthocyanins from Eugenia brasiliensis Edible Fruits as Potential Therapeutics for COPD Treatment. Food Chem. 2012, 134, 1256–1262. [Google Scholar] [CrossRef] [Green Version]
- Lamarca, E.V.; de Oliveira Júnior, C.J.F.; Barbedo, C.J. Etnobotânica Na Conservação de Espécies Com Sementes Sensíveis à Dessecação: O Exemplo da Eugenia brasiliensis Lam. Hoehnea 2020, 47, e372019. [Google Scholar] [CrossRef]
- Ramos, A.L.C.C.; Mendes, D.D.; Silva, M.R.; Augusti, R.; Melo, J.O.F.; de Araújo, R.L.B.; Lacerda, I.C.A. Chemical Profile of Eugenia brasiliensis (Grumixama) Pulp by PS/MS Paper Spray and SPME-GC/MS Solid-Phase Microextraction. Res. Soc. Dev. 2020, 9, e318974008. [Google Scholar] [CrossRef]
- de Lira Teixeira, L.; Bertoldi, F.C.; Lajolo, F.M.; Hassimotto, N.M.A. Identification of Ellagitannins and Flavonoids from Eugenia brasilienses Lam. (Grumixama) by HPLC-ESI-MS/MS. J. Agric. Food Chem. 2015, 63, 5417–5427. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; De Oliveira, B.D.; Da Silva, E.R.; Sacramento, N.T.B.; Bertoldi, M.C.; Pinto, U.M. Anti-Quorum Sensing Activity of Phenolic Extract from Eugenia brasiliensis (Brazilian Cherry). Food Sci. Technol. 2016, 36, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Alves, G.; Franco, M.R. Headspace Gas Chromatography–Mass Spectrometry of Volatile Compounds in Murici (Byrsonima crassifolia L. Rich). J. Chromatogr. A 2003, 985, 297–301. [Google Scholar] [CrossRef]
- da Silva, A.P.G.; Spricigo, P.C.; Purgatto, E.; de Alencar, S.M.; Jacomino, A.P. Volatile Compounds Determined by SPME-GC, Bioactive Compounds, In Vitro Antioxidant Capacity and Physicochemical Characteristics of Four Native Fruits from South America. Plant Foods Hum. Nutr. 2019, 74, 358–363. [Google Scholar] [CrossRef]
- García, Y.M.; de Lemos, E.E.P.; Ramos, A.L.C.C.; Reina, L.D.C.B.; de Oliveira, A.F.; de Paula, A.C.C.F.F.; de Melo, A.C.; Augusti, R.; de Araújo, R.L.B.; Melo, J.O.F. Extração e Análise de Compostos Orgânicos Voláteis por SPME-HS e GC-MS—Um Breve Referencial Teórico. In Ciências Agrárias: O Avanço da Ciência no Brasil; Guarujá, Editora Científica: São Paulo, Brazil, 2021; Volume 1, pp. 68–83. [Google Scholar]
- García, Y.M.; de Lemos, E.E.P.; Augusti, R.; Melo, J.O.F. Optimization of Extraction and Identification of Volatile Compounds from Myrciaria floribunda. Rev. Ciênc. Agron. 2021, 52, e20207199. [Google Scholar] [CrossRef]
- Mariano, A.P.X.; Ramos, A.L.C.C.; de Oliveira Júnior, A.H.; García, Y.M.; de Paula, A.C.C.F.F.; Silva, M.R.; Augusti, R.; de Araújo, R.L.B.; Melo, J.O.F. Optimization of Extraction Conditions and Characterization of Volatile Organic Compounds of Eugenia klotzschiana O. Berg Fruit Pulp. Molecules 2022, 27, 935. [Google Scholar] [CrossRef]
- Pasternak, T.; Potters, G.; Caubergs, R.; Jansen, M.A.K. Complementary Interactions between Oxidative Stress and Auxins Control Plant Growth Responses at Plant, Organ, and Cellular Level. J. Exp. Bot. 2005, 56, 1991–2001. [Google Scholar] [CrossRef]
- García, Y.M.; Rufini, J.C.M.; Campos, M.P.; Guedes, M.N.S.; Augusti, R.; Melo, J.O.F. SPME Fiber Evaluation for Volatile Organic Compounds Extraction from Acerola. J. Braz. Chem. Soc. 2019, 30, 55–70. [Google Scholar] [CrossRef]
- Barros Neto, B.; Scarminio, I.S.; Bruns, R.E. Como Fazer Experimentos: Aplicações Na Ciência e Na Indústria, 2nd ed.; Editora UNICAMP: Campinas, Brazil, 2003. [Google Scholar]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of Solid-Phase Microextraction in Food Analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; de Mendonça, H.O.P.; Nogueira, L.A.; de Paula, A.C.C.F.F.; de Melo, A.C.; Reina, L.D.C.B.; Silva, M.R.; de Araújo, R.L.B.; Augusti, R.; Melo, J.O.F. Caracterização de Compostos Voláteis e Compostos Bioativos da Polpa e Geleia de Cagaita Por Microextração Em Fase Sólida No Modo Headspace e Espectrometria de Massa Por Paper Spray. Res. Soc. Dev. 2021, 10, e25610111735. [Google Scholar] [CrossRef]
- Silva, M.; Bueno, G.; Araújo, R.; Lacerda, I.; Freitas, L.; Morais, H.; Augusti, R.; Melo, J. Evaluation of the Influence of Extraction Conditions on the Isolation and Identification of Volatile Compounds from Cagaita (Eugenia dysenterica) Using HS-SPME/GC-MS. J. Braz. Chem. Soc. 2019, 30, 379–387. [Google Scholar] [CrossRef]
- Netto, D.C.; Reis, R.M.; Mendes, C.B.; Gomes, P.C.F.L.; Martins, I.; Siqueira, M.E.P.B. Headspace Solid-Phase Microextraction Procedure for Gas-Chromatography Analysis of Toluene in Urine. J. Braz. Chem. Soc. 2008, 19, 1201–1206. [Google Scholar] [CrossRef]
- Francisco, V.; Almeida, L.; Bogusz Junior, S.; Oiano Neto, J.; Nassu, R. Optimization of Extraction Conditions of Volatile Compounds of Roasted Beef by Solid-Phase Microextraction. Quim. Nova 2020, 43, 435–441. [Google Scholar] [CrossRef]
- Santos Silva, J.; Damiani, C.; da Cunha, M.C.; Nunes Carvalho, E.E.; de Barros Vilas Boas, E.V. Volatile Profiling of Pitanga Fruit (Eugenia uniflora L.) at Different Ripening Stages Using Solid-Phase Microextraction and Mass Spectrometry Coupled with Gas Chromatography. Sci. Hortic. 2019, 250, 366–370. [Google Scholar] [CrossRef]
- Siebert, D.A.; Tenfen, A.; Yamanaka, C.N.; de Cordova, C.M.M.; Scharf, D.R.; Simionatto, E.L.; Alberton, M.D. Evaluation of Seasonal Chemical Composition, Antibacterial, Antioxidant and Anticholinesterase Activity of Essential Oil from Eugenia brasiliensis Lam. Nat. Prod. Res. 2015, 29, 289–292. [Google Scholar] [CrossRef]
- Santana, D.V.S.; Trindade, I.A.S.; Carvalho, Y.M.B.G.; Carvalho-Neto, A.G.; Silva, E.C.D.; Silva-Júnior, E.F.; Leite, R.F.S.; Quintans-Júnior, L.J.; Aquino, T.M.; Serafini, M.R.; et al. Analytical Techniques to Recognize Inclusion Complexes Formation Involving Monoterpenes and Cyclodextrins: A Study Case with (–) Borneol, a Food Ingredient. Food Chem. 2021, 339, 127791. [Google Scholar] [CrossRef]
- Magina, M.D.A.; Dalmarco, E.M.; Wisniewski, A.; Simionatto, E.L.; Dalmarco, J.B.; Pizzolatti, M.G.; Brighente, I.M.C. Chemical Composition and Antibacterial Activity of Essential Oils of Eugenia Species. J. Nat. Med. 2009, 63, 345–350. [Google Scholar] [CrossRef]
- Lado, J.; Gurrea, A.; Zacarías, L.; Rodrigo, M.J. Influence of the Storage Temperature on Volatile Emission, Carotenoid Content and Chilling Injury Development in Star Ruby Red Grapefruit. Food Chem. 2019, 295, 72–81. [Google Scholar] [CrossRef]
- Batur, Ö.Ö.; Kıran, İ.; Berger, R.G.; Demirci, B. Microbial Transformation of β-Caryophyllene and Longifolene by Wolfiporia Extensa. Nat. Volatiles Essent. Oils 2019, 6, 8–15. [Google Scholar]
- da Silva Oliveira, G.L.; Machado, K.C.; Machado, K.C.; Feitosa, C.M.; de Castro Almeida, F.R. Non-Clinical Toxicity of β-Caryophyllene, a Dietary Cannabinoid: Absence of Adverse Effects in Female Swiss Mice. Regul. Toxicol. Pharmacol. 2018, 92, 338–346. [Google Scholar] [CrossRef]
- Abdulra’uf, L.B.; Tan, G.H. Chemometric Approach to the Optimization of HS-SPME/GC–MS for the Determination of Multiclass Pesticide Residues in Fruits and Vegetables. Food Chem. 2015, 177, 267–273. [Google Scholar] [CrossRef]
- STATSOFT, Stat-Soft Inc.: Tulsa, OK, USA, 2011.
- Figueiredo, Y.G.; Corrêa, E.A.; de Oliveira Junior, A.H.; Mazzinghy, A.C.d.C.; Mendonça, H.d.O.P.; Lobo, Y.J.G.; García, Y.M.; Gouvêia, M.A.d.S.; de Paula, A.C.C.F.F.; Augusti, R.; et al. Profile of Myracrodruon urundeuva Volatile Compounds Ease of Extraction and Biodegradability and In Silico Evaluation of Their Interactions with COX-1 and INOS. Molecules 2022, 27, 1633. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).