Abstract
COVID-19 is the name of the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection that occurred in 2019. The virus–host-specific interactions, molecular targets on host cell deaths, and the involved signaling are crucial issues, which become potential targets for treatment. Spike protein, angiotensin-converting enzyme 2 (ACE2), cathepsin L-cysteine peptidase, transmembrane protease serine 2 (TMPRSS2), nonstructural protein 1 (Nsp1), open reading frame 7a (ORF7a), viral main protease (3C-like protease (3CLpro) or Mpro), RNA dependent RNA polymerase (RdRp) (Nsp12), non-structural protein 13 (Nsp13) helicase, and papain-like proteinase (PLpro) are molecules associated with SARS-CoV infection and propagation. SARS-CoV-2 can induce host cell death via five kinds of regulated cell death, i.e., apoptosis, necroptosis, pyroptosis, autophagy, and PANoptosis. The mechanisms of these cell deaths are well established and can be disrupted by synthetic small molecules or natural products. There are a variety of compounds proven to play roles in the cell death inhibition, such as pan-caspase inhibitor (z-VAD-fmk) for apoptosis, necrostatin-1 for necroptosis, MCC950, a potent and specific inhibitor of the NLRP3 inflammasome in pyroptosis, and chloroquine/hydroxychloroquine, which can mitigate the corresponding cell death pathways. However, NF-κB signaling is another critical anti-apoptotic or survival route mediated by SARS-CoV-2. Such signaling promotes viral survival, proliferation, and inflammation by inducing the expression of apoptosis inhibitors such as Bcl-2 and XIAP, as well as cytokines, e.g., TNF. As a result, tiny natural compounds functioning as proteasome inhibitors such as celastrol and curcumin can be used to modify NF-κB signaling, providing a responsible method for treating SARS-CoV-2-infected patients. The natural constituents that aid in inhibiting viral infection, progression, and amplification of coronaviruses are also emphasized, which are in the groups of alkaloids, flavonoids, terpenoids, diarylheptanoids, and anthraquinones. Natural constituents derived from medicinal herbs have anti-inflammatory and antiviral properties, as well as inhibitory effects, on the viral life cycle, including viral entry, replication, assembly, and release of COVID-19 virions. The phytochemicals contain a high potential for COVID-19 treatment. As a result, SARS-CoV-2-infected cell death processes and signaling might be of high efficacy for therapeutic targeting effects and yielding encouraging outcomes.
1. Introduction
Since December 2019, a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic has emerged, posing a global public health threat never seen before [,]. This virus caused over 239 million infection cases and 4.88 million deaths till 14 October 2021 []. Acute respiratory distress syndrome and severe cytokine release syndrome are the main causes of COVID-19 mortality [,]. It shares similarities with previous severe acute respiratory syndrome CoV infections such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) []. COVID-19 is an acronym that stands for coronavirus disease of 2019. SARS-CoV-2 was classified in Coronaviruses (CoVs), which are a group of enveloped, single-stranded positive-sense RNA viruses with club-like spikes on their surface [,]. During replication, CoVs are prone to mutation and recombination, which has led to the diversity and uncontrollability of coronaviruses by the former developed vaccines. SARS-CoV-2 infects the host cell via interacting of its spike protein with angiotensin-converting enzyme 2 (ACE2) receptors on a host cell membrane [,]. Virus entry into cells can directly induce host cell death after the virions are enormously produced. Additionally, an excessive release of cytokines and chemokines, known as a COVID-19-related cytokine storm, is also a cause of host cell death [].
Fever, dry cough, and lethargy are the most common respiratory symptoms of a patient infected with coronavirus. Other symptoms include loss of taste and smell, nasal congestion, conjunctivitis, sore throat, muscular and joint discomfort, diarrhea, shortness of breath, and hyperthermia in certain individuals. Most infected people have mild to moderate symptoms []; however, approximately 33 percent of hospitalized COVID-19 patients develop acute respiratory distress syndrome (ARDS) []. Currently, there is no standard treatment available. Pre-existing medications could be repositioned as a quick and appealing strategy with well-known safety, features, and dosage. Several medications have been tested for efficacy and safety in the treatment of COVID-19, with most of them still in clinical trials [].
Aside from vaccine research, much work has been performed towards finding effective COVID-19 prophylactics for high-risk populations, but only a few studies have yielded positive results. Several clinical examples and in vivo studies have lately suggested that some anti-inflammation and anti-virus medications could be used as preventive possibilities []. Natural products and herbal medications have been utilized to prevent and treat viral infection for thousands of years in oriental traditional medicine []. Those compounds have a high efficiency and a low toxicity. Herbal medicine is undeniably a valuable resource for therapeutic development, and its low toxicity makes it a viable prophylactic candidate against COVID-19 [,]. The antiviral action mechanisms of these natural agents on the influence of the viral life cycle, such as viral entrance, replication, assembly, and release, as well as virus–host-specific interactions, have been studied intensively over the last few decades []. In this review, we aim to provide an update on the molecular mechanisms of coronavirus 2 infection process and SARS-CoV2-induced cell death and signaling, new therapeutic strategies, and remarkable natural compounds regarding prevention and treatment of COVID-19.
2. Life Cycle of SARS-CoV-2 and the Viral Proteins Involved in the Infection
The primary site of attachment and replication of inhaled SARS-CoV-2 is epithelial cells in the nasal cavity. Then virus spreads and migrates across the respiratory tract, following the conducting airways []. Virus can infect the host cell by interacting of its spike protein with angiotensin-converting enzyme receptor 2 (ACE2) on a host cell membrane []. It requires the host cell’s transmembrane protease, serine 2 (TMPRSS2), to activate the spike protein and cleave the ACE2 receptor, which then allows it to attach to the host cell membrane and induce viral and plasma membrane fusion. In comparison with ACE2 expression in lung-specific pulmonary alveolar type II cells, ACE2 is abundantly expressed in the bladder, ileum, kidney, and liver. However, the renin–angiotensin system and the PPAR signaling pathway, as well as a few basic metabolic and influential pathways, such as insulin resistance, play an important role in enhancing the infection following its entry via ACE2 []. Viral RNA is released into the host cell after entry, and viral polyproteins are translated using the ribosome of the host cell. Polyproteins (PP) PP1a and PP1ab are formed during translation and are then cleaved by viral proteases, papain-like protease (PLPRO), and 3C-like protease (3CLPRO) to produce functional non-structural proteins (Nsp). Two types of proteins are encoded by SARS-CoV-2 genomic RNA. Nsp and structural proteins are essential for viral RNA synthesis and virion assembly, respectively []. The RNA-dependent RNA polymerase (RdRp) (NSP12), helicase (NSP13), and other subunits such as NSP7 and NSP8 comprise the replicase–transcriptase complex (RTC). The complex also transcribes a viral genome template containing negative-sense genes, as well as progeny genome and subgenomic RNA as intermediary products, before directing RdRp to transcribe positive-sense mRNAs []. After transcription and translation, subgenomic proteins form structural and accessory proteins such as M, S, and E proteins, which are then encased in the endoplasmic reticulum and transported to the endoplasmic reticulum–Golgi intermediate compartment (ERGIC). The viral genome can then bind the N protein directly to create the nucleocapsid, which is then insulated into the ERGIC. Finally, nucleocapsids and numerous other structural proteins combine to create vesiculated virion particles, which are then exocytosed out of the cell in the form of virions [,].
SARS-CoV and SARS-CoV-2 are both zoonotic coronaviruses belonging to the Betacoronavirus genus [,]. The novel SARS-CoV-2 genome was found to share 82 percent nucleotide similarity with SARS-CoV. The membrane, envelope, spike, nucleoprotein, and orf1a/b polyproteins clustered closely together, according to phylogenetic research []. It was also confirmed that, similar to SARS-CoV, the angiotensin-converting enzyme 2 (ACE2) is the major host receptor for SARS-CoV-2 [,]. Furthermore, the spike-receptor binding domain (RBD) sequences of SARS-CoV-2 and SARS-CoV were discovered to be 76 percent similar, and the two viruses’ major proteases were determined to be closely related (96 percent identity) [,]. The proteins that are involved in the infection of SARS-CoV-2 are summarized in Table 1.

Table 1.
The proteins involved in the infection of SARS-CoV-2.
4. The Possible Mechanisms of Cell Death Regulation in SARS-CoV-2 Infection
In genomic sequences, the catalytic sites of the four SARS-CoV2 enzymes are strikingly similar to those of SARS-CoV and MERS. Furthermore, among the three coronaviruses, the architectures of the main drug-binding pockets are remarkably conserved []. As a result, existing anti-SARS-CoV and anti-MERS medications that target these enzymes can be repurposed to treat SARS-CoV-2. According to Chen et al. in 2020, the SARS-CoV-2 genome encodes four structural proteins, sixteen non-structural proteins (nsp), and auxiliary proteins, identical to SARS and MERS. Spike (S), envelope (E), membrane (M), and nucleoprotein (N) are all structural proteins that have been used to build anti-COVID-19 medicines [].
SARS-CoV is thought to have a two-part pathogenic mechanism: (1) the virus directly injures target cells, and (2) causes immune system malfunction. T cells and macrophages are the most important cells in this step. These circulating immune cells subsequently carry the viruses to other tissues, including secondary lymphoid organs. Because SARS-CoV is similar to HIV, both viruses assault immune cells and produce immunodeficiency []. Normally, pattern recognition receptors in the host detect viral pathogen-associated molecular patterns (PAMPs). However, the virus can utilize a variety of tactics to evade the innate immune response. The nuclear factor-κB (NF-κB) pathway stimulates the production of type I IFN. Signal transducer and activator of transcription (STAT) proteins are activated when IFN binds to the IFN receptor, increasing the synthesis of other antiviral proteins and then blocking SARS-CoV replication []. MERS-cell CoV receptor DPP4 is widely expressed on epithelial cells in the prostate, alveoli, kidneys, liver, and small intestine, as well as on active leukocytes. Hence, MERS-tissue CoV’s tropism is broader than any other coronavirus []. The clinical antiviral drugs that target structural and nonstructural proteins of SARS-CoV-2 are employed to develop the following drugs. Lopivir, ritonavir, darunavir, cobicistat, and ASC09F are the most likely inhibitors of 3CLpro (phase III, in combination with oseltamivir, for SARS-CoV-2) []. Among the possible inhibitors of RdRp are favipiravir, ribavirin (randomized study for SARS-CoV-2), and remdesivir []. Previously, chemicals such as bananins that may interfere with ATPase and helicase activity have been reported (before COVID-19). Another possibility is the creation of antibodies to disrupt the ACE2 receptor, which has a considerable affinity for the RBD of SARS-CoV-2. The second way is blocking the spike protein directly with a significant dose of soluble ACE2. The third way is developing a medicine that directly blocks the spike membrane fusion process, such as enfuvirtide. The fourth strategy is to find an agent that inhibits the action of disintegrin and metalloproteinase 17 (ADAM17), a proteinase that plays a role in the formation of fibrosis or scar tissue [].
Because SARS-CoV-2 is the third virus of its kind to cause an outbreak, and because it shares similarities with the previous two viruses, SARS-CoV-1 and MERS-CoV, it is critical to determine whether SARS-CoV-2-induced lymphopenia or lymphocyte killing mechanism is the same. COVID-19-associated lymphopenia is a significant pathological finding and severity criterion that can be used as a biomarker and a target for intervention to reduce the likelihood of severe disease. The transition of lymphocyte subsets and variations in peripheral lymphocyte numbers could point to plausible pathways in the pathophysiology of SARS-CoV-2 infection. Only a few studies have looked into severe COVID-19 cases, which have a specific profile of reduced memory T cells. Flow cytometry was used to assess the levels of peripheral lymphocyte subsets in 60 individuals with COVID-19 over the course of their illness. There was a decrease in total lymphocytes, CD4+ T cells, CD8+ T cells, B cells, and natural killer (NK) cells []. In a previous study evaluating laboratory indices, twelve patients with COVID-19 were found to have a lower percentage of lymphocytes and a lower CD8 cell count []. T cells were found to be more affected by SARS-CoV-2 in 286 patients with COVID-19, with T cell counts about half the lower reference level and tending to be more reduced in severe cases []. In a study of severely ill SARS-CoV-2 pneumonia patients, 85 percent of the patients had lymphopenia [].
The processes underlying the substantial lymphocyte depletion seen in severe instances are established. In COVID-19, if the kind of lymphocyte death and its mechanisms are better understood, it can be a treatment option for severe cases. SARS and MERS have been linked to lymphopenia, which has been linked to apoptosis in the liver, lung, and T lymphocytes []. The role of apoptosis in lymphopenia patients with SARS was studied by looking at plasma soluble Fas-ligand levels and cleaved caspase-3 activation in fifteen individuals. In the acute phase of SARS, patients with greater plasma Fas-ligand levels were shown to have more intracellular cleaved caspase-3–positive CD4 and CD8 cells [].
Apoptosis of lymphocytes is thought to cause lymphopenia in SARS-CoV-2-infected critically sick individuals. It is worth noting that evidence is mounting for direct SARS-CoV-2 infection of T cells, which could result in a cytopathic effect on infected T cells. COVID-19 was found to possess a decrease in the number of circulating Treg cells (CD3+CD4+CD25+CD127low+) []. A large drop in lymphocyte numbers, as well as their fast depletion, can play a role in the etiology of COVID-19 and contribute to its development to severe COVID-19. As a result, medications that target lymphocyte growth or apoptosis, such as interleukin IL-2, IL-7, or programmed cell death protein 1 (PD1/PD-L1) inhibitors, may be able to assist in avoiding lymphopenia or in restoring lymphocyte levels in very ill patients [].
MERS-CoV causes apoptosis in human kidney cells, lung cells, and primary T lymphocytes, most likely through inducing Smad7 and FGF2. SARS-CoV proteins including ORF3a, ORF3b, ORF7a, ORF8a, ORF9b, and E proteins have been shown to be pro-apoptotic proteins. ORF7a promotes the intrinsic apoptotic pathway in human embryonic kidney cell line by interacting with anti-apoptotic protein Bcl-XL in the endoplasmic reticulum, thus sequestering a crucial suppressor of apoptosis. Because MERS and SARS-CoV-2 have similar structural and non-structural proteins in infecting renal and lung epithelial cells, and all types of cells have the cell surface receptors for viral S protein binding and signaling, it is thought that the apoptotic cell death pathway is responsible for all T lymphocytes, kidney cells, and lung epithelial cells [].
The structural proteins and signals of MERS-CoV, SARS-CoV-1, and SARS-CoV-2 are all similar. Through intrinsic and extrinsic signaling mechanisms, MERS-CoV can infect T cells and trigger apoptosis, bypassing the immune system and allowing for fast spread []. SARS-CoV2 may cause T cell cytotoxicity by causing apoptotic-regulated cell death. One method of bypassing the host cell’s immune system has been discovered by MERS-CoV. When host sensors engage signaling pathways, type I IFN genes are transcribed, and the type I IFN response, a key component of antiviral innate immunity, begins. IFN activates the transcription of numerous IFN-stimulated genes (ISGs) and produces 2′,5′-oligoadenylate, which promotes RNase L (2-5A) [,,]. Activated RNase L can cleave both viral and host ssRNA, resulting in translation standstill and death, as well as limiting virus reproduction and transmission [].
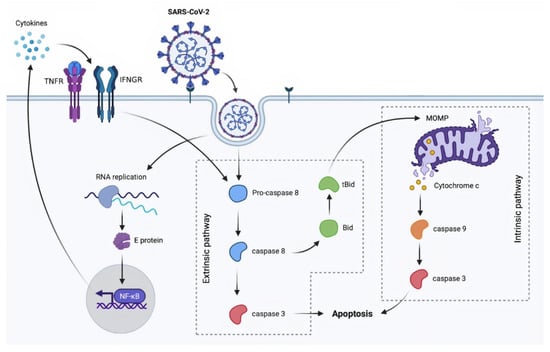
Apoptosis is a well-characterized cell death mechanism caused by viral infection including HIV, MERS, SARS-Co, and SARS-CoV-2, according to various studies [,,,,,,,,]. As a result, because apoptosis includes a vast number of molecules and processes, multiple means for influencing these mechanisms could be applied as follows. Caspase-8 activation initiates an apoptotic cell death via cascades leading to the extrinsic apoptosis pathway. In the extrinsic pathway, FLIP exhibits inhibitory effects on apoptosis.
Since the development of understanding and knowledge of FLIP has a long history of insight and concept concerning the death receptor pathway, it has the following scientific evidence-based data, which is support by much research. The death domain (DD) plays a pivotal role because it is found in the cytosolic part of death receptors, viz., Fas, TNF-R, TRAIL-R1, and TRAIL-R2; adaptor proteins, including FADD (Fas-associated protein with death domain), TRADD (TNF-receptor-associated protein with death domain); and RIP1. Moreover, death effector domain (DED) is in the adaptor proteins, viz., FADD, TRADD, (pro)caspase-8/10, and FADD-like IL-1-converting enzyme (FLICE) []. Hence, it was found that in the death-inducing signaling complex (DISC), it comprises death receptor and adaptor protein(s) binding via DD, together with (pro)caspase-8/10, leading to autoactivate the procaspase-8/10 themselves to be active caspase-8/10 that further induces caspases-3, 6, and 7, then finally apoptosis. However, in cells that have cFLIP with DED domain, cFLIP binds to DED of pro-caspase-8/10 and inhibits the induction of the extrinsic apoptosis pathway [,]. The roles of apoptosis have been found in physiology, such as in development and pathophysiology, e.g., neurodegenerative diseases [], viral infection [], and even cancer []. Restraining of SARS-CoV-2 viral replication and virus-induced tissue damage through modulation of the DD network may pave the way towards new therapeutic approaches by inhibiting caspase-8 activity, at death-inducing signaling complex (DISC) formation. Therefore, it was assumed that FLIP containing DED may play a pivotal role in apoptosis induction in COVID-19 patient cells/tissues [].
Ivanisenko et al. (2020) investigated the pathogen penetration and regulating immune response by creating a mouse model that mimicked a regulated cytokine storm. As indicated by important clinical phenotypes, it was uncovered that SARS-CoV-2 induces pandemic, dysregulated anti-pathogen immune responses, known as cytokine release syndrome (CRS), which can produce life-threatening inflammatory illnesses. FLIP, a protein that regulates caspase-8 death pathways, was found to be highly expressed in the myeloid cells of COVID-19 lungs. FLIP regulates CRS via inducing an inflammatory response that is dependent on STAT3. Consistent expression of a viral FLIP homolog in myeloid cells generates a STAT3-linked, progressive, and fatal inflammatory disease in mice that imitates human CRS and includes elevated cytokine output, lymphopenia, lung injury, and multiple organ dysfunctions. Because STAT3-targeting strategies reduce inflammation, immunological issues, and organ failures in these mice, it is feasible that blocking this pathway can reduce CRS’s lethal inflammatory condition [].
FLICE (FADD-like IL-1-converting enzyme)-like inhibitory proteins (referred to as c-FLIP and v-FLIP) functions as an anti-apoptotic cellular and viral factor capable of reprogramming monocytes []. FLIP isoforms are known to inhibit caspase-8 and/or activate NF-κB to regulate cell survival and proliferation [,,,]. FLIPs, on the other hand, regulate different biological processes depending on their protein structure (for example, the presence of several death effector domains in a single protein) [], cellular location (for example, nucleocytoplasmic trafficking) [], and nuclear localization of c-FLIPL and its regulation of AP-1 activity. Upregulation of FLIP proteins in monocytes leads to the development of an unusual phenotype characterized by the expression of immunosuppressive (e.g., programmed death-ligand 1 (PD-L1), interleukin (IL)-10) and pro-inflammatory (e.g., IL-1, IL-6, tumor necrosis factor (TNF)-α) features, which is partially dependent on the nuclear translocation of the complex FLIP/nuclear factor complex [].
FLIP- and pSTAT3-expressing myeloid cells have also been linked to COVID-19-associated CRS, since both human ACE2-expressing transgenic mice and SARS-CoV-2 patients possess a high level of FLIP. Furthermore, monocytes isolated from COVID-19 patients exhibit high levels of myeloid c-FLIP and pSTAT3, which is linked to their immunosuppressive properties. Immunological dysfunctions and the bronchoalveolar immune landscape of patients with severe COVID-19 were discovered to be reflected in vFLIP transgenic mice. This one-of-a-kind model was used to investigate STAT3 inhibition approaches for treating uncontrolled inflammation and acute sickness symptoms in systemic and myeloid-targeted mice. Finally, it was revealed that a novel pathway of FLIP’s role is its critical function and expression in myeloid cells as a direct means to stimulate a deadly inflammatory state by fueling an abnormal STAT3-dependent signaling pathway. Furthermore, the therapeutic efficacy of the STAT3 on-target strategy in reducing uncontrolled inflammation and acute disease becomes the laying of a groundwork for the development of more precise and evidence-based therapies to treat CRS disorders and severe clinical aspects of the ongoing COVID-19 pandemic crisis. The importance of FLIP and the proteins involved in the extrinsic route in limiting the DD-mediated signaling network to potentially suppress viral replication and reduce tissue damage was highlighted in this study [].
As mentioned, the participation of cellular FLICE-like inhibitory protein (cFLIP) in the death-inducing signaling complex (DISC) is required for its activation. At the DISC, the long isoform of cFLIP (cFLIPL) can operate in both a pro- and anti-apoptotic manner [,]. Because it operates as an anti-apoptotic factor by inhibiting procaspase-8 activation, viruses upregulate cFLIP expression as a strategy to prevent extrinsic apoptosis in order to continue their replication []. High amounts of cFLIP were found in the pulmonary myeloid cells of COVID-19 patients as well as during the start of SARS-CoV-2 infection []. As a result, the use of small molecules that act against cFLIP to activate caspase 8 and induce extrinsic apoptosis has been proposed as an important strategy for combating virus replication []. 4-(4-Chloro-2-methylphenoxy)-N-hydroxybutanamide (CMH) and droxinostat, the two small molecule inhibitors of cFLIP, have been found to reduce c-FLIPL and c-FLIPS mRNA and protein levels, which can inhibit cell viability and promote apoptosis []. Inhibiting apoptosis in T cells by using small molecules such as pancaspase inhibitor (z-VAD-fmk) or TRADD inhibitors (ICCB-19 and Apt-1) [] seemed to be an appealing strategy for preventing lymphopenia in COVID-19 patients [].
Furthermore, either VDAC1 oligomerization inhibitors (VBIT-4, VBIT-3, and AKOS-022) [] or a pan-caspase inhibitor may rescue the survival of T cells that showed characteristics of apoptosis caused by mitochondrial degeneration as well []. These findings are consistent with the fact that VDAC1 oligomerization and interaction with Bcl-2 family proteins are thought to be responsible for the formation of pores in the outer membrane of mitochondria, allowing for cytochrome c release and the activation of the caspase cascade, which induces cellular apoptosis [,].
The NF-κB pathway is another important anti-apoptotic route mediated by SARS-CoV-2. When NF-κB is activated, apoptosis inhibitors such as cFLIP, B-cell lymphoma 2 (Bcl-2), and X-linked inhibitor of apoptosis protein (XIAP) are upregulated. This strategy is critical for promoting viral infection, survival, and inflammation [,]. Therefore, targeted inhibition of the NF-κB signaling with small molecule drugs such as proteasome inhibitors suggests yet another potential strategy for combating SARS-CoV-2 []. Celastrol and curcumin are natural compounds that disrupt the ubiquitin–proteasome system (UPS), which inhibits the NF-κB pathway []. In addition, there are several small molecule inhibitors of the NF-κB pathway from cell surface receptor stimulation to nuclear signaling that were approved by the US FDA. Acalabrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor, and selinexor, a selective inhibitor of nuclear export (SINE) acting via NF-κB inactivation, are currently being evaluated in COVID-19 clinical trials [,,,].
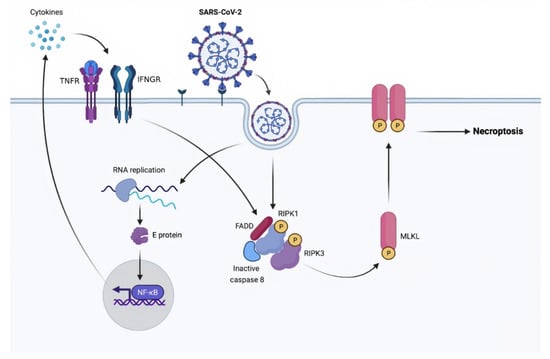
The use of necrostatin-1 (Nec-1) and Nec-1 analogues may relieve cytokine storm, systemic inflammation, and COVID-19 infection by inhibiting RIPK1, the molecule that interacts with RIPK3 forming rippoptosome and promotes MLKL phosphorylation []. Because RNA viruses can activate the inflammasome via RIPK1/RIPK3 [], Nec-1 may aid in the regulation of this process by decreasing inflammation and cytokine release caused by COVID-19. To prevent necroptosis, researchers used the RIPK1-specific inhibitor necrostatin-1 (Nec-1), RIPK3 inhibitor GSK’872, MLKL inhibitor necrosulfonamide (NSA), and small interfering RNA (siRNA) []. It is unclear whether inhibiting necroptosis improves the host’s antiviral response or worsens tissue inflammation and damage. In fact, necroptosis can both inhibit viral replication by causing cell death and can enhance viral dissemination by causing host cell rupture.
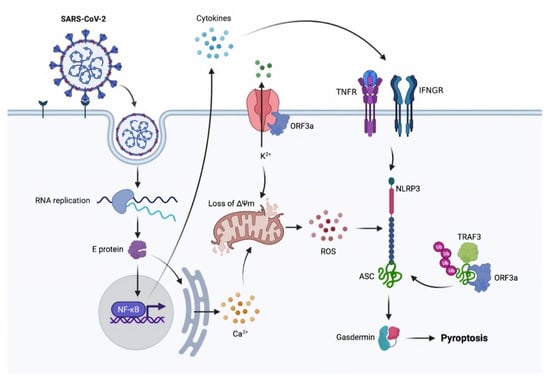
SARS-CoV-2 activates the inflammasome and causes pyroptosis in human monocytes. As a result, the use of MCC950, a potent and specific inhibitor of the NLRP3 inflammasome, may alleviate cytokine storm and systemic inflammation caused by SARS-CoV-2-induced immunogenic cell death [,].
In different disease models, such as cancer [], neurological illnesses, cardiovascular disease, and inflammatory diseases [], several plant extracts such as Tiliacora racemosa leaf methanolic extract and natural constituents such as fisetin and quercetin can inhibit the controlled apoptotic cell death pathways by targeting multiple death-inducing molecules. As a result, we hypothesize that the plant-derived phyto-chemicals may have a similar effect on viral infection-mediated apoptotic diseases (including SARS-CoV2).
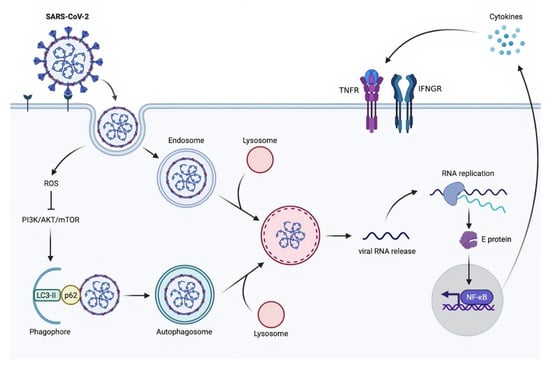
The FDA-approved anti-malarial medications chloroquine and hydroxychloroquine have been proposed for the treatment of COVID-19 via autophagy inhibition [,,]; however, this is extensively debated [,]. Although chloroquine is a lysosomotropic drug that inhibits autophagic degradation, potentially by inhibiting autophagosome fusion with lysosomes [], the putative autophagic effects may not be responsible for the antiviral action. Indeed, endosomal acidification following endocytosis is required for SARS-CoV-2 entry [], and chloroquine suppresses this acidification []. Furthermore, chloroquine inhibits the terminal glycosylation of the metallopeptidase ACE2, which serves as a functional receptor for SARS-CoV and SARS-CoV-2 cell entry [,]. Non-glycosylated ACE2 appears to interact with the SARS-CoV spike protein less efficiently, leading to a decrease in viral entry [].
6. Conclusions
Globally, SARS-CoV-2 is a virus that causes severe COVID-19 pandemic fatalities. Extensive research is being performed to investigate drugs or natural products for prophylaxis and treatment, while the vaccine development is still developing simultaneously to prevent the infection. The viruses cause host cell death and inflammation due to the cytokine storm. Numerous medicinal herbs and the phyto-chemicals possess anti-inflammatory and antiviral effects on the progression and amplification of numerous viruses. Natural compounds targeting against coronaviruses including SARS-Co-V2 via different molecules involving in the processes of viral entry, amplification, replication, and protein synthesis were provided in detail. Such components included alkaloids, flavonoids, terpenoids, diarylheptanoids, polyphenolic acids, saponins, and anthraquinones from nature with fewer unfavorable side effects, as expected. The targeted molecules of SARS-CoV-2 for infection are as follows: spike (S), TMPRSS2, papain-like proteinase (PLpro), viral main protease (3C-like protease (3CLpro or Mpro)), non-structural protein 13 (Nsp13) helicase, open reading frame 7a (ORF7a), and RNA-dependent RNA polymerase (RdRp) (Nsp12), which are the proteins needed for making new virions because the host cells are killed by five different types of regulated cell death, viz., apoptosis, necroptosis, pyroptosis, autophagy, and PANoptosis. The natural inhibitors or small molecules modifying the cell death pathways or signalings may be of high efficacy for the treatment and prevention of SARS-CoV2 infection. For example, pancaspase inhibitor (z-VAD-fmk) (for apoptosis intervention); necrostatin-1 (a necroptosis inhibitor), MCC950, a specific small-molecule inhibitor of the NLRP3 inflammasome (as a pyroptosis inhibitor); and chloroquine/hydroxychloroquine (as an autophagy inhibitors) may be beneficial. The NF-κB pathway is another important anti-apoptotic and inflammatory route mediated by the viruses since NF-κB signaling promotes viral proliferation, survival, and inflammation via the enhancing of anti-apoptosis proteins such as Bcl-2 and XIAP expression and cytokine release, e.g., TNF and interleukin-1. As a result, tiny compounds functioning as controlling the NF-κB-mediated proteasome inhibition, such as celastrol and curcumin, can be applied to target the ubiquitin–proteasome system (UPS), then affecting NF-κB signaling to provide another reasonable method for prophylaxis and therapy of COVID-19 patients. The linkages of cell death signaling, the mechanism of viral entry/replication, and the natural products are still scarce, and research in this field is advancing and progressing at the molecular level to understand more of the mechanisms of phyto-chemicals and natural compounds as cell death inducers of virus-infected host cells and to understand the use of such strategic targets for orchestrating and obtaining an effective high potential treatment and prevention for the rescue of COVID-19 patients. Nevertheless, acalabrutinib (a tyrosine kinase inhibitor) and selinexor (acting through deactivation of NF-κB signaling) have been investigated in clinical trials for COVID-19 therapy and chloroquine and hydroxychloroquine have been approved by the US FDA for SARS-CoV-2 patients. Various natural compounds’ in vitro cytotoxicity effects have been recorded, although animal model and human tests are still lacking. We anticipate that natural products that target multiple cell death signaling molecules will be examined and authorized in clinical trials with supporting scientific data in the near future. Rather than using COVID-19 therapy, the best course of action is to prevent SARS-CoV-2 infection.
Author Contributions
Conceptualization, R.Y., P.K.-o. and R.B.; drafting of the manuscript, R.Y. and R.B.; reviewing and editing of the paper, R.Y., R.B. and P.K-o. All authors have read and agreed to the published version of the manuscript.
Funding
This review paper received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
We thank Pre-Square Wellness Co., Ltd., and Chiang Mai Genetics Laboratory Co., Ltd., for supporting the present study. We are grateful to Faculty of Medicine, Chiang Mai University.
Conflicts of Interest
The authors declared no conflict of interest.
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Roser, M.; Ritchie, H.; Ortiz-Ospina, E.; Hasell, J. Coronavirus Pandemic (COVID-19). Available online: Ourworldindata.org (accessed on 11 September 2021).
- Hirano, T.; Murakami, M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity 2020, 52, 731–733. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Guan, Z.; Li, H.; Ye, M.; Chen, X.; Shen, J.; Zhou, Y.; Shi, Z.-L.; Zhou, P. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target. Ther. 2020, 5, 235. [Google Scholar] [CrossRef]
- Chauhan, S. Comprehensive review of coronavirus disease 2019 (COVID-19). Biomed. J. 2020, 43, 334–340. [Google Scholar] [CrossRef]
- Tzotzos, S.J.; Fischer, B.; Fischer, H.; Zeitlinger, M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit. Care 2020, 24, 516. [Google Scholar] [CrossRef] [PubMed]
- Tarighi, P.; Eftekhari, S.; Chizari, M.; Sabernavaei, M.; Jafari, D.; Mirzabeigi, P. A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur. J. Pharmacol. 2021, 895, 173890. [Google Scholar] [CrossRef]
- Bartoszko, J.J.; Siemieniuk, R.A.; Kum, E.; Qasim, A.; Zeraatkar, D.; Ge, L.; Han, M.A.; Sadeghirad, B.; Agarwal, A.; Agoritsas, T. Prophylaxis against covid-19: Living systematic review and network meta-analysis. BMJ 2021, 373, n949. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.C. Traditional Chinese medicine and clinical pharmacology. Drug Discov. Eval. Methods Clin. Pharmacol. 2020, 455–482. [Google Scholar] [CrossRef]
- Chakravarti, R.; Singh, R.; Ghosh, A.; Dey, D.; Sharma, P.; Velayutham, R.; Roy, S.; Ghosh, D. A review on potential of natural products in the management of COVID-19. RSC Adv. 2021, 11, 16711–16735. [Google Scholar] [CrossRef]
- Ospanov, M.; Leó, F.; Janar, J.; Khan, I.A.; Ibrahim, M.A. Challenges and future directions of potential natural products leads against 2019-nCoV outbreak. Curr. Plant Biol. 2020, 24, 100180. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Mandalari, G.; Sciortino, M.T. Antiviral Activity Exerted by Natural Products against Human Viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. Soc. 2020, 55, 2000607. [Google Scholar] [CrossRef]
- Yang, J.; Petitjean, S.J.; Koehler, M.; Zhang, Q.; Dumitru, A.C.; Chen, W.; Derclaye, S.; Vincent, S.P.; Soumillion, P.; Alsteens, D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020, 11, 4541. [Google Scholar] [CrossRef]
- Dey, A.; Sen, S.; Maulik, U. Unveiling COVID-19-associated organ-specific cell types and cell-specific pathway cascade. Brief. Bioinform. 2021, 22, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Coronaviruses 2015, 1–23. [Google Scholar] [CrossRef]
- Salata, C.; Calistri, A.; Parolin, C.; Palu, G. Coronaviruses: A paradigm of new emerging zoonotic diseases. Pathog. Dis. 2019, 77, ftaa006. [Google Scholar] [CrossRef]
- Kannan, S.; Ali, P.S.S.; Sheeza, A.; Hemalatha, K. COVID-19 (Novel Coronavirus 2019)-recent trends. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2006–2011. [Google Scholar] [PubMed]
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yiu, C.-P.B.; Wong, K.-Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL pro) structure: Virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research 2020, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Lung, J.; Lin, Y.S.; Yang, Y.H.; Chou, Y.L.; Shu, L.H.; Cheng, Y.C.; Liu, H.T.; Wu, C.Y. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J. Med. Virol. 2020, 92, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, T.M.; Buchmeier, M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology 2001, 279, 371–374. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011, 85, 873–882. [Google Scholar] [CrossRef]
- Kamitani, W.; Narayanan, K.; Huang, C.; Lokugamage, K.; Ikegami, T.; Ito, N.; Kubo, H.; Makino, S. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc. Natl. Acad. Sci. USA 2006, 103, 12885–12890. [Google Scholar] [CrossRef]
- Taylor, J.K.; Coleman, C.M.; Postel, S.; Sisk, J.M.; Bernbaum, J.G.; Venkataraman, T.; Sundberg, E.J.; Frieman, M.B. Severe acute respiratory syndrome coronavirus ORF7a inhibits bone marrow stromal antigen 2 virion tethering through a novel mechanism of glycosylation interference. J. Virol. 2015, 89, 11820–11833. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Harcourt, B.H.; Jukneliene, D.; Kanjanahaluethai, A.; Bechill, J.; Severson, K.M.; Smith, C.M.; Rota, P.A.; Baker, S.C. Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity. J. Virol. 2004, 78, 13600–13612. [Google Scholar] [CrossRef]
- Anand, K.; Ziebuhr, J.; Wadhwani, P.; Mesters, J.R.; Hilgenfeld, R. Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science 2003, 300, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Shum, K.T.; Tanner, J.A. Differential inhibitory activities and stabilisation of DNA aptamers against the SARS coronavirus helicase. Chembiochem 2008, 9, 3037. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Imre, G. Cell death signalling in virus infection. Cell. Signal. 2020, 76, 109772. [Google Scholar] [CrossRef]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.-D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Garron, T.M.; Chang, Q.; Su, Z.; Zhou, C.; Qiu, Y.; Gong, E.C.; Zheng, J.; Yin, Y.W.; Ksiazek, T. Cell-type apoptosis in lung during SARS-CoV-2 infection. Pathogens 2021, 10, 509. [Google Scholar] [CrossRef]
- Ren, Y.; Shu, T.; Wu, D.; Mu, J.; Wang, C.; Huang, M.; Han, Y.; Zhang, X.-Y.; Zhou, W.; Qiu, Y. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell. Mol. Immunol. 2020, 17, 881–883. [Google Scholar] [CrossRef]
- Bianchi, M.; Borsetti, A.; Ciccozzi, M.; Pascarella, S. SARS-Cov-2 ORF3a: Mutability and function. Int. J. Biol. Macromol. 2021, 170, 820–826. [Google Scholar] [CrossRef]
- Issa, E.; Merhi, G.; Panossian, B.; Salloum, T.; Tokajian, S. SARS-CoV-2 and ORF3a: Nonsynonymous mutations, functional domains, and viral pathogenesis. Msystems 2020, 5, e00266-20. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, B.-J.; Xu, K.; Schwarz, W.; Du, L.; Wong, C.K.; Chen, J.; Duan, S.; Deubel, V.; Sun, B. Severe acute respiratory syndrome-associated coronavirus 3a protein forms an ion channel and modulates virus release. Proc. Natl. Acad. Sci. USA 2006, 103, 12540–12545. [Google Scholar] [CrossRef]
- Scott, C.; Griffin, S. Viroporins: Structure, function and potential as antiviral targets. J. Gen. Virol. 2015, 96, 2000–2027. [Google Scholar] [CrossRef]
- Thompson, E.A.; Cascino, K.; Ordonez, A.A.; Zhou, W.; Vaghasia, A.; Hamacher-Brady, A.; Brady, N.R.; Sun, I.-H.; Wang, R.; Rosenberg, A.Z. Metabolic programs define dysfunctional immune responses in severe COVID-19 patients. Cell Rep. 2021, 34, 108863. [Google Scholar] [CrossRef]
- Castaño-Rodriguez, C.; Honrubia, J.M.; Gutiérrez-Álvarez, J.; DeDiego, M.L.; Nieto-Torres, J.L.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Fernandez-Delgado, R.; Verdia-Báguena, C.; Queralt-Martín, M. Role of severe acute respiratory syndrome coronavirus viroporins E, 3a, and 8a in replication and pathogenesis. MBio 2018, 9, e02325-17. [Google Scholar] [CrossRef]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Lavrik, I. Systems biology of death receptor networks: Live and let die. Cell Death Dis. 2014, 5, e1259. [Google Scholar] [CrossRef] [PubMed]
- Bellesi, S.; Metafuni, E.; Hohaus, S.; Maiolo, E.; Marchionni, F.; D’Innocenzo, S.; La Sorda, M.; Ferraironi, M.; Ramundo, F.; Fantoni, M. Increased CD95 (Fas) and PD-1 expression in peripheral blood T lymphocytes in COVID-19 patients. Br. J. Haematol. 2020, 191, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Kellert, B.; Dimitrova, D.P.; Langlais, C.; Hupe, M.; Cain, K.; MacFarlane, M.; Häcker, G.; Leverkus, M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 2011, 43, 449–463. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Chen, I.-Y.; Moriyama, M.; Chang, M.-F.; Ichinohe, T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef] [PubMed]
- DeDiego, M.L.; Nieto-Torres, J.L.; Regla-Nava, J.A.; Jimenez-Guardeño, J.M.; Fernandez-Delgado, R.; Fett, C.; Castaño-Rodriguez, C.; Perlman, S.; Enjuanes, L. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014, 88, 913–924. [Google Scholar] [CrossRef]
- Kanzawa, N.; Nishigaki, K.; Hayashi, T.; Ishii, Y.; Furukawa, S.; Niiro, A.; Yasui, F.; Kohara, M.; Morita, K.; Matsushima, K. Augmentation of chemokine production by severe acute respiratory syndrome coronavirus 3a/X1 and 7a/X4 proteins through NF-κB activation. FEBS Lett. 2006, 580, 6807–6812. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.L.; Yuen, K.S.; Castano-Rodriguez, C.; Ye, Z.W.; Yeung, M.L.; Fung, S.Y.; Yuan, S.; Chan, C.P.; Yuen, K.Y.; Enjuanes, L. Severe acute respiratory syndrome Coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019, 33, 8865–8877. [Google Scholar] [CrossRef]
- Yap, J.K.; Moriyama, M.; Iwasaki, A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J. Immunol. 2020, 205, 307–312. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Singh, K.D.; Karnik, S.S. Angiotensin receptors: Structure, function, signaling and clinical applications. J. Cell Signal. 2016, 1, 111. [Google Scholar]
- Mori, J.; Oudit, G.Y.; Lopaschuk, G.D. SARS-CoV-2 perturbs the renin-angiotensin system and energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E43–E47. [Google Scholar] [CrossRef]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 inflammasome in severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Kim, J. Autophagy: An essential degradation program for cellular homeostasis and life. Cells 2018, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Gutierrez, D.; Bauer, M.A.; Zimmermann, A.; Kainz, K.; Hofer, S.J.; Kroemer, G.; Madeo, F. Digesting the crisis: Autophagy and coronaviruses. Microb. Cell 2020, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-S.; Nabar, N.R.; Huang, N.-N.; Kehrl, J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019, 5, 101. [Google Scholar] [CrossRef]
- Gassen, N.C.; Niemeyer, D.; Muth, D.; Corman, V.M.; Martinelli, S.; Gassen, A.; Hafner, K.; Papies, J.; Mösbauer, K.; Zellner, A. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun. 2019, 10, 5770. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Wang, P.-H.; Yang, N.; Huang, J.; Ou, J.; Xu, T.; Zhao, X.; Liu, T.; Huang, X. SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2021, 1867, 166260. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.S. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 2021, 184, 149–168. [Google Scholar] [CrossRef]
- He, W.-T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef]
- Orning, P.; Weng, D.; Starheim, K.; Ratner, D.; Best, Z.; Lee, B.; Brooks, A.; Xia, S.; Wu, H.; Kelliher, M.A. Pathogen blockade of TAK1 triggers caspase-8–dependent cleavage of gasdermin D and cell death. Science 2018, 362, 1064–1069. [Google Scholar] [CrossRef]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Li, P.; Nilson, R.; Tang, A.Y.; Rongvaux, A.; Bunnell, S.C.; Shao, F.; Green, D.R. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. USA 2018, 115, E10888–E10897. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020, 368, aaz7548. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Lakhani, S.A.; Masud, A.; Kuida, K.; Porter, G.A.; Booth, C.J.; Mehal, W.Z.; Inayat, I.; Flavell, R.A. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science 2006, 311, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflamm. 2018, 15, 199. [Google Scholar] [CrossRef]
- Giampietri, C.; Starace, D.; Petrungaro, S.; Filippini, A.; Ziparo, E. Necroptosis: Molecular signalling and translational implications. Int. J. Cell Biol. 2014, 2014, 490275. [Google Scholar] [CrossRef]
- Murphy, J.M.; Czabotar, P.E.; Hildebrand, J.M.; Lucet, I.S.; Zhang, J.-G.; Alvarez-Diaz, S.; Lewis, R.; Lalaoui, N.; Metcalf, D.; Webb, A.I. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 2013, 39, 443–453. [Google Scholar] [CrossRef]
- Malireddi, R.S.; Tweedell, R.E.; Kanneganti, T.-D. PANoptosis components, regulation, and implications. Aging 2020, 12, 11163. [Google Scholar] [CrossRef]
- Malireddi, R.S.; Karki, R.; Sundaram, B.; Kancharana, B.; Lee, S.; Samir, P.; Kanneganti, T.-D. Inflammatory cell death, PANoptosis, mediated by cytokines in diverse cancer lineages inhibits tumor growth. Immunohorizons 2021, 5, 568–580. [Google Scholar] [CrossRef]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S. Identification of the PANoptosome: A molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Malireddi, R.S.; Kanneganti, T.-D. Caspases in cell death, inflammation, and pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef]
- Malireddi, R.; Gurung, P.; Kesavardhana, S.; Samir, P.; Burton, A.; Mummareddy, H.; Vogel, P.; Pelletier, S.; Burgula, S.; Kanneganti, T.-D. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity–independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J. Exp. Med. 2020, 217, e20191644. [Google Scholar] [CrossRef]
- Malireddi, R.; Kesavardhana, S.; Kanneganti, T.-D. ZBP1 and TAK1: Master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis). Front. Cell. Infect. Microbiol. 2019, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Samir, P.; Malireddi, R.; Kanneganti, T.-D. The PANoptosome: A deadly protein complex driving pyroptosis, apoptosis, and necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Kanneganti, T.D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 2020, 297, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Kanneganti, T.-D. Newly Identified Function of Caspase-6 in ZBP1-mediated Innate Immune Responses, NLRP3 Inflammasome Activation, PANoptosis, and Host Defense. J. Cell. Immunol. 2020, 2, 341. [Google Scholar]
- Chen, K.W.; Demarco, B.; Heilig, R.; Shkarina, K.; Boettcher, A.; Farady, C.J.; Pelczar, P.; Broz, P. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP 3 inflammasome assembly. EMBO J. 2019, 38, e101638. [Google Scholar] [CrossRef]
- Taabazuing, C.Y.; Okondo, M.C.; Bachovchin, D.A. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem. Biol. 2017, 24, 507–514. [Google Scholar] [CrossRef]
- Laing, A.G.; Lorenc, A.; Del Barrio, I.D.M.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef]
- Moulian, N.; Truffault, F.; Gaudry-Talarmain, Y.M.; Serraf, A.; Berrih-Aknin, S. In vivo and in vitro apoptosis of human thymocytes are associated with nitrotyrosine formation. Blood J. Am. Soc. Hematol. 2001, 97, 3521–3530. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.S.; Lalonde, T.; Xu, S.; Liu, W.R. Learning from the past: Possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem 2020, 21, 730. [Google Scholar] [CrossRef]
- Chen, B.; Tian, E.-K.; He, B.; Tian, L.; Han, R.; Wang, S.; Xiang, Q.; Zhang, S.; El Arnaout, T.; Cheng, W. Overview of lethal human coronaviruses. Signal Transduct. Target. Ther. 2020, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Korteweg, C.; McNutt, M.A.; Gu, J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008, 133, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, C.; Li, X.; Song, Y.; Yao, X.; Wu, X.; Duan, Y.; Zhang, H.; Wang, Y.; Qian, Z. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020, 7, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Ibrahim, I.M.; Amin, F.G.; Magdy, M.; Elgharib, A.M.; Azzam, E.B.; Nasser, F.; Yousry, K.; Shamkh, I.M.; Mahdy, S.M. A Review of Human Coronaviruses’ Receptors: The Host-Cell Targets for the Crown Bearing Viruses. Molecules 2021, 26, 6455. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Fung, S.-Y.; Yuen, K.-S.; Ye, Z.-W.; Chan, C.-P.; Jin, D.-Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: Lessons from other pathogenic viruses. Emerg. Microbes Infect. 2020, 9, 558–570. [Google Scholar] [CrossRef]
- Chen, R.-F.; Chang, J.-C.; Yeh, W.-T.; Lee, C.-H.; Liu, J.-W.; Eng, H.-L.; Yang, K.D. Role of vascular cell adhesion molecules and leukocyte apoptosis in the lymphopenia and thrombocytopenia of patients with severe acute respiratory syndrome (SARS). Microbes Infect. 2006, 8, 122–127. [Google Scholar] [CrossRef]
- Garassino, M.C.; Ribas, A. At the crossroads: COVID-19 and immune-checkpoint blockade for cancer. Cancer Immunol. Res. 2021, 9, 261–264. [Google Scholar] [CrossRef]
- Tan, Y.-X.; Tan, T.H.; Lee, M.J.-R.; Tham, P.-Y.; Gunalan, V.; Druce, J.; Birch, C.; Catton, M.; Fu, N.Y.; Yu, V.C. Induction of apoptosis by the severe acute respiratory syndrome coronavirus 7a protein is dependent on its interaction with the Bcl-XL protein. J. Virol. 2007, 81, 6346–6355. [Google Scholar] [CrossRef]
- Chu, H.; Zhou, J.; Wong, B.H.-Y.; Li, C.; Chan, J.F.-W.; Cheng, Z.-S.; Yang, D.; Wang, D.; Lee, A.C.-Y.; Li, C. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 2016, 213, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.A.; Jha, B.K.; Silverman, R.H.; Hesselberth, J.R.; Barton, D.J. Ribonuclease L and metal-ion–independent endoribonuclease cleavage sites in host and viral RNAs. Nucleic Acids Res. 2014, 42, 5202–5216. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H. Viral encounters with 2′, 5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J. Virol. 2007, 81, 12720–12729. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zeqiraj, E.; Dong, B.; Jha, B.K.; Duffy, N.M.; Orlicky, S.; Thevakumaran, N.; Talukdar, M.; Pillon, M.C.; Ceccarelli, D.F. Dimeric structure of pseudokinase RNase L bound to 2-5A reveals a basis for interferon-induced antiviral activity. Mol. Cell 2014, 53, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Mbita, Z.; Hull, R.; Dlamini, Z. Human immunodeficiency virus-1 (HIV-1)-mediated apoptosis: New therapeutic targets. Viruses 2014, 6, 3181–3227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, L.; Jiang, D.; Wang, J.; Cong, X.; Fei, R. SARS-CoV nucleocapsid protein induced apoptosis of COS-1 mediated by the mitochondrial pathway. Artif. Cells Blood Substit. Biotechnol. 2007, 35, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, A.; Fang, Y.; Shu, T.; Wu, D.; Wang, C.; Huang, M.; Min, J.; Jin, L.; Zhou, W. SARS-CoV-2 Membrane Glycoprotein M Triggers Apoptosis with the Assistance of Nucleocapsid Protein N in Cells. Front. Cell. Infect. Microbiol. 2021, 11, 627. [Google Scholar] [CrossRef]
- Barhoumi, T.; Alghanem, B.; Shaibah, H.; Mansour, F.A.; Alamri, H.S.; Akiel, M.A.; Alroqi, F.; Boudjelal, M. SARS-CoV-2 Coronavirus Spike Protein-Induced Apoptosis, Inflammatory, and Oxidative Stress Responses in THP-1-Like-Macrophages: Potential Role of Angiotensin-Converting Enzyme Inhibitor (Perindopril). Front. Immunol. 2021, 12, 728896. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Maynes, M.; Natesampillai, S.; Shweta, F.; Badley, A.; Cummins, N. SARS-CoV-2 spike protein induces monocyte apoptosis and interleukin-8 production. Top. Antivir. Med. 2021, 29, 61. [Google Scholar]
- Ivanisenko, N.V.; Seyrek, K.; Kolchanov, N.A.; Ivanisenko, V.A.; Lavrik, I.N. The role of death domain proteins in host response upon SARS-CoV-2 infection: Modulation of programmed cell death and translational applications. Cell Death Discov. 2020, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Hillert, L.K.; Ivanisenko, N.V.; Busse, D.; Espe, J.; König, C.; Peltek, S.E.; Kolchanov, N.A.; Ivanisenko, V.A.; Lavrik, I.N. Dissecting DISC regulation via pharmacological targeting of caspase-8/c-FLIP L heterodimer. Cell Death Differ. 2020, 27, 2117–2130. [Google Scholar] [CrossRef]
- Seyrek, K.; Ivanisenko, N.V.; Richter, M.; Hillert, L.K.; König, C.; Lavrik, I.N. Controlling cell death through post-translational modifications of DED proteins. Trends Cell Biol. 2020, 30, 354–369. [Google Scholar] [CrossRef]
- Ma, Y.; Walsh, M.J.; Bernhardt, K.; Ashbaugh, C.W.; Trudeau, S.J.; Ashbaugh, I.Y.; Jiang, S.; Jiang, C.; Zhao, B.; Root, D.E. CRISPR/Cas9 screens reveal Epstein-Barr virus-transformed B cell host dependency factors. Cell Host Microbe 2017, 21, 580–591. [Google Scholar] [CrossRef]
- Safa, A.R.; Pollok, K.E. Targeting the anti-apoptotic protein c-FLIP for cancer therapy. Cancers 2011, 3, 1639–1671. [Google Scholar] [CrossRef]
- Fiore, A.; Ugel, S.; De Sanctis, F.; Sandri, S.; Fracasso, G.; Trovato, R.; Sartoris, S.; Solito, S.; Mandruzzato, S.; Vascotto, F. Induction of immunosuppressive functions and NF-κB by FLIP in monocytes. Nat. Commun. 2018, 9, 5193. [Google Scholar] [CrossRef] [PubMed]
- Thome, M.; Schneider, P.; Hofmann, K.; Fickenscher, H.; Meinl, E.; Neipel, F.; Mattmann, C.; Burns, K.; Bodmer, J.-L.; Schröter, M. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 1997, 386, 517–521. [Google Scholar] [CrossRef]
- Irmler, M.; Thome, M.; Hahne, M.; Schneider, P.; Hofmann, K.; Steiner, V.; Bodmer, J.-L.; Schröter, M.; Burns, K.; Mattmann, C. Inhibition of death receptor signals by cellular FLIP. Nature 1997, 388, 190–195. [Google Scholar] [CrossRef]
- Sadek, J.; Wuo, M.G.; Rooklin, D.; Hauenstein, A.; Hong, S.H.; Gautam, A.; Wu, H.; Zhang, Y.; Cesarman, E.; Arora, P.S. Modulation of virus-induced NF-κB signaling by NEMO coiled coil mimics. Nat. Commun. 2020, 11, 1786. [Google Scholar] [CrossRef]
- Golks, A.; Brenner, D.; Krammer, P.H.; Lavrik, I.N. The c-FLIP–NH2 terminus (p22-FLIP) induces NF-κB activation. J. Exp. Med. 2006, 203, 1295–1305. [Google Scholar] [CrossRef]
- Shisler, J.L. Viral and cellular FLICE-inhibitory proteins: A comparison of their roles in regulating intrinsic immune responses. J. Virol. 2014, 88, 6539–6541. [Google Scholar] [CrossRef][Green Version]
- Katayama, R.; Ishioka, T.; Takada, S.; Takada, R.; Fujita, N.; Tsuruo, T.; Naito, M. Modulation of Wnt signaling by the nuclear localization of cellular FLIP-L. J. Cell Sci. 2010, 123, 23–28. [Google Scholar] [CrossRef]
- Chang, D.W.; Xing, Z.; Pan, Y.; Algeciras-Schimnich, A.; Barnhart, B.C.; Yaish-Ohad, S.; Peter, M.E.; Yang, X. c-FLIPL is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002, 21, 3704–3714. [Google Scholar] [CrossRef]
- Fricker, N.; Beaudouin, J.; Richter, P.; Eils, R.; Krammer, P.H.; Lavrik, I.N. Model-based dissection of CD95 signaling dynamics reveals both a pro-and antiapoptotic role of c-FLIPL. J. Cell Biol. 2010, 190, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Musiu, C.; Caligola, S.; Fiore, A.; Lamolinara, A.; Frusteri, C.; Del Pizzo, F.D.; De Sanctis, F.; Canè, S.; Adamo, A.; Hofer, F. Fatal cytokine release syndrome by an aberrant FLIP/STAT3 axis. Cell Death Differ. 2021, 27, 6083–6094. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhao, H.; Jin, M.; Zhu, H.; Shan, B.; Geng, J.; Dziedzic, S.A.; Amin, P.; Mifflin, L.; Naito, M.G. Modulating TRADD to restore cellular homeostasis and inhibit apoptosis. Nature 2020, 587, 133–138. [Google Scholar] [CrossRef]
- Ben-Hail, D.; Begas-Shvartz, R.; Shalev, M.; Shteinfer-Kuzmine, A.; Gruzman, A.; Reina, S.; De Pinto, V.; Shoshan-Barmatz, V. Novel compounds targeting the mitochondrial protein VDAC1 inhibit apoptosis and protect against mitochondrial dysfunction. J. Biol. Chem. 2016, 291, 24986–25003. [Google Scholar] [CrossRef]
- Abu-Hamad, S.; Arbel, N.; Calo, D.; Arzoine, L.; Israelson, A.; Keinan, N.; Ben-Romano, R.; Friedman, O.; Shoshan-Barmatz, V. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J. Cell Sci. 2009, 122, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Ide, T.; Yanagida, T.; Tsujimoto, Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J. Biol. Chem. 2000, 275, 12321–12325. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ye, F.; Wu, A.; Yang, R.; Pan, M.; Sheng, J.; Zhu, W.; Mao, L.; Wang, M.; Xia, Z. Comparative transcriptome analysis reveals the intensive early stage responses of host cells to SARS-CoV-2 infection. Front. Microbiol. 2020, 11, 2881. [Google Scholar] [CrossRef]
- Miller, S.C.; Huang, R.; Sakamuru, S.; Shukla, S.J.; Attene-Ramos, M.S.; Shinn, P.; Van Leer, D.; Leister, W.; Austin, C.P.; Xia, M. Identification of known drugs that act as inhibitors of NF-κB signaling and their mechanism of action. Biochem. Pharmacol. 2010, 79, 1272–1280. [Google Scholar] [CrossRef]
- Mofers, A.; Selvaraju, K.; Gubat, J.; D’Arcy, P.; Linder, S. Identification of proteasome inhibitors using analysis of gene expression profiles. Eur. J. Pharmacol. 2020, 889, 173709. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, V.; Vaiyapuri, T.; Tergaonkar, V. Small molecule NF-κB pathway inhibitors in clinic. Int. J. Mol. Sci. 2020, 21, 5164. [Google Scholar] [CrossRef]
- Acerta Pharma, B. A Phase 2, Open Label, Randomized Study of the Efficacy and Safety of Acalabrutinib with Best Supportive Care versus Best Supportive Care in Subjects Hospitalized with COVID-19. 2020. Available online: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/ictrp-PER-018-20 (accessed on 1 October 2021).
- Roschewski, M.; Lionakis, M.S.; Sharman, J.P.; Roswarski, J.; Goy, A.; Monticelli, M.A.; Roshon, M.; Wrzesinski, S.H.; Desai, J.V.; Zarakas, M.A. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020, 5, eabd0110. [Google Scholar] [CrossRef]
- Agree, I. Karyopharm to Evaluate Low Dose Selinexor as a Potential Treatment for Hospitalized Patients with COVID-19. Available online: https://stockhouse.com/news/press-releases/2020/04/07/karyopharm-to-evaluate-low-dose-selinexor-as-a-potential-treatment-for (accessed on 2 October 2021).
- Cao, L.; Mu, W. Necrostatin-1 and necroptosis inhibition: Pathophysiology and therapeutic implications. Pharmacol. Res. 2020, 163, 105297. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, W.; Yan, Y.; Gong, T.; Han, J.; Tian, Z.; Zhou, R. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat. Immunol. 2014, 15, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lv, X.; Hu, B.; Shao, Z.; Wang, B.; Ma, K.; Lin, H.; Cui, M. RIPK1/RIPK3/MLKL-mediated necroptosis contributes to compression-induced rat nucleus pulposus cells death. Apoptosis 2017, 22, 626–638. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Soares, V.C.; de Azevedo-Quintanilha, I.G.; Dias, S.D.S.G.; Fintelman-Rodrigues, N.; Sacramento, C.Q.; Mattos, M.; de Freitas, C.S.; Temerozo, J.R.; Teixeira, L. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.-Y.; Zhu, Y.; Yang, J.; Xi, S.-S.; Wen, X.; Gu, Q.; Hu, W. Hypothermia protects neurons against ischemia/reperfusion-induced pyroptosis via m6A-mediated activation of PTEN and the PI3K/Akt/GSK-3β signaling pathway. Brain Res. Bull. 2020, 159, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Sengupta, S.; Kundu, R. Tiliacora racemosa leaves induce oxidative stress mediated DNA damage leading to G2/M phase arrest and apoptosis in cervical cancer cells SiHa. J. Ethnopharmacol. 2021, 269, 113686. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Bonam, S.R.; Muller, S.; Bayry, J.; Klionsky, D.J. Autophagy as an emerging target for COVID-19: Lessons from an old friend, chloroquine. Autophagy 2020, 16, 2260–2266. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef]
- Jaffe, S. Regulators split on antimalarials for COVID-19. Lancet 2020, 395, 1179. [Google Scholar] [CrossRef]
- Ferner, R.E.; Aronson, J.K. Chloroquine and hydroxychloroquine in covid-19. Br. Med. J. Publ. Group 2020, 369, m1432. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Al-Bari, M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017, 5, e00293. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, F.; Comunale, M.A.; Mehta, A.; Sehgal, M.; Jain, P.; Cuconati, A.; Lin, H.; Block, T.M.; Chang, J. Inhibition of endoplasmic reticulum-resident glucosidases impairs severe acute respiratory syndrome coronavirus and human coronavirus NL63 spike protein-mediated entry by altering the glycan processing of angiotensin I-converting enzyme 2. Antimicrob. Agents Chemother. 2015, 59, 206–216. [Google Scholar] [CrossRef]
- Katia, F.; Myriam, D.P.; Ucciferri, C.; Auricchio, A.; Di Nicola, M.; Marchioni, M.; Eleonora, C.; Emanuela, S.; Cipollone, F.; Vecchiet, J. Efficacy of canakinumab in mild or severe COVID-19 pneumonia. Immun. Inflamm. Dis. 2021, 9, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Tan, X. Current Strategies of Antiviral Drug Discovery for COVID-19. Front. Mol. Biosci. 2021, 8, 310. [Google Scholar] [CrossRef]
- Jordan, S.C.; Zakowski, P.; Tran, H.P.; Smith, E.A.; Gaultier, C.; Marks, G.; Zabner, R.; Lowenstein, H.; Oft, J.; Bluen, B. Compassionate use of tocilizumab for treatment of SARS-CoV-2 pneumonia. Clin. Infect. Dis. 2020, 71, 3168–3173. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Valoriani, B.; Barbieri, C.; Occhineri, S.; Mazzetti, P.; Vatteroni, M.L.; Suardi, L.R.; Riccardi, N.; Pistello, M. Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe covid-19 and role of variants of concern. Infect. Dis. Ther. 2021, 10, 2479–2488. [Google Scholar] [CrossRef]
- Chau, A.S.; Weber, A.G.; Maria, N.I.; Narain, S.; Liu, A.; Hajizadeh, N.; Malhotra, P.; Bloom, O.; Marder, G.; Kaplan, B. The longitudinal immune response to coronavirus disease 2019: Chasing the cytokine storm. Arthritis Rheumatol. 2021, 73, 23–35. [Google Scholar] [CrossRef]
- Huang, J.; Tao, G.; Liu, J.; Cai, J.; Huang, Z.; Chen, J.-X. Current prevention of COVID-19: Natural products and herbal medicine. Front. Pharmacol. 2020, 11, 588508. [Google Scholar] [CrossRef]
- Boozari, M.; Hosseinzadeh, H. Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother. Res. 2021, 35, 864–876. [Google Scholar] [CrossRef]
- Mohammadi Pour, P.; Fakhri, S.; Asgary, S.; Farzaei, M.H.; Echeverria, J. The signaling pathways, and therapeutic targets of antiviral agents: Focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Walls, A.C.; Tortorici, M.A.; Snijder, J.; Xiong, X.; Bosch, B.-J.; Rey, F.A.; Veesler, D. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc. Natl. Acad. Sci. USA 2017, 114, 11157–11162. [Google Scholar] [CrossRef]
- Cheng, P.W.; Ng, L.T.; Chiang, L.C.; Lin, C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin. Exp. Pharmacol. Physiol. 2006, 33, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-Y.; Wu, S.-L.; Chen, J.-C.; Li, C.-C.; Hsiang, C.-Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007, 74, 92–101. [Google Scholar] [CrossRef]
- Schwarz, S.; Wang, K.; Yu, W.; Sun, B.; Schwarz, W. Emodin inhibits current through SARS-associated coronavirus 3a protein. Antivir. Res. 2011, 90, 64–69. [Google Scholar] [CrossRef]
- Li, S.-Y.; Chen, C.; Zhang, H.-Q.; Guo, H.-Y.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.-N.; Yu, J.; Xiao, P.-G. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Jan, J.-T.; Ma, S.-H.; Kuo, C.-J.; Juan, H.-F.; Cheng, Y.-S.E.; Hsu, H.-H.; Huang, H.-C.; Wu, D.; Brik, A. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. USA 2004, 101, 10012–10017. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Jeon, S.; Jang, Y.; Gotina, L.; Won, J.; Ju, Y.H.; Kim, S.; Jang, M.W.; Won, W.; Park, M.G. Platycodin D, a natural component of Platycodon grandiflorum, prevents both lysosome-and TMPRSS2-driven SARS-CoV-2 infection by hindering membrane fusion. Exp. Mol. Med. 2021, 53, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Basharat, Z.; Yousuf, M.; Castaldo, G.; Rastrelli, L.; Khan, H. Virtual screening of natural products against type II transmembrane serine protease (TMPRSS2), the priming agent of coronavirus 2 (SARS-CoV-2). Molecules 2020, 25, 2271. [Google Scholar] [CrossRef] [PubMed]
- Chikhale, R.V.; Gupta, V.K.; Eldesoky, G.E.; Wabaidur, S.M.; Patil, S.A.; Islam, M.A. Identification of potential anti-TMPRSS2 natural products through homology modelling, virtual screening and molecular dynamics simulation studies. J. Biomol. Struct. Dyn. 2020, 37, 6660–6675. [Google Scholar] [CrossRef] [PubMed]
- Durai, P.; Batool, M.; Shah, M.; Choi, S. Middle East respiratory syndrome coronavirus: Transmission, virology and therapeutic targeting to aid in outbreak control. Exp. Mol. Med. 2015, 47, e181. [Google Scholar] [CrossRef]
- Ratia, K.; Kilianski, A.; Baez-Santos, Y.M.; Baker, S.C.; Mesecar, A. Structural basis for the ubiquitin-linkage specificity and deISGylating activity of SARS-CoV papain-like protease. PLoS Pathog. 2014, 10, e1004113. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Tsai, F.-J.; Tsai, C.-H.; Lai, C.-C.; Wan, L.; Ho, T.-Y.; Hsieh, C.-C.; Chao, P.-D.L. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005, 68, 36–42. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Park, S.-J.; Kim, Y.M.; Lee, J.-Y.; Seo, W.D.; Chang, J.S.; Park, K.H.; Rho, M.-C.; Lee, W.S. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg. Med. Chem. Lett. 2010, 20, 1873–1876. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Luo, C.; Liu, H.; Xu, W.; Chen, G.; Liew, O.W.; Zhu, W.; Puah, C.M.; Shen, X. Binding interaction of quercetin-3-β-galactoside and its synthetic derivatives with SARS-CoV 3CLpro: Structure–activity relationship studies reveal salient pharmacophore features. Bioorg. Med. Chem. 2006, 14, 8295–8306. [Google Scholar] [CrossRef] [PubMed]
- Enmozhi, S.K.; Raja, K.; Sebastine, I.; Joseph, J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach. J. Biomol. Struct. Dyn. 2021, 39, 3092–3098. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, J.H.; Kim, Y.M.; Jeong, H.J.; Kim, D.W.; Park, K.H.; Kwon, H.-J.; Park, S.-J.; Lee, W.S.; Ryu, Y.B. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 2012, 20, 5928–5935. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, S.-J.; Kim, D.; Park, K.H.; Lee, W.S.; Ryu, Y.B. Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol. Pharm. Bull. 2012, 35, b12-00623. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Gutti, G.; Singh, S. Viral Polymerases: Structures, Functions and Roles as Antiviral Drug Targets; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Allam, A.E.; Amen, Y.; Ashour, A.; Assaf, H.K.; Hassan, H.A.; Abdel-Rahman, I.M.; Sayed, A.M.; Shimizu, K. In silico study of natural compounds from sesame against COVID-19 by targeting M pro, PL pro and RdRp. RSC Adv. 2021, 11, 22398–22408. [Google Scholar] [CrossRef]
- Cho, J.K.; Curtis-Long, M.J.; Lee, K.H.; Kim, D.W.; Ryu, H.W.; Yuk, H.J.; Park, K.H. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg. Med. Chem. 2013, 21, 3051–3057. [Google Scholar] [CrossRef]
- Yu, M.-S.; Lee, J.; Lee, J.M.; Kim, Y.; Chin, Y.-W.; Jee, J.-G.; Keum, Y.-S.; Jeong, Y.-J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012, 22, 4049–4054. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Min, J.S.; Jeon, S.; Lee, J.; Kim, S.; Park, T.; Park, D.; Jang, M.S.; Park, C.M.; Song, J.H. Lycorine, a non-nucleoside RNA dependent RNA polymerase inhibitor, as potential treatment for emerging coronavirus infections. Phytomedicine 2021, 86, 153440. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Dwivedi, A.; Du Plessis, J. Sinigrin and its therapeutic benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-J.; Chao, T.-L.; Kao, H.-C.; Tsai, Y.-M.; Liu, Y.-K.; Wang, L.H.-C.; Hsieh, M.-C.; Chang, S.-Y.; Liang, P.-H. Kinetic characterization and inhibitor screening for the proteases leading to identification of drugs against SARS-CoV-2. Antimicrob. Agents Chemother. 2021, 65, e02577-20. [Google Scholar] [CrossRef]
- Fan, H.-H.; Wang, L.-Q.; Liu, W.-L.; An, X.-P.; Liu, Z.-D.; He, X.-Q.; Song, L.-H.; Tong, Y.-G. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin. Med. J. 2020, 133, 1051. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, H.; Watashi, K.; Saso, W.; Shionoya, K.; Iwanami, S.; Hirokawa, T.; Shirai, T.; Kanaya, S.; Ito, Y.; Kim, K.S. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. Iscience 2021, 24, 102367. [Google Scholar] [CrossRef]
- Dejani, N.N.; Elshabrawy, H.A.; Bezerra Filho, C.D.S.M.; de Sousa, D.P. Anticoronavirus and Immunomodulatory Phenolic Compounds: Opportunities and Pharmacotherapeutic Perspectives. Biomolecules 2021, 11, 1254. [Google Scholar] [CrossRef]
- Guler, H.I.; Fulya, A.; Zehra, C.; Yakup, K.; Belduz, A.O.; Canakci, S.; Kolayli, S. Targeting CoV-2 Spike RBD and ACE-2 Interaction with Flavonoids of Anatolian Propolis by in silico and in vitro Studies in terms of possible COVID-19 therapeutics. Turk J. Biol. 2021, 45, 530–548. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.-Y.; Kim, D.; Naguyen, T.T.H.; Park, S.-J.; Chang, J.S.; Park, K.H. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H. Anti–SARS-CoV-2 Natural Products as Potentially Therapeutic Agents. Front. Pharmacol. 2021, 12, 590509. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Li, S.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzym. Inhib. Med. Chem. 2021, 36, 497–503. [Google Scholar] [CrossRef]
- Chen, C.-N.; Lin, C.P.; Huang, K.-K.; Chen, W.-C.; Hsieh, H.-P.; Liang, P.-H.; Hsu, J.T.-A. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3, 3’-digallate (TF3). Evid.-Based Complement. Altern. Med. 2005, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Chen, Y.; Wang, Y.-C.; Wang, W.-J.; Yang, C.-S.; Tsai, C.-L.; Hou, M.-H.; Chen, H.-F.; Shen, Y.-C.; Hung, M.-C. Tannic acid suppresses SARS-CoV-2 as a dual inhibitor of the viral main protease and the cellular TMPRSS2 protease. Am. J. Cancer Res. 2020, 10, 4538. [Google Scholar]
- Jang, M.; Park, Y.-I.; Cha, Y.-E.; Park, R.; Namkoong, S.; Lee, J.I.; Park, J. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid.-Based Complement. Altern. Med. 2020, 2020, 5630838. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential use of polyphenols in the battle against COVID-19. Curr. Opin. Food Sci. 2020, 32, 149–155. [Google Scholar] [CrossRef]
- Park, J.Y.; Yuk, H.J.; Ryu, H.W.; Lim, S.H.; Kim, K.S.; Park, K. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 504–512. [Google Scholar] [CrossRef]
- Park, J.-Y.; Ko, J.-A.; Kim, D.W.; Kim, Y.M.; Kwon, H.-J.; Jeong, H.J.; Kim, C.Y.; Park, K.H.; Lee, W.S.; Ryu, Y.B. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzym. Inhib. Med. Chem. 2016, 31, 23–30. [Google Scholar] [CrossRef]
- Van de Sand, L.; Bormann, M.; Alt, M.; Schipper, L.; Heilingloh, C.S.; Steinmann, E.; Todt, D.; Dittmer, U.; Elsner, C.; Witzke, O. Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by Inhibiting the Viral Main Protease. Viruses 2021, 13, 609. [Google Scholar] [CrossRef]
- Wen, C.-C.; Kuo, Y.-H.; Jan, J.-T.; Liang, P.-H.; Wang, S.-Y.; Liu, H.-G.; Lee, C.-K.; Chang, S.-T.; Kuo, C.-J.; Lee, S.-S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Sayed, A.M.; Sharif, A.M.; Azhar, E.I.; Rateb, M.E. Olive-Derived Triterpenes Suppress SARS COV-2 Main Protease: A Promising Scaffold for Future Therapeutics. Molecules 2021, 26, 2654. [Google Scholar] [CrossRef]
- Sa-Ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Chaopreecha, J. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J. Nat. Prod. 2021, 84, 1261–1270. [Google Scholar] [CrossRef]
- Kanjanasirirat, P.; Suksatu, A.; Manopwisedjaroen, S.; Munyoo, B.; Tuchinda, P.; Jearawuttanakul, K.; Seemakhan, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Rangkasenee, N. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci. Rep. 2020, 10, 19963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Yap, Y.L. Old drugs as lead compounds for a new disease? Binding analysis of SARS coronavirus main proteinase with HIV, psychotic and parasite drugs. Bioorg. Med. Chem. 2004, 12, 2517–2521. [Google Scholar] [CrossRef]
- Guy, R.K.; DiPaola, R.S.; Romanelli, F.; Dutch, R.E. Rapid repurposing of drugs for COVID-19. Science 2020, 368, 829–830. [Google Scholar] [CrossRef] [PubMed]
- El Mazouri, S.; Aanniz, T.; Touhtouh, J.; Kandoussi, I.; Hakmi, M.; Belyamani, L.; Ibrahimi, A.; Ouadghiri, M. Anthraquinones: A Promising Multi-target Therapeutic Scaffold To Treat Covid-19. Int. J. Appl. Biol. Pharm. Technol. 2021, 12, 338–355. [Google Scholar] [CrossRef]
- Stewart, C.A.; Gay, C.M.; Ramkumar, K.; Cargill, K.R.; Cardnell, R.J.; Nilsson, M.B.; Heeke, S.; Park, E.M.; Kundu, S.T.; Diao, L. Lung cancer models reveal SARS-CoV-2-induced EMT contributes to COVID-19 pathophysiology. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhang, W.; Roehrl, M.W.; Roehrl, V.B.; Roehrl, M.H. An autoantigen profile of human A549 lung cells reveals viral and host etiologic molecular attributes of autoimmunity in COVID-19. J. Autoimmun. 2021, 120, 102644. [Google Scholar] [CrossRef]
- Liu, F.; Ma, J.; Shi, Z.; Zhang, Q.; Wang, H.; Li, D.; Song, Z.; Wang, C.; Jin, J.; Xu, J. Clerodane diterpenoids isolated from the leaves of Casearia graveolens. J. Nat. Prod. 2020, 83, 36–44. [Google Scholar] [CrossRef]
- Luo, W.; Sun, R.; Chen, X.; Li, J.; Jiang, J.; He, Y.; Shi, S.; Wen, H. ERK activation-mediated autophagy induction resists licochalcone A-induced anticancer activities in lung cancer cells in vitro. OncoTargets Ther. 2020, 13, 13437. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Q.; Feng, J.; Yan, L.; Sun, Y.; Liu, S.; Xiang, Y.; Zhang, M.; Pan, T.; Chen, X. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Target. Ther. 2020, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Boonjing, S.; Pothongsrisit, S.; Wattanathamsan, O.; Sritularak, B.; Pongrakhananon, V. Erianthridin induces non-small cell lung cancer cell apoptosis through the suppression of extracellular signal-regulated kinase activity. Planta Med. 2021, 87, 283–293. [Google Scholar] [CrossRef]
- Yang, J.; Cao, L.; Li, Y.; Liu, H.; Zhang, M.; Ma, H.; Wang, B.; Yuan, X.; Liu, Q. Gracillin isolated from Reineckia carnea induces apoptosis of A549 Cells via the mitochondrial pathway. Drug Des. Dev. Ther. 2021, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhang, W.; Li, T.; Jiang, L.; Lu, X.; Lin, J. Hispidulin exhibits potent anticancer activity in vitro and in vivo through activating ER stress in non-small-cell lung cancer cells. Oncol. Rep. 2020, 43, 1995–2003. [Google Scholar] [CrossRef]
- Sheng, H.; Lv, W.; Zhu, L.; Wang, L.; Wang, Z.; Han, J.; Hu, J. Liriopesides B induces apoptosis and cell cycle arrest in human non-small cell lung cancer cells. Int. J. Mol. Med. 2020, 46, 1039–1050. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).