Formation and Physical Stability of Zanthoxylum bungeanum Essential Oil Based Nanoemulsions Co-Stabilized with Tea Saponin and Synthetic Surfactant
Abstract
:1. Introduction
2. Results and Discussion
2.1. ZBEO Chemical Compositions
2.2. Preparation of ZBEO Nanoemulsions
2.2.1. Effect of Surfactant Type
2.2.2. Effect of Surfactant Concentration
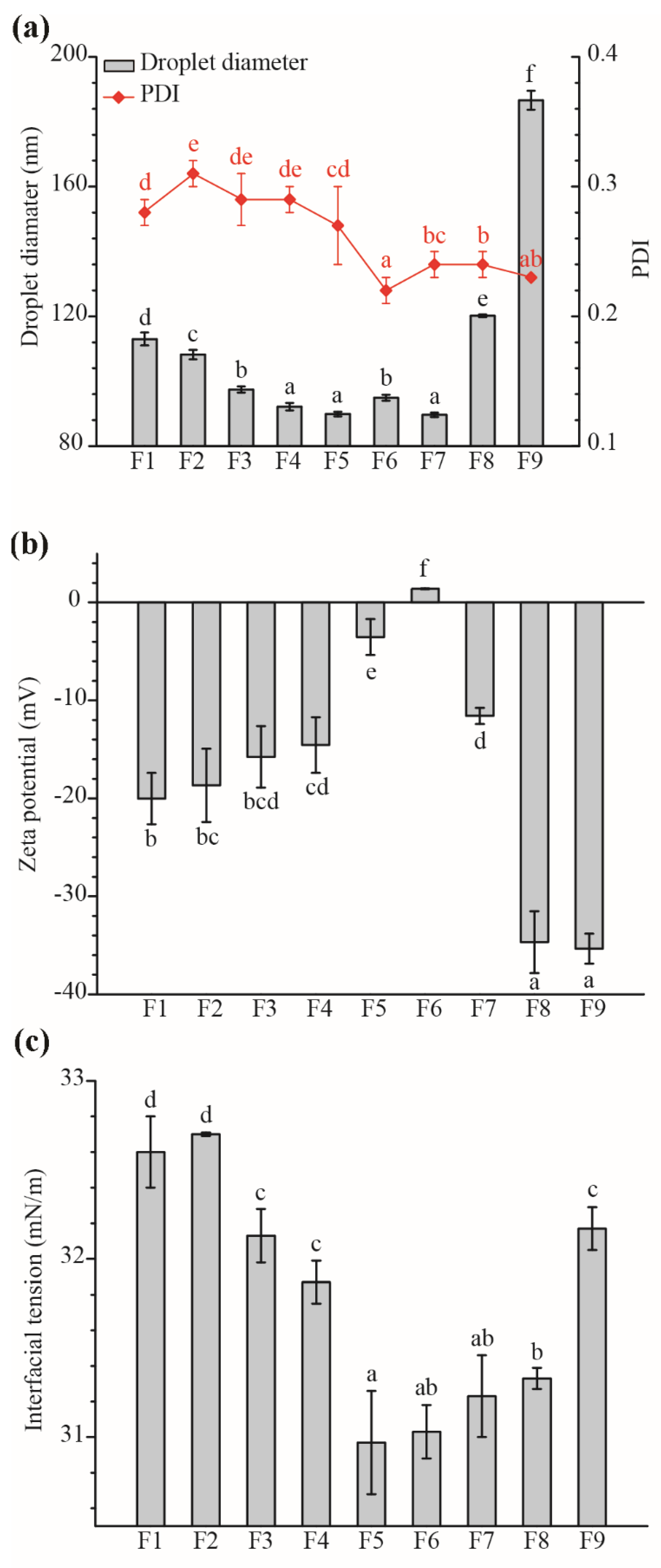
2.3. Influence of TS on The ZBEO Nanoemulsions Formation
2.4. TEM
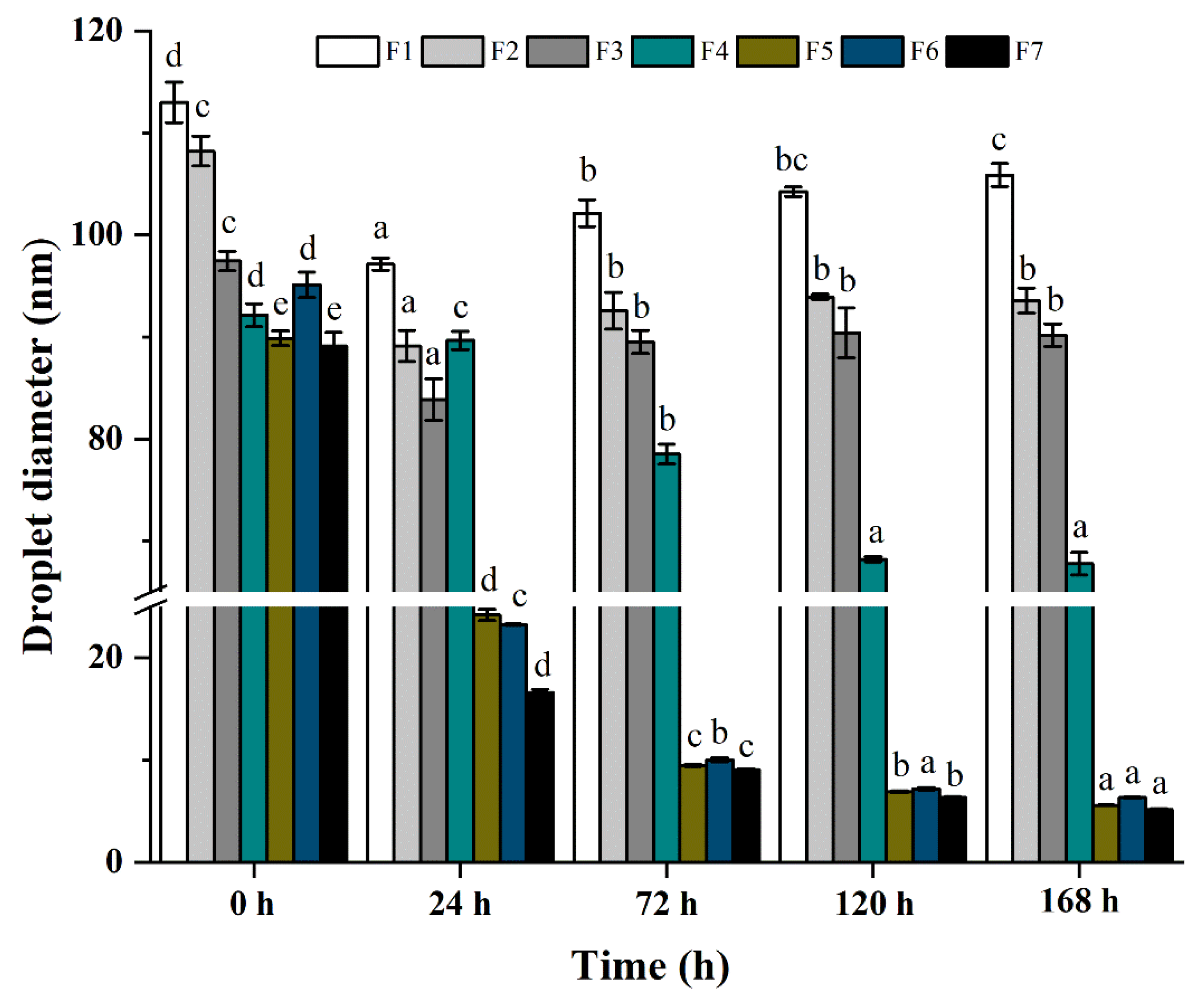
2.5. Influence of TS on The ZBEO Nanoemulsions Stability
3. Materials and Methods
3.1. ZBEO Chemical Compositions
3.2. GC-MS Analysis of ZBEO
3.3. Nanoemulsion Preparation
3.4. Droplet Size and Zeta Potential Determination
3.5. Interfacial Tension Measurement
3.6. Transmission Electron Microscopy (TEM)
3.7. Stability Evaluation
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface 2004, 108, 303–318. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; González, C.; Maestro, A.; Solè, I.; Pey, C.M.; Nolla, J. Nano-emulsions: New applications and optimization of their preparation. Curr. Opin. Colloid Interface Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- Ryu, V.; McClements, D.J.; Corradini, M.G.; McLandsborough, L. Effect of ripening inhibitor type on formation, stability, and antimicrobial activity of thyme oil nanoemulsion. Food Chem. 2018, 245, 104–111. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef]
- Cuomo, F.; Perugini, L.; Marconi, E.; Messia, M.C.; Lopez, F. Enhanced curcumin bioavailability through nonionic surfactant/caseinate mixed nanoemulsions. J. Food Sci. 2019, 84, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, L.; Zhang, W. Influence of ionic surfactants on the properties of nanoemulsions emulsified by nonionic surfactants Span 80/Tween 80. J. Disper. Sci. Technol. 2016, 37, 1511–1517. [Google Scholar] [CrossRef]
- Uluata, S.; Decker, E.A.; McClements, D.J. Optimization of nanoemulsion fabrication using microfluidization: role of surfactant concentration on formation and stability. Food Biophys. 2016, 11, 52–59. [Google Scholar] [CrossRef]
- Yao, M.; Li, Z.; Julian McClements, D.; Tang, Z.; Xiao, H. Design of nanoemulsion-based delivery systems to enhance intestinal lymphatic transport of lipophilic food bioactives: Influence of oil type. Food Chem. 2020, 317, 126229. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surface B 2019, 180, 127–140. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers-Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Enhancement of curcumin bioavailability by encapsulation in sophorolipid-coated nanoparticles: an in vitro and in vivo study. J. Agric. Food Chem. 2018, 66, 1488–1497. [Google Scholar] [CrossRef]
- Reichert, C.L.; Salminen, H.; Weiss, J. Quillaja saponin characteristics and functional properties. Annu. Rev. Food Sci. Technol. 2019, 10, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Tong, Z.; Dai, L.; Ma, P.; Zhang, M.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Novel colloidal particles and natural small molecular surfactants co-stabilized Pickering emulsions with hierarchical interfacial structure: Enhanced stability and controllable lipolysis. J. Colloid Interface Sci. 2020, 563, 291–307. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Devlieghere, F.; Dirckx, A.; Van der Meeren, P. Fabrication of Origanum compactum essential oil nanoemulsions stabilized using quillaja saponin biosurfactant. J. Food Process Pres. 2018, 42, e13668. [Google Scholar] [CrossRef]
- Riquelme, N.; Zúñiga, R.N.; Arancibia, C. Physical stability of nanoemulsions with emulsifier mixtures: Replacement of tween 80 with quillaja saponin. LWT-Food Sci. Technol. 2019, 111, 760–766. [Google Scholar] [CrossRef]
- Guo, N.; Tong, T.; Ren, N.; Tu, Y.; Li, B. Saponins from seeds of Genus Camellia: phytochemistry and bioactivity. Phytochemistry 2018, 149, 42–55. [Google Scholar] [CrossRef]
- Yang, X. Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef]
- Xia, L.; You, J.; Li, G.; Sun, Z.; Suo, Y. Compositional and antioxidant activity analysis of Zanthoxylum bungeanum seed oil obtained by supercritical CO2 fluid extraction. J. Am. Oil Chem. Soc. 2011, 88, 23–32. [Google Scholar] [CrossRef]
- Jing, N.; Wang, M.; Gao, M.; Zhong, Z.; Ma, Y.; Wei, A. Color sensory characteristics, nutritional components and antioxidant capacity of Zanthoxylum bungeanum Maxim. as affected by different drying methods. Ind. Crop. Prod. 2021, 160, 113167. [Google Scholar] [CrossRef]
- Ma, M.; Yuan, Y.; Yang, S.; Wang, Y.; Lv, Z. Fabrication and characterization of zein/tea saponin composite nanoparticles as delivery vehicles of lutein. LWT-Food Sci. Technol. 2020, 125, 109270. [Google Scholar] [CrossRef]
- Long, J.; Song, J.; Zhang, X.; Deng, M.; Xie, L.; Zhang, L.; Li, X. Tea saponins as natural stabilizers for the production of hesperidin nanosuspensions. Int. J. Pharmaceut. 2020, 583, 119406. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xiao, J.; Zhang, P.; Ma, M.; Wang, D.; Xu, Y. Development of pH-driven zein/tea saponin composite nanoparticles for encapsulation and oral delivery of curcumin. Food Chem. 2021, 364, 130401. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Stevens, C.V.; Claeys, M.; Van Der Meeren, P. Fundamental study on the salt tolerance of oregano essential oil-in-water nanoemulsions containing Tween 80. Langmuir 2019, 35, 10572–10581. [Google Scholar] [CrossRef]
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: Impact of oil compositions and processing parameters. Food Chem. 2019, 291, 199–206. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, L.; Li, T.; Zhou, X.; Wang, L.; Zhang, H.; Liu, L.; Li, Y.; Liu, Z.; Wang, H.; et al. Improved solvent-free microwave extraction of essential oil from dried Cuminum cyminum L. and Zanthoxylum bungeanum Maxim. J. Chromatogr. A 2006, 1102, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wu, Q.; Mao, Y.-q.; Wang, Q.; An, J.; Chen, Y.; Wang, W.; Zhao, B.; Liu, N.; Zhang, Y. Cytotoxicity and enhancement activity of essential oil from Zanthoxylum bungeanum Maxim. as a natural transdermal penetration enhancer. J. Zhejiang Univ. Sci. B 2014, 15, 153–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Guo, S.; You, C.; Geng, Z.; Liang, J.; Deng, Z.; Wang, C.; Du, S.; Wang, Y. Chemical composition of essential oils from Zanthoxylum bungeanum Maxim. and their bioactivities against Lasioderma serricorne. J. Oleo Sci. 2016, 65, 871–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adak, T.; Barik, N.; Patil, N.B.; Govindharaj, G.P.P.; Gadratagi, B.G.; Annamalai, M.; Mukherjee, A.K.; Rath, P.C. Nanoemulsion of eucalyptus oil: An alternative to synthetic pesticides against two major storage insects (Sitophilus oryzae (L.) and Tribolium castaneum (Herbst)) of rice. Ind. Crop. Prod. 2020, 143, 111849. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods: Influence of the oil phase, surfactant, and temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef]
- Lima, L.A.; Ferreira-Sá, P.S.; Garcia Jr, M.D.N.; Pereira, V.L.P.; Carvalho, J.C.T.; Rocha, L.; Fernandes, C.P.; Souto, R.N.P.; Araújo, R.S.; Botas, G.; et al. Nano-emulsions of the essential oil of Baccharis reticularia and its constituents as eco-friendly repellents against Tribolium castaneum. Ind. Crop. Prod. 2021, 162, 113282. [Google Scholar] [CrossRef]
- Feng, J.; Wang, R.; Chen, Z.; Zhang, S.; Yuan, S.; Cao, H.; Jafari, S.M.; Yang, W. Formulation optimization of D-limonene-loaded nanoemulsions as a natural and efficient biopesticide. Colloids Surface A 2020, 596, 124746. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Food-grade microemulsions and nanoemulsions: Role of oil phase composition on formation and stability. Food HydroColloids 2012, 29, 326–334. [Google Scholar] [CrossRef]
- Mayer, S.; Weiss, J.; McClements, D.J. Vitamin E-enriched nanoemulsions formed by emulsion phase inversion: Factors influencing droplet size and stability. J. Colloid Interface Sci. 2013, 402, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, P.; André, V.; Rieger, J.; Kühnle, A. Nano-emulsion formation by emulsion phase inversion. Colloids Surface A 2004, 251, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Saberi, A.H.; Fang, Y.; Mcclements, D.J. Fabrication of vitamin E-enriched nanoemulsions: Factors affecting particle size using spontaneous emulsification. J. Colloid Interface Sci. 2013, 391, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, O.; Sanromán, M.A.; Pazos, M. Surfactant-enhanced solubilization and simultaneous degradation of phenanthrene in marine sediment by electro-fenton treatment. Ind. Eng. Chem. Res. 2014, 53, 2917–2923. [Google Scholar] [CrossRef]
- Surassmo, S.; Min, S.; Bejrapha, P.; Choi, M. Effects of surfactants on the physical properties of capsicum oleoresin-loaded nanocapsules formulated through the emulsion–diffusion method. Food Res. Int. 2010, 43, 8–17. [Google Scholar] [CrossRef]
- Nazarzadeh, E.; Anthonypillai, T.; Sajjadi, S. On the growth mechanisms of nanoemulsions. J. Colloid Interface Sci. 2013, 397, 154–162. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Wagner, C. Theorie der Alterung von Niederschlägen durch Umlösen (Ostwald-Reifung). Z. Elektrochem. Ber. Bunsenges. Phys. Chem. 1961, 65, 581–591. [Google Scholar] [CrossRef]
- Bibette, J.; Leal-Calderon, F. Surfactant-stabilized emulsions. Curr. Opin. Colloid Interface 1996, 1, 746–751. [Google Scholar] [CrossRef]
- Wang, H.; Davis, R.H. Droplet growth due to brownian, gravitational, or thermocapillary motion and coalescence in dilute dispersions. J. Colloid Interface Sci. 1993, 159, 108–118. [Google Scholar] [CrossRef]
- Walstra, P. Principles of emulsion formation. Chem. Eng. Sci. 1993, 48, 333–349. [Google Scholar] [CrossRef]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-energy formation of edible nanoemulsions: Factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef]



| Compounds | Retention Time (min) | Peak Area | Relative Amount (%) |
|---|---|---|---|
| β-Pinene | 8.191 | 107,140,447.22 | 6.18 |
| D-Limonene | 8.987 | 232,870,450.99 | 13.42 |
| Eucalyptol | 9.062 | 29,745,305.23 | 1.71 |
| β-Ocimene | 9.113 | 30,355,650.51 | 1.75 |
| Linalool | 11.966 | 155,003,077.43 | 8.94 |
| Linalyl acetate | 12.282 | 195,163,631.66 | 11.25 |
| Germacrene D | 15.772 | 32,742,265.62 | 1.89 |
| Palmitelaidic acid | 23.190 | 48,072,287.94 | 2.77 |
| Palmitic Acid | 23.482 | 119,511,013.73 | 6.89 |
| 9,12-Octadecadienoic acid (Z,Z)- | 26.162 | 111,333,343.35 | 6.42 |
| Oleic Acid, (Z)- | 26.247 | 133,153,719.97 | 7.68 |
| β-Eudesmol | 31.397 | 55,400,844.42 | 4.43 |
| Sample | ZBEO (g) | Tween 40 (g) | TS (g) | Droplet Size (nm) | PDI |
|---|---|---|---|---|---|
| F1 | 0.25 | 0.25 | 0.5 | 112.99 ± 2.00 | 0.28 ± 0.01 |
| F2 | 0.25 | 0.25 | 0.25 | 108.21 ± 1.44 | 0.31 ± 0.01 |
| F3 | 0.25 | 0.25 | 0.1 | 97.46 ± 0.94 | 0.29 ± 0.02 |
| F4 | 0.25 | 0.25 | 0.05 | 92.15 ± 1.14 | 0.29 ± 0.01 |
| F5 | 0.25 | 0.25 | 0.01 | 89.88 ± 0.72 | 0.27 ± 0.03 |
| F6 | 0.25 | 0.25 | 0 | 94.90 ± 0.94 | 0.22 ± 0.01 |
| F7 | 0.25 | 0.20 | 0.05 | 89.63 ± 0.67 | 0.24 ± 0.01 |
| F8 | 0.25 | 0.15 | 0.10 | 120.17 ± 0.39 | 0.24 ± 0.01 |
| F9 | 0.25 | 0.10 | 0.15 | 186.65 ± 2.92 | 0.23 ± 0 |
| F10 | 0.25 | 0.05 | 0.20 | - | - |
| Sample | Ostwald Ripening Rate (nm3/s) | Correlation (r2) | Coalesence (nm−2/s) | Correlation (r2) |
|---|---|---|---|---|
| F1 | −51.96 | −0.30 | 2.01 × 10−8 | −0.33 |
| F2 | −185.46 | −0.05 | 3.29 × 10−7 | −0.15 |
| F3 | −44.93 | −0.28 | 6.36 × 10−8 | −0.32 |
| F4 | −438.45 | 0.20 | 2.69 × 10−6 | 0.92 |
| F5 | −373.73 | 0.21 | 7.83 × 10−4 | 0.98 |
| F6 | −385.91 | 0.90 | 6.28 × 10−4 | 0.99 |
| F7 | −361.13 | 0.19 | 9.00 × 10−4 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Liu, Y.; Yuan, Z.; Wang, Z. Formation and Physical Stability of Zanthoxylum bungeanum Essential Oil Based Nanoemulsions Co-Stabilized with Tea Saponin and Synthetic Surfactant. Molecules 2021, 26, 7464. https://doi.org/10.3390/molecules26247464
Zeng L, Liu Y, Yuan Z, Wang Z. Formation and Physical Stability of Zanthoxylum bungeanum Essential Oil Based Nanoemulsions Co-Stabilized with Tea Saponin and Synthetic Surfactant. Molecules. 2021; 26(24):7464. https://doi.org/10.3390/molecules26247464
Chicago/Turabian StyleZeng, Liya, Yongchang Liu, Zhihui Yuan, and Zhe Wang. 2021. "Formation and Physical Stability of Zanthoxylum bungeanum Essential Oil Based Nanoemulsions Co-Stabilized with Tea Saponin and Synthetic Surfactant" Molecules 26, no. 24: 7464. https://doi.org/10.3390/molecules26247464
APA StyleZeng, L., Liu, Y., Yuan, Z., & Wang, Z. (2021). Formation and Physical Stability of Zanthoxylum bungeanum Essential Oil Based Nanoemulsions Co-Stabilized with Tea Saponin and Synthetic Surfactant. Molecules, 26(24), 7464. https://doi.org/10.3390/molecules26247464





