Abstract
This study’s aim was to determine the pesticide residues in 10 different vegetable commodities from the Asir region, Saudi Arabia. We evaluated 211 vegetable samples, collected from supermarkets between March 2018 and September 2018, for a total of 80 different pesticides using ultrahigh-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) and gas chromatography–tandem mass spectrometry (GC-MS/MS) after extraction with a multi-residue method (the QuEChERS method). The results were assessed according to the maximum residue limit (MRL) provided by European regulations for each pesticide in each commodity. All lettuce, cauliflower, and carrot samples were found to be free from pesticide residues. A total of 145 samples (68.7%) contained detectable pesticide residues at or lower than MRLs, and 44 samples (20.9%) contained detectable pesticide residues above MRLs. MRL values were exceeded most often in chili pepper (14 samples) and cucumber (10 samples). Methomyl, imidacloprid, metalaxyl, and cyproconazole were the most frequently detected pesticides. Based on the results of this study, we recommend that a government-supported program for the monitoring of pesticide residues in vegetables be established to promote consumers’ health and achieve sustainable farming systems.
1. Introduction
Maintaining high agricultural output requires the use of pesticides, since, in high-input agricultural production systems, pests, among other crop invaders, including herbs and fungi, inevitably need to be managed []. However, reliance on pesticides is unsustainable due to their harmful effects on the environment and human health. The risk to human health comes from direct or indirect exposure to pesticide residues in primary or derived agricultural products []. Pesticides play a role in many human health problems, and can exert acute effects, such as dizziness, headaches, rashes, and nausea, and chronic effects, such as cancers, neurotoxicity, genotoxicity, birth defects, impaired fertility, and endocrine system disruption []. Children are particularly susceptible to exposure to pesticides []. Consequently, governments of different countries have enacted legislation in order to reduce consumer exposure to harmful pesticides, and regulate the appropriate use of pesticides in terms of the authorization that is granted, the type of registration (application rates and pre-harvest intervals), and allowing for free deliberation as to which products are to be treated with pesticides as long as the treatment complies with the established maximum residue limits (MRLs) []. For a specific pesticide applied to a certain food item, there is a tolerance level that, when exceeded, is called ‘violative residue’. Commonly, violation takes place when residues that exceed the established tolerance for a specific food item are detected. Tolerances may be not an accurate standard for health-related levels, but are at least suitable for the maximum residue limits that have been set for the use of pesticides by law []. Furthermore, violation rates do not consider the degree of consumption of various food items and the existing levels of pesticide residues [].
The detection of pesticide residues in vegetable commodities, for the purpose of optimally evaluating vegetables’ quality and mitigating potential risks to human health, is a predominant aim of pesticide research. The most common extraction procedure for a wide range of pesticide classes is the Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method. In this method, liquid–liquid extraction (LLE) with salting-out (MgSO4 and NaCl salts) is first performed, followed by a cleanup using primary secondary amine (PSA)-bonded silica with dispersive solid phase extraction (dSPE). This method was proposed for the extraction of pesticide residues from food commodities []. Gas chromatographic and Liquid chromatographic methods coupled with mass spectrometric detection (GC-MS/MS and LC-MS/MS, respectively) are among the most highly selective and sensitive instruments for determining the residues of pesticides in a variety of food commodities. They also allow for a simultaneous quantitative and qualitative analysis of the targeted analytes and have excellent separation efficiency and a high speed of analysis. Several multi-residue methods, and selective and sensitive detectors, for detecting different classes of pesticides with different chemical and physical properties and separating individual compounds have been proposed [,,]. There is a limited amount of information about the contamination of food, particularly vegetables, with pesticide residues in the Asir region, Saudi Arabia. There is no published literature on the contamination of vegetables with pesticide residues in Asir, which is of concern when taking into consideration the fact that vegetables are prone to being contaminated with higher pesticide levels when compared to other food groups []. Thus, the purpose of this study was to monitor pesticide residues in vegetables collected from supermarkets in the Asir region in order to establish a database that includes the levels of these residues in this region. We employed highly sensitive and selective multi-residue methods for the quantitative and qualitative determination of pesticides from several compound classes with different chemicals and physical properties using GC-MS/MS and LC-MS/MS. Then, we evaluated whether the results complied with existing regulations, particularly the European ones. Finally, we considered the appropriateness of the studied commodities for human consumption with respect to the official MRLs.
2. Results
2.1. Verification of the Analytical Method
The procedure for extracting multi-residue pesticides in vegetable samples was carried out using the rapid, sensitive, and rugged QuEChERS method. The method was validated under optimal conditions by investigating the recovery, precision, and detection limits. The recovery values at two fortification levels ranged from 70.5% to 126.6%, and the precision values (expressed as RSD, %) were below 20% for all of the investigated analytes (Table 1), which satisfies the criteria for quantitative methods for pesticide residues in food []. The limit of detection (LOD) and limit of quantitation (LOQ) were calculated by multiplying the standard deviation of repeatability by factors of 3 and 6, respectively []. All pesticide LOD (0.0004–0.0023 mg kg−1) and LOQ (0.0008–0.0047 mg kg−1) (Table 1) values were less than the maximum residue levels (MRLs) appointed for each analyte in each commodity. In this study, 80 pesticides from different chemical classes were deemed to be among those that are commonly used in vegetable production in Saudi Arabia. A total of 51 pesticides were analyzed by LC-MS/MS, and the remainder were analyzed by GC-MS/MS.

Table 1.
Recovery, precision, and detection limit ranges for selected pesticides that exceeded the maximum residue levels (MRLs) in different commodities.
2.2. Evaluation by Commodity
The concentrations of pesticide residues in 211 vegetable samples from the Asir region, southwest Saudi Arabia, were determined. Detectable residues were found in 145 samples (68.7%), while 66 samples (31.3%) were found to be residue-free. The percentage of detected residues was high for all analyzed vegetables except carrot, cauliflower, and lettuce. All samples of cucumber (100%) and chili pepper (100%) were contaminated with pesticide residues, while none of the carrot, cauliflower, and lettuce samples contained pesticide residues. Only 3.9% of tomato samples, 10% of cabbage samples, 15% of eggplant samples, 18.2% of potato samples, and 25% of onion samples were pesticide-free. Cucumber (100%), chili pepper (100%), tomato (96.1%), and cabbage (90%) had the highest percentage of detected residues (Table 2).

Table 2.
Frequency of samples with pesticide residues in the Asir region, Saudi Arabia from March 2018 to September 2018.
2.3. The Frequency of Detection and Exceedance of MRLs
Pesticide residue concentrations above the MRLs stipulated by EU regulations [] were detected in a total of 44 samples (20.9%). MRL values were surpassed most often in chili pepper and cucumber; 50% of the chili pepper samples and 41.7% of the cucumber samples were found to contain pesticide residue concentrations above the MRL values. Table 3 presents the frequency and ranges of the detectable residues in the tested commodities.

Table 3.
Pesticide concentration ranges, frequencies, and MRLs in the analyzed vegetable samples.
2.4. Evaluation by Pesticide Residue
In this study, the concentrations of 80 different pesticides were determined in 10 different vegetable commodities. Of the 80 pesticides, 37 were detected in the tested samples. Of the detected substances, 20 were insecticides (54.1%), 12 were fungicides (32.4%), 4 were herbicides (10.8%), and 1 was a growth regulator (2.7%). Thirty percent (30%) of the detected insecticides (6 of 20) exceeded the MRL, and the insecticide methomyl was found to most frequently exceed the MRL. Of the detected fungicides, 41.7% (5 of 12) exceeded the MRL, and the fungicide cyproconazole was found to most frequently exceed the MRL. Of all detected pesticides, methomyl, imidacloprid, metalaxyl, cyproconazole, carbendazim, triadimenol, profenofos, chlorpyrifos-methyl, malathion, and acetamiprid were found the most often. Figure 1 shows the detection frequency of the pesticides that frequently occurred in the analyzed samples.
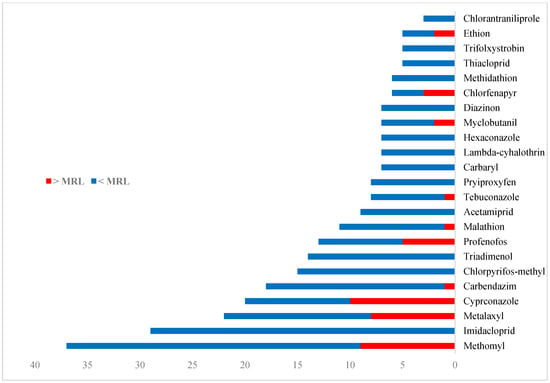
Figure 1.
Frequency of the most-often-detected pesticides in the analyzed samples.
As shown in Figure 1, methomyl was the most frequently detected pesticide in all tested commodities. Residues of methomyl were detected in tomato, chili pepper, cucumber, cabbage, onion, potato, and eggplant in the concentration range 0.005–0.307 mg kg−1 and exceeded the MRL in all of these commodities except for tomato and potato, which contained residues at or below the MRL values. Imidacloprid was the second most frequently detected pesticide in the vegetable commodities and was found in the concentration range 0.014–0.199 mg kg−1. Residues of imidacloprid were found in tomato, cucumber, cabbage, onion, eggplant, and potato; however, they did not exceed the MRLs in any of these commodities. Metalaxyl was detected in tomato, potato, cucumber, and chili pepper in the concentration range 0.007–0.419 mg kg−1, and exceeded the MRL values in only tomato and potato. Residues of cyproconazole and carbendazim were detected in cucumber, chili pepper, eggplant, and cabbage in the concentration range 0.008–0.541 mg kg−1 and 0.004–0.158 mg kg−1, respectively. Cyproconazole exceeded the MRLs in all four of these commodities, while carbendazim exceeded the MRLs only in cabbage (a concentration of 0.158 mg kg−1). Triadimenol and chlorpyrifos-methyl were found in cabbage, onion, potato, eggplant, chili pepper, and tomato in the concentration range 0.004–0.044 mg kg−1 and 0.004–0.061 mg kg−1, respectively. Profenofos exceeded the MRLs in cabbage and chili pepper with a concentration of 0.496 mg kg−1 and 0.041 mg kg−1, respectively. Profenofos was also detected in tomato; however, the concentration was within the MRL. Malathion and myclobutanil were detected in eggplant, cabbage, and cucumber in the concentration range 0.007–0.273 mg kg−1 and 0.010–0.470 mg kg−1, respectively. Myclobutanil exceeded the MRL in eggplant with a concentration of 0.470 mg kg−1 and in cucumber with a concentration of 0.436 mg kg−1. Malathion exceeded the MRL only in cucumber with a concentration of 0.273 mg kg−1. Chlorantraniliprole and tebuconazole exceeded the MRLs in potato with a concentration of 0.031 mg kg−1 and 0.039 mg kg−1, respectively. Chlorfenapyr exceeded the MRLs in cucumber and chili pepper with a concentration of 0.034 mg kg−1 and 0.026 mg kg−1, respectively. Ethion was detected only in chili pepper and exceeded the MRL with a concentration of 0.061 mg kg−1. Acetamiprid residues were found to fall within the MRL in tomato, chili pepper, and eggplant. Diazinon residues were found to fall within the MRL in chili pepper and cucumber. Additionally, measurable residues of hexaconazole were detected in tomato, chili pepper, cucumber, and potato. Of all detected pesticides, the highest concentration levels were found in chili pepper (0.541 mg kg−1, cyproconazole), cabbage (0.496 mg kg−1, profenofos), cucumber (0.436 mg kg−1, myclobutanil), tomato (0.419 mg kg−1, metalaxyl), and eggplant (0.307 mg kg−1, methomyl).
2.5. The Co-Occurrence of Pesticide Residues
The incidence of multiple residues in the tested commodities is shown in Figure 2. Of the tested commodities, 12.8% (27 samples) contained a single residue, 41.7% (88 samples) contained two residues, 10.4% (22 samples) contained three residues, and 3.79% (eight samples) contained four residues. The presence of multiple pesticide residues was observed most frequently in chili pepper, tomato, cucumber, potato, cabbage, and eggplant (Figure 3).
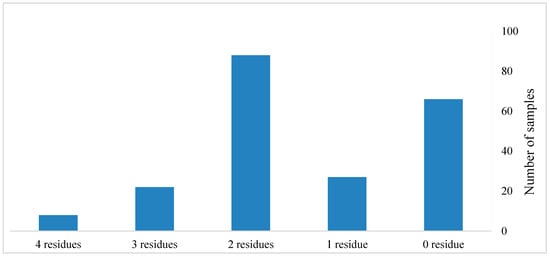
Figure 2.
The co-occurrence of pesticide residues in the tested samples.
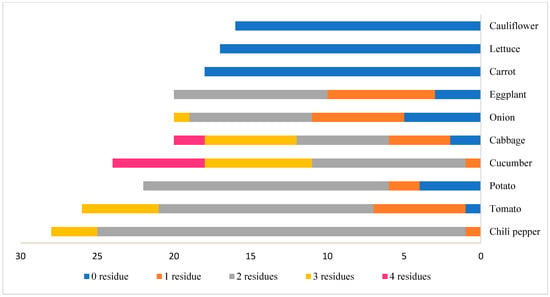
Figure 3.
The occurrence of multiple residues in different vegetables.
3. Discussion
This study, to our knowledge, is the first to monitor the concentration of 80 pesticide residues in different vegetable commodities from the southwest region of Saudi Arabia. Saudi Arabia’s southwest region is considered to be an important agricultural area due to its fertile ground, suitable climate, and torrential rain throughout the year. The three main agricultural areas in Saudi Arabia’s southwest region are located in Jizan, Baha, and Asir []. In this study, we tested 211 vegetable samples for pesticide residues. Of all tested samples, 66 samples (31.3%) were found to be residue-free, while 145 samples (68.7%) were found to contain a detectable amount of pesticide residue. Of the analyzed samples, 20.9% contained pesticide residues whose concentration exceeded the MRLs. Similarly, Osman et al. (2010) analyzed 160 vegetable samples collected from supermarkets in the Al-Qassim region, Saudi Arabia and found that 44.4% of the tested samples were free of pesticide residues, 55.6% contained detectable amounts of pesticide residues, and 59.6% (53 of 89) of the pesticide-contaminated samples had a residue concentration greater than the MRL values. Also, Jallow et al. (2017) analyzed 150 vegetable and fruit samples from Kuwait and found that 42% of the tested samples were residue-free, 58% contained a detectable amount of residue, and 21% contained pesticide residues whose concentration was greater than the MRL values. The incidence of pesticide residues in the tested vegetables may be due to vegetable crops being damaged by many pests and their various species [,] (Table 4); therefore, different pesticides are applied to protect these crops against pests and diseases, particularly vegetable crops that are cultivated under greenhouse conditions [,]. The humid conditions and large amount of food in greenhouse environments make them ideal habitats for pests and make crops in these environments more susceptible to pests such that successive applications of pesticide treatments are required to prevent considerable crop losses [,].

Table 4.
Common vegetable crop pests.
The highest concentrations of detected pesticides were recorded for the fungicide cyproconazole (in chili pepper), followed by the insecticide profenofos (in cabbage), the fungicide myclobutanil (in cucumber), the fungicide metalaxyl (in tomato), and the insecticide methomyl (in eggplant). The pesticide residue levels were found to vary among the vegetable types, and are greatly dependent on the harvest time, size of the fruit, and pesticide application mechanism [,,]. Cyproconazole most frequently exceeded the MRL values (10 samples), followed by methomyl (nine samples), metalaxyl (eight samples), profenofos (five samples), chlorfenapyr (three samples), myclobutanil and ethion (two samples), and malathion and chlorantraniliprole (one sample). MRLs are typically set by using a scientific risk assessment [] and dominate pesticide residue standards, which may differ from one country to another [] due to different agricultural and climatic conditions and directly reflect the pesticide application rate []. MRL exceedance may be due to GAP non-compliance, cross-contamination or spray drift, contamination from a previous use of persistent pesticides, and/or unexpectedly slow degradation of residues []. Cyproconazole is a broad-spectrum fungicide and acts as a sterol biosynthesis inhibitor (a demethylation inhibitor) in fungi. It has moderate mobility in soil (KFoc = 173–711 mL g−1), moderate to high persistence in soil (DT50 = 72.4–347 days), and high residue stability. Cyproconazole has moderate acute toxicity when inhaled and is very highly toxic to organic organisms. The FAO/WHO set the ADI to 0.02 mg/kg bw/day and the ARfD to 0.06 mg/kg bw with a safety factor (SF) of 100 [,]. Methomyl is an oxime carbamate and works by inhibiting acetylcholinesterase (AChE) enzymes. The overuse of methomyl may be due to its effectiveness as a contact and systemic broad-spectrum insecticide against organophosphorus-resistant pests and foliar treatment. It also has very high mobility in soil (KFoc = 13.3–42.8 mL/g), low to moderate persistence in soil (DT50 lab 20 °C = 4.6–11.5 days), high solubility in water, and high stability. However, it was classified by the EPA as a restricted-use pesticide (RUP) due to its high acute toxicity to humans. The European Food Safety Authority (EFSA) and FAO/WHO set the ADI, ARfD, and NOAEL of methomyl to 0.0025 mg/kg bw with a safety factor (SF) of 100 [,,]. In the present study, the MRL values were exceeded most often in chili pepper (14 samples), cucumber (10 samples), tomato (five samples), potato (five samples), cabbage (four samples), and eggplant (four samples). All of the tested commodities were cultivated in Saudi Arabia except for chili pepper, which was imported mainly from India. Among the tested samples, chili pepper was found to be the most highly contaminated commodity that exceeded the MRL. On May 2014, the ministry of agriculture in Saudi Arabia decided to ban the import of chili pepper from India after detecting a high level of pesticide residue in this commodity. Saudi Arabia lifted the ban after confirmation that exporters had complied with regulations on the permissible levels of pesticide residues in chili pepper. High levels of contamination with pesticide residues may be due to overuse of pesticides to control pests and/or farmers having a lack of awareness about pesticide application doses, mechanisms, and standard pre-harvest intervals (PHIs). Additionally, the non-availability of proper guidance about pesticides’ application, inadequate supervision by relevant departments, and non-compliance with best agricultural practices may lead to contaminated vegetables, which are considered to be a potential source of health hazards to consumers [,]. Household processing is needed to reduce the intake of pesticide residues. Washing, the most prevalent form of processing, can more effectively remove water-soluble pesticides than low-polarity materials. Peeling can also be used to reduce pesticide residue intake, particularly the intake of non-systemic pesticides that remain in the peel [,].
In terms of pesticide residues, some vegetables were found to contain more than one type of residue, particularly those vegetables that were cultivated under greenhouse conditions, which require consecutive applications of pesticides. In recent years, the decrease in pests’ susceptibility to pesticides has led to changes in the global chemical pesticide market and widespread use of mixtures, such as binary pesticide mixtures. Insufficient knowledge about the proper use of pesticides, a lack of awareness about integrated pest management (IPM) methods, and a desire to increase the attractiveness of a product may be additional reasons for the harmful co-occurrence of pesticide residues []. The occurrence of multiple residues does not entail non-compliance with MRL legislation if the individual pesticide concentrations do not exceed permissible limits. The existing law does not establish limits for those cases where pesticides co-occur. However, products with multiple pesticide residues should be evaluated carefully in order to be sure that a combination of pesticides was not used intentionally to circumvent MRL limits on single substances. The EFSA developed a software tool, called the Monte Carlo risk assessment (MCRA) tool, that is able to assess the cumulative risks arising from exposure to multiple pesticides []. From a toxicological viewpoint, if it has not been observed that the incidence of multiple residues could have additive or synergic effects, they may still affect the overall quality of the food. The quality index for residue (IqR) can be used to evaluate how multiple residues affect the quality of the commodity [,,]. The IqR is calculated as the sum of the ratios between the residue concentrations and the corresponding MRLs (Equation (1)):
This index considers the ratio of residue concentrations to the allowable limits in order to observe the degree of contamination as compared to the MRLs (see Figure 4). The Iqr divides the quality of fruit and vegetables into four groups: optimal (IqR = 0), good (IqR 0–0.6), adequate (IqR = 0.6–1), and inadequate (IqR > 1). The results presented in Table 5 show that 31.28%, 22.27%, and 15.17% of the tested samples were of optimal, good, and adequate quality, respectively, while 31.28% of the tested samples were of inadequate quality.
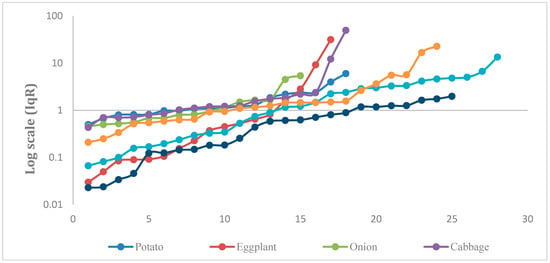
Figure 4.
The calculated quality index for residue (IqR) for the selected vegetable commodities on a Log scale.

Table 5.
The quality of the selected vegetables according to the calculated IqR.
The excessive use of pesticides in Saudi agriculture, particularly in greenhouse crop production, is a serious problem. Precedence should be given to improving strategies for the reduction of pesticides in agriculture through tighter government regulations, including the implementation of laws in relation to pesticide use, the control of pesticide sales, adherence to pesticide label instructions, the application of appropriate pre-harvest intervals, compliance with integrated pest management approaches, and best agricultural practices [,]. Organic farming may be an effective and safe way to reduce excessive pesticide use. In April 2005, Saudi Arabia started an organic farming project in cooperation with the Research Institute of Organic Agriculture (FiBL) and the German Society for International Cooperation (GIZ). The project’s aim was to develop a functioning and sustainable organic farming sector. According to the GIZ report, the southwest region is a reduced organic surface region []. Therefore, the Saudi organic farming association (SOFA) should implement programs that help farmers convert to organic farming, which is a holistic and environmentally friendly agricultural production system.
4. Materials and Methods
4.1. Chemicals and Reagents
Pesticide active ingredients were obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany) with certified purities greater than 95%. The monitored pesticides, their classification [,], and technical data for the LC-MS/MS pesticides and the GC-MS/MS pesticides are listed in Table 6 and Table 7, respectively. As shown in Figure 5, the set of selected pesticides includes most insecticides. As the standards have different purities, the concentration was corrected individually for each one. Methanol and acetonitrile (pesticide-grade) were obtained from Fischer company, Dallas, TX, USA. Ultra-pure deionized water (18 MΩ cm) was obtained from a water purification system (PURELAB Option-R, ELGA, BUCKS, UK). Magnesium sulfate (MgSO4), sodium chloride (NaCl), Sodium Citrate, disodium citrate sesquihydrate, PSA, and graphite carbon black (GCB) were obtained from Agilent (Santa Clara, CA, USA).

Table 6.
Summary of LC-MS/MS pesticides (properties and use).

Table 7.
Summary of GC-MS/MS pesticides (properties and use).
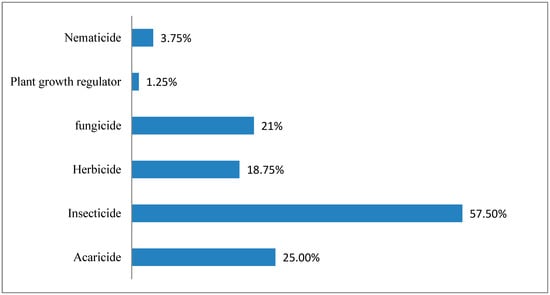
Figure 5.
Distribution of selected pesticides according to usage.
4.2. Preparation of Intermediate, Working Solutions, and Calibration Curves
By dissolving a corrected weight of each compound (according to its purity) into 10 mL of acetonitrile, standard stock solutions were prepared at 1000 mg kg−1. An intermediate mix of standards with a concentration of 5 mg L−1 was then prepared. Lastly, the working standard solutions were used to prepare matrix-matched calibrations between 2.5 and 200 μg L−1.
4.3. Sample Collection
According to the 2002/63/EC [] regulation, a total of 211 different vegetable samples covering 10 commodities that are frequently consumed by local people (tomato, cucumber, cabbage, eggplant, chili pepper, onion, potato, carrot, lettuce, and cauliflower) were collected from supermarkets in Asir, Saudi Arabia in the period from March 2018 to September 2018. These samples were transported under cold conditions to the laboratory and kept at 4 °C. Shortly after their arrival, they were analyzed for pesticide residues following the QuEChERS method described below.
4.4. LC-MS/MS Analysis
LC-MS/MS analysis was conducted using a liquid chromatograph (Thermo ultimate 3000, Dionex Softron GmbH, Rohrbach, Germany) combined with a triple quadruple mass detector with a heated electrospray ionization (HESI) source (Thermo, TSQ Quantum Access Max, San Jose, CA, USA) and a Thermo Scientific Hypersil GOLD aQ column (100 × 2.1 mm; 1.9 μm particles). Time-specific SRM (t-SRM) windows were used at the target compound’s retention time to maximize the performance of the mass spectrometer. The sheath gas flow rate was 55 units, the AUX gas flow rate was 15 units, the capillary temperature and the heater temperature were 280 °C and 295 °C, respectively, the spray voltage was 3500 V, and the cycle time was 0.2 s. Water containing 0.1% formic acid and 4 mM ammonium formate (mobile phase A) and methanol containing 0.1% formic acid and 4 mM ammonium formate (mobile phase B) were used for the gradient program, which started with 2% B and sharply increased to 30% B over 0.25 min, then linearly increased to 100% B over 19.75 min, and finally maintained 100% B for 6 min. The column was then reconditioned to 2% B for 4 min. The column’s temperature was set at 40 °C. The injection volume was 10 μL at a flow rate of 0.3 mL/min. At least two multi-reaction monitoring (MRM) transitions were monitored for each compound.
4.5. GC-MS/MS Analysis
All samples were analyzed using a TSQ Quantum XLS GC-MS/MS system equipped with a Thermo Scientific TRACE GC Ultra gas chromatograph with a programmable split/splitless injector. The capillary column was a Thermo Scientific TRACE TR-Pesticide II (30 m × 0.25 mm × 0.25 µm) with a 5 m guard column. Sample volumes of 1.0 μL were injected in split/splitless injection mode, and a deactivated fused-silica liner with a diameter of 2 mm was used. The temperature of the injection port was set at 240 °C (isothermal). A constant velocity of 1 mL/min was used for the helium carrier gas. The oven temperature program was initially set to hold at 80 °C for 1 min, then ramp with no hold to 140 °C at 25 °C/min, and finally ramp to 200 °C with no hold at 5 °C/min. The oven program’s total length was 39 min with an injection-to-injection time of 10 min. The transfer line and the ion source of the mass spectrometer were heated to 280 °C. A higher-level standard was used to optimize transitions in the positive electron ionization (EI)-SRM mode on the TSQ Quantum XLS GC-MS/MS. The t-SRM function tool allows one to monitor SRM transitions more effectively by monitoring only the analyzed compounds at specific elution times, allowing for partial overlap. The collision gas (Argon) pressure was 1.2 mTorr, and the Q1/Q3 resolution was 0.7 u (full width at half maximum (FWHM)). Electron ionization was set at −70 eV and the emission current was 30 µA.
4.6. Extraction Procedure
The acetate-buffered QuEChERS method was applied to determine the concentration of pesticides in the vegetable samples (AOAC 133 Official Method 2007.01) []. Homogenization for more than 1 min was carried out using a blender (Waring, DCA, Torrington, CT, USA) to obtain thoroughly mixed homogenates. A 15 g portion of the homogenized sample was weighed in a 50 mL PTFE tube and 15 mL of acetonitrile containing 1% acetic acid was added. Then, 6 g of MgSO4 and 2.5 g of sodium acetate trihydrate were added and the sample was shaken for 4 min. The sample was then centrifuged at 4000 rpm for 5 min (Eppendorf 5804 R, Hamburg, Germany) and 5 mL of the supernatant was transferred to a 15 mL PTFE tube containing 750 mg MgSO4 and 250 mg PSA. Furthermore, graphitized carbon was used to clean up the chili pepper (10). The extract was shaken for 20 s using a vortex mixer and then centrifuged for 5 min at 4000 rpm. Approximately 3 mL of the supernatant was filtered through a 0.45 μm PTFE filter (13 mm in diameter).
4.7. Quality Control
Recovery tests were done using blank samples that were free from pesticide. Subsamples of those blanks from the different studied commodities were spiked with two levels (0.010 and 0.1 mg kg−1) of each compound. Then, they were extracted in accordance with the above-described QuEChERS procedure. Recovery and precision (expressed as RSD, %) were measured by analyzing three samples of each commodity individually.
5. Conclusion
This study presented evidence of the incidence of pesticide residues in vegetable commodities from the southwest region of Saudi Arabia. The most highly contaminated commodities were found to be chili pepper and cucumber. Methomyl, imidacloprid, metalaxyl, and cyproconazole were the most frequently detected pesticide residues in the tested commodities. The high observed levels of pesticide residues may represent a potential health risk for consumers. As most of these vegetables are consumed raw, household processing, including washing, peeling, and cooking, is necessary in order to reduce the amount of pesticide residues in them. Based on our findings, we recommend that pesticide residues in a greater number of crops be regularly monitored over long periods in order to better protect consumers’ health.
Author Contributions
M.F.A.R.: Ideas; formulation and evolution of overarching research goals and aims. M.M.A.A.-H.: Performing the experiments, instrumentation and data interpretation, application of statistical and computational techniques to analyze and study data. M.M.F.A.: Application of statistical, mathematical, computational, and other formal techniques to analyze or synthesize study data, or data/evidence collection. H.A.A.: Management activities to annotate (produce metadata), scrub data and maintain research data. M.A.A.: Conducting a research and investigation process. Provision of study materials, laboratory samples. A.A.S.: Application of statistical, mathematical, computational, and other formal techniques to analyze or synthesize study data. H.A.S.: Development and design of methodology; creation of models. N.S.A.: Acquisition of the financial support for the project leading to this publication. Management and coordination responsibility for the research activity planning and execution. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research Center for Advanced Materials (RCAMS) at King Khalid University, grant number RCAMS/KKU/006‑19.
Acknowledgments
The authors thank the Research Center for Advanced Materials (RCAMS) at King Khalid University for supporting this work through the research project program under grant number RCAMS/KKU/006‑19.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| UHPLC-MS/MS (LC-MS/MS) | ultrahigh-performance liquid chromatography-tandem mass spectrometry |
| GC-MS/MS | gas chromatography-tandem mass spectrometry |
| QuEChERS | quick, easy, cheap, effective, rugged, safe |
| MRL | maximum residue limit |
| LLE | liquid–liquid extraction |
| dSPE | dispersive solid phase extraction |
| PSA | primary secondary amine |
| GCB | graphite carbon black |
| t-SRM | time-specific selected reaction monitoring |
| MRM | multi-reaction monitoring |
| FWHM | full width at half maximum |
| PTFE | polytetrafluoroethylene |
| LOD | limit of detection |
| LOQ | limit of quantification |
| RSD | relative standard deviation |
| GAP | good agricultural practice |
| KFoc | Freundlich organic carbon adsorption coefficient |
| DT50 | time required for 50% disappearance |
| FAO | food and agriculture organization |
| WHO | World Health Organization |
| ADI | acceptable daily intake |
| ARfD | acute reference dose |
| AChE | Acetylcholinesterase |
| EPA | environmental protection agency |
| EFSA | European food safety agency |
| NOAEL | non-observable adverse effect level |
| PHI | pre-harvest interval |
| IPM | integrated pest management |
| MCRA | Monte Carlo risk assessment |
| IqR | residue quality index |
| FiBL | research institute of organic agriculture |
| GIZ | German society for international co-operation |
| SOFA | Saudi organic farming association |
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Jeyaratnam, J. Acute pesticide poisoning: A major global health problem. World Health Stat. Q. Rapp. Trimest. Stat. Sanit. Mond. 1990, 43, 139–144. [Google Scholar]
- Alavanja, M.C.R.; Matthew, K.R.; Matthew, R.B. Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA Cancer J. Clin. 2013, 63, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Jallow, M.F.A.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Thomas, B.M. Monitoring of pesticide residues in commonly used fruits and vegetables in Kuwait. Int. J. Environ. Res. Public Health 2017, 14, 833. [Google Scholar] [CrossRef]
- Winter, C.K. Pesticide tolerances and their relevance as safety standards. Reg. Toxicol. Pharmacol. 1992, 15, 137–150. [Google Scholar] [CrossRef]
- Katz, J.M.; Winter, C.K. Comparison of pesticide exposure from consumption of domestic and imported fruits and vegetables. Food Chem. Toxicol. 2009, 47, 335–338. [Google Scholar] [CrossRef]
- Lehotay, S.J. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Efficiency of QuEChERS approach for determining 52 pesticide residues in honey and honey bees. MethodsX 2016, 3, 452–458. [Google Scholar] [CrossRef]
- Adalberto, F.M.; Dos Santos, F.N.; Pereira, P.A. Development, validation and application of a methodology based on solid-phase micro extraction followed by gas chromatography coupled to mass spectrometry (SPME/GC-MS) for the determination of pesticide residues in mangoes. Talanta 2010, 81, 346–354. [Google Scholar]
- Tankiewicz, M. Determination of selected priority pesticides in high water fruits and vegetables by modified QuEChERS and GC-ECD with GC-MS/MS confirmation. Molecules 2019, 24, 417. [Google Scholar] [CrossRef]
- Bargańska, Z.; Konieczka, P.; Namieśnik, J. Comparison of two methods for the determination of selected pesticides in honey and honeybee samples. Molecules 2018, 23, 2582. [Google Scholar] [CrossRef]
- Chen, C.; Qian, Y.; Chen, Q.; Tao, C.; Li, C.; Li, Y. Evaluation of pesticide residues in fruits and vegetables from Xiamen, China. Food Control 2011, 22, 1114–1120. [Google Scholar] [CrossRef]
- Directorate General Health and Food Safety (DG SANTE). Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed.SANTE/11813/2017; Directorate General Health and Food Safety: Luxembourg, 2017. [Google Scholar]
- Hovind, H.; Magnusson, B.; Krysell, M.; Lund, U.; Mäkinen, I. Internal quality control—Handbook for chemical laboratories. NT TR 2012, 569, 04038. [Google Scholar]
- European Commission-EU Pesticides Database-Pesticides MRL According to Regulation (EC) No 396/2005. Available online: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=pesticide.residue.selection&language=EN (accessed on 1 May 2018).
- AQUASTAT—FAO’s Information System on Water and Agriculture. Available online: http://www.fao.org/nr/water/aquastat/countries_regions/SAU/ (accessed on 15 July 2018).
- Osman, K.A.; Al-Humaid, A.M.; Al-Rehiayani, S.M.; Al-Redhaiman, K.N. Monitoring of pesticide residues in vegetables marketed in Al-Qassim region, Saudi Arabia. Ecotoxicol. Environ. Saf. 2010, 73, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Rinehold, J.; Bell, N.; Waters, T. Common Pests of Vegetable Crops. Available online: https://pnwhandbooks.org/insect/vegetable/vegetable-pests/common-vegetable (accessed on 15 July 2018).
- Paradjikovic, N.; Hrlec, G.; Horvat, D. Residues of vinclozolin and procymidone after treatment of greenhouse grown lettuce, tomato and cucumber. Acta Agric. Scand. Sect. B Soil Plant Sci. 2004, 54, 241–248. [Google Scholar] [CrossRef]
- Li, W.; Tai, L.; Liu, J.; Gai, Z.; Ding, G. Monitoring of pesticide residues levels in fresh vegetable form Heibei Province, North China. Environ. Monit. Assess. 2014, 186, 6341–6349. [Google Scholar] [CrossRef]
- Qin, G.; Zou, K.; Li, Y.; Chen, Y.; He, F.; Ding, G. Pesticide residue determination in vegetables from Western China applying gas chromatography with mass spectrometry. Biomed. Chromatogr. 2016, 30, 1430–1440. [Google Scholar] [CrossRef]
- Lozowicka, B.; Abzeitova, E.; Sagitov, A.; Kaczynski, P.; Toleubayev, K.; Li, A. Studies of Pesticide residues in tomatoes and cucumbers from Kazakhstan and the associated health risks. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Cancho-Grande, B.; Rial-Otero, R.; Simal-Gándara, J. The dissipation rates of cyprodinil, fludioxonil, procymidone and vinclozoline during storage of grape juice. Food Control 2006, 17, 1012–1017. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.M.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. Determination of 23 pesticide residues in leafy vegetables using gas chromatography-ion trap mass spectrometry and analyte protectants. J. Chromatogr. A 2008, 1196, 100–109. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Wang, Z.; Pan, C.; Jin, R. Residue dynamics of procymidone in leeks and soil in greenhouses by smoke generator application. Ecotoxicol. Environ. Saf. 2009, 73, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.M.; Pico, Y.; Manes, J. Determination of pesticide residues in fruit and vegetables. J. Chromatogr A 1996, 754, 301–331. [Google Scholar] [CrossRef]
- Abelson, P.H. Risk assessments of low-level exposures. Science 1994, 265, 1507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.; Wang, Z.; Zhang, Y.; Wang, J.; Guo, R. Pesticide residues in market foods in Shaanxi Province of China in 2010. Food Chem. 2013, 138, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). National summary reports on pesticide residue analysis performed in 2016. Efsa Support. Publ. 2018. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance cyproconazole. EFSA J. 2010, 8, 1897. [Google Scholar] [CrossRef]
- FAO/WHO. Pesticide Residues in Food 2010—Joint FAO/WHO Meeting on Pesticide Residues; FAO/WHO: Rome, Italy, 2010. [Google Scholar]
- European Food Safety Authority (EFSA). Peer review of the pesticide risk assessment of the active substance methomyl. EFSA Sci. Rep. 2008, 222, 1–99. [Google Scholar]
- FAO/WHO. JMPR Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group. In Pesticide Residues in Food—2001; Food and Agriculture Organization: Geneva, Switzerland, 2001; Volume 167, pp. 20–29. [Google Scholar]
- US EPA, OCSPP, OPP, Methomyl. Available online: https://www.epa.gov/ingredients-used-pesticide-products/methomyl (accessed on 15 June 2018).
- Yu, Y.; Hu, S.; Yang, Y.; Zhao, X.; Xue, J.; Zhang, J.; Gao, S.; Yang, A. Successive monitoring surveys of selected banned and restricted pesticide residues in vegetables from the northwest region of China from 2011 to 2013. BMC Public Health 2017, 18, 91. [Google Scholar] [CrossRef]
- Li, Z.; Nie, J.; Yan, Z.; Cheng, Y.; Lan, F.; Huang, Y.; Chen, Q.; Zhao, X.; Li, A. A monitoring survey and dietary risk assessment for pesticide residues on peaches in China. Regul. Toxicol. Pharmacol. 2018, 97, 152–162. [Google Scholar] [CrossRef]
- Hassanzadeh, N.; Bahramifar, N.; Esmaili-Sari, A. Residue content of carbaryl applied on greenhouse cucumbers and its reduction by duration of a pre-harvest interval and post-harvest household processing. J. Sci. Food Agric. 2010, 90, 2249–2253. [Google Scholar] [CrossRef]
- Saeedi Saravi, S.S.; Shokrzadeh, M. Effects of washing, peeling, storage, and fermentation on residue contents of carbaryl and mancozeb in cucumbers grown in greenhouses. Toxicol. Ind. Health 2016, 32, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Latif, Y.; Sherazi, S.T.H.; Bhanger, M.I. Assessment of pesticide residues in commonly used vegetables in Hyderabad, Pakistan. Ecotoxicol. Environ. Saf. 2011, 74, 2299–2303. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Pesticides-Breakthrough on Cumulative Risk Assessment|European. Available online: https://www.efsa.europa.eu/en/press/news/160127 (accessed on 27 June 2017).
- Arienzo, M.; Cataldo, D.; Ferrara, L. Pesticide residues in fresh-cut vegetables from integrated pest management by ultra performance liquid chromatography coupled to tandem mass spectrometry. Food Control 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Lorenzin, M. Un nuovo parametro per una valutazione della qualità degli alimenti: Indice di qualità per i Residui (IqR). Sci. Degli. Aliment. 1998, 27, 175–180. [Google Scholar]
- Mac Loughlin, T.M.; Peluso, L.; Etchegoyen, A.; Alonso, L.L.; Castro, C.; Percudani, C.; Marino, J.G. Pesticide residues in fruits and vegetables of the argentine domestic market: Occurrence and quality. Food Control 2018, 93, 129–138. [Google Scholar] [CrossRef]
- Chowdhury, M.A.Z.; Fakhruddin, A.N.M.; Islam, N.; Moniruzzaman, M.; Gan, S.H.; Alam, K. Detection of the residues of nineteen pesticides in fresh vegetable samples using gas chromatography–mass spectrometry. Food Control 2013, 34, 457–465. [Google Scholar] [CrossRef]
- Bakirci, G.T.; Acay, D.B.Y.; Bakirci, F.; Otles, S. Pesticide residues in fruits and vegetables from the Aegean Region, Turkey. Food Chem. 2014, 160, 379–392. [Google Scholar] [CrossRef]
- German Society for International Cooperation (GIZ). Development of Organic Farming Markets in Saudi Arabia, 2012. Available online: https://Www.Giz.de/En/Downloads/Giz2012-Organic-Agriculture-Saudi-Arabia-En.Pdf (accessed on 15 July 2018).
- Wood, A.M. Compendium of Pesticide Common Names According to ISO 1750. Available online: http://www.alanwood.net/pesticides/ (accessed on 15 June 2018).
- Pesticide Properties DataBase A to Z List of Pesticide Active Ingredients. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/atoz.htm (accessed on 15 July 2018).
- EU Commission. Commission directive 2002/63/EC of 11 July 2002 establishing community methods of sampling for the official control of pesticide residues in and on products of plant and animal origin and repealing directive 79/700/EEC. OJEC 2002, 2, 30–43. [Google Scholar]
- Kmellar, B.L.; Abranko, P.F.; Lehotay, S.J. Routine approach to qualitatively screening 300 pesticides and quantification of those frequently detected in fruit and vegetables using liquid chromatography tandem mass spectrometry (LC-MS/MS). Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 1415–1430. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).