Evaluation of Isoflavones as Bone Resorption Inhibitors upon Interactions with Receptor Activator of Nuclear Factor-κB Ligand (RANKL)
Abstract
1. Introduction
2. Results and Discussion
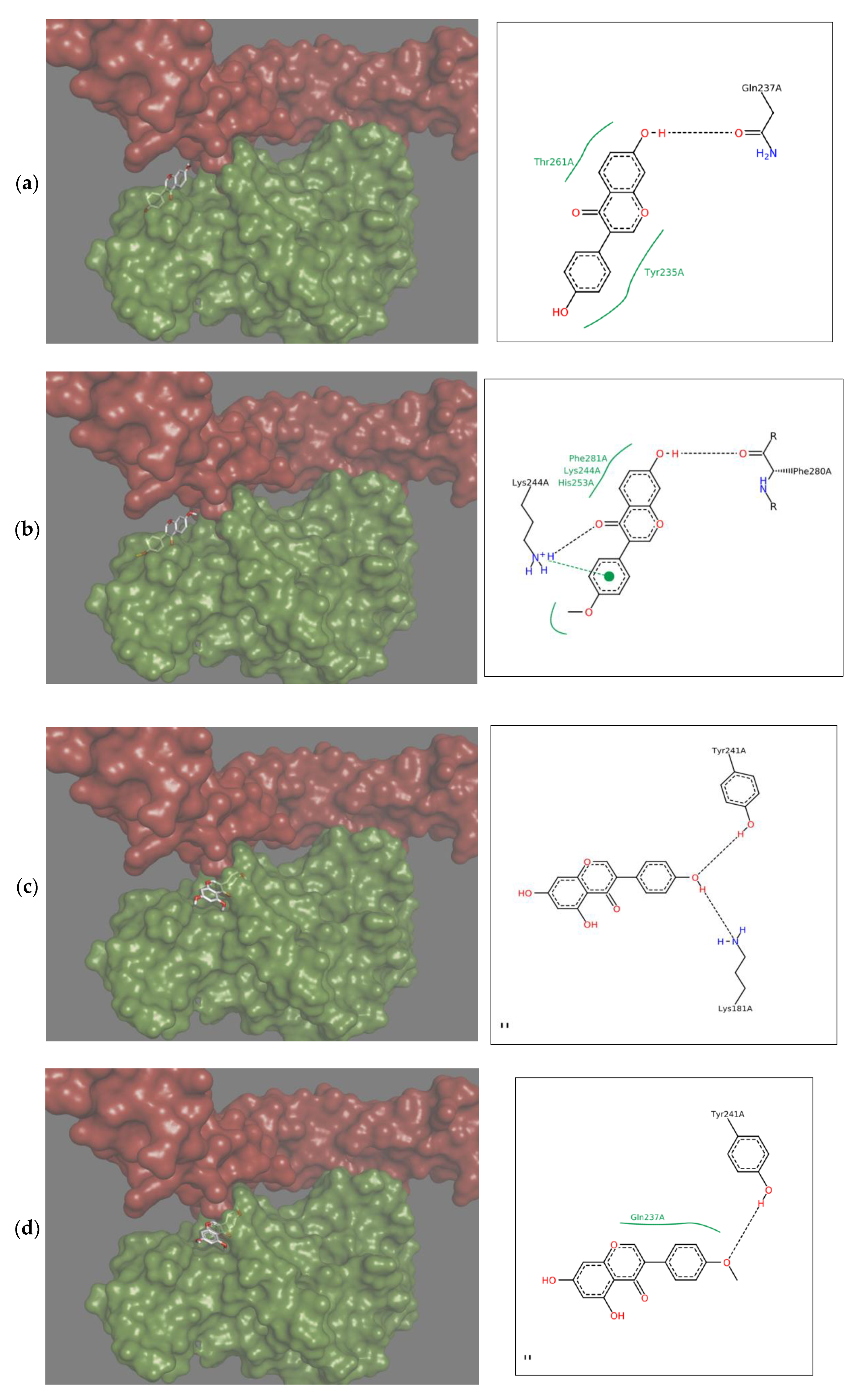
2.1. Characteristic of Complexes of Isoflavones with RANKL
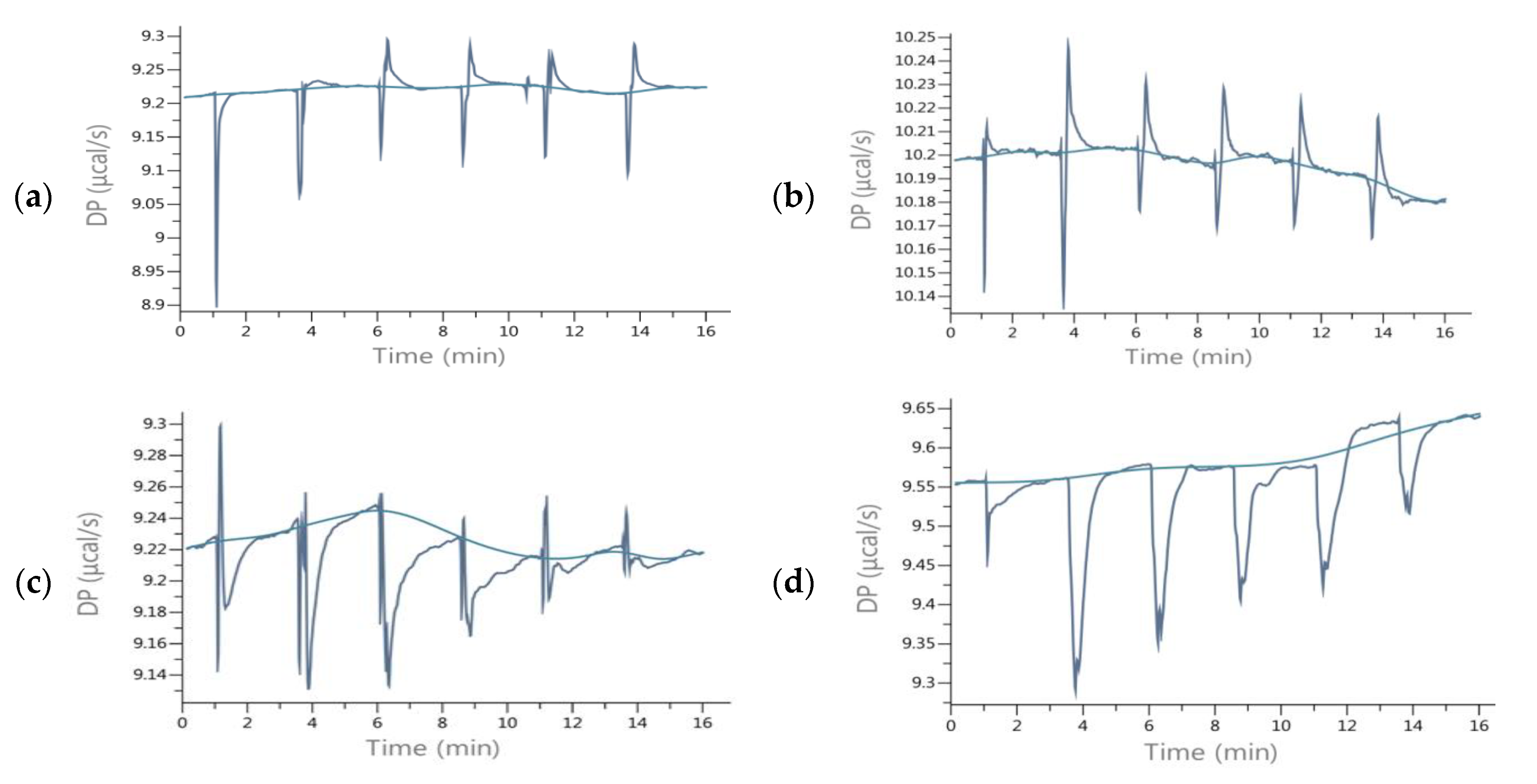
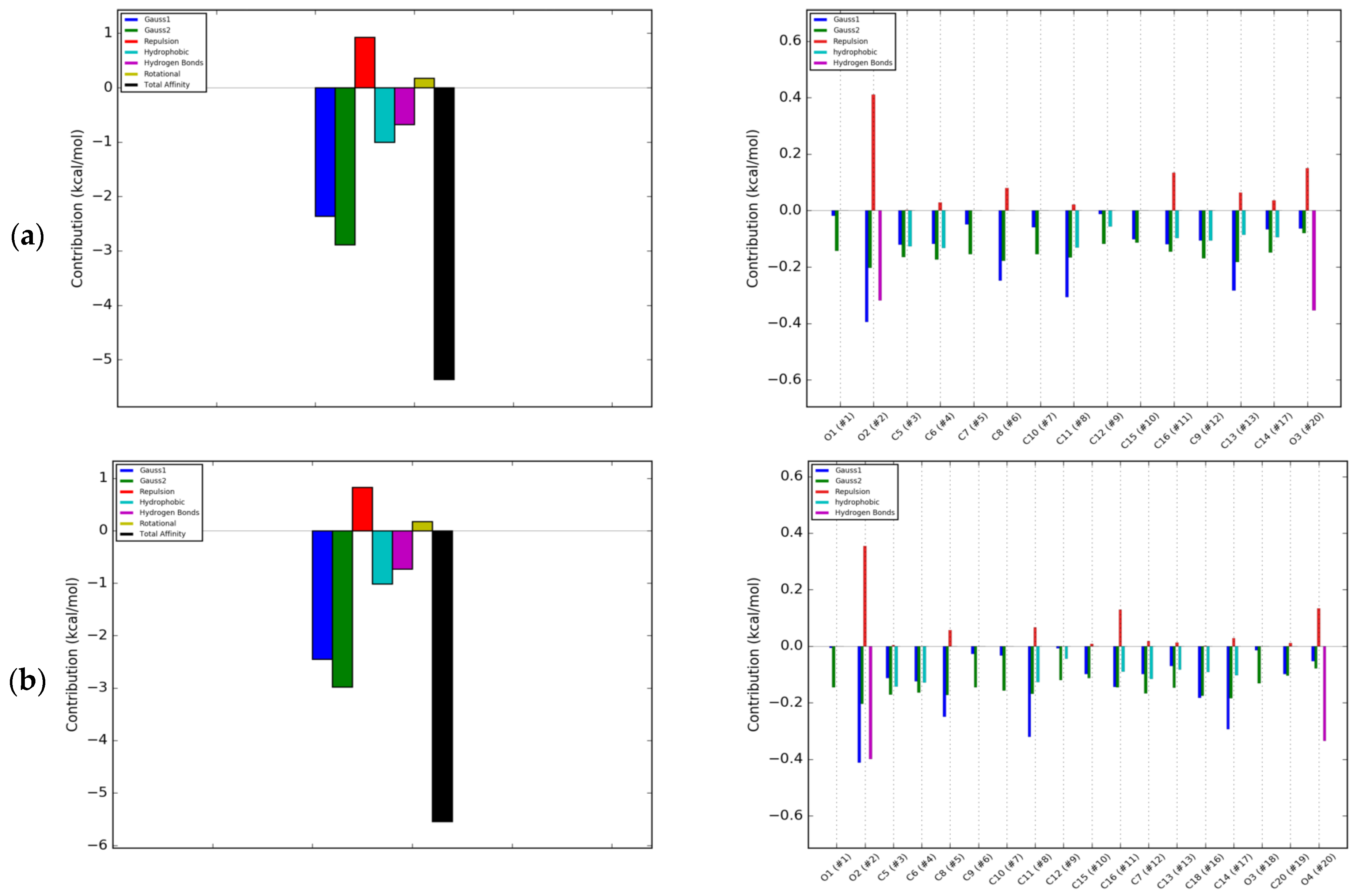
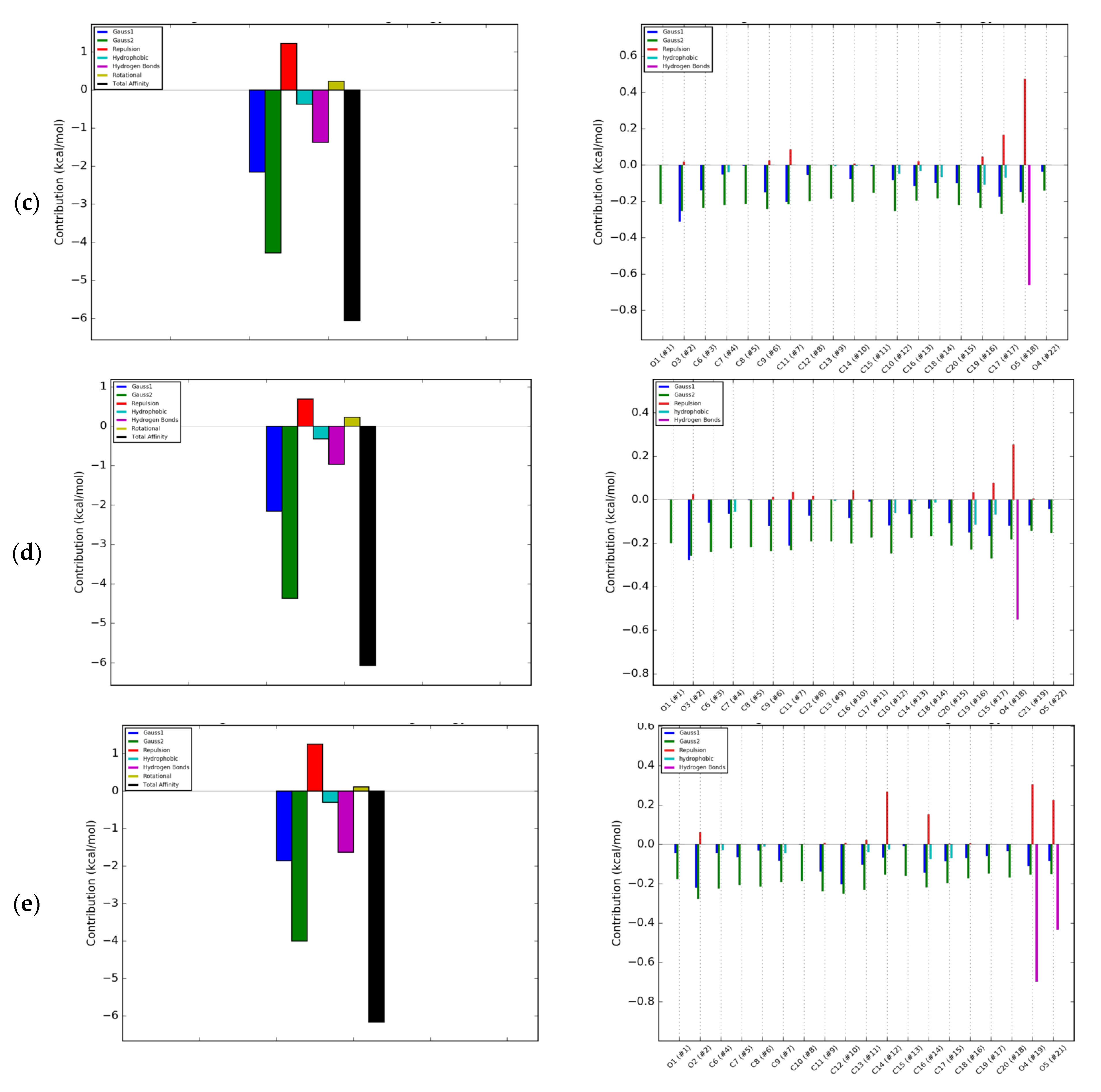
2.2. Energetic Effects of RANKL-Isoflavones Interactions
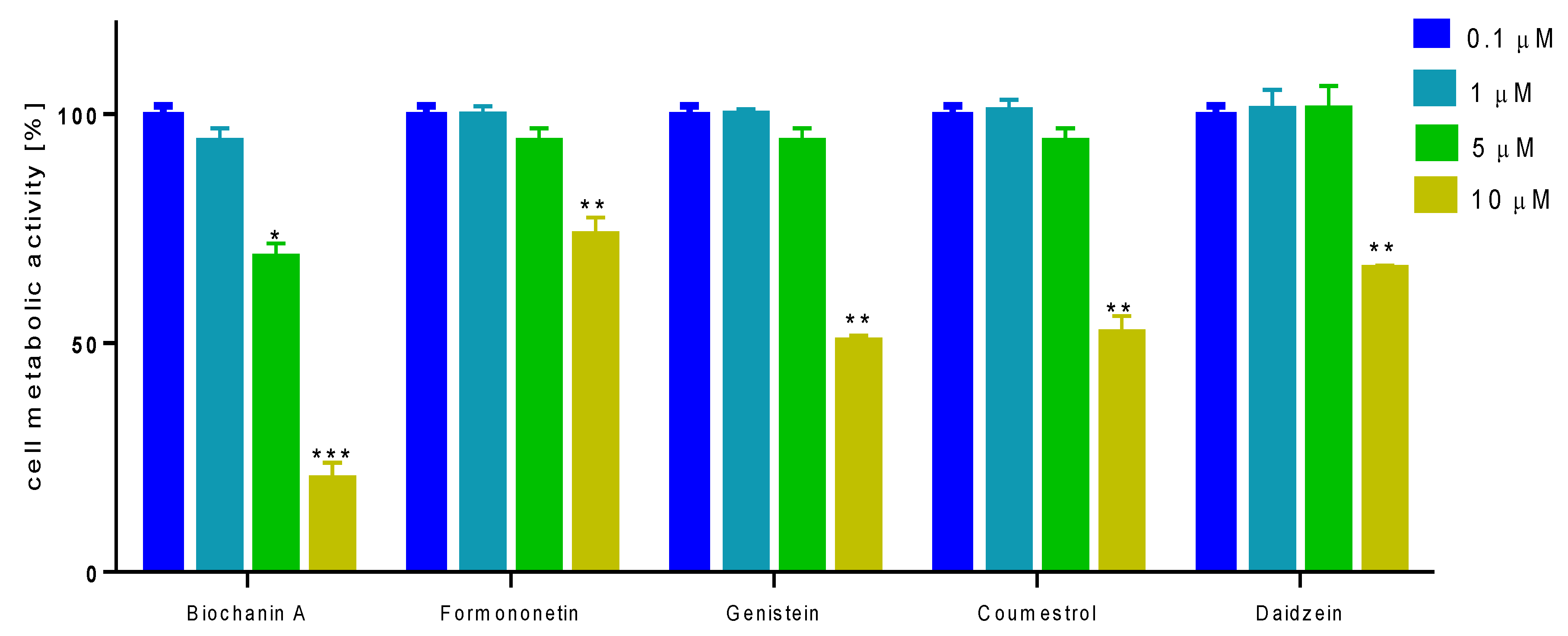
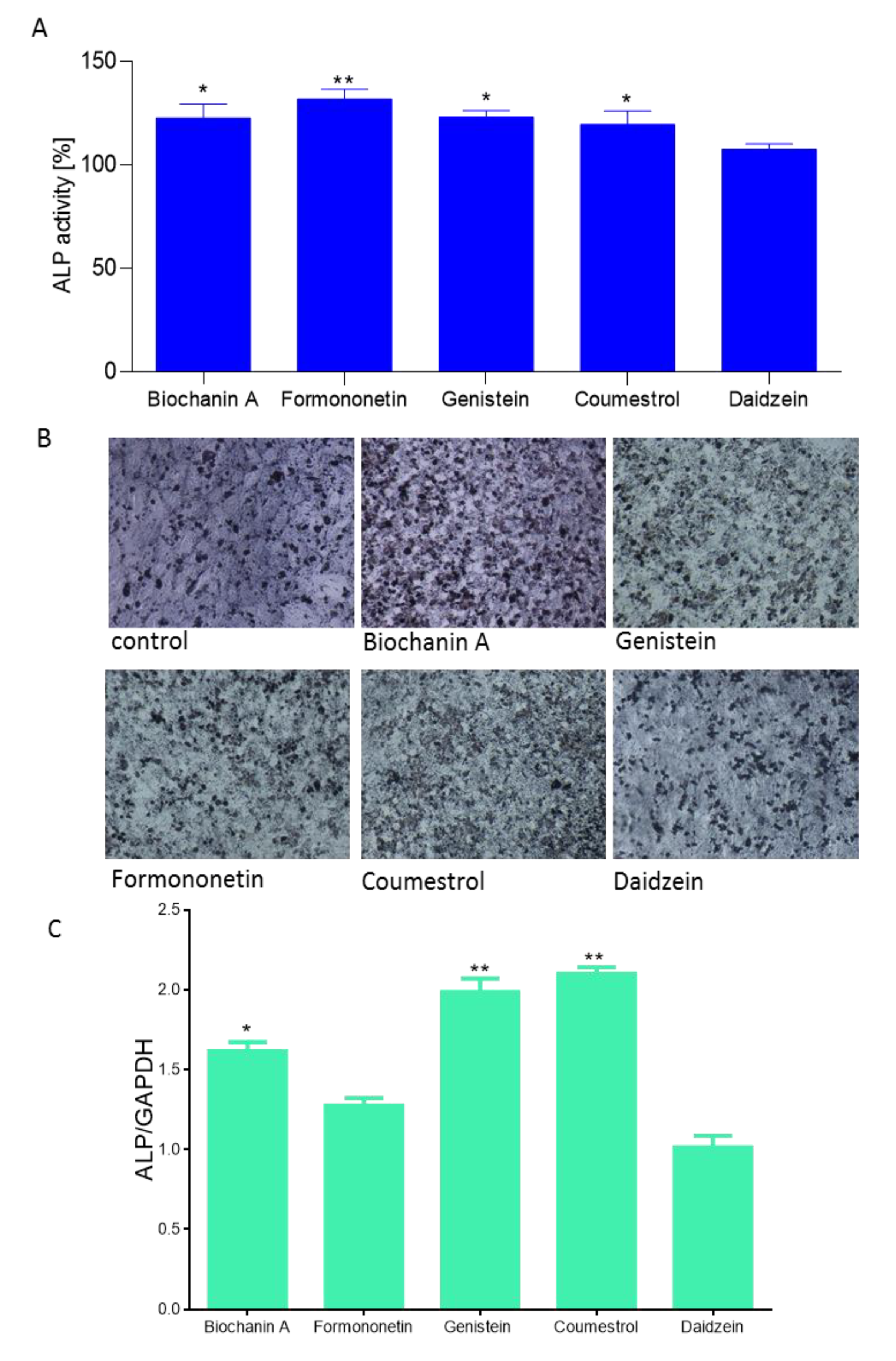
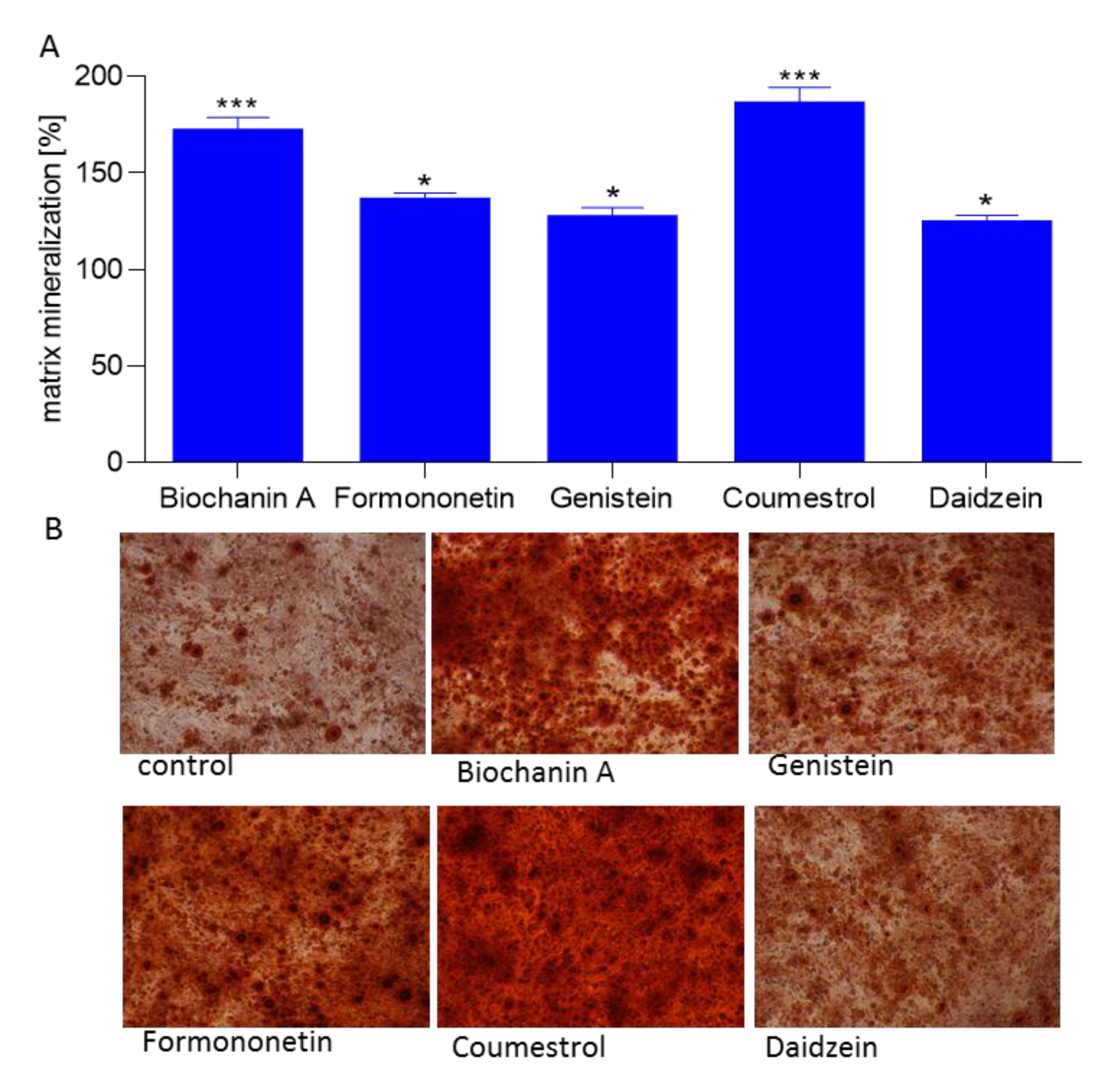
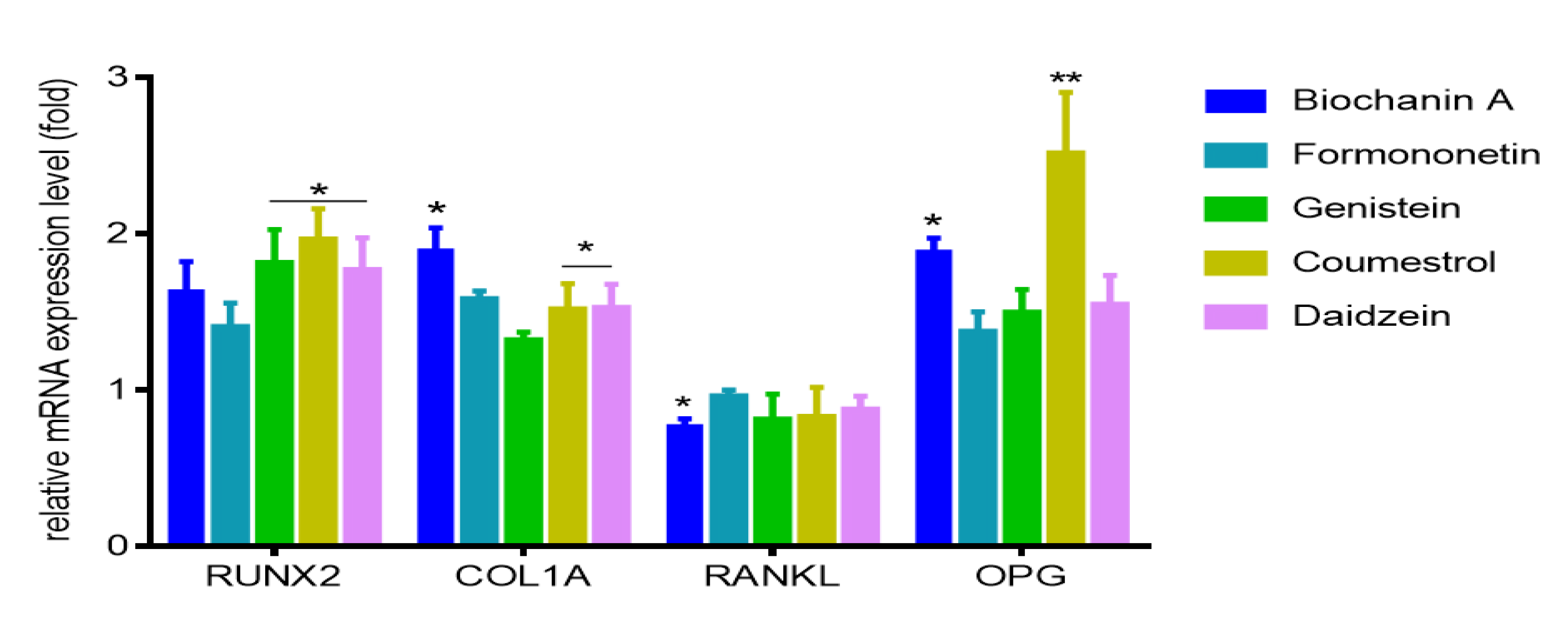
2.3. Effect of Isoflavones on Saos-2 Viability and Mineralization
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Isothermal Titration Calorimetry
3.3. Molecular Modeling
3.4. Cell Culture
3.5. Cell Viability
3.6. Alizarin Red Cells Staining
3.7. Estimation of ALP Activity
3.8. Gene Expresssion Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stawińska, N.; Ziętek, M.; Kochanowska, I. Molecular aspects of bone resorption and their therapeutical capability. Dent. Med. Probl. 2005, 42, 627–635. [Google Scholar]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.T.; Zou, W.; Decker, C.E.; Rohadki, N.; Nelson, C.A.; Fremont, D.H.; Teitelbaum, S.L. Correlating RANK ligand/RANK binding kinetics with osteoclast formation and function. J. Cell. Biochem. 2015, 116, 2476–2483. [Google Scholar] [CrossRef] [PubMed]
- Bekker, P.J.; Holloway, D.; Nakanishi, A.; Arrighi, M.; Leese, P.T.; Dunstan, C.R. The effect of a single dose of osteoprotegerin in postmenopausal women. J. Bone Miner. Res. 2001, 16, 348–360. [Google Scholar] [CrossRef]
- Boyce, F.B.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Cheng, X.; Kinosaki, M.; Takami, M.; Choi, Y.; Zhang, H.; Murali, R. Disabling of receptor activator of nuclear factor−kappa B (RANK) receptor complex by novel osteoprotegerin−like peptidomimetics restores bone loss in vivo. J. Biol. Chem. 2004, 279, 8269–8277. [Google Scholar] [CrossRef]
- Kaczmarczyk-Sedlak, I.; Wojnar, W.; Zych, M.; Ozimina-Kamińska, E.; Taranowicz, J.; Siwek, A. Effekt of formononetin on mechanical properties and chemical composition of bones in rats with ovariectomy-induced osteoporosis. Evid. Based Complement. Altern. Med. 2013, 2013, 457052. [Google Scholar] [CrossRef]
- García Palacios, V.; Robinson, L.J.; Borysenko, C.W.; Lehmann, T.; Kalla, S.E.; Blair, H.C. Negative regulation of RANKL-induced osteoclastic differentiation in RAW264.7 cells by estrogen and phytoestrogens. J. Biol. Chem. 2005, 280, 13720–13727. [Google Scholar] [CrossRef]
- Su, S.J.; Yeh, Y.T.; Shyu, H.W. The preventive effect of biochanin A on bone loss in ovariectomized rats: Involvement in regulation of growth and activity of osteoblasts and osteoclasts. Evid. Based Complement. Altern. Med. 2013, 2013, 594857. [Google Scholar] [CrossRef]
- Singh, B.K.; Dixit, M.; Dev, K.; Maurya, R.; Singh, D. Formononetin, a methoxy isoflavone, enhances bone regeneration in a mouse model of cortical bone defect. Br. J. Nutr. 2017, 117, 1511–1522. [Google Scholar] [CrossRef]
- Ayoub, N.; Singab, A.N.; El-Naggar, M.; Lindequist, U. Investigation of phenolic leaf extract of Heimia myrtifolia (Lythraceae): Pharmacological properties (stimulation of mineralization of SaOS-2 osteosarcoma cells) and identification of polyphenols. Drug Discov. Ther. 2010, 4, 341–348. [Google Scholar] [PubMed]
- Aricov, L.; Angelescu, D.G.; Băran, A.; Leontieş, A.R.; Popa, V.T.; Precupaş, A.; Sandu, R.; Stîngă, G.; Anghel, D.F. Interaction of piroxicam with bovine serum albumin investigated by spectroscopic, calorimetric and computational molecular methods. J. Biomol. Struct. Dyn. 2019, in press. [Google Scholar] [CrossRef]
- Dan, Q.; Xiong, W.; Liang, H.; Wu, D.; Zhan, F.; Chen, Y.; Ding, S.; Li, B. Characteristic of interaction mechanism between β-lactoglobulin and nobiletin: A multi-spectroscopic, thermodynamics methods and docking study. Food Res. Int. 2019, 120, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qin, P.; Zheng, X.; Hu, Z.; Zong, W.; Zhang, D.; Yang, B. Characterizing the noncovalent binding behavior of tartrazine to lysozyme: A combined spectroscopic and computational analysis. J. Biochem. Mol. Toxicol. 2019, 33, e22258. [Google Scholar] [CrossRef]
- Budryn, G.; Gałązka-Czarnecka, I.; Brzozowska, E.; Grzelczyk, J.; Mostowski, R.; Żyżelewicz, Ż.; Cerón-Carrasco, J.P.; Pérez-Sánchez, H. Evaluation of estrogenic activity of red clover (Trifolium pratense L.) sprouts cultivated under different conditions by content of isoflavones, calorimetric study and molecular modeling. Food Chem. 2018, 245, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Grzelczyk, J.; Jaśkiewicz, A.; Żyżelewicz, D.; Pérez-Sánchez, H.; Cerón-Carrasco, J.P. Evaluation of butyrylcholinesterase inhibitory activity by chlorogenic acids and coffee extracts assed in ITC and docking simulation models. Food Res. Int. 2018, 109, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Grzelczyk, J.; Pérez-Sánchez, H. Binding of red clover isoflavones to actin as a potential mechanism of anti-metastatic activity restricting the migration of cancer cells. Molecules 2018, 23, 2471. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Warren, J.T.; Wang, M.W.H.; Teitelbaum, S.L.; Fremont, D.H. RANKL employs distinct binding modes to engage RANK and the osteoprotegerin decoy receptor. Structure 2012, 20, 1971–1982. [Google Scholar] [CrossRef]
- Heinonen, S.M.; Wähälä, K.; Adlercreutz, H. Identification of urinary metabolites of the red clover isoflavones formononetin and biochanin A in human subjects. J. Agric. Food Chem. 2004, 52, 6802–6809. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G. A review of dietary polyphenol-plasma protein interactions: Characterization, influence on the bioactivity, and structure-affinity relationship. Crit. Rev. Food Sci. Nutr. 2012, 52, 85–101. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Gardner, P.T.; McPhail, D.B.; Morrice, F.C.; Collins, A.R.; Duthie, G.G. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch. Biochem. Biophys. 1998, 360, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Frazier, R.A.; Papadopoulou, A.; Green, R.J. Isothermal titration calorimetry study of epicatechin binding to serum albumin. J. Pharm. Biochem. Anal. 2006, 28, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into protein–ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, P.; Lou, L.; Wang, Y. Perspectives regarding the role of biochanin A in humans. Front. Pharmacol. 2019, 10, 793. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Prideaux, M.; Wijenayaka, A.R.; Kumarasinghe, D.D.; Ormsby, R.T.; Evdokiou, A.; Findlay, D.M.; Atkins, G.J. SaOS2 Osteosarcoma cells as an in vitro model for studying the transition of human osteoblasts to osteocytes. Calcif. Tissue Int. 2014, 95, 183–193. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cell Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Zheng, X.; Lee, S.K.; Chun, O.K. Soy isoflavones and osteoporotic bone loss: A review with an emphasis on modulation of bone remodeling. J. Med. Food 2016, 19, 1–14. [Google Scholar] [CrossRef]
- Uehara, M. Isoflavone metabolism and bone sparing effects of daidzein metabolites. J. Clin. Biochem. Nutr. 2013, 52, 193–201. [Google Scholar] [CrossRef]
- Moon, Y.J.; Sagawa, K.; Frederick, K.; Zhang, S.; Morris, M.E. Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J. 2006, 8, E433–E442. [Google Scholar] [CrossRef]
- Torre, E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-F.; Wong, M.-S. Genistein modulates the effects of parathyroid hormone in human osteoblastic SaOS-2 cells. Br. J. Nutr. 2006, 95, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-J.; Yeh, Y.-T.; Su, S.-H.; Chang, K.-L.; Shyu, H.-W.; Chen, K.-M.; Yeh, H. Biochanin A promotes osteogenic but inhibits adipogenic differentiation: Evidence with primary adipose-derived stem cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 846039. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-K.; Choi, I.-W.; Hong, G.-E.; Pyun, C.-W.; Park, K.; Park, P.-J.; Seo, S.-K.; Choi, Y.H.; Lee, C.-H. Effects of isoflavone aglycone-rich fermented soybean paste extracts on osteoblastic differentiation of MG-63 Cells. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 803–809. [Google Scholar] [CrossRef]
- Ho, M.-X.; Poon, C.C.; Wong, K.-C.; Qiu, Z.-C.; Wong, M.-S. Icariin, but not genistein, exerts osteogenic and anti-apoptotic effects in osteoblastic cells by selective activation of non-genomic ERα Signaling. Front. Pharmacol. 2018, 9, 474. [Google Scholar] [CrossRef]
- Bitto, A.; Burnett, B.P.; Polito, F.; Marini, H.; Levy, R.M.; Armbruster, M.A.; Minutoli, L.; Di Stefano, V.; Irrera, N.; Antoci, S.; et al. Effects of genistein aglycone in osteoporotic, ovariectomized rats: A comparison with alendronate, raloxifene and oestradiol. Br. J. Pharmacol. 2008, 155, 896–905. [Google Scholar] [CrossRef]
- Jin, X.; Sunb, J.; Yuc, B.; Wanga, Y.; Sund, W.J.; Yangc, J.; Huangc, S.H.; Xieb, W. Daidzein stimulates osteogenesis facilitating proliferation, differentiation, and antiapoptosis in human osteoblast-like MG-63 cells via estrogen receptor–dependent MEK/ERK and PI3K/Akt activation. Nutr. Res. 2017, 42, 20–30. [Google Scholar] [CrossRef]
- Ha, H.; Lee, H.Y.; Lee, J.-H.; Jung, D.; Choi, J.; Song, K.-Y.; Jung, H.J.; Choi, J.S.; Chang, S.-I.; Kim, C. Formononetin prevents ovariectomy-induced bone loss in rats. Arch. Pharm. Res. 2010, 33, 625–632. [Google Scholar] [CrossRef]
- Huh, J.-E.; Seo, D.-M.; Baek, Y.-H.; Choi, D.-Y.; Park, D.-S.; Lee, J.-E. Biphasic positive effect of formononetin on metabolic activity of human normal and osteoarthritic subchondral osteoblasts. Int. Immunopharmacol. 2010, 10, 500–507. [Google Scholar] [CrossRef]
- Sun, J.-S.; Li, Y.-Y.; Liu, M.-H.; Sheu, S.-Y. Effects of coumestrol on neonatal and adult mice osteoblasts activities. J. Biomed. Mater. Res. 2007, 81, 214–223. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity—A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Ghiacci, G.; Lumetti, S.; Manfredi, E.; Mori, D.; Macaluso, G.M.; Sala, R. Stanozolol promotes osteogenic gene expression and apposition of bone mineral in vitro. J. Appl. Oral Sci. 2019, 27, e20180014. [Google Scholar] [CrossRef] [PubMed]
- Muthusami, S.; Senthilkumar, K.; Vignesh, C.; Ilangovan, R.; Stanley, J.; Selvamurugan, N.; Srinivasan, N. Effects of Cissus quadrangularis on the proliferation, differentiation and matrix mineralization of human osteoblast like SaOS-2 Cells. J. Cell. Biochem. 2011, 112, 1035–1045. [Google Scholar] [CrossRef] [PubMed]


| Isoflavone | KA × 103 (L/mol) | ∆H (kJ/mol) | ∆S (J/mol*K) | ∆G (kJ/mol) | ∆Gpredicted (kJ/mol) |
|---|---|---|---|---|---|
| Daidzein | 15.95 ± 4.05 | −0.05 ± 0.01 b | 78.94 ± 10.12 a | −22.82 | −23.65 a |
| Formononetin | 3.14 ± 0.72 a | 0.19 ± 0.04 a | 67.61 ± 7.92 | −19.30 c | −23.23 a |
| Genistein | 3.15 ± 0.59 a | 2.47 ± 0.48 | 75.69 ± 9.09 a | −19.34 c | −25.32 b |
| Biochanin A | 1.20 ± 0.47 | 22.90 ± 4.09 | 138.42 ± 21.28 b | −17.00 | −25.53 b |
| Coumestrol | 0.70 ± 0.11 | 18.55 ± 2.17 | 118.92 ± 17.11 | −15.74 a | −25.11 b |
| OPG | 720.15 ± 95.41 | −10.80 ± 1.77 | 17.15 ± 2.54 | −15.72 a | - |
| Daidzein + Formononetin | - | −21.94 ± 3.54 e | −19.47 ± 3.21 d | −16.33 | - |
| Daidzein + Genistein | - | 21.10 ± 2.08 | 137.89 ± 18.03 b | −18.63 | - |
| Daidzein + Biochanin A | - | −20.05 ± 4.17 e | −17.29 ± 4.19 d | −15.07 | - |
| Daidzein + Coumestrol | - | −5.74 ± 0.90 c | 41.25 ± 5.12 | −17.63 b | - |
| Formononetin + Genistein | - | 0.05 ± 0.02 a,b | 76.75 ± 15.07 a | −22.06 | - |
| Formononetin + Biochanin A | - | −5.40 ± 0.82 c | 106.94 ± 11.34 | −36.22 | - |
| Formononetin + Coumestrol | - | −8.42 ± 1.93 d | 32.98 ± 4.18 c | −17.92 | - |
| Genistein + Biochanin A | - | −21.56 ± 5.73 e | −20.78 ± 3.05 | −15.57 a | - |
| Genistein + Coumestrol | - | −15.37 ± 3.03 | 7.85 ± 1.83 | −17.63 b | - |
| Biochanin A + Coumestrol | - | −8.50 ± 2.15 d | 30.66 ± 5.22 c | −17.33 b | - |
| Isoflavone | IC50 [µM] |
|---|---|
| Biochanin A | 7.0 ± 0.3 |
| Formononetin | 25 ± 1.5 |
| Genistein | 10 ± 0.6 |
| Coumestrol | 12 ± 0.3 |
| Daidzein | 20 ± 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakłos-Szyda, M.; Budryn, G.; Grzelczyk, J.; Pérez-Sánchez, H.; Żyżelewicz, D. Evaluation of Isoflavones as Bone Resorption Inhibitors upon Interactions with Receptor Activator of Nuclear Factor-κB Ligand (RANKL). Molecules 2020, 25, 206. https://doi.org/10.3390/molecules25010206
Zakłos-Szyda M, Budryn G, Grzelczyk J, Pérez-Sánchez H, Żyżelewicz D. Evaluation of Isoflavones as Bone Resorption Inhibitors upon Interactions with Receptor Activator of Nuclear Factor-κB Ligand (RANKL). Molecules. 2020; 25(1):206. https://doi.org/10.3390/molecules25010206
Chicago/Turabian StyleZakłos-Szyda, Małgorzata, Grażyna Budryn, Joanna Grzelczyk, Horacio Pérez-Sánchez, and Dorota Żyżelewicz. 2020. "Evaluation of Isoflavones as Bone Resorption Inhibitors upon Interactions with Receptor Activator of Nuclear Factor-κB Ligand (RANKL)" Molecules 25, no. 1: 206. https://doi.org/10.3390/molecules25010206
APA StyleZakłos-Szyda, M., Budryn, G., Grzelczyk, J., Pérez-Sánchez, H., & Żyżelewicz, D. (2020). Evaluation of Isoflavones as Bone Resorption Inhibitors upon Interactions with Receptor Activator of Nuclear Factor-κB Ligand (RANKL). Molecules, 25(1), 206. https://doi.org/10.3390/molecules25010206







