Abstract
Silymarin flavonolignans are well-known agents that typically possess antioxidative, anti-inflammatory, and hepatoprotective functions. Recent studies have also documented the antiviral activities of silymarin and its derivatives against several viruses, including the flaviviruses (hepatitis C virus and dengue virus), togaviruses (Chikungunya virus and Mayaro virus), influenza virus, human immunodeficiency virus, and hepatitis B virus. This review will describe some of the latest preclinical and clinical studies detailing the antiviral profiles of silymarin and its derivatives, and discuss their relevance for antiviral drug development.
1. Silymarin, Its Components, and Derivatives
Silymarin, an extract from the seed of the milk thistle plant (Silybum marianum [S. marianum]) is widely known for its hepatoprotective functions, mainly due to its anti-oxidative, anti-inflammatory, and immunomodulatory effects [1]. The primary bioactive components of the extract consist of several flavonolignans (silybin, silychristin, silydianin, isosilybin, and dehydrosilybin), and a few flavonoids, mainly taxifolin [2]. The mixture of silybin A and silybin B (1:1) is also known as silibinin (C25H22O10, PubChem CID: 31553; Figure 1), which makes up the major active ingredient (roughly 50%) of silymarin [2,3]. Although silymarin is known mostly for its hepatoprotective functions, accumulating evidence now suggests that the extract possesses potent antiviral activities against numerous viruses, particularly hepatitis C virus (HCV). Consequently, silymarin is the most commonly consumed herbal product among HCV-infected patients in western countries [4]. Despite its potent medicinal effects, silymarin suffers from poor solubility which affects its bioavailability in vivo. To improve the issue, the chemically-hydrophilized silibinin, Legalon® SIL (C66H56Na4O32, PubChem CID: 76956344), was developed by the pharmaceutical company Rottapharm Madaus (Monza, Italy) for the administration by intravenous infusion, and the drug was further granted orphan medicinal product designation (EU/3/10/828) from the European Medicines Agency (EMA) for the prevention of recurrent hepatitis C in liver transplant recipients in 2010 [5]. To date, silymarin and its derivatives have been examined for potential bioactivities against several viruses and various strategies to address its drug delivery challenges have also been explored. This review examines the current literature concerning the antiviral effects of silymarin and silymarin-derived compounds used in preclinical and clinical studies, the challenges to clinical application, as well as its prospects as clinically applicable antiviral agents.
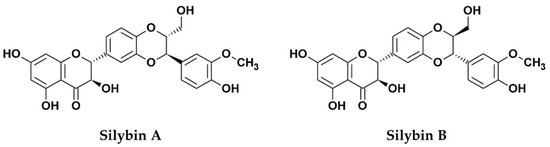
Figure 1.
Chemical structures of silibinin, the 1:1 mixture of silybin A and silybin B.
4. Challenges to Clinical Application and the Need to Enhance Bioavailability
Drug solubility has an important influence over drug absorption, and hence bioavailability. Despite the wide range of biological and pharmacological effects of silymarin, the extract is relatively insoluble in water (0.4 mg/mL), and the use of other solvents such as ethanol, glyceryl monooleate, polysorbate 20, and transcutol may help increase its solubility ranging from 33–350 mg/mL [59]. Studies based on silymarin’s primary active molecular component silybin indicate extensive enterohepatic circulation following oral administration, rapid excretion in bile and urine with an elimination half-life of about 6 h, and a low absorption from the gastrointestinal tract with a reported 0.73% of oral bioavailability in rat plasma [60,61]. In addition, the silybin content is particularly susceptible to conjugation reactions in phase II metabolism in the human liver, yielding various silybin metabolites conjugated with sulfates and glucuronides [61], and an observed average of 10% of the silybin isomers as unconjugated form in the plasma of orally-administered healthy volunteers [62]. The recent phase II trial demonstrated in chronic hepatitis C patients who received silymarin capsules that serum level of silybin varied significantly from 2.1 to 2048 ng/mL despite the high dose range used (420–700 mg, 3x daily) in the patient, indicating absorption and bioavailability issues which likely affected the efficacy outcome of the drug against hepatitis C [35]. The above factors contribute to the poor oral bioavailability of silymarin and, likewise, of its active constituent silybin. For this reason, most clinical trials and case studies, including those against chronic hepatitis C and HIV/HCV coinfection, employed the more water-soluble salt-derivatives such as Legalon® SIL (silibinin-C-2′,3-dihydrogen succinate, disodium salt) [39,40,41,42,43,44,46,47,48,51,52,53,56,57,58]. However, Legalon® SIL is inconvenient for administration, because it is given by i.v. infusion and cannot be administered orally. The available pharmacokinetic and clinical studies highlight the need to overcome drug delivery problems and formulate or modify silymarin and its active derivatives into more soluble forms that can achieve higher bioavailability.
To address this challenge, several methods have also been explored to increase the bioavailability of silymarin and its constituents. These include combination with phosphatidylcholine [63] or β-cyclodextrins [64], formation of salts and glycoside derivatives [65,66], liposome delivery [67,68], solid dispersion incorporation [69,70], self-microemulsifying drug delivery systems (SMEDDS) [59,71,72], and nanoformulations [73,74,75], which can all improve the solubility of silymarin as well as enhance the prolonged and sustained release of silybin. As an example, we have recently employed a nano-emulsification strategy in addressing the solubility and bioavailability issue of the standardized silibinin (silybin isomers). Specifically, silibinin-loaded nanoparticles (SB-NP) with diameters <200 nm were successfully developed using the hydrophilic carrier polyvinylpyrrolidone (PVP), which resulted in the transition of the silibinin crystalline structure into an amorphous state in the SB-NP and demonstrated a significantly enhanced solubility [15]. Interestingly, free silibinin was efficiently released from the nanoformulation at pH 7.4 but was prohibited at pH 1.2, indicating that the drug would be released extensively in the alkaline intestine rather than the acerbic stomach, thus favoring intestinal absorption. Importantly, the SB-NP retained their antioxidant activity and antiviral function against HCV infection in vitro, and were safe and orally bioavailable in vivo [15]. Enhanced serum concentration and superior biodistribution to the liver was observed compared to non-modified silibinin following oral administration in rats [15]. The orally applicable SB-NP with its improved solubility, absorption, and higher accumulation in the liver highlight an advantage for application against viral hepatitis, including hepatitis C, and underscores its potency for further development as a promising candidate drug agent.
Altogether, due to the widely known pharmacological effects but low solubility and bioavailability of silymarin and its derivatives, the above suggests that increasing the oral bioavailability is critical to their development and application in clinical settings. This is attested by the numerous studies to date, as mentioned above, aiming to address these challenges.
Author Contributions
Conceptualization, C.-H.L. and L.-T.L.; investigation, C.-H.L. and A.J.; writing—original draft preparation, C.-H.L., A.J., H.-Y.H., and L.-T.L.; writing—review and editing, C.-H.L., A.J., and L.-T.L.; visualization, C.-H.L. and L.-T.L.; supervision, L.-T.L.; project administration, L.-T.L.; funding acquisition, L.-T.L.
Funding
This research was supported in part by funding from the Ministry of Science and Technology of Taiwan (MOST107-2320-B-038-034-MY3) and a grant from Chi-Mei Medical Center and Taipei Medical University (108CM-TMU-02). C.-H.L. is a recipient of the Canadian Network on Hepatitis C (CanHepC) PhD Fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Federico, A.; Dallio, M.; Loguercio, C.; Tsai, T.-H.; Jeon, Y. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef]
- Dixit, N.; Kohli, K.; Ahmad, S.; Baboota, S.; Ali, J. Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches. Indian J. Pharmacol. 2007, 39, 172. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Strader, D.B.; Bacon, B.R.; Lindsay, K.L.; Brecque, D.R.; Morgan, T.; Wright, E.C.; Allen, J.; Khokar, M.F.; Hoofnagle, J.H.; Seeff, L.B.; et al. Use of complementary and alternative medicine in patients with liver disease. Am. J. Gastroenterol. 2002, 97, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Silibinin-C-2′,3-dihydrogensuccinate, Disodium Salt for the Prevention of Recurrent Hepatitis C in Liver Transplant Recipients. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu310828 (accessed on 23 March 2019).
- Alter, H.J.; Seeff, L.B. Recovery, persistence, and sequelae in hepatitis C virus infection: A perspective on long-term outcome. Semin. Liver Dis. 2000, 20, 17–35. [Google Scholar]
- Franco, L.; Palacios, G.; Martinez, J.A.; Vázquez, A.; Savji, N.; De Ory, F.; Sanchez-Seco, M.P.; Martín, D.; Lipkin, W.I.; Tenorio, A. First report of sylvatic DENV-2-associated dengue hemorrhagic fever in West Africa. PLoS Negl. Trop. Dis. 2011, 5, e1251. [Google Scholar] [CrossRef] [PubMed]
- Polyak, S.J.; Morishima, C.; Shuhart, M.C.; Wang, C.C.; Liu, Y.; Lee, D.Y. Inhibition of T-Cell Inflammatory Cytokines, Hepatocyte NF-κB Signaling, and HCV Infection by Standardized Silymarin. Gastroenterology 2007, 132, 1925–1936. [Google Scholar] [CrossRef]
- Morishima, C.; Lohmann, V.; Pal, S.; Liu, Y.; Polyak, S.J.; Lee, D.Y.W.; Graf, T.N.; Oberlies, N. Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. USA 2010, 107, 5995–5999. [Google Scholar]
- Wagoner, J.; Negash, A.; Kane, O.J.; Martinez, L.E.; Nahmias, Y.; Bourne, N.; Owen, D.M.; Grove, J.; Brimacombe, C.; McKeating, J.A.; et al. Multiple Effects of Silymarin on the Hepatitis C Virus Lifecycle. Hepatology 2010, 51, 1912–1921. [Google Scholar] [CrossRef]
- Ahmed–Belkacem, A.; Ahnou, N.; Barbotte, L.; Wychowski, C.; Pallier, C.; Brillet, R.; Pohl, R.; Pawlotsky, J. Silibinin and Related Compounds Are Direct Inhibitors of Hepatitis C Virus RNA-Dependent RNA Polymerase. Gastroenterology 2010, 138, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Blaising, J.; Lévy, P.L.; Gondeau, C.; Phelip, C.; Varbanov, M.; Teissier, E.; Ruggiero, F.; Polyak, S.J.; Oberlies, N.H.; Ivanovic, T.; et al. Silibinin inhibits hepatitis C virus entry into hepatocytes by hindering clathrin-dependent trafficking. Cell. Microbiol. 2013, 15, 1866–1882. [Google Scholar] [CrossRef]
- Esser-Nobis, K.; Romero-Brey, I.; Ganten, T.M.; Gouttenoire, J.; Harak, C.; Klein, R.; Schemmer, P.; Binder, M.; Schnitzler, P.; Moradpour, D.; et al. Analysis of hepatitis C virus resistance to Silibinin in vitro and in vivo points to a novel mechanism involving nonstructural protein 4B. Hepatology 2013, 57, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Gosert, R.; Egger, D.; Lohmann, V.; Bartenschlager, R.; Blum, H.E.; Bienz, K.; Moradpour, D. Identification of the Hepatitis C Virus RNA Replication Complex in Huh-7 Cells Harboring Subgenomic Replicons. J. Virol. 2003, 77, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Lin, C.C.; Hsu, W.C.; Chung, C.Y.; Lin, C.C.; Jassey, A.; Chang, S.P.; Tai, C.J.; Tai, C.J.; Shields, J.; et al. Highly bioavailable silibinin nanoparticles inhibit HCV infection. Gut 2017, 66, 1853–1861. [Google Scholar] [CrossRef]
- DebRoy, S.; Hiraga, N.; Imamura, M.; Hayes, C.N.; Akamatsu, S.; Canini, L.; Perelson, A.S.; Pohl, R.T.; Persiani, S.; Uprichard, S.L. Hepatitis C virus dynamics and cellular gene expression in uPA-SCID chimeric mice with humanized livers during intravenous silibinin monotherapy. J. Viral Hepat. 2016, 23, 708–717. [Google Scholar] [CrossRef]
- Wagoner, J.; Morishima, C.; Graf, T.N.; Oberlies, N.H.; Teissier, E.; Pécheur, E.-I.; Tavis, J.E.; Polyak, S.J. Differential In Vitro Effects of Intravenous versus Oral Formulations of Silibinin on the HCV Life Cycle and Inflammation. PLoS ONE 2011, 6, e16464. [Google Scholar] [CrossRef] [PubMed]
- Qaddir, I.; Rasool, N.; Hussain, W.; Mahmood, S. Computer-aided analysis of phytochemicals as potential dengue virus inhibitors based on molecular docking, ADMET and DFT studies. J. Vector Borne Dis. 2017, 54, 255. [Google Scholar]
- Webster, R.G.; Govorkova, E.A. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014, 1323, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Sugaya, N.; Hirotsu, N.; Lee, N.; De Jong, M.D.; Hurt, A.C.; Ishida, T.; Sekino, H.; Yamada, K.; Portsmouth, S.; et al. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N. Engl. J. Med. 2018, 379, 913–923. [Google Scholar] [CrossRef]
- Gažák, R.; Purchartová, K.; Marhol, P.; Živná, L.; Sedmera, P.; Valentova, K.; Kato, N.; Matsumura, H.; Kaihatsu, K.; Křen, V. Antioxidant and antiviral activities of silybin fatty acid conjugates. Eur. J. Med. Chem. 2010, 45, 1059–1067. [Google Scholar] [CrossRef]
- Song, J.; Choi, H. Silymarin efficacy against influenza A virus replication. Phytomedicine 2011, 18, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-P.; Wu, L.-Q.; Li, R.; Zhao, X.-F.; Wan, Q.-Y.; Chen, X.-X.; Li, W.-Z.; Wang, G.-F.; Li, K.-S. Identification of 23-(S)-2-Amino-3-Phenylpropanoyl-Silybin as an Antiviral Agent for Influenza A Virus Infection In Vitro and In Vivo. Antimicrob. Agents Chemother. 2013, 57, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase II enzymes: Implications in cancer chemoprevention. Carcinogenesis 1999, 20, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Roe, B.; Hall, W.W. Cellular and molecular interactions in coinfection with hepatitis C virus and human immunodeficiency virus. Expert Rev. Mol. Med. 2008, 10, 10. [Google Scholar] [CrossRef]
- McClure, J.; Lovelace, E.S.; Elahi, S.; Maurice, N.J.; Wagoner, J.; Dragavon, J.; Mittler, J.E.; Kraft, Z.; Stamatatos, L.; Horton, H.; et al. Correction: Silibinin Inhibits HIV-1 Infection by Reducing Cellular Activation and Proliferation. PLoS ONE 2012, 7, 41832. [Google Scholar] [CrossRef]
- McClure, J.; Margineantu, D.H.; Sweet, I.R.; Polyak, S.J. Inhibition of HIV by Legalon-SIL is independent of its effect on cellular metabolism. Virology 2014, 449, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Lani, R.; Hassandarvish, P.; Chiam, C.W.; Moghaddam, E.; Chu, J.J.H.; Rausalu, K.; Merits, A.; Higgs, S.; VanLandingham, D.; Abu Bakar, S.; et al. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015, 5, 11421. [Google Scholar] [CrossRef]
- Camini, F.C.; Da Silva, T.F.; Caetano, C.C.D.S.; Almeida, L.T.; Ferraz, A.C.; Vitoreti, V.M.A.; Silva, B.D.M.; Silva, S.D.Q.; De Magalhães, J.C.; Magalhães, C.L.D.B. Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress. Antivir. Res. 2018, 158, 8–12. [Google Scholar] [CrossRef]
- Hyun, S.; Lee, S.; Ventura, W.R.; McMenamin, J. Knowledge, Awareness, and Prevention of Hepatitis B Virus Infection Among Korean American Parents. J. Immigr. Minor. Heal. 2017, 20, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, T.; Inoue, J.; Kogure, T.; Kakazu, E.; Ninomiya, M.; Iwata, T.; Takai, S.; Nakamura, T.; Sano, A.; Shimosegawa, T. Inhibitory effect of silibinin on hepatitis B virus entry. Biochem. Biophys. Rep. 2018, 14, 20–25. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Fu, S.-L.; Kao, C.-H.; Yang, C.-W.; Lin, C.-H.; Hsu, M.-T.; Tsai, T.-F. Chemopreventive Effect of Silymarin on Liver Pathology in HBV X Protein Transgenic Mice. Cancer Res. 2008, 68, 2033–2042. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanamly, M.; Tadros, F.; Labeeb, S.; Makld, H.; Mikhail, N.; Abdel-Hamid, M.; Shehata, M.; Abu-Baki, L.; Medhat, A.; Magder, L.; et al. Randomised double-blinded trial evaluating silymarin for chronic hepatitis C in an Egyptian village: Study description and 12-month results. Dig. Liver Dis. 2004, 36, 752–759. [Google Scholar] [CrossRef]
- Gabbay, E.; Zigmond, E.; Pappo, O.; Hemed, N.; Rowe, M.; Zabrecky, G.; Cohen, R.; Ilan, Y. Antioxidant therapy for chronic hepatitis C after failure of interferon: Results of phase II randomized, double-blind placebo controlled clinical trial. World J. Gastroenterol. 2007, 13, 5317–5323. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.W.; Navarro, V.J.; Afdhal, N.; Belle, S.H.; Wahed, A.S.; Hawke, R.L.; Doo, E.; Meyers, C.M.; Reddy, K.R.; Silymarin, N.; et al. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: A randomized controlled trial. JAMA 2012, 308, 274–282. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Motta, M.; Vacante, M.; Malaguarnera, G.; Caraci, F.; Nunnari, G.; Gagliano, C.; Greco, C.; Chisari, G.; Drago, F.; et al. Silybin-vitamin E-phospholipids complex reduces liver fibrosis in patients with chronic hepatitis C treated with pegylated interferon alpha and ribavirin. Am. J. Transl. Res. 2015, 7, 2510–2518. [Google Scholar]
- Malaguarnera, G.; Bertino, G.; Chisari, G.; Motta, M.; Vecchio, M.; Vacante, M.; Caraci, F.; Greco, C.; Drago, F.; Nunnari, G.; et al. Silybin supplementation during HCV therapy with pegylated interferon-α plus ribavirin reduces depression and anxiety and increases work ability. BMC Psychiatry 2016, 16, 398. [Google Scholar] [CrossRef]
- Loguercio, C.; Festi, D.; Loguercio, C. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P.; Scherzer, T.M.; Kerschner, H.; Rutter, K.; Beinhardt, S.; Hofer, H.; Schöniger–Hekele, M.; Holzmann, H.; Steindl–Munda, P. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology 2008, 135, 1561–1567. [Google Scholar] [CrossRef]
- Guedj, J.; Dahari, H.; Pohl, R.-T.; Ferenci, P.; Perelson, A.S. Understanding silibinin’s modes of action against HCV using viral kinetic modeling. J. Hepatol. 2012, 56, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Biermer, M.; Berg, T. Rapid Suppression of Hepatitis C Viremia Induced by Intravenous Silibinin Plus Ribavirin. Gastroenterology 2009, 137, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Rutter, K.; Scherzer, T.-M.; Beinhardt, S.; Kerschner, H.; Stättermayer, A.F.; Hofer, H.; Popow-Kraupp, T.; Steindl-Munda, P.; Ferenci, P. Intravenous silibinin as ‘rescue treatment’ for on-treatment non-responders to pegylated interferon/ribavirin combination therapy. Antivir. Ther. 2011, 16, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Biermer, M.; Schlosser, B.; Fülöp, B.; van Bömmel, F.; Brodzinski, A.; Heyne, R.; Keller, K.; Sarrazin, C.; Berg, T. High-dose silibinin rescue treatment for HCV-infected patients showing suboptimal virologic response to standard combination therapy. J. Viral Hepat. 2012, 19, 547–553. [Google Scholar] [CrossRef]
- Dahari, H.; Shteingart, S.; Gafanovich, I.; Cotler, S.J.; D’Amato, M.; Pohl, R.T.; Weiss, G.; Ashkenazi, Y.J.; Tichler, T.; Goldin, E.; et al. Sustained virological response with intravenous silibinin: Individualized IFN-free therapy via real-time modelling of HCV kinetics. Liver Int. 2015, 35, 289–294. [Google Scholar] [CrossRef]
- Verna, E.C.; Brown, R.S., Jr. Hepatitis C Virus Infection in Liver Transplant Candidates and Recipients. Available online: https://www.uptodate.com/contents/hepatitis-c-virus-infection-in-liver-transplant-candidates-and-recipients (accessed on 10 March 2019).
- Neumann, U.; Biermer, M.; Eurich, D.; Neuhaus, P.; Berg, T. Successful prevention of hepatitis C virus (HCV) liver graft reinfection by silibinin mono-therapy. J. Hepatol. 2010, 52, 951–952. [Google Scholar] [CrossRef] [PubMed]
- Beinhardt, S.; Rasoul-Rockenschaub, S.; Scherzer, T.M.; Ferenci, P. Silibinin monotherapy prevents graft infection after orthotopic liver transplantation in a patient with chronic hepatitis C. J. Hepatol. 2011, 54, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Eurich, D.; Bahra, M.; Berg, T.; Boas-Knoop, S.; Biermer, M.; Neuhaus, R.; Neuhaus, P.; Neumann, U. Treatment of hepatitis C-virus-reinfection after liver transplant with silibinin in nonresponders to pegylated interferon-based therapy. Exp. Clin. Transplant. 2011, 9, 1–6. [Google Scholar] [PubMed]
- Aghemo, A.; Bhoori, S.; De Nicola, S.; Mazzaferro, V.; Colombo, M. Failure of Intravenous Silibinin Monotherapy to Prevent Hepatitis C Genotype 2A Liver Graft Reinfection. Hepat. Mon. 2012, 12, 411–414. [Google Scholar] [CrossRef]
- Knapstein, J.; Wörns, A.M.; Galle, P.R.; Zimmermann, T. Combination therapy with silibinin, pegylated interferon and ribavirin in a patient with hepatitis C virus genotype 3 reinfection after liver transplantation: A case report. J. Med. Case Rep. 2014, 8, 257. [Google Scholar] [CrossRef][Green Version]
- Mariño, Z.; Crespo, G.; D’Amato, M.; Brambilla, N.; Giacovelli, G.; Rovati, L.; Costa, J.; Navasa, M.; Forns, X. Intravenous silibinin monotherapy shows significant antiviral activity in HCV-infected patients in the peri-transplantation period. J. Hepatol. 2013, 58, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Rendina, M.; D’Amato, M.; Castellaneta, A.; Castellaneta, N.M.; Brambilla, N.; Giacovelli, G.; Rovati, L.; Rizzi, S.F.; Zappimbulso, M.; Bringiotti, R.S.; et al. Antiviral activity and safety profile of silibinin in HCV patients with advanced fibrosis after liver transplantation: A randomized clinical trial. Transpl. Int. 2014, 27, 696–704. [Google Scholar] [CrossRef]
- Bárcena, R.; Moreno, A.; Rodriguez-Gandia, M.A.; Albillos, A.; Arocena, C.; Blesa, C.; García-Hoz, F.; Graus, J.; Nuño, J.; López-Hervás, P.; et al. Safety and anti-HCV effect of prolonged intravenous silibinin in HCV genotype 1 subjects in the immediate liver transplant period. J. Hepatol. 2013, 58, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Maier, I.; Wu, G.Y.; Maier, G.Y.W.I. Hepatitis C and HIV co-infection: A review. World J. Gastroenterol. 2002, 8, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Meissner, E.G. Update in HIV/HCV Co-Infection in the Direct Acting Antiviral Era. Curr. Opin. Gastroenterol. 2017, 33, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Payer, B.; Reiberger, T.; Rutter, K.; Beinhardt, S.; Staettermayer, A.; Peck-Radosavljevic, M.; Ferenci, P. Successful HCV eradication and inhibition of HIV replication by intravenous silibinin in an HIV–HCV coinfected patient. J. Clin. Virol. 2010, 49, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Rauch, A.; Durisch, N.; Eberhard, N.; Anagnostopoulos, A.; Ledergerber, B.; Metzner, K.J.; Böni, J.; Weber, R.; Fehr, J. Efficacy of lead-in silibinin and subsequent triple therapy in difficult-to-treat HIV/hepatitis C virus-coinfected patients. HIV Med. 2014, 15, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.L.; Rauch, A.; Aouri, M.; Durisch, N.; Eberhard, N.; Anagnostopoulos, A.; Ledergerber, B.; Müllhaupt, B.; Metzner, K.J.; Decosterd, L.; et al. A Lead-In with Silibinin Prior to Triple-Therapy Translates into Favorable Treatment Outcomes in Difficult-To-Treat HIV/Hepatitis C Coinfected Patients. PLoS ONE 2015, 10, e0133028. [Google Scholar] [CrossRef]
- Woo, J.S.; Kim, T.-S.; Park, J.-H.; Chi, S.-C. Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch. Pharmacal. Res. 2007, 30, 82–89. [Google Scholar] [CrossRef]
- Wu, J.-W.; Lin, L.-C.; Hung, S.-C.; Chi, C.-W.; Tsai, T.-H. Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. J. Pharm. Biomed. Anal. 2007, 45, 635–641. [Google Scholar] [CrossRef]
- Wu, J.-W.; Lin, L.-C.; Tsai, T.-H. Drug–drug interactions of silymarin on the perspective of pharmacokinetics. J. Ethnopharmacol. 2009, 121, 185–193. [Google Scholar] [CrossRef]
- Weyhenmeyer, R.; Mascher, H.; Birkmayer, J. Study on dose-linearity of the pharmacokinetics of silibinin diastereomers using a new stereospecific assay. Int. J. Clin. Pharmacol. Ther. Toxicol. 1992, 30, 134–138. [Google Scholar]
- Kidd, P.; Head, K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (Siliphos). Altern. Med. A J. Clin. Ther. 2005, 10, 193–203. [Google Scholar]
- Voinovich, D.; Perissutti, B.; Grassi, M.; Passerini, N.; Bigotto, A. Solid State Mechanochemical Activation of Silybum marianum Dry Extract with Betacyclodextrins: Characterization and Bioavailability of the Coground Systems. J. Pharm. Sci. 2009, 98, 4119–4129. [Google Scholar] [CrossRef]
- Kosina, P.; Kren, V.; Gebhardt, R.; Grambal, F.; Ulrichova, J.; Walterova, D. Antioxidant properties of silybin glycosides. Phytother. Res. 2002, 16 (Suppl. 1), S33–S39. [Google Scholar] [CrossRef]
- Mira, L.; Silva, M.; Manso, C. Scavenging of reactive oxygen species by silibinin dihemisuccinate. Biochem. Pharmacol. 1994, 48, 753–759. [Google Scholar] [CrossRef]
- Maheshwari, H.; Agarwal, R.; Patil, C.; Katare, O.P. Preparation and pharmacological evaluation of silibinin liposomes. Arzneimittelforschung 2003, 53, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Rai, A.; Reddy, N.D.; Raj, P.V.; Jain, P.; Deshpande, P.; Mathew, G.; Kutty, N.G.; Udupa, N.; Rao, C.M. Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharmacol. Rep. 2014, 66, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Q.; Hu, J.-H. Improvement of the Dissolution Rate of Silymarin by Means of Solid Dispersions. Chem. Pharm. Bull. (Tokyo) 2004, 52, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.-F.; Jia, W.; Li, S.-S.; Xu, Z.-H.; Sun, X.; Wang, X.-R.; Zhang, Y.-Y.; Xie, G.-X. A new silymarin preparation based on solid dispersion technique. Adv. Ther. 2005, 22, 595–600. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Q.; Huang, Y.; Zhou, Y.; Liu, Y. Development of Silymarin Self-Microemulsifying Drug Delivery System with Enhanced Oral Bioavailability. AAPS PharmSciTech 2010, 11, 672–678. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Que, L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur. J. Pharm. Biopharm. 2006, 63, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Baboota, S.; Ali, J.; Ahuja, A.; Ahmad, S. Stability studies of silymarin nanoemulsion containing Tween 80 as a surfactant. J. Pharm. Bioallied Sci. 2015, 7, 321–324. [Google Scholar] [PubMed]
- Wang, Y.; Zhang, D.; Liu, Z.; Liu, G.; Duan, C.; Jia, L.; Feng, F.; Zhang, X.; Shi, Y.; Zhang, Q. In vitroandin vivoevaluation of silybin nanosuspensions for oral and intravenous delivery. Nanotechnology 2010, 21, 155104. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Ng, L.-T.; Wu, T.-H.; Lin, L.-T.; Yen, F.-L.; Lin, C.-C.; Huang, L.-T. Characteristics and Antioxidant Activities of Silymarin Nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Polyak, S.J.; Ferenci, P.; Pawlotsky, J.M. Hepatoprotective and antiviral functions of silymarin components in hepatitis C virus infection. Hepatology 2013, 57, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
