Abstract
Bacteraemia brought on by Clostridium perfringens has a very low incidence but is severe and fatal in fifty per cent of cases. C. perfringens is a commensal anaerobic bacterium found in the environment and in the intestinal tracts of animals; it is known to produce six major toxins: α-toxin, β-toxin, ε-toxin, and others. C. perfringens is classified into seven types, A, B, C, D, E, F and G, according to its ability to produce α-toxin, enterotoxin, and necrotising enterotoxin. The bacterial isolates from humans include types A and F, which cause gas gangrene, hepatobiliary infection, and sepsis; massive intravascular haemolysis (MIH) occurs in 7–15% of C. perfringens bacteraemia cases, resulting in a rapid progression to death. We treated six patients with MIH at a single centre in Japan; however, unfortunately, they all passed away. From a clinical perspective, MIH patients tended to be younger and were more frequently male; however, there was no difference in the toxin type or genes of the bacterial isolates. In MIH cases, the level of θ-toxin in the culture supernatant of clinical isolates was proportional to the production of inflammatory cytokines in the peripheral blood, suggesting the occurrence of an intense cytokine storm. Severe and systemic haemolysis is considered an evolutionary maladaptation as it leads to the host’s death before the bacterium obtains the benefit of iron utilisation from erythrocytes. The disease’s extraordinarily quick progression and dismal prognosis necessitate a straightforward and expedient diagnosis and treatment. However, a reliable standard of diagnosis and treatment has yet to be put forward due to the lack of sufficient case analysis.
1. Introduction
The anaerobic bacterium Clostridium perfringens, widespread in the environment and gastrointestinal tract, infrequently produces bacteraemia. However, when it does occur, the fatality rate can exceed 52 per cent, making it one of the most severe types of bacteraemia []. C. perfringens infection is more lethal when massive intravascular haemolysis (MIH) develops, with fatality rates ranging from 70 to 100 per cent [,,,,]. At Nihon University Hospital in Tokyo, we observed six cases of C. perfringens-related MIH.
To clarify the clinical and bacteriological profiles of MIH caused by C. perfringens bacteraemia, a systematic search of Pubmed over the past seven decades was conducted in this study. We searched for every English-language case report with C. perfringens and hemolysis (haemolysis) in the title, abstract, or main body. Then, we compared our findings to those of earlier studies.
Recent advancements in highly sensitive molecular detection techniques, such as polymerase chain reaction (PCR), have permitted the detection of bacterial genes in peripheral blood samples. After brushing or dental treatment, susceptible bacteria, such as Polyphylomonas gingivalis, are often detected. Nevertheless, culture tests are essential for accurately diagnosing bacteraemia, in which living bacteria are discovered in the blood. Even in developed countries, 30-day death rates range from 3 to 47% when a definitive diagnosis of clinically significant bacteraemia caused by Staphylococcus aureus, Escherichia coli, Klebsiella spp., and Pseudomonas spp. is made [,]. In most cases of bacteraemia, the bacteria are rapidly eliminated from the bloodstream; however, patients with sepsis have a poor prognosis due to systemic infection and damage to several organs. Understanding sepsis’s pathophysiology is essential, and we must investigate the specific features of relevant bacteria and host immune response.
2. Clostridium Perfringens
C. perfringens is a Gram-positive anaerobic spore-forming bacterium frequently isolated from soil and human and animal intestinal tracts. Interestingly, C. perfringens is also a component of the normal genital flora of 1–10% of healthy women []. C. perfringens is classified into seven types, A, B, C, D, E, F and G, according to the production of six major toxins: α-toxin, β-toxin, ε-toxin, ι-toxin, enterotoxin, and necrotising enterotoxin. All seven types produce α-toxin [,]. In addition to these toxins, C. perfringens secretes more than 20 pathogenic substances. The C. perfringens subtypes usually isolated from humans are type A and type F. Type A organisms only produce α-toxin (phospholipase C, CPA), which causes gas gangrene, hepatobiliary infections, sepsis [,], and foodborne diarrhoea []. Type F organisms produce CPA and enterotoxins, which cause food poisoning and nonfoodborne diarrhoea []. In clinical practice, bacteraemia occurs much less often than food poisoning or gas gangrene. Only approximately 0.12–0.16% of blood culture-positive samples in clinical laboratories show Clostridium spp., with C. perfringens found in 22–42% of them [,,]. Clinically relevant is the fact that 7–15% of patients with C. perfringens bacteraemia suffer massive intravascular haemolysis (MIH) [,], which is characterised by the severe and systemic destruction of red blood cells. MIH is brought on by many pathogeneses [], including immune-mediated and microangiopathic illnesses, malaria, and babesiosis [,]. MIH is characterised by bright red serum (Figure 1) [,].

Figure 1.
Macroscopic appearance of massive haemolysis caused by C. perfringens bacteraemia. Bright red appearance of the serum (arrow).
Though the laboratory criteria for MIH have yet to be established, the clinical diagnosis is simple: due to the release of large amounts of haemoglobin from red blood cells into plasma, the serum of patients with MIH becomes very bright red in appearance. Among various infections that cause MIH, C. perfringens is one of the most critical causative organisms [,].
3. C. perfringens Infection with MIH
Epidemiology
The median age of patients with C. perfringens bacteraemia is reported to be relatively old, ranging from 70.7 to 75.6 years [,,,]. Therefore, older age has been reported to be a risk factor for bacteraemia [,]. Interestingly, in our clinical cohort, the median age of bacteraemia patients with MIH was 61–66.5 years. As reported by us and others, MIH patients are suggested to be significantly younger than non-MIH patients [,,]. In addition, it appears to be more prevalent in men; as reported previously, 60% [] and 58.1% [] were males, while the molecular basis of these gender differences is so far unknown.
C. perfringens bacteraemia is more prevalent in patients with diabetes, malignant neoplasms, biliary tract illness, renal failure, cirrhosis, and/or those being treated with immunosuppressive formulas [,,,]. Community-acquired infections are considered to be more common than hospital-based infections [,,]. C. perfringens-related MIH is typically accompanied by intra-abdominal infections, liver and biliary tract infections, and lower respiratory tract infections. However, 20–30% of cases have an unknown focus [,,], with no significant difference between MIH and non-MIH groups [].
4. Pathogenic Factors
There have been approximately 100 case reports of C. perfringens bacteraemia with MIH over the past 60 years, and the number of cases has been increasing in recent years (Table 1 and Table 2). Nevertheless, because most of these cases are found in single case reports, there has been little research on causative organisms. In six cases, multiplex PCR was applied for typing toxins produced by the causative organism, all of which were type A bacteria that produce only CPA [,,,,]. Type A C. perfringens bacteria, on the other hand, are common and have been linked to hepatobiliary infections, gas gangrene, and sepsis in humans. Furthermore, CPA produced by type A bacteria is produced by all types of C. perfringens []; assuming that the alpha toxin is the main pathogenic toxin in MIH is unreasonable. We typed eleven C. perfringens bacteraemia blood-derived clinical isolates (five from the MIH group and six from the non-MIH group) []. Four of the five C. perfringens strains that caused MIH were type A, one was type F, four of the six non-MIH strains were type A, and two were type F. Involvement of type A and F strains suggests that both may be responsible for MIH. There was no difference in the type of bacteria between the two groups.

Table 1.
Reported case numbers of C. perfringens bacteraemia with MIH from 1951 to 2022.

Table 2.
All reported cases of C. perfringens bacteraemia with MIH from 1991 to 2022.
Next, we examined the virulence factors other than the six toxins used in A-G typing for MIH. We extracted chromosomal DNA from eleven clinical isolates and compared the repertoire of known virulence-related genes between five MIH and six non-MIH groups []. However, there were no differences in the variation of genes considered to encode virulence factors between the groups [].
We then compared the biological characteristics of isolates between MIH and non-MIH groups, and the growth rate of the isolates and the production of CPA did not differ []. In vitro human erythrocyte haemolysis experiments showed that erythrocyte haemolytic elements were present in the culture supernatants of the MIH group bacteria, with significant differences. A significant correlation was found between the erythrocyte haemolytic effect of the culture supernatant of the bacteria and the amount of θ toxin (perfringolysin O, PFO) in the culture supernatant. The amount of PFO in the culture supernatant of this clinical isolate also correlated with cytotoxicity towards human peripheral blood mononuclear cells (PBMCs) and production of interleukin-6 (IL-6) and interleukin-8 (IL-8) by human PBMCs []. However, there was no correlation between CPA in culture supernatants and erythrocyte haemolytic effects, cytotoxicity towards human PBMCs, or production of IL-6 and IL-8 by human PBMCs. These findings suggest that PFO is one of the most important virulence factors in C. perfringens bacteraemia with MIH [].
PFO and CPA produced by C. perfringens have potent cytotoxic and proinflammatory cytokine-inducing effects on human blood cells []. PFO is produced from human PBMCs via induction of tumour necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-13, and macrophage inflammatory protein-1β (MIP-1β), inducing human erythrocyte haemolysis. PFO induces TNF-α, IL-5, IL-6, and IL-8 production more strongly than CPA. CPA induces production of IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, granulocyte macrophage colony-stimulating factor (GM-CSF), and MIP-1β []. PFO, which induces a potent haemolytic and acute inflammatory response, plus the proinflammatory cytokine-producing effect of the CPA produced by all C. perfringens strains, may lead to a rapid and lethal course.
PFO is a cholesterol-dependent cytolysin (CDC) toxin family member that forms pores in membranes containing cholesterol []. PFO has been demonstrated to promote the development of human gas gangrene by synergistically enhancing the action of the main toxin CPA [,,,]. In animal studies, it has been documented that PFO synergistically amplifies the effects of other toxins and contributes to disease progression, such as in bovine necrohemorrhagic enteritis in combination with CPA [] and in enterotoxaemia of sheep and goats in cooperation with ε-toxin []. Despite the ubiquitous occurrence of C. perfringens strains that produce PFO, no illnesses in which PFO has been identified as a major virulence factor have been reported [,,]. More case series and research are required to establish that PFO is the primary virulence factor for MIH in C. perfringens bacteraemia.
5. Symptoms and Laboratory Findings
In MIH patients, severe primary symptoms such as altered consciousness, severe pain, shock, haematuria, and gas formation occur more frequently than in those without MIH []. Sudden onset of severe pain is also characteristic [,,,,], which is difficult to distinguish from myocardial infarction or aortic dissection [,]. Due to the high incidence of intra-abdominal, hepatic, and biliary tract infections, significant abdominal pain is frequently observed. However, the pain may involve the entire abdomen, not just the pericardium, the right upper abdomen, the lower abdomen, or the suprapubic region. In cases of unknown aetiology, abdominal pain, chest pain, back pain, and headache could be present. Serum ALT levels are higher in those with MIH than those without MIH, despite no difference in underlying disease, foci of infection, or total bilirubin levels []; this may reflect a severe inflammatory response and progressive shock [,]. MIH patients present with tachypnoea and subsequent rapid respiratory failure, although the focus is not a respiratory infection. Initial blood gas analysis reveals acidaemia due to metabolic acidosis, followed by further hypoxaemia and respiratory acidosis, often resulting in death from acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) despite ventilatory management. MIH patients may present with metabolic acidosis at an early stage, even in the absence of hypoxemia or chest X-ray abnormalities [,,,,,,,,], and some documented patients were acidotic before the onset of intravascular haemolysis []. A comparison of the symptoms of C. perfringens sepsis with/without MIH is listed in Table 3.

Table 3.
Clinical profiles of C.perfringens infection with/without MIH.
High cytokine levels in the blood have been reported to rapidly cause metabolic acidosis and multiorgan failure with ARDS, acute liver failure, and acute renal failure []. Cytokines are also known to be induced by exotoxins of pathogenic microorganisms, such as Streptococcus pyrogenic exotoxins (SPE) [] and Staphylococcus aureus toxic shock syndrome toxin []. Autopsy findings in reported MIH patients show simple oedema; however, without pathological findings suggestive of so-called bacterial pneumonia, such as inflammatory reactions or massive bacterial growth in the alveoli of the lungs [,]. These findings suggest that inflammatory cytokine levels are strongly related to the rapid progression of C. perfringens bacteraemia with MIH. The significantly higher age group among non-MIH patients with C. perfringens bacteraemia [] may be because the production of inflammatory cytokines decreases with age [,]. The induction of cytokines may be related to PFO and CPA produced by C. perfringens. In particular, TNF-α and IL-6, which PFO strongly induces, produce pathological pain as well as fever [], which may explain the characteristic severe pain, and it has been reported that ALI/ARDS is induced by IL-6, IL-8, and IL-10 [,]. However, because the sera from patients with MIH were strongly haemolytic, it was difficult to measure cytokine levels or C. perfringens-produced toxins in serum samples using ELISA or other laboratory methods. This was also the case at other centres, which may have hindered pathophysiologic analysis.
6. Diagnosis with/without Bacterial Culture
Among patients with C. perfringens bacteraemia, 40–55% have polymicrobial bacteraemia [,], with no difference between the MIH and non-MIH groups. Regarding susceptibility to antimicrobial agents, both groups were found to be sensitive to penicillin and carbapenem, while susceptibility to clindamycin tended to be lower in the MIH group []. Blood culture tests for C. perfringens provide positive results in an average of 16.9 hours, which is faster than those for other Clostridium species []. This is owing to a doubling period of around 7 minutes, which is significantly faster than other bacteria. Due to the rapid progression of MIH, however, patients cannot be treated based on culture results, and many perish by the time the results are acquired. Gram staining of the buffy coat and the presence of Gram-positive rods will lead to an early diagnosis if MIH is suspected based on patient serum results (Figure 2) [,].
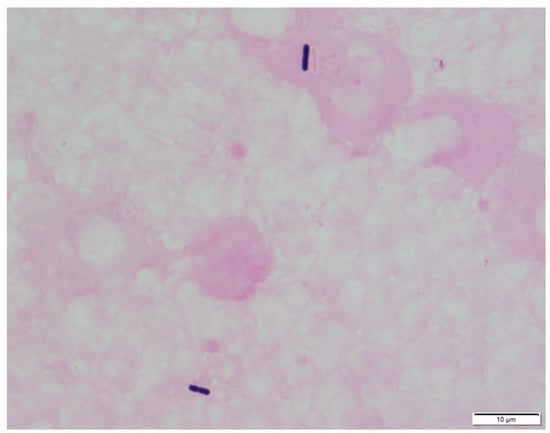
Figure 2.
Microscopic appearance of C. perfringens prepared from the buffy coat of a patient’s peripheral blood and Gram staining (×1000).
7. State-of-the-Art Treatment and Prognosis
C. perfringens bacteraemia with MIH has a poor prognosis. Because reported cases suggested that life expectancy was significantly lower among MIH patients, with attributed mortality of 6/6, or 100%, compared to 13/54, or 24.1%, among non-MIH patients (p 0.001), the mean time between bacteraemia and death was 0.18 days (range: 0.04–1.08 days) in MIH versus 32 days (5–73 days) in controls (p 0.001).
Therefore, it is strongly recommended that a clinician who encounters such cases begin potent systemic antibacterial therapy as soon as it is suspected. Although there are few reports, some suggest that penicillin plus clindamycin lowers the risk of death compared to penicillin alone or other antimicrobial agents []. In association with antimicrobial therapy, surgical resection of infected lesions has been reported to improve survival [] significantly. However, patients with C. perfringens bacteraemia with MIH are already in a state of shock when the disease is suspected. Many patients die before they can benefit from treatment [,], making the choice of surgical intervention difficult. Patients who can undergo surgery may have MIH but are relatively haemodynamically stable and have a high chance of survival []. Blood purification [] and hyperbaric oxygen therapy (HBOT) [] have also been attempted. However, the prognosis for C. perfringens bacteraemia with MIH is extremely poor, with a median time from admission to death of only 10 hours, despite intensive care [,,]. Even when apparently susceptible antimicrobial agents are used, mortality rates range from 70 to 100% [,,,,], and there has been no decrease in mortality over the past 30 years. So far, antimicrobials have been used to treat cases of other Clostridium pathogens, including C. difficile infection (CDI), while C. tetani and C. botulinum infections are already effectively treated with antitoxins. []. Antitoxin therapy has also been utilised to treat gas gangrene induced by C. perfringens []. In newborn piglets infected with C. perfringens type C, commercial swine anti-beta toxoid vaccinations have also been reported to be efficacious against necrotising enterocolitis []. To treat C. perfringens bacteremia with MIH, we suggest using anti-PFO toxin therapy and establishing cytokine-targeted treatment with anti-IL-6 antibodies []. We suggest this because anti-IL-6 monoclonal antibody medicines were initially developed to treat persistent inflammation, such as that caused by autoimmune disorders. Furthermore, multiple data suggest that they are efficacious for COVID-19-induced cytokine storms.
8. Inflammatory Foci or Bacterial Translocation Preceding C. pefringens Invasion Pathways
Bacterial translocation or inflammatory foci can come before C. pefringens invasion routes. Numerous anaerobic bacteria are present in the commensal flora on the skin, oral cavity, gastrointestinal tract, and vagina. Anaerobic bacteria can infect wounded tissues even in the presence of a normally functioning host immune system. In some instances, the illness is caused by a combination of aerobic and anaerobic bacteria, as opposed to anaerobic bacteria alone. The main focus of mixed infection is necrotic tissue resulting from trauma, ischaemia, or malignancies. Neoangiogenesis is induced by inflamed tissue, which boosts blood flow and makes it possible for aerobic or anaerobic bacteria to enter the bloodstream and cause bacteremia. As a result, after an infection has established itself at the primary site, it may spread to other areas through the bloodstream and induce systemic effects, such as disseminated intravascular coagulation (DIC), cytokine storms, and MIH. Most anaerobic infections do not result in DIC when bacteraemia occurs. However, clostridial infections can infrequently result in coagulopathy related to sepsis. In 20–30% of the 60 cases of C. perfringens bacteraemia we documented, the main inflammatory lesion of the bacterial entrance was difficult to identify. This result is reflected in reports from other centres.
This means that C. perfringens can enter the bloodstream without necessitating the formation of inflammatory foci anywhere in the body, which could have catastrophic effects. In such circumstances, the most likely entrance route is bacterial translocation from the gastrointestinal system. Small amounts of enteric bacteria can enter the bloodstream even in healthy people; however, they have been detoxified in the liver via the portal vein. The reticuloendothelial system also processes them at the spleen. However, for unknown reasons, such as an abnormality in the intestinal microbiota or a disruption in the intestinal mucosal barrier, more bacteria are allowed to enter into circulation. Inadequate processing of blood bacteria causes systemic bacteraemia. Furthermore, as previously reported, C. perfringens-produced cholesterol-dependent cytolysin (CDC) is another candidate mucosal disruptor []. This bacterial species damages the mucosal function to prevent bacterial entry into the systemic circulation by silencing mucosal macrophages that protect the intestinal barrier function.
9. Evolutionary Significance of Bacterial Haemolysis
Haemolysis is the breakdown of red blood cells and derives from the Greek word αιμόλυση, meaning “destruction of blood.” It emerged in evolution because of the use of host animals with red blood cells as a source of nutrients. Iron is an essential component for bacterial growth, and it is believed that the breakdown of erythrocytes, which contain large amounts of iron, promotes bacterial growth. Many human infections, particularly Gram-positive cocci, produce haemolysin, a substance that induces haemolysis. In clinical bacteriology, bacteria can also be categorised based on their haemolysis pattern. The haemolytic pattern of bacterial colonies grown on blood agar media determines whether they cause alpha- or beta-haemolysis. Alpha-haemolysis, in which hydrogen peroxide produced by the bacteria oxidises haemoglobin to become methaemoglobin, a green oxidised derivative, is indicative of Streptococcus pneumoniae and Streptococcus viridans. Group A streptococci (GAS) and Streptococcus dysgalactae produce beta-haemolysis (complete haemolysis), a condition in which red blood cells in the medium around and under the colony fully decompose and become transparent. Streptolysin O (SLO) and streptolysin S (SLS), both of which are generated by bacteria, are responsible. SLS specifically damages immunological cells, including polymorphonuclear leukocytes and lymphocytes. For convenience, bacteria that do not cause haemolysis are referred to as “gamma-haemolytic”, which include Enterococcus faecalis and Staphylococcus epidermidis, commensal bacteria of the gastrointestinal system and skin that have no direct contact with red blood cells. C. perfringens is a common bacterium in the gastrointestinal tract and on the skin. As mentioned previously, C. perfringens is found in the environment, intestines, and vagina. It rarely interacts with erythrocytes. Therefore, it is unlikely that C. perfringens actively uses the iron in erythrocytes released by haemolysis, and the damaging haemolysis that occurs with bacteraemia is either incidental or an overreaction by the host. We believe that the haemolysis associated with bacteraemia is either an evolutionary accident or an overreaction by the host, as killing the host would render the parasite bacteria unable to thrive.
10. Involvement of Haemolysis in Pathophysiology of C. Perfringens MIH and Potential for Iron/Haem Scavenging Therapy
Haemolysis is caused by various factors; however, there is growing evidence that free haem and iron can harm the body by activating endothelial and immune cells []. The haem and iron released from erythrocytes as a result of haemolysis promote leukocyte adhesion to endothelial cells, causing damage not only to blood vessels but also to the various functions of systemic organs. Increased circulating free Hb concentrations reduce the Hb scavenger haptoglobin (Hp), part of a critical detoxification system in mammals, scavenging haemolytic by-products in the blood and maintaining normal intracellular metabolism [,]. Furthermore, the haemopexin (Hx) scavenger and the intracellular enzymes haem oxygenase (HO-1 and -2) are involved []. Hp and Hx bind to Hb and haem with high affinity and transport Hb to macrophages and haem to hepatocytes, respectively, preventing oxidative enhancement in the circulation and non-specific uptake in non-target cells; HO degrades haemo porphyrins to iron-carbon monoxide and biliverdin, which have anti-inflammatory, anti-oxidative, and anti-apoptotic effects. Released iron, on the other hand, forms ferritin-heavy chains (H-ferritin). It is oxidised to ferrous iron (Fe2+) by ferroxidase activity; if the haem detoxification system is saturated with high levels of haemolysis, these systems fail []. Increased oxidative stress and elevated levels of soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble endothelial selectin (sE-selectin), tumour necrosis factor (TNF), interleukin-6 (IL-6), and vascular endothelial growth factor (VEGF) result from scavenger depletion []. Soluble haem is a potent inducer of type I IFN, which causes haemophagocytic syndrome and worsens the patient’s prognosis. These findings suggest that C. perfringens-caused severe haemolysis has a common aetiology, with non-infectious haemolysis as a cause of death in patients. In this context, we advocate for early iron/haem scavenging therapy in non-infectious haemolytic diseases.
11. Local or Systemic Modulation of Immune Function by Genus Clostridium and How to Control Them
Bacteria of the genus Clostridium are known for their strong immunoregulatory effects. Regulatory T cells, essential for maintaining pregnancy and preventing the onset of autoimmune diseases, are induced by bacteria of the genus Clostridium living in the intestinal tract []. The number of Treg cells in the large intestines of mice raised in a normal environment is drastically higher than in the intestines of mice raised in a sterile environment (aseptic mice). When sterile mice are inoculated with various intestinal bacteria, Clostridium spp. markedly increase the number of Treg cells in the colon. It has long been known that mice with high levels of Clostridium spp. are less prone to develop enteritis and allergic reactions; however, when the intestinal bacteria are eradicated with antimicrobial agents, the incidence of these reactions increases. Similarly, in humans, Clostridia in the intestinal tract are supposed to induce regulatory T cells. In our study of the intestinal microbiota in children, we reported that intestinal Clostridia are closely related to the development of orthostatic dysregulation and allergic diseases []. It is thought that there are two types of clostridia: so-called “good” clostridia, which are useful for our intestinal environment and immune function, and “bad” clostridia, which are the focus of inflammation and induce excessive immunosuppression. It is difficult to speculate whether the bias in the intestinal microflora can be corrected simply by the administration of probiotics or antibiotics. The most probable candidate is breastfeeding during the neonatal period. This is because C. perfringens colonisation occurs early after birth and persists for an extended period of time throughout life but is reported to be more frequent in children born by caesarean section []. Breastfeeding is strongly recommended to maintain a healthy gut microbiota throughout life [] because it effectively prevents necrotising enterocolitis caused by C. perfringens in infants born by either vaginal delivery or caesarean section.
12. Conclusions
Bacteraemia associated with MIH advances rapidly, and patients with suspected cases frequently die before blood cultures can be completed because they are in serious shock condition when they arrive at the hospital. The clinical features of C. perfringens bacteraemia are severe pain at onset, impaired consciousness, shock, haematuria, metabolic acidosis, and gas formation. When a blood sample from a person with these symptoms shows intravascular haemolysis, the physician should be ready for a very fulminant case outcome. Future multicentre, case-intensive clinical studies using, if possible, the prospective approach is desirable to elucidate the pathophysiology of this rare but fatal disease and to establish treatment for it.
In addition, the identification of the molecular backgrounds of the MIH-causing C. perfringens substrains is required.
There have been numerous instances of various Clostridium species causing haemolysis []. However, this is uncommon, indicating that MIH-related C. perfringens may have a particular mechanism. Moreover, C. perfringens occasionally inhabits the digestive and vaginal tracts. While the number of clinical cases in humans is exceedingly low, it may be possible to clarify the aetiology of MIH by using animal models to identify the strains prone to cause MIH, the relevant genes, and the host immune response and cytokine patterns.
Author Contributions
Conceptualization, S.H. and A.S.; Analysis of clinical data: A.S.; Collection of published materials and analysis A.S.; writing—original draft preparation, A.S.; writing—review and editing, S.H. project administration, S.H.; funding acquisition, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Nihon University Research Grant for emerging infectious diseases (Satoshi Hayakawa 2022–23).
Acknowledgments
The Nihon University research grant for Emerging Infectious Diseases partially supported this study (Satoshi Hayakawa).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morel, G.; Mulier, G.; Ghrenassia, E.; Abdel-Nabey, M.; Tandjaoui, Y.; Kouatchet, A.; Platon, L.; Pène, F.; Moreau, A.S.; Seguin, A.; et al. Non-C. difficile Clostridioides bacteremia in intensive care patients, France. Emerg. Infect. Dis. 2021, 27, 1840–1849. [Google Scholar] [CrossRef]
- Van Bunderen, C.C.; Bomers, M.K.; Wesdorp, E.; Peerbooms, P.; Veenstra, J. Clostridium perfringens septicaemia with massive intravascular haemolysis: A case report and review of the literature. Neth. J. Med. 2010, 68, 343–346. [Google Scholar]
- Simon, T.G.; Bradley, J.; Jones, A.; Carino, G. Massive intravascular hemolysis from Clostridium perfringens septicemia: A review. J. Intensive Care Med. 2014, 29, 327–333. [Google Scholar] [CrossRef]
- Liu, F.; Xue, S.; Zhang, Y.; Yang, J.; Hu, J.; Li, D.; Ma, X.; Wang, J. Clostridium perfringens sepsis in three patients with acute leukemia and review of the literature. Int. J. Hematol. 2021, 113, 508–517. [Google Scholar] [CrossRef]
- Suzaki, A.; Komine-Aizawa, S.; Nishiyama, H.; Hayakawa, S. Massive intravascular hemolysis is an important factor in Clostridium perfringens-induced bacteremia. Intern. Emerg. Med. 2022, 17, 1959–1967. [Google Scholar] [CrossRef]
- Woittiez, N.J.C.; Van Prehn, J.; Van Immerseel, F.; Goossens, E.; Bauer, M.P.; Ramspek, C.L.; Slangen, R.M.E.; Purmer, I.M.; Ludikhuize, J. Toxinotype A Clostridium perfringens causing septicaemia with intravascular haemolysis: Two cases and review of the literature. Int. J. Infect. Dis. 2022, 115, 224–228. [Google Scholar] [CrossRef]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019-results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Kern, W.V.; Rieg, S. Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrug-resistant pathogens. Clin. Microbiol. Infect. 2020, 26, 151–157. [Google Scholar] [CrossRef]
- Adams, B.N.; Lekovic, J.P.; Robinson, S. Clostridium perfringens sepsis following a molar pregnancy. Am. J. Obstet. Gynecol. 2014, 210, e13–e14. [Google Scholar] [CrossRef]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef]
- Uzal, F.A.; Freedman, J.C.; Shrestha, A.; Theoret, J.R.; Garcia, J.; Awad, M.M.; Adams, V.; Moore, R.J.; Rood, J.I.; McClane, B.A. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 2014, 9, 361–377. [Google Scholar] [CrossRef]
- Rechner, P.M.; Agger, W.A.; Mruz, K.; Cogbill, T.H. Clinical features of clostridial bacteremia: A review from a rural area. Clin. Infect. Dis. 2001, 33, 349–353. [Google Scholar] [CrossRef]
- Stabler, S.; Titécat, M.; Duployez, C.; Wallet, F.; Loïez, C.; Bortolotti, P.; Faure, E.; Faure, K.; Kipnis, E.; Dessein, R.; et al. Clinical relevance of Clostridium bacteremia: An 8-year retrospective study. Anaerobe 2020, 63, 102202. [Google Scholar] [CrossRef]
- Leal, J.; Gregson, D.B.; Ross, T.; Church, D.L.; Laupland, K.B. Epidemiology of Clostridium species bacteremia in Calgary, Canada, 2000–2006. J. Infect. 2008, 57, 198–203. [Google Scholar] [CrossRef]
- Martín, S.; Pérez, A.; Aldecoa, C. Sepsis and immunosenescence in the elderly patient: A review. Front. Med. 2017, 4, 20. [Google Scholar] [CrossRef]
- Dhaliwal, G.; Cornett, P.A.; Tierney, L.M., Jr. Hemolytic anemia. Am. Fam. Phys. 2004, 69, 2599–2606. [Google Scholar]
- Ou, T.Y.; Chuang, C.Y.; Chen, C.D.; Cheng, C.Y. Therapeutic plasma exchange in the treatment of complicated Plasmodium falciparum malaria: A case report. J. Clin. Apher. 2018, 33, 419–422. [Google Scholar] [CrossRef]
- Setty, S.; Khalil, Z.; Schori, P.; Azar, M.; Ferrieri, P. Babesiosis: Two atypical cases from Minnesota and a review. Am. J. Clin. Pathol. 2003, 120, 554–559. [Google Scholar] [CrossRef]
- Jakharia, N.; Hossain, A.; Luethy, P.; Riedel, D.J. 48-year-old male with febrile neutropenia and massive hemolysis. Clin. Infect. Dis. 2019, 69, 2193–2194. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsu, P.C.; Chang, H.J.; Cheng, C.W.; Lee, M.H. Clinical significance and outcomes of Clostridium perfringens bacteremia—A 10-year experience at a tertiary care hospital. Int. J. Infect. Dis. 2013, 17, e955–e960. [Google Scholar] [CrossRef]
- Shindo, Y.; Dobashi, Y.; Sakai, T.; Monma, C.; Miyatani, H.; Yoshida, Y. Epidemiological and pathobiological profiles of Clostridium perfringens infections: Review of consecutive series of 33 cases over a 13-year period. Int. J. Clin. Exp. Pathol. 2015, 8, 569–577. [Google Scholar] [PubMed]
- Haddy, R.I.; Nadkarni, D.D.; Mann, B.L.; Little, D.R.; Domers, T.D.; Clover, R.D.; Silvers, M.J. Clostridial bacteremia in the community hospital. Scand. J. Infect. Dis. 2000, 32, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, M.; Ota, K.; Nakamura, E.; Nitta, M.; Oka, M.; Oishi, Y.; Sano, Y.; Yonogi, S.; Takasu, A. Type A fuluminant Clostridium perfringens sepsis indicated RBC/Hb discrepancy; a case report. BMC Infect. Dis. 2019, 19, 719. [Google Scholar] [CrossRef]
- Hashiba, M.; Tomino, A.; Takenaka, N.; Hattori, T.; Kano, H.; Tsuda, M.; Takeyama, N. Clostridium perfringens infection in a febrile patient with severe hemolytic anemia. Am. J. Case Rep. 2016, 17, 219–223. [Google Scholar] [CrossRef]
- Wild, W.; Bormann, F.; Sweiti, H.; Tamimi, N.; Pichulek, D.; Divo, M.; Dörr, P.; Schwarzbach, M. Clostridium perfringens septicemia and a bleeding ulcer of a jejunal interposition: A case report and short review of the literature. Case Rep. Med. 2018, 2018, 4278904. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, A.; Ohtani, K.; Komine-Aizawa, S.; Matsumoto, A.; Kamiya, S.; Hayakawa, S. Pathogenic characterization of Clostridium perfringens strains isolated from patients with massive intravascular hemolysis. Front. Microbiol. 2021, 12, 713509. [Google Scholar] [CrossRef]
- Ohtani, K.; Hirakawa, H.; Tashiro, K.; Yoshizawa, S.; Kuhara, S.; Shimizu, T. Identification of a two-component VirR/VirS regulon in Clostridium perfringens. Anaerobe 2010, 16, 258–264. [Google Scholar] [CrossRef]
- Popoff, M.R. Clostridial pore-forming toxins: Powerful virulence factors. Anaerobe 2014, 30, 220–238. [Google Scholar] [CrossRef]
- Bryant, A.E.; Bergstrom, R.; Zimmerman, G.A.; Salyer, J.L.; Hill, H.R.; Tweten, R.K.; Sato, H.; Stevens, D.L. Clostridium perfringens invasiveness is enhanced by effects of theta toxin upon PMNL structure and function: The roles of leukocytotoxicity and expression of CD11/CD18 adherence glycoprotein. FEMS Immunol. Med. Microbiol. 1993, 7, 321–336. [Google Scholar] [CrossRef]
- Awad, M.M.; Ellemor, D.M.; Boyd, R.L.; Emmins, J.J.; Rood, J.I. Synergistic effects of α-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 2001, 69, 7904–7910. [Google Scholar] [CrossRef]
- Verherstraeten, S.; Goossens, E.; Valgaeren, B.; Pardon, B.; Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Deprez, P.; Wade, K.R.; Tweten, R.; et al. Perfringolysin O: The underrated Clostridium perfringens toxin? Toxins 2015, 7, 1702–1721. [Google Scholar] [CrossRef] [PubMed]
- Verherstraeten, S.; Goossens, E.; Valgaeren, B.; Pardon, B.; Timbermont, L.; Vermeulen, K.; Schauvliege, S.; Haesebrouck, F.; Ducatelle, R.; Deprez, P.; et al. The synergistic necrohemorrhagic action of Clostridium perfringens perfringolysin and alpha toxin in the bovine intestine and against bovine endothelial cells. Vet. Res. 2013, 44, 45. [Google Scholar] [CrossRef] [PubMed]
- Chinen, K. Sudden death caused by Clostridium perfringens sepsis presenting as massive intravascular hemolysis. Autops. Case Rep. 2020, 10, e2020185. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Kishimoto, T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int. Immunol. 2014, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Makino, Y.; Chiba, F.; Motomura, A.; Inokuchi, G.; Yajima, D.; Iwase, H. Fatal Clostridium perfringens septicemia suggested by postmortem computed tomography: A medico-legal autopsy case report. Forensic Sci. Int. 2015, 253, e4–e9. [Google Scholar] [CrossRef]
- Uppal, A.; Hymes, K.; Schwartz, D.R. A 61-year-old-man with massive intravascular hemolysis. Chest 2009, 136, 1424–1427. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef]
- Woźnica, E.A.; Inglot, M.; Woźnica, R.K.; Łysenko, L. Liver dysfunction in sepsis. Adv. Clin. Exp. Med. 2018, 27, 547–551. [Google Scholar] [CrossRef]
- Ng, H.; Lam, S.M.; Shum, H.P.; Yan, W.W. Clostridium perfringens liver abscess with massive haemolysis. Hong Kong Med. J. 2010, 16, 310–312. [Google Scholar]
- Cécilia, R.; Baptiste, V.; Benjamin, C.; Virginie, H.; Guillaume, V.; Philippe, R.; Matthieu, B. Acute hemolysis in the emergency department: Think about Clostridium perfringens! Case Rep. Emerg. Med. 2013, 2013, 948071. [Google Scholar] [CrossRef]
- Ohtani, S.; Watanabe, N.; Kawata, M.; Harada, K.; Himei, M.; Murakami, K. Massive intravascular hemolysis in a patient infected by Clostridium perfringens. Acta. Med. Okayama 2006, 60, 357–360. [Google Scholar] [CrossRef] [PubMed]
- McArthur, H.L.; Dalal, B.I.; Kollmannsberger, C. Intravascular hemolysis as a complication of Clostridium perfringens sepsis. J. Clin. Oncol. 2006, 24, 2387–2388. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int. Immunopharmacol. 2020, 89 Pt B, 107087. [Google Scholar] [CrossRef]
- Hackett, S.P.; Stevens, D.L. Streptococcal toxic shock syndrome: Synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J. Infect. Dis. 1992, 165, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Ikejima, T.; Dinarello, C.A.; Gill, D.M.; Wolf, S.M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J. Clin. Investig. 1984, 73, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, K.P.; Vasas, B.; Kiss, I.; Lazar, A.; Horvath, I.; Simon, M.; Peto, Z.; Urban, E. Fatal Clostridium perfringens sepsis due to emphysematous gastritis and literature review. Anaerobe 2016, 40, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Plowden, J.; Renshaw-Hoelscher, M.; Engleman, C.; Katz, J.; Sambhara, S. Innate immunity in aging: Impact on macrophage funcion. Aging Cell 2004, 3, 161–167. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, infammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Tan, F.; Zhang, J.; Zhu, W. Mechanism of IL-8-induced acute lung injury through pulmonary surfactant proteins A and B. Exp. Ther. Med. 2020, 19, 287–293. [Google Scholar] [CrossRef]
- Aisiku, I.P.; Yamal, J.M.; Doshi, P.; Benoit, J.S.; Gopinath, S.; Goodman, J.C.; Robertson, C.S. Plasma cytokines IL-6, IL-8, and IL-10 are associated with the development of acute respiratory distress syndrome in patients with severe traumatic brain injury. Crit. Care 2016, 20, 288. [Google Scholar] [CrossRef]
- Fujikawa, H.; Araki, M. Clostridium perfringens septicemia with massive intravascular hemolysis. Intern. Med. 2020, 59, 591. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S. Antibody responses to clostridial infection in humans. Clin. Infect. Dis. 1997, 25 (Suppl. S2), S173–S177. [Google Scholar] [CrossRef] [PubMed]
- McSwain, B.; Sawyers, J.L.M.; Lawler, R., Jr. Clostridial infections of the abdominal wall: Review of 10 cases. Ann. Surg. 1966, 163, 859–865. [Google Scholar] [CrossRef]
- Richard, O.K.; Grahofer, A.; Nathues, H.; Posthaus, H. Vaccination against Clostridium perfringens type C enteritis in pigs: A field study using an adapted vaccination scheme. Porcine Health Manag. 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.; Ray, S.; Keyel, P.A. Interaction of macrophages and cholesterol-dependent cytolysins: The impact on immune response and cellular survival. Toxins 2020, 12, 531. [Google Scholar] [CrossRef]
- Vinchi, F.; Sparla, R.; Passos, S.T.; Sharma, R.; Vance, S.Z.; Zreid, H.S.; Juaidi, H.; Manwani, D.; Yazdanbakhsh, K.; Nandi, V.; et al. Vasculo-toxic and pro-inflammatory action of unbound haemoglobin, haem and iron in transfusion-dependent patients with haemolytic anaemias. Br. J. Haematol. 2021, 193, 637–658. [Google Scholar] [CrossRef]
- Vinchi, F.; Tolosano, E. Therapeutic approaches to limit hemolysis-driven endothelial dysfunction: Scavenging free heme to preserve vasculature homeostasis. Oxid. Med. Cell. Longev. 2013, 2013, 396527. [Google Scholar] [CrossRef]
- Chiabrando, D.; Vinchi, F.; Fiorito, V.; Mercurio, S.; Tolosano, E. Heme in pathophysiology: A matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 2014, 5, 61. [Google Scholar] [CrossRef]
- Belcher, J.D.; Mahaseth, H.; Welch, T.E.; Otterbein, L.E.; Hebbel, R.P.; Vercellotti, G.M. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J. Clin. Investig. 2006, 116, 808–816. [Google Scholar] [CrossRef]
- Smith, A.; McCulloh, R.J. Hemopexin and haptoglobin: Allies against heme toxicity from hemoglobin not contenders. Front. Physiol. 2015, 6, 187. [Google Scholar] [CrossRef]
- Liu, Y.; Pal, M.; Bao, W.; Shi, P.A.; Lobo, C.A.; An, X.; Manwani, D.; Zhong, H.; Yazdanbakhsh, K. Type I interferon is induced by hemolysis and drives antibody-mediated erythrophagocytosis in sickle cell disease. Blood 2021, 138, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ishii, W.; Komine-Aizawa, S.; Takano, C.; Fujita, Y.; Morioka, I.; Hayakawa, S. Relationship between the fecal microbiota and depression and anxiety in pediatric patients with orthostatic intolerance. Prim. Care Companion CNS Disord. 2019, 21, 25523. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Tsuji, H.; Takahashi, T.; Nomoto, K.; Kawashima, K.; Nagata, S.; Yamashiro, Y. Gut dysbiosis following C-section instigates higher colonisation of toxigenic Clostridium perfringens in infants. Benef. Microbes 2017, 8, 353–365. [Google Scholar] [CrossRef]
- Yadav, J.P.; Kaur, S.; Dhaka, P.; Vijay, D.; Bedi, J.S. Prevalence, molecular characterization, and antimicrobial resistance profile of Clostridium perfringens from India: A scoping review. Anaerobe 2022, 77, 102639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).