Abstract
In the era of increasing availability of high-resolution chest computed tomography, the diagnosis and management of solitary pulmonary nodules (SPNs) has become a common challenging clinical problem. Meanwhile, surgical techniques have improved, and minimally invasive approaches such as robot- and video-assisted surgery are becoming standard, rendering the palpation of such lesions more difficult, not to mention pure ground-glass opacities, which cannot be felt even in open surgery. In this article, we explore the role of bronchoscopy in helping surgeons achieve successful minimally invasive resections in such cases.
1. Surgery in Lung Cancer
The prevalence of solitary pulmonary nodules (SPNs) can be as high as 50% in lung screening programs [], and their successful management can decrease lung-related mortality by 20%, as shown by The National Lung Screening Trial [].
These results are confirmed by the NELSON study, where 15,792 participants were randomly assigned to either periodic low-dose CT screening or no screening. After 10 years of follow-up, lung cancer mortality was lower in the screening group than in the control group, both among men (lower by 24%) and among women (lower by 33%) []. These numbers underline the importance of early diagnosis and treatment.
While lobectomy is still the gold-standard treatment for early-stage non-small-cell lung carcinoma (NSCLC) in patients with normal pulmonary function tests, segmentectomy has become an established choice of treatment in those with limited functioning, advanced age, and important comorbidities. Its role can also be diagnostic, notably in the case of infra-centimetric lesions, ground-glass opacities (GGOs), or lesions located deeply within the lung parenchyma.
Literature review and metanalysis show that survival is comparable whether the patient is treated by segmentectomy or lobectomy, for early-stage lung cancer [].
Wedge resections can also be considered to treat lung cancer in patients with limited functioning or lung metastases, or to diagnose a lesion of an unknown histopathology [,,].
The key to a successful infra-lobar resection lies in preoperative planning, the correct localization of the lesion, and securing good margins around it [,].
In a multicenter study by Sato et al. published in 2018, the authors highlighted the importance of this concept, and that the depth of the required margin was the most significant factor associated with resection failure in a series of 203 lesions requiring sublobar resections [].
While traditional surgical teaching emphasizes the importance of bimanual palpation of such lesions, minimally invasive techniques have become more and more popular, and do not allow for palpation.
For example, in France, the number of segmentectomies performed via minimally invasive surgery, compared to open thoracotomy, has rapidly increased over the past 10 years (Figure 1).
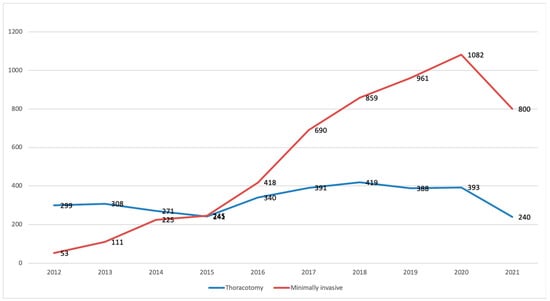
Figure 1.
Number of segmentectomies based on a surgical approach in France between 2009 and September 2021. Data extracted from Epithor database [], in red: minimally invasive surgeries.
To overcome these difficulties, various methods of SPN localization have been developed, including bronchoscopy-based techniques, or percutaneous ones, such as CT-guided radiotracer injection [], CT-guided wire localization [], CT-guided fiducial placement and fluoroscopic localization [], ultrasonography [], and percutaneous methylene blue (MB) injection [].
Practically speaking, CT-guided procedures are cumbersome, and require a high degree of coordination between surgeons, anesthetists, and radiologists, as the technical platform of CT-guided procedures is often located outside the operative theater.
Furthermore, complications such as pneumo- or hemothorax can occur. In a series of 174 CT-guided lipiodol markings reported by Watanabe et al., 16 patients suffered from pain requiring analgesia, 11 from bloody sputum, and 30 from pneumothorax including 11 requiring chest drainage; additionally, 1 patient required an emergency surgery for hemopneumothorax [].
In this review, we explore the different bronchoscopy-based methods, as well as their respective efficacies, which have been the subject of many publications; of these, we cite a recent metanalysis by Yanagia et al. in 2020 [].
2. A Word on Endobronchially Delivered Markers
Endobronchially delivered markers range from dye agents (indigo carmine (IC), Methylene blue (MB), and indocyanine green (ICG)), to the placement of coils and fiducials [,,].
The choice of dye agent depends on many factors, including the time between the localization procedure and surgery, as some agents such as indigo carmine last 2–3 days [], while others such as methylene blue can diffuse to adjacent tissues rapidly [,].
The use of ICG, on the other hand, requires a near-infra-red (NIR) camera. It has been reported as an intrabronchial tattooing agent []. Moreover, it can be used intravenously, either intraoperatively to delaminate the intersegmental plane [], or 24 h prior to surgery to detect nodules as small as 0.2 cm and as deep as 1.3 cm from the pleural surface [].
4. Virtual-Assisted Lung Mapping (VAL-MAP)
Sato et al. reported the use of virtual bronchoscopy and a bronchoscopic multispot dye-marking technique using three-dimensional virtual imaging, for precise thoracoscopic sublobar lung resection with safe surgical margins [].
Three-dimensional reconstruction of computed tomography (CT) data is first performed to generate virtual bronchoscopy images, as this helps the operator design multiple locations for dye marking on the lung surface and define their different routes.
The procedure is achieved under local anesthesia and sedation. Once the bronchoscope reaches the target bronchus, using the predefined virtual bronchoscopy route, a metal-tip catheter is inserted into the bronchus and advanced to the pleura, under fluoroscopic guidance, followed by injection of 1 mL of indigo carmine. This is repeated to complete all the planned markings. The procedure is usually performed within 48 h before surgery, and is followed by a CT scan and 3D reconstructions to confirm the localization of markings within 2 h after the mapping procedure.
In the original article published in 2014, out of the 95 marking attempts for 37 tumors in 30 patients, 88 (92.6%) were identified and contributed to the surgery, with a successful resection rate of 100%. No clinical complications were reported.
In clinical practice, dye marking is not always successful; sometimes, the planned location is not correctly marked and/or not visible on the lung surface. Hence, the role of the post-mapping CT scan in conventional VAL-MAP is significant. Indeed, Sato et al. analyzed 43 markings in 11 patients and found that the average difference between the predicted and actual marking locations was 30 mm. Despite this discrepancy, all lesions were successfully removed thanks to the use of post-mapping CT scan guidance, as it gave the surgeons a 3D understanding of the actual location of the marks and their distances from the lesion, rather than their planned virtual locations [].
This technique also presents logistical challenges regarding the post-dye-marking CT scan. The authors have reported a simpler modified method that uses ENB in order to avoid post-mapping CT scans []. All procedures were performed under general anesthesia in the operative room. Two groups were compared: The first was the non-adjustment group, where surgery was performed immediately after ENB dye marking, while in the second group, the locational information was transferred to a radiology workstation to construct an adjusted 3D image.
The accuracy was graded intraoperatively.
The authors conclude that ENB VAL-MAP quality was improved by adding on-site adjustment, i.e., without post-mapping CT scans, achieving clinical outcomes similar to conventional VAL-MAP.
One of this technique’s limitations (as for ENB) is that the 2D tattooing on the lung surface jeopardizes acceptable resection margins, as the lesion is located deeper into the lung parenchyma. In order to overcome this, VAL-MAP 2.0 was developed. With VAL-MAP 2.0, the difference lies in placing one or two microcoils (visible on intraoperative fluoroscopy) in the most peripheral bronchus, leading to the tumor, thus securing better margins [].
The first clinical trial to investigate the effectiveness and safety of VAL-MAP 2.0 is currently underway, and it is hoped that it will enable more accurate resectioning with sufficient margins, even of deeply located lesions []. The study, however, is a single-arm study, containing biases in patient selection and outcome comparison, compared to other techniques.
While short-term results have been repeatedly reported, Yamaguchi et al. recently published a study focusing on the long term. The authors retrieved clinical data pertaining to the 264 patients (sublobar resection after VAL-MAP) eligible of the 663 ones enrolled in two prospective short-term studies. The 5-year local recurrence-free rate was 98.4%, and the 5-year overall survival (OS) rate was 94.5% [].
To date, VAL-MAP appears to be the most precise technique for preoperative localization of small peripheral lung nodules.
However, it cannot be performed immediately before the surgery or during the same surgical procedure, leading to increased time and resources for each procedure. Therefore, even if it is highly efficient, this technique appears difficult to implement, at least in the near future, in most surgical centers, which conflicts with the growing issue of peripheral lung nodules that need to be removed.
5. Virtual Bronchoscopy Combined with Radial EBUS
Radial EBUS combined with virtual bronchoscopy can easily be used for pleural dye marking, as it is easy to perform in the operative room, relatively cheaper than other techniques, and reproducible, especially for wedge resections or segmentectomies.
The procedure starts with uploading the CT scan into a virtual bronchoscopy program, allowing the pulmonologist, after careful study of the frontal, coronal, and sagittal sections, to mark the target. The software then reconstructs a pathway leading to the lesion. This technique does not provide real-time navigation, and the pulmonologist memorizes the pathway and can consult it on the computer at all times in case of doubt; it is a peri-operative form of assistance. Note that this is the main difference between virtual navigation and augmented navigation (i.e., EMN).
Then, the bronchoscopist follows the predefined route, and reaches the most distal bronchus. The guide sheath with the r-EBUS probe is then inserted into the working channel and pushed towards the lesion in order to reach the subpleural space. The probe is then removed and 1 mL of methylene blue (5 mg/1 mL) is injected and rinsed with 20 mL of air. When the NIR camera is available in the operative room, double dye marking using 0.5 ml of MB and 0.5 of IGG can be used, giving the surgeon full visibility on the lesion at all times, during normal as well as infra-red vision (Figure 3).
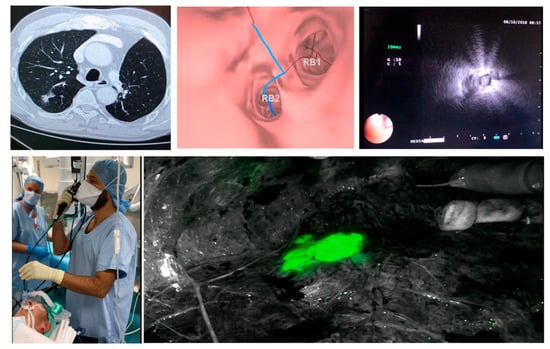
Figure 3.
Dye marking using virtual bronchoscopy + R EBUS, courtesy of Dr. Samy Lachkar.
In our institutional experience, this method adds no more than 10 min of procedure time to the surgery in experienced hands. In fact, between April 2016 and June 2017, all anticipated difficult minimally invasive sublobar resections of peripheral lesions (n = 25) were marked using this method.
The dye was visible on the pleural surface in 24 cases. Histological diagnosis and free margin resection were obtained in all cases [].
Note that the purpose of the procedure is to mark the nodule’s area rather than the nodule itself (which is far from the pleura in the majority of cases), thus helping the surgeon to be more confident regarding the nodule localization.
7. Conclusions
Bronchoscopic dye marking represents a valuable and safe solution to localize small lung nodules in the setting of minimally invasive surgery.
The authors believe that it represents one of the cornerstones of modern surgical management, alongside 3D reconstructions and virtual reality.
The best technique should be precise, time-efficient, and easily reproducible in one’s own center. The future seems promising with the development of robot-assisted techniques and new dyes.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ost, D.; Fein, A.M.; Feinsilver, S.H. Clinical practice. The solitary pulmonary nodule. N. Engl. J. Med. 2003, 348, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Bedetti, B.; Bertolaccini, L.; Rocco, R.; Schmidt, J.; Solli, P.; Scarci, M. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: A systematic review and meta-analysis. J. Thorac. Dis. 2017, 9, 1615–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, S.; Stiles, B.; Altorki, N. What is the role of wedge resection for T1a lung cancer? J. Thorac. Dis. 2018, 10, S1157–S1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Shen, J.; Ren, Y.; Zhong, S.; Zheng, H.; He, J.; Xie, J.H.; Fei, K.; Liang, W.; Jiang, G. Choice of Surgical Procedure for Patients with Non–Small-Cell Lung Cancer ≤1 cm or >1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J. Clin. Oncol. 2016, 34, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Shiono, S.; Okumura, T.; Boku, N.; Hishida, T.; Ohde, Y.; Sakao, Y.; Yoshiya, K.; Hyodo, I.; Mori, K.; Kondo, H. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur. J. Cardio Thorac. Surg. 2017, 51, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Sawabata, N.; Kawase, A.; Takahashi, N.; Kawaguchi, T.; Woo, T.; Saito, Y.; Shiono, S.; Matsutani, N.; From The International Lung-Clinical-Study Organization (ILO). Validating margin status in lung wedge resection for clinical stage I non-small cell lung cancer. Surg. Today 2018, 48, 963–967. [Google Scholar] [CrossRef]
- Schuchert, M.J.; Normolle, D.P.; Awais, O.; Pennathur, A.; Wilson, D.O.; Luketich, J.D.; Landreneaua, R.J. Factors influencing recurrence following anatomic lung resection for clinical stage I non-small cell lung cancer. Lung Cancer Amst. Neth. 2019, 128, 145–151. [Google Scholar] [CrossRef]
- Sato, M.; Kobayashi, M.; Kojima, F.; Tanaka, F.; Yanagiya, M.; Kosaka, S.; Ryuta, F.; Nakajima, J. Effect of virtual-assisted lung mapping in acquisition of surgical margins in sublobar lung resection. J. Thorac. Cardiovasc. Surg. 2018, 156, 1691–1701.e5. [Google Scholar] [CrossRef] [Green Version]
- S.F.C.T.C.V. Société Française de Chirurgie Thoracique et Cardio-Vasculaire [Internet]. Société Française de Chirurgie Thoracique et Cardio-Vasculaire. Available online: https://www.sfctcv.org (accessed on 10 February 2020).
- Grogan, E.L.; Jones, D.R.; Kozower, B.D.; Simmons, W.D.; Daniel, T.M. Identification of Small Lung Nodules: Technique of Radiotracer-Guided Thoracoscopic Biopsy. Ann. Thorac. Surg. 2008, 85, S772–S777. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, J.; Zhang, J.; Hu, H.; Luo, X.; Zhang, Y.; Chen, H. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg. Endosc. 2011, 25, 1723–1729. [Google Scholar] [CrossRef]
- Sancheti, M.S.; Lee, R.; Ahmed, S.U.; Pickens, A.; Fernandez, F.G.; Small, W.C.; Nour, S.G.; Force, S.D. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann. Thorac. Surg. 2014, 97, 1914–1918, discussion 1919. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hirata, T.; Ogawa, E.; Fukuse, T.; Ueda, H.; Koyama, T.; Nakamura, T.; Wada, H. Ultrasonographic evaluation of small nodules in the peripheral lung during video-assisted thoracic surgery (VATS). Eur. J. Cardio Thorac. Surg. 2004, 26, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Wicky, S.; Mayor, B.; Cuttat, J.F.; Schnyder, P. CT-guided localizations of pulmonary nodules with methylene blue injections for thoracoscopic resections. Chest 1994, 106, 1326–1328. [Google Scholar] [CrossRef]
- Watanabe, K.I.; Nomori, H.; Ohtsuka, T.; Kaji, M.; Naruke, T.; Suemasu, K. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: Experience with 174 nodules. J. Thorac. Cardiovasc. Surg. 2006, 132, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Yanagiya, M.; Kawahara, T.; Ueda, K.; Yoshida, D.; Yamaguchi, H.; Sato, M. A meta-analysis of preoperative bronchoscopic marking for pulmonary nodules. Eur. J. Cardio Thorac. Surg. 2020, 58, 40–50. [Google Scholar] [CrossRef]
- Sharma, A.; McDermott, S.; Mathisen, D.J.; Shepard, J.A.O. Preoperative Localization of Lung Nodules with Fiducial Markers: Feasibility and Technical Considerations. Ann. Thorac. Surg. 2017, 103, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Finley, R.J.; Mayo, J.R.; Grant, K.; Clifton, J.C.; English, J.; Leo, J.; Lam, S. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: A prospective randomized controlled trial. J. Thorac. Cardiovasc. Surg. 2015, 149, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Takada, Y.; Endoh, M.; Matsuoka, H.; Tsubota, N. Bronchoscopic dye injection for localization of small pulmonary nodules in thoracoscopic surgery. Ann. Thorac. Surg. 2001, 72, 296–297. [Google Scholar] [CrossRef]
- Krimsky, W.S.; Minnich, D.J.; Cattaneo, S.M.; Sarkar, S.A.; Harley, D.P.; Finley, D.J.; Browning, R.F.; Parrish, S.C. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 23084. [Google Scholar] [CrossRef] [Green Version]
- Vandoni, R.E.; Cuttat, J.F.; Wicky, S.; Suter, M. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur. J. Cardio Thorac. Surg. 1998, 14, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Anayama, T.; Qiu, J.; Chan, H.; Nakajima, T.; Weersink, R.; Daly, M.; McConnell, J.; Waddell, T.; Keshavjee, S.; Jaffray, D. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann. Thorac. Surg. 2015, 99, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Misaki, N.; Chang, S.S.; Gotoh, M.; Yamamoto, Y.; Satoh, K.; Yokomise, H. A novel method for determining adjacent lung segments with infrared thoracoscopy. J. Thorac. Cardiovasc. Surg. 2009, 138, 613–618. [Google Scholar] [CrossRef] [Green Version]
- Okusanya, O.T.; Holt, D.; Heitjan, D.; Deshpande, C.; Venegas, O.; Jiang, J.; Judy, R.; de Jesus, E.; Madajewski, B.; Oh, K. Intraoperative Near-Infrared Imaging Can Identify Pulmonary Nodules. Ann. Thorac. Surg. 2014, 98, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, Y.; Greif, J.; Becker, H.D.; Ernst, A.; Mehta, A. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: The first human study. Chest 2006, 129, 988–994. [Google Scholar] [CrossRef]
- Khandhar, S.J.; Bowling, M.R.; Flandes, J.; Gildea, T.R.; Hood, K.L.; Krimsky, W.S.; Minnich, D.J.; Murgu, S.D.; Pritchett, M.; Toloza, E.M. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: First results of the prospective, multicenter NAVIGATE study. BMC Pulm. Med. 2017, 17, 59. [Google Scholar] [CrossRef]
- Folch, E.E.; Pritchett, M.A.; Nead, M.A.; Bowling, M.R.; Murgu, S.D.; Krimsky, W.S.; Murillo, B.A.; LeMense, G.P.; Minnich, D.J.; Bansal, S.; et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J. Thorac. Oncol. 2019, 14, 445–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folch, E.E.; Bowling, M.R.; Pritchett, M.A.; Murgu, S.D.; Nead, M.A.; Flandes, J.; Krimsky, W.S.; Mahajan, A.K.; LeMense, G.P.; Murillo, B.A.; et al. NAVIGATE 24-Month Results: Electromagnetic Navigation Bronchoscopy for Pulmonary Lesions at 37 Centers in Europe and the United States. J. Thorac. Oncol. 2022, 17, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Bowling, M.R.; Folch, E.E.; Khandhar, S.J.; Arenberg, D.A.; Awais, O.; Minnich, D.J.; Rickman, O.B.; Sztejman, E.; Anciano, C.J. Pleural dye marking of lung nodules by electromagnetic navigation bronchoscopy. Clin. Respir. J. 2019, 13, 700–707. [Google Scholar] [CrossRef]
- Sato, M.; Omasa, M.; Chen, F.; Sato, T.; Sonobe, M.; Bando, T.; Date, H. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J. Thorac. Cardiovasc. Surg. 2014, 147, 1813–1819. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Nagayama, K.; Kuwano, H.; Nitadori, J.I.; Anraku, M.; Nakajima, J. Role of post-mapping computed tomography in virtual-assisted lung mapping. Asian Cardiovasc. Thorac. Ann. 2017, 25, 123–130. [Google Scholar] [CrossRef]
- Sato, M.; Shinohara, Y.; Yanagiya, M.; Karasaki, T.; Kitano, K.; Nagayama, K.; Nakajima, J. Use of electromagnetic navigation bronchoscopy in virtual-assisted lung mapping: The effect of on-site adjustment. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 1062–1069. [Google Scholar] [CrossRef]
- Sato, M. Precise sublobar lung resection for small pulmonary nodules: Localization and beyond. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Uemura, Y.; Sato, M. Protocol for the VAL-MAP 2.0 trial: A multicentre, single-arm, phase III trial to evaluate the effectiveness of virtual-assisted lung mapping by bronchoscopic dye injection and microcoil implementation in patients with small pulmonary nodules in Japan. BMJ Open 2019, 9, e028018. Available online: https://bmjopen.bmj.com/content/9/9/e028018 (accessed on 10 February 2020). [CrossRef] [PubMed] [Green Version]
- Yamaguchi, H.; Sato, M.; Yamamoto, K.; Ueda, K.; Date, H.; Chen-Yoshikawa, T.; Yamada, Y.; Tokuno, J.; Yanagiya, M.; Kojima, F.; et al. Virtual-assisted lung mapping in sublobar resection of small pulmonary nodules, long-term results. Eur. J. Cardio Thorac. Surg. 2022, 61, 761–768. [Google Scholar] [CrossRef]
- Lachkar, S.; Baste, J.M.; Thiberville, L.; Peillon, C.; Rinieri, P.; Piton, N.; Guisier, F.; Salaun, M. Pleural Dye Marking Using Radial Endobronchial Ultrasound and Virtual Bronchoscopy before Sublobar Pulmonary Resection for Small Peripheral Nodules. Respir. Int. Rev. Thorac. Dis. 2018, 95, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Gillespie, C.T. Robotic Endoscopic Airway Challenge: REACH Assessment. Ann. Thorac. Surg. 2018, 106, 293–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarmus, L.; Hospital, F.J.H.; Wahidi, M.; Lee, H.; Chen, A.; Molena, D.; Yu, D.; Akulian, J.; Maldonado, F.; Vachani, A. The Precision-1 Study: A Prospective Single-Blinded Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules. Chest 2019, 156, A2256–A2257. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

