Abstract
Extracellular vesicles (EVs) are lipid bilayer-delimited particles. According to their size and synthesis pathway, EVs can be classified into exosomes, ectosomes (microvesicles), and apoptotic bodies. Extracellular vesicles are of great interest to the scientific community due to their role in cell-to-cell communication and their drug-carrying abilities. The study aims to show opportunities for the application of EVs as drug transporters by considering techniques applicable for loading EVs, current limitations, and the uniqueness of this idea compared to other drug transporters. In addition, EVs have therapeutic potential in anticancer therapy (especially in glioblastoma, pancreatic cancer, and breast cancer).
1. Introduction
Extracellular vesicles (EVs) are a heterogeneous population of cell-derived membrane particles, and are carriers for transferring substances, such as proteins, lipids, RNA, or DNA, at higher concentrations than in their environment (Figure 1).
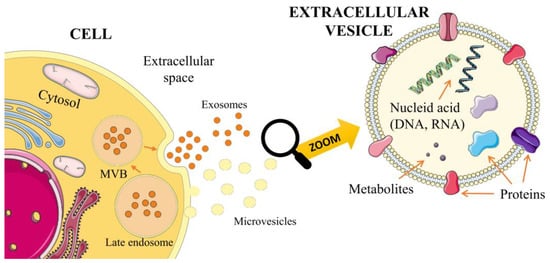
Figure 1.
Biogenesis of exosomes and microvesicles.
Moreover, they can adhere to particular cells or tissues in a receptor-controlled manner to release their contents into the corresponding target structures. Therefore, they play an essential role in intercellular communication [,]. EVs are released from cell surfaces by normal, cancerous, and apoptotic cells [,]. They have also been found in many body fluids, including saliva, urine, milk, and amniotic fluid []. The current classification of EVs is based mainly on their size and the cellular compartment from which they come. EVs can be divided into three major categories: exosomes, ectosomes, and apoptotic bodies [,]. These are characterized in the table below (Table 1).

Table 1.
Characterization of subtypes of EVs: exosomes, ectosomes, and apoptotic bodies.
Exosomes are produced within multivesicular endosomes in the process of MVB maturation, in which the endosomal membrane buds inward. They are secreted as a result of MVB fusion with the cell membrane.
Ectosomes are believed to be formed by pinching off the plasma membrane as the cell contracts in unique spaces. This process is thought to be dependent on certain cytoskeleton components, i.e., microtubules and actin fibers with molecular motors and SNARE proteins.
Apoptotic bodies are formed during apoptosis as a result of blebbing, fragmenting, and dividing of the cell membrane [].
EVs are signal carriers involved in the homeostasis of many bodily functions and participate in cell development, such as cell differentiation []. They are used for cell interactions, which can occur unilaterally or bilaterally. They can both deliver EV cargo and modulate target cells, for example, by activating immune cells []. Extracellular vesicles can fuse with target cells in various ways, including endocytosis, phagocytosis, or direct fusion with the plasma membrane. That process can occur through receptor–ligand interactions []. EVs induce profound phenotypic changes in the tumor microenvironment (TME) by transferring molecules, including oncoproteins and oncopeptides, from donor cells to recipient cells. Tumor cells secrete more EVs than non-malignant cells. Cancer cell growth, invasion, and metastasis depend on bidirectional cell-to-cell communication in complex tissue environments. A growing body of research has focused on assessing the role of EVs in tumor growth and metastasis []. The effect of exosome-derived miRNAs on angiogenesis necessary for cancerous tumor growth has also been pointed out. One example is miRNAs from exosomes released from CD105+ renal cancer cells []. The miRNA molecules contained in exosomes are often specific for a particular type of cancer, such as breast cancer with miR-21 and miR-1246 (exosomes from plasma) [], and Squamous cell carcinoma of the esophagus with miR-21 (serum exosomes) []. Therefore, EVs are considered good diagnostic markers for detecting early cancers in a minimally invasive manner []. A growing body of evidence points to the potential of EVs as carriers for effective drug delivery, which carries excellent pharmacological prospects and is still being developed [].
In order to bring EVs into clinical settings, they can be isolated by several methods, including the most common, namely ultracentrifugation. These methods can be divided into the following categories: density-based, size-based, affinity-based, exosome precipitation, and microfluidic-based isolation []. These are outlined in the table below (Table 2).

Table 2.
Characterization of techniques used for EVs isolation.
2. EVs as Transporters—Opportunities and Limitations
The EV group includes exosomes, ectosomes, and apoptotic bodies, which are naturally responsible for the transport and communication between cells. On this basis, EVs can perfectly function as drug transporters. However, recent years of research on the concept have made it clear that the idea has several limitations, which despite extensive research, still limit the clinical use of EVs.
The EVs have a diverse and heterogeneous structure consisting of proteins, lipids, and nucleic acids. Their size and characteristics vary depending on biogenesis pathways and mother cells []. They are present in almost all body fluids and cells, allowing them to differentiate and create targeted transport on an extensive scale [,]. Moreover, peptide ligand surface modification of the EVs can be successfully carried out using genetic engineering, resulting in the conjugation of additional fusion proteins []. It targets specific tissues and allows signaling pathways to inhibit or induce events []. The small size of the EVs (30 nm–5 μm) and their biocompatible, differentiated structure also creates the possibility of transporting drugs, enzymes, or nucleic acids across the blood–brain barrier [,,]. This extraordinary feature makes the EVs of great importance, since most drugs cannot enter the brain.
In addition, the transport of substances through the EVs, for instance, dopamine, increases their distribution to the brain with minimized toxicity []. Furthermore, it has been shown that significantly lower immune clearance is observed during therapy with EVs than with artificial liposomes. This fact applies to the passage of nanocarriers into cells and cargo insertion [,]. The EVs characterized by excellent transcellular permeability []. Phagocytosis, macropinocytosis, lipid raft interactions, caveolae, receptor-mediated endocytosis, clathrin interactions, direct fusion, or binding are possible cellular pathways for the EVs to enter a cell in a variety of tissues [,]. Depending on the cargo, the possible loading methods can be passive, mechanical, or chemical. However, no universal procedure has been found to reproducibly charge the EVs with different cargos. In addition, the EVs’ lipid membrane structure facilitates the charging of many hydrophobic therapeutics [,]. Cell-free vaccines are developed based on bacterial EVs with antigens and virulence factors [].
On the other hand, EVs have limited loading capacity due to different sizes, which poses problems with planning therapeutic applications []. Furthermore, various EV sizes cause difficulty in standardization and high-throughput production processes. Another area for improvement is the short half-life of the EVs, much faster than for liposomes, complicating the cargo delivery and accumulation in target tissues [].
Moreover, the rapid accumulation of unmodified EVs in the elimination organs (liver, spleen, lungs) is a highly limiting factor for drug distribution []. This problem has been observed for EVs regardless of their tissue origin. Transported cargo must also face possible endosomal escape, which is related to the acidic environment of the lysosomal pathway [,]. Differential expression of cell surface receptors in the recipient’s body is another challenge to overcome in designing EV drug therapies. Furthermore, EVs’ functional heterogeneity also affects cellular pathways, such as immunomodulation []. The lack of understanding of EVs is blamed on the difficulty of experimental observation, structural complexities, and the need for appropriate research methods []. However, despite all these problems, using EVs in drug therapy is promising. Therefore, it is extensively researched worldwide. The opportunities and limitations of EVs as drug transporters are illustrated in Figure 2.
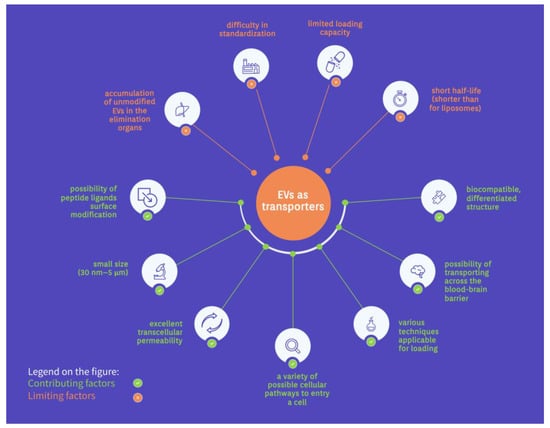
Figure 2.
The opportunities and limitations of EVs as drug transporters.
3. Techniques Applicable for Loading EVs
There are several methods of introducing the drug into EVs, as listed in Figure 3.
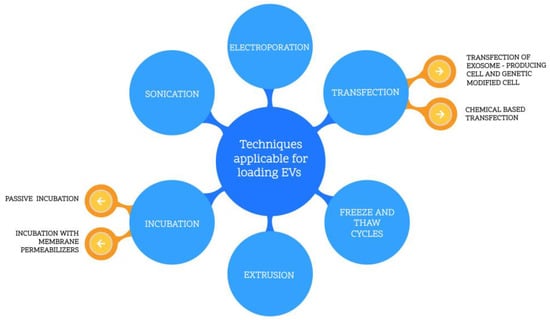
Figure 3.
Techniques applicable for loading EVs.
3.1. Chemical Based Transfection
Chemical reactants can be successfully utilized to insert nucleic acids into the EVs by transfection. Zhang et al. [] presented a modified method protocol for loading miRNAs into the EVs using calcium chloride. First, the miRNAs were mixed with exosomes in a PBS buffer. Subsequently, CaCl2 solution was gradually added until it reached a concentration of 0.1 M. Next, the sample was cooled in ice, then rapidly heated at 42 °C and cooled again in ice. The final step was to isolate the exosomes from the mixture. In this case, it was suspected that the formed CaCl2–RNA complex might be introduced into the exosome under heat shock conditions, resulting in membrane liquefaction. The authors compared the results of this method with electroporation, performed as a reference method []. In both cases, miRNAs were successfully loaded into the EVs with similar efficiency. However, the simplicity and the lack of necessary advanced equipment are in favor of the incubation method. In another paper [], commercial reagent lipofectamine was used as a chemical transfection reactant for loading siRNA into the EVs. The siRNA was mixed with lipofectamine and incubated for 10 min at room temperature. Subsequently, exosome suspension was added and incubated for 30 min. In the end, the solution was filtered from the excess micelles. The transfection reagent HiPerFect was applied likewise to transfect double-stranded siRNA into exosomes. The siRNA was incubated in PBS solution with HiPerFect (QIAGEN, 19300 Germantown, MD 20874, USA) for 10 min at room temperature. Finally, the excess siRNA was purified using latex beads []. The following method is fast and uncomplicated. However, it requires additional purification processes and a specific reagent, which increases the cost.
3.2. Incubation (Permeabilized Membrane or Passive)
Incubation is the simplest method to load EVs. Two types of incubation can be distinguished: incubation utilizing a permeabilized membrane or passive incubation. Permeabilizers are often surfactants that form complexes or interact with the EVs’ membrane, resulting in pore formation and enhanced cargo loading []. Sun et al. [] incubated curcumin with a solution of the EVs in PBS for 5 min at 22 °C, then centrifuged the solution in a sucrose gradient for 1.5 h at 36,000 rpm. The co-incubation method facilitated loading the EVs. Nevertheless, its efficiency is cargo-dependent. In this case, it was hydrophobic curcumin, which interacts with lipid membranes to allow the process. Various drug substances were loaded into the milk-derived exosomes: withaferin A, bilberry-derived anthocyanidins, curcumin, paclitaxel, and docetaxel []. Each was dissolved in ethanol, or a mixture of ethanol and acetonitrile, then added to the exosome solution and incubated at 22 °C. The samples were centrifuged for over two h to purify the loaded EVs. Ultimately the drug loading for the obtained exosomes varied from 10–40%. The value depended on the type and size of the drug. However, it was concluded that the solvent in a concentration of up to 10% did not affect the efficiency of the process []. In other studies [], EVs, porphyrins, and saponins were incubated at room temperature for 10 min. It was concluded that the saponin-assisted method allows for an 11-fold increase in porphyrin loading compared to other passive incubation methods. The result was influenced by increased permeabilization caused by cholesterol complexes in the EVs’ membrane with saponins [].
3.3. Extrusion
The extrusion method involves mixing a cargo solution with the EVs, then introducing the solution into an extruder as a syringe blocked with a porous membrane. It results in the membrane disruption and formation of the EVs with a size appropriate to the membrane used (usually 100–400 nm) []. Fuhrmann et al. [] prepared EVs with porphyrin using a polycarbonate membrane with 400 nm pores in a syringe-based extruder at 42 °C. However, this procedure was repeated 31 times to obtain a single sample. Smaller-sized EVs were presented in Haney et al. [], wherein authors applied a membrane with 200 nm pores for catalase loading. This extrusion was characterized by high efficiency. However, it caused numerous structural changes in EVs. In some cases, cytotoxicity is observed, in contrast to other methods of loading the same cargo into the EVs [,]. Fuhrmann et al. [] observed the changing zeta potential for EVs received by the extrusion method.
3.4. Freeze and Thaw Cycles
The freeze and thaw cycles method typically consists of alternating cycles of temperature shocks: incubation at room temperature and freezing at −80 °C. The cycles are often repeated for enhanced loading efficiency. Haney et al. [] applied the procedure to insert the catalase enzyme into macrophage-derived exosomes. The process consisted of three repeated cycles: 30 min of exosomes incubation at room temperature in PBS buffer with catalase, then rapid freezing of the samples at −80 °C, and re-thawing. The results were not satisfactory compared to other methods (sonication, extrusion, incubation). The loading efficiency obtained for freeze–thaw cycles was 14.7%, and the sonication and extrusion method results were more promising (26.1% and 22.2%, respectively). Nevertheless, incubation at room temperature yielded a much lower result of 4.7%. Furthermore, the catalytic activity of the catalase-loaded EVs prepared by different methods followed the same listed order. An analogous procedure was carried out to load the EVs with peptides composed of 34 amino acids []. Among the mentioned conditions, only the incubation time at room temperature was extended to 2 h. The method showed satisfactory results over various EV sizes (26 to 295 nm).
3.5. Electroporation
Electroporation uses an electrical field to disturb the EV membrane, thus, creating pores through which active agents can be loaded into EVs []. Electroporation can load nucleic acids [,] and drugs [] into EVs. Isolated EVs are mixed with cargo in an electroporation buffer, which can be either sucrose-based [], trehalose-based [], or contain 1.15 mM (pH = 7.2) potassium phosphate, 25 mM potassium chloride, and 21% OptiPrep (Sigma-Aldrich, 14508 St. Louis, MO 68178, United States) [,,,,], 272 mM sucrose, 7 mM (pH = 7.40 di-Potassium hydrogen phosphate, and 1 mM magnesium chloride []. Depending on cargo type, electroporation is carried out to load cargo into EVs at a voltage and capacitance. Then, EVs are isolated using centrifugation and microfiltration [,,,,]. To maximize cargo loading, EV concentration, cargo concentration, electric pulse voltages, and capacitances must be optimized []. Lennard et al. [] proposed the most effective doxorubicin loading method using a doxorubicin–EV ratio of 1 mM:5 × 1011 incubated in a buffer of 400 mM sucrose and electroporated at 950 V and 50 µF.
3.6. Sonication
The first step in the sonication method is incubating cargo with EVs at room temperature for 30 min in 1.5 mL tubes. After that, sonication is performed in a water bath sonicator at 35 kHZ for the 30 s and repeated after placing tubes on ice for 1 min []. Loading of siRNA, miRNA, and ssDNA was greater than passive loading control (325%, 267%, and 225%, respectively) (Table 3) [].

Table 3.
Characterization of techniques applicable for loading EVs.
4. Extracellular Vesicles as Drug Transporters—Practical Examples
Recent findings have pointed out extracellular vesicles (EVs) as potential therapeutic tools to attack HIV infection, given their pivotal role in mediating important cell-to-cell communication mechanisms. EVs may block HIV infectivity, control infection, modulate the immune response, and deliver anti-HIV factors to target cells []. For example, research conducted by Elsharkasy et al. [] showed that cells treated with special vesicles (EVs expressing vesicular stomatitis virus glycoprotein) displayed a reduction in the copy numbers of HIV provirus and the viral protein Nef. Moreover, EVs from other body fluids have also shown anti-HIV effects. EVs isolated from healthy donors’ breast milk have a protective role in vitro. EVs isolated from vaginal fluid could block HIV in vitro at post-entry steps, most likely by halving the reverse transcription and the integration processes [,]. This evidence shows the potential application of extracellular vesicles in treating HIV infection as a novel solution to combination antiretroviral therapy (cART).
The therapeutic potential of EVs is also supported by clinical data emerging from cancer. For example, in glioblastoma (GBM) patients, the levels of PD-L1 DNA in serum-and plasma-derived EVs correlated with tumor volume []. Khan et al. [] showed that poloxamer 188-coated NPs could be used for intracranial glioblastoma treatment. Poloxamers are nonionic triblock copolymers composed of a central hydrophobic chain of polypropylene flanked by two hydrophilic polyoxyethylene chains. Clathrin-mediated endocytosis is the primary mechanism involved in the internalization of NPs, depending on the surface characteristics of NPs. After entry to U87 glioma cells, NPs accessed and released doxorubicin (DOX) in lysosomes, and the drug was transported to nuclei for its cytotoxicity. These results were consistent with the potential of poloxamer 188 binding to Apo proteins and facilitating receptor-mediated endocytosis of nanocarriers [].
Pascucci et al. [] demonstrated that MSC-derived EVs could package and deliver paclitaxel (PTX), and PTX-containing EVs have a strong anti-proliferative activity on human pancreatic cancer cells. Study carried out by Haney et al., showed the high anticancer efficacy of macrophage-derived EVs loaded with PTX (EV-PTX) and Dox (EV-Dox) in a mouse model of pulmonary metastases []. Another study [] which focused on EVs from red blood cells (RBC-EVs) loaded with doxorubicin or sorafenib (SRF) showed enhanced therapeutic effects on a murine model of orthotopic liver cancer through a mechanism dependent on macrophages—the growth of orthotopic liver cancer was inhibited. RBC-Evs loaded with drug showed no systematic toxicity, whereas routine doses of DOX and SRF showed systemic toxicity at therapeutically effective doses. Furthermore, Zhang et al., showed that drug-loaded RBC-EVs have a simple production process, so they are promising for the treatment of liver diseases [].
Kim et al. [] demonstrated that PTX-loaded, macrophage-derived EVs result in more cytotoxicity in P-gp-positive drug-resistant MDCK cells than the free drug alone. Moreover, PTX prodrug and cucurbitacin B-loaded nano micelles caused the inhibition of tumor growth and captured CTCs to suppress cancer metastasis in breast cancer mouse models []. Dendritic cell-derived EV (DEX) administration into patients with melanoma and non-small cell lung cancer (NSCLC) shows modest T-cell activation in clinical trials [,]. DEX from interferon-γ maturated DCs boosts the anti-tumor response of NK cells and achieves better progression-free survival in advanced unresectable NSCLC patients []. However, receiving highly efficient anti-tumor effects in cancer patients is difficult, probably due to the complicated tumor microenvironment. More research is needed to find the possibility of engineering DEX to improve their therapeutic efficacy. In summary, utilizing engineered EVs as drug transporters in therapy is promising.
Utilizing engineered EVs as drug transporters in therapy is promising. The specificity of using exosomes as a drug carrier creates opportunities for treatments of many inflammation-related diseases. Research carried out by Sun et al., showed that incorporation of curcumin into exosomes can increase the solubility, stability, and bioavailability of curcumin []. Exosomal curcumin-treated macrophages produced significantly less IL-6 and TNF-α in comparison with curcumin treatment alone. A study investigating the ability of plant exosomes to deliver curcumin to normal and colon cancer tissue is in phrase 1 trial [].
Research using mesenchymal stromal cell-derived exosomes with KRAS G12D siRNA in treating 28 patients with metastatic pancreas cancer with KrasG12D mutation is in phrase 1 []. Another trial using tumor antigen-loaded dendritic cell-derived exosomes on patients with non-small cell lung cancer is in phase 2 []. It is need to be highlighted that the production of artificial EVs can overcome challenges related to sterility, mass production, and regulation [].
Preclinical studies of EVs as drug delivery systems for cardiovascular disease treatment include acute myocardial infarction, myocardial ischemia reperfusion injury, cerebral ischemia, and cardiotoxicity [,,]. Most of these studies showed the potential therapeutic effects of EVs.
EVs can also be potentially used in treatment of neurological diseases, such as Alzheimer’s disease (curcumin as active pharmaceutical ingredient—API) and Parkinson’s disease (Anti-alpha-synuclein shRNA-minicircle as API) [,].
Despite these promising results, more insights are needed to unlock EVs; full potential, comprehensively assess the risk–benefit ratio, and to establish final conclusions.
5. Conclusions
Despite the difficulties in standardization and high-throughput production processes, a growing body of evidence indicates that using EVs in drug therapy is prospective. The therapeutic potential of EVs is supported by clinical data emerging from the field of cancer, for example, glioblastoma, pancreatic cancer, lung cancer, or breast cancer. EVs as drug delivery systems may be use for cardiovascular and neurological disease treatment. Nevertheless, more research is needed to find the possibility of receiving highly efficient anti-tumor effects in cancer patients.
Author Contributions
Conceptualization, M.N., J.G. and A.C.; investigation, M.N., J.G., K.K. and J.R.; resources, A.C.; writing—original draft preparation, M.N., J.G., K.K. and J.R.; writing—review and editing, A.C.; supervision, A.C.; project administration, A.C.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
Scientific Students’ Group no. 148 of the Department of Molecular and Cellular Biology, Wroclaw Medical University, and partially by the Statutory Subsidy Funds of the Department of Molecular and Cellular Biology no. SUBZ.D260.23.018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Foster, B.P.; Balassa, T.; Benen, T.D.; Dominovic, M.; Elmadjian, G.K.; Florova, V.; Fransolet, M.D.; Kestlerova, A.; Kmiecik, G.; Kostadinova, I.A.; et al. Extracellular vesicles in blood, milk and body fluids of the female and male urogenital tract and with special regard to reproduction. Crit. Rev. Clin. Lab. Sci. 2016, 53, 379–395. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.L.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Chang, W.-H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. Methods Mol. Biol. 2021, 2174, 143–170. [Google Scholar] [CrossRef]
- Sedgwick, A.E.; D’Souza-Schorey, C. The biology of extracellular microvesicles. Traffic 2018, 19, 319–327. [Google Scholar] [CrossRef]
- Szwedowicz, U.; Łapińska, Z.; Gajewska-Naryniecka, A.; Choromańska, A. Exosomes and Other Extracellular Vesicles with High Therapeutic Potential: Their Applications in Oncology, Neurology, and Dermatology. Molecules 2022, 27, 1303. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Fang, H.; Li, Q.; Wang, G. Extracellular vesicles in Inflammatory Skin Disorders: From Pathophysiology to Treatment. Theranostics 2020, 10, 9937–9955. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Regnault, A.; Garin, J.; Wolfers, J.; Zitvogel, L.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999, 147, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef]
- Gross, J.C.; Chaudhary, V.; Bartscherer, K.; Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 2012, 14, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles Released from Human Renal Cancer Stem Cells Stimulate Angiogenesis and Formation of Lung Premetastatic Niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Trigoso, Y.D.; Calloway, C.L.; Zhao, Y.D.; Lum, D.H.; Welm, A.L.; Zhao, Z.J.; Blick, K.E.; Dooley, W.C.; Ding, W.Q. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016, 18, 90. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kamohara, H.; Kinoshita, K.; Kurashige, J.; Ishimoto, T.; Iwatsuki, M.; Watanabe, M.; Baba, H. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer 2013, 119, 1159–1167. [Google Scholar] [CrossRef]
- Campoy, I.; Lanau, L.; Altadill, T.; Sequeiros, T.; Cabrera, S.; Cubo-Abert, M.; Pérez-Benavente, A.; Garcia, A.; Borrós, S.; Santamaria, A.; et al. Exosome-like vesicles in uterine aspirates: A comparison of ultracentrifugation-based isolation protocols. J. Transl. Med. 2016, 14, 180. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.-Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E.; et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, K.; Gao, L.; Zhang, Z.; Li, F.; Zhou, F.; Zhang, L. Methods and Technologies for Exosome Isolation and Characterization. Small Methods 2018, 2, 1800021. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V.; Nagaeva, O.; Dehlin, E. Isolation and Characterization of Exosomes from Cultures of Tissue Explants and Cell Lines. Curr. Protoc. Immunol. 2016, 115, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef]
- Zeringer, E.; Barta, T.; Li, M.; Vlassov, A.V. Strategies for isolation of exosomes. Cold Spring Harb. Protoc. 2015, 2015, 319–323. [Google Scholar] [CrossRef]
- Liu, F.; Vermesh, O.; Mani, V.; Ge, T.J.; Madsen, S.J.; Sabour, A.; Hsu, E.-C.; Gowrishankar, G.; Kanada, M.; Jokerst, J.V.; et al. The Exosome Total Isolation Chip. ACS Nano 2017, 11, 10712–10723. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.-L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; La Franquesa, M.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef]
- Gheinani, A.H.; Vögeli, M.; Baumgartner, U.; Vassella, E.; Draeger, A.; Burkhard, F.C.; Monastyrskaya, K. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 2018, 8, 3945. [Google Scholar] [CrossRef]
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. (Eds.) Enzyme-Linked Immunosorbent Assay (ELISA): From A to Z; Springer: Singapore, 2018; ISBN 978-981-10-6766-2. [Google Scholar]
- Hong, C.S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS ONE 2014, 9, e103310. [Google Scholar] [CrossRef]
- Samsonov, R.; Shtam, T.; Burdakov, V.; Glotov, A.; Tsyrlina, E.; Berstein, L.; Nosov, A.; Evtushenko, V.; Filatov, M.; Malek, A. Lectin-induced agglutination method of urinary exosomes isolation followed by mi-RNA analysis: Application for prostate cancer diagnostic. Prostate 2016, 76, 68–79. [Google Scholar] [CrossRef]
- Lee, K.; Shao, H.; Weissleder, R.; Lee, H. Acoustic purification of extracellular microvesicles. ACS Nano 2015, 9, 2321–2327. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 2016, 16, 489–496. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Fitts, C.A.; Ji, N.; Li, Y.; Tan, C. Exploiting Exosomes in Cancer Liquid Biopsies and Drug Delivery. Adv. Healthc. Mater. 2019, 8, 1801268. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, M.; Koch, M.; Lehr, C.-M.; Fuhrmann, G. Streptococcal Extracellular Membrane Vesicles Are Rapidly Internalized by Immune Cells and Alter Their Cytokine Release. Front. Immunol. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, Z.; Jiang, X.; Yu, X. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Fliervoet, L.A.L.; van der Meel, R.; Fens, M.H.A.M.; Heijnen, H.F.G.; van Bergen En Henegouwen, P.M.P.; Vader, P.; Schiffelers, R.M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release 2016, 224, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, H.; Zhu, Z.; Minhas, J.K.; Jin, Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L110–L121. [Google Scholar] [CrossRef]
- Shtam, T.A.; Kovalev, R.A.; Varfolomeeva, E.Y.; Makarov, E.M.; Kil, Y.V.; Filatov, M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013, 11, 88. [Google Scholar] [CrossRef]
- Wahlgren, J.; de Karlson, T.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Lee, B.-H.; Chen, B.-R.; Huang, C.-T.; Lin, C.-H. The Immune Activity of PT-Peptide Derived from Anti-Lipopolysaccharide Factor of the Swimming Crab Portunus trituberculatus Is Enhanced when Encapsulated in Milk-Derived Extracellular Vesicles. Mar. Drugs 2019, 17, 248. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Lennaárd, A.J.; Mamand, D.R.; Wiklander, R.J.; El Andaloussi, S.; Wiklander, O.P.B. Optimised Electroporation for Loading of Extracellular Vesicles with Doxorubicin. Pharmaceutics 2021, 14, 38. [Google Scholar] [CrossRef]
- El-Andaloussi, S.; Lee, Y.; Lakhal-Littleton, S.; Li, J.; Seow, Y.; Gardiner, C.; Alvarez-Erviti, L.; Sargent, I.L.; Wood, M.J.A. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 2012, 7, 2112–2126. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Ther. Nucleic Acids 2013, 2, e126. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Bai, M.; Wang, J.; Zhu, K.; Liu, R.; Ge, S.; Li, J.; Ning, T.; Deng, T.; et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018, 109, 629–641. [Google Scholar] [CrossRef]
- Navarrete-Muñoz, M.A.; Llorens, C.; Benito, J.M.; Rallón, N. Extracellular Vesicles as a New Promising Therapy in HIV Infection. Front. Immunol. 2021, 12, 811471. [Google Scholar] [CrossRef]
- Smith, J.A.; Daniel, R. Human vaginal fluid contains exosomes that have an inhibitory effect on an early step of the HIV-1 life cycle. AIDS 2016, 30, 2611–2616. [Google Scholar] [CrossRef]
- Näslund, T.I.; Paquin-Proulx, D.; Paredes, P.T.; Vallhov, H.; Sandberg, J.K.; Gabrielsson, S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS 2014, 28, 171–180. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C.; et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent progress of drug nanoformulations targeting to brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Zhao, Y.; Jin, Y.S.; Li, S.M.; Bago, J.R.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy. J. Neuroimmune Pharmacol. 2020, 15, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, X.; Xiu, H.; Sun, Y.; Chen, J.; Cheng, G.; Song, Z.; Peng, Y.; Shen, Y.; Wang, J.; et al. Extracellular vesicles: Natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. J. Extracell. Vesicles 2020, 10, e12030. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Wang, K.; Ye, H.; Zhang, X.; Wang, X.; Yang, B.; Luo, C.; Zhao, Z.; Zhao, J.; Lu, Q.; Zhang, H.; et al. An exosome-like programmable-bioactivating paclitaxel prodrug nanoplatform for enhanced breast cancer metastasis inhibition. Biomaterials 2020, 257, 120224. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.-P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
- Available online: https://Clinicaltrials.gov/ct2/show/NCT01294072 (accessed on 4 May 2023).
- Available online: https://Clinicaltrials.gov/ct2/show/NCT03608631 (accessed on 4 May 2023).
- Available online: https://Clinicaltrials.gov/ct2/show/NCT01159288 (accessed on 4 May 2023).
- Escudé Martinez de Castilla, P.; Tong, L.; Huang, C.; Sofias, A.M.; Pastorin, G.; Chen, X.; Storm, G.; Schiffelers, R.M.; Wang, J.-W. Extracellular vesicles as a drug delivery system: A systematic review of preclinical studies. Adv. Drug Deliv. Rev. 2021, 175, 113801. [Google Scholar] [CrossRef]
- Yang, J.; Wu, S.; Hou, L.; Zhu, D.; Yin, S.; Yang, G.; Wang, Y. Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol. Ther. Nucleic Acids 2020, 21, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.-H.; Liu, H.; Wang, S.-J.; Liang, S.-W.; Wang, G.-G. Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. J. Cell. Physiol. 2019, 234, 13878–13893. [Google Scholar] [CrossRef] [PubMed]
- Izco, M.; Blesa, J.; Schleef, M.; Schmeer, M.; Porcari, R.; Al-Shawi, R.; Ellmerich, S.; de Toro, M.; Gardiner, C.; Seow, Y.; et al. Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology. Mol. Ther. 2019, 27, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).