Abstract
(1) Background: One mechanism through which physical activity (PA) provides benefits is by triggering activity at a molecular level, where neurotrophins (NTs) are known to play an important role. However, the expression of the circulating levels of neurotrophic factors, brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT-4/5), in response to exercise, is not fully understood. Therefore, the aim was to provide an updated overview on the neurotrophin (NT) variation levels of BDNF and NT-4/5 as a consequence of a long-term aerobic exercise intervention, and to understand and describe whether the upregulation of circulating NT levels is a result of neurotrophic factors produced and released from the brain, and/or from neurotrophic secreting peripheral organs. (2) Methods: The articles were collected from PubMed, SPORTDiscus, Web of Science, MEDLINE, and Embase. Data were analyzed through a narrative synthesis. (3) Results: 30 articles studied humans who performed training protocols that ranged from 4 to 48 weeks; 22 articles studied rodents with an intervention period that ranged from 4 to 64 weeks. (4) Conclusions: There is no unanimity between the upregulation of BDNF in humans; conversely, concerning both BDNF and NT-4/5 in animal models, the results are heterogeneous. Whilst BDNF upregulation appears to be in relative agreement, NT-4/5 seems to display contradictory and inconsistent conclusions.
1. Introduction
There is growing evidence that insufficient physical activity (PA), highly linked with society’s modern-day sedentary lifestyle, is a major contributor to the increased risk of various health-related problems [1]. On the other hand, it has been well established that PA provides various stimuli that are capable of enhancing both the metabolic and functional status of the human body [2]. Regular PA has evidenced an improvement of the physiological performance of the skeletal and cardiac muscles and, in addition, the decrease of the incidence of a wide range of diseases, including multiple types of cancers, type 2 diabetes, osteoporosis, and brain disease [3]. PA has particularly been known to provoke a cascade of molecular and cellular processes that naturally support brain plasticity [4]. One central mechanism which accounts for the modifications that occur in brain morphology and plasticity is through PA and its ability to produce, release, and regulate growth factors that influence the development of structural and functional changes on the brain [5].
Growth factors are proteins that control various aspects of cellular function, involving survival, proliferation, migration, and differentiation [6]. Subsequently, when considered in the context of the nervous system, the formerly cited growth factors are regularly referred to as neurotrophic factors [7]. To date, four members have been identified as belonging to the mammalian neurotrophic family; nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4, also referred to as neurotrophin-4/5 (NT-4/5) [8]. Each of these neurotrophic factors bind to one or more cellular receptors and mediate their physiological functions [9].
BDNF, one of the most widely reviewed neurotrophic factors belonging to the mammalian family, and NT-4/5, bind to the tyrosine kinase B receptor (trkB) [10]. Moreover, since both bind to the same receptor, it is thought that their roles may be, to a certain extent, similar [11]. BDNF and NT-4/5 also share more than 50% of amino acid homology [12].
Originally, neurotrophins (NTs) were characterized and limited to their role in initial development, growth, maintenance, and the plasticity of the nervous system throughout development, however, current up-to-date literature has indicated the complex role they fulfill during normal physiology in numerous cell populations across both central and peripheral nervous systems, as well as in neuronal and non-neuronal tissues [10,13,14,15]. Although literature in this respect has been fairly inconsistent, recent reports on human and animal models have attempted to examine the impact and link between PA and the upregulation of neurotrophic factor expression and concentrations in multiple body compartments such as the brain, blood, and muscle. Among the numerous well-known exercise training interventions developed throughout these past years, there seems to be a body of evidence reporting that aerobic exercise appears to increase the expression of NTs, particularly BDNF [9,16,17,18]. Contrarily, less clear, and a rather limited number of studies have evaluated the influence of PA on other NTs, such as NT-4/5 [9]. To date, the evidence seems to suggest that there is no sort of unanimity within the relationship between PA, more specifically aerobic exercise, and a consequential NT-4/5 upregulation within compartments.
One of the main reasons related to the inconsistencies between neurotrophic factor upregulation results and PA is mainly due to discrepancies linked with the heterogeneity between participants characteristics (age, health, status, diseases, etc.), study duration, different intervention exercise programs [4,19], as well as specific methodological considerations, such as blood processing conditions (plasma, serum or whole blood) and timing of collection [4,20], among others. As aforementioned, taken into account that both BDNF and NT-4/5 initiate their intracellular signaling via identical cell surface receptors, it is of interest the reason NT-4/5 has not been evaluated following similar exercise conditions when compared with its neurotrophic counterpart, BDNF [21]. Secondly, according to Pan and colleagues (1998) [22], most members of the mammalian NT family, including BDNF and NT-4/5, appear to cross the blood-brain barrier (BBB), through saturable transport systems, to arrive intact in the central nervous system (CNS), suggesting the possible existence of an exercise-induced neurotrophic crosstalk [23]. Consequently, this increases the difficulty to identify whether in human and animal models these neurotrophic factor fluctuations in serum levels result from changes in central or peripheral tissues. Therefore, the current review aims to provide an updated overview of the NT variation levels of both BDNF and NT-4/5 as a consequence of a long-term aerobic exercise intervention. We will also discuss whether the upregulation of circulating NT levels is a result of the neurotrophic factors produced and released from the brain (cerebral circulation) or, under other conditions, from peripheral organs (peripheral circulation).
2. Methods
The manuscript was authored in agreement with the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [24], having partially adopted the checklist. The protocol was not pre-registered, but was written and approved by the authors before the collection process. Multiple electronic databases were systematically searched from the commencement until 7 July 2020, correspondingly identifying articles published before this date (no restriction was placed on how far back the articles were published). The electronic databases PubMed, SPORTDiscus, Web of Science, MEDLINE, and Embase were used. The following search-term categories were used independently as well as in combination: neurotrophins (e.g., BDNF and neurotrophin-4/5), exercise (e.g., aerobic exercise, treadmill, cycling, swimming), and circulation (e.g., cerebral and peripheral circulation).
The Population, Intervention, Comparator, Outcomes, and Study design (PICOS) framework design was adopted to create the eligibility criteria for the inclusion of articles in this review. This review included adults (>18 years) and animal models with no restrictions regarding physical or cognitive conditions. Therefore, studies involving individuals with risk factors (e.g., overweight, obesity, diabetes), with known cardiometabolic diseases (e.g., Type 2 diabetes, coronary heart disease), or neurological disorders (e.g., Alzheimer’s disease, depression, mild cognitive impairment) were, too, eligible for inclusion. This review included experimental studies that focused on aerobic exercise intervention models. Any other form of exercise (strength training, HIIT, etc.) was not contemplated in this review. Concerning animal protocols, albeit voluntary wheel-running exercise has been associated in diminishing the potential confounding factor of stress, only forced, involuntary exercise protocols were included as they allow control of the intensity, duration, and timing of the exercise. Restrictions regarding the duration of the intervention were considered. A 4-week training protocol was defined as being the minimum duration-period to be contemplated in this review. Control groups and baseline measurements were considered comparators for intervention effect values. Only original, peer-reviewed, English-language literature was examined. Reviews, meta-analyses, books, book reviews, abstracts, scientific conference abstracts, opinion articles, statements, editorials, letters, and commentaries were excluded.
Data Collection, Extraction, and Analysis
To identify potentially eligible articles, two investigators independently screened the titles and abstracts acquired from the retrieved articles through the electronic search according to the inclusion and exclusion criteria. Subsequently, after this first selection, full-length articles were read by the same investigators to define which articles consequently met all the inclusion criteria. Nonetheless, in the case of hypothetical inconsistencies or disagreements concerning inclusion or data extraction, a third independent investigator was consulted. Investigators were not blinded to the titles, authors, institutions or manuscript content during the selection process.
Within each of the identified studies, multiple variables were evaluated to determine if they deliberated the association between an aerobic exercise intervention and the changes in BDNF and NT-4/5 levels from pre-exercise to post-exercise. A standardized electronic data extraction method form was developed to obtain information pertinent to this review. Data withdrawal for each article, extracted into the preformatted spreadsheet by two raters, include information regarding: population studied (exercise group vs. control group), sample size, BDNF & NT-4/5 measurement technique used (analysis technique), time of measure, duration, type, and intensity of exercise, as well as the main outcomes. The last item—main outcomes—involves the variation levels of BDNF and NT-4/5 concentrations (increased, decreased, or no change) between pre- and post-exercise intervention values. A subset analysis has also been conducted examining the effects of interventions of less than 12 weeks, between 12 and 36 weeks, and more than 36 weeks. Furthermore, an analysis based on the exercise type and population of the study has also been performed. Data were analyzed through a narrative synthesis.
3. Results
An initial raw screening resulted in a selection of 907 articles. Following the titles, abstracts, and a full-text article selection, based on specific predetermined criteria, our search resulted in a final selection of 49 reports (Figure 1).
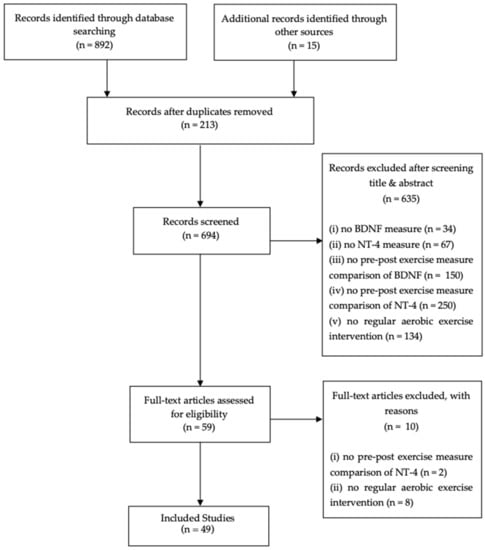
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart.
Regarding the relationship between chronic aerobic exercise interventions and the variation of BDNF blood concentration levels on human subjects, 30 human studies (Table 1) were detected, with a heterogeneous study population varying from young individuals to elderly groups; as well as from healthy study populations to groups with a wide spectrum of medical conditions (e.g., multiple sclerosis [MS], Parkinson’s and Alzheimer’s disease, major depressive disorder [MDD], obese and overweight individuals, etc.). The duration of the exercise protocols varied from 4- to 48-week exercise intervention programs. A wide repertoire of exercise modalities were observed between reports, varying from swimming, treadmill running, cycling, rowing, etc., with the intensity of exercise being relatively similar between the below-mentioned screened articles (moderate-to-vigorous aerobic intensity varying from ±50–80% of VO2max). Most of the analyzed reports (n = 24) assessed BDNF measurements from serum samples, whilst some (n = 8) used plasma as an alternative measure, and one study, alone, adopted platelet BDNF as an assessment sample. Between all 30 studies conducted on human subjects, the conclusions and main-outcomes varied considerably; within the 30 analyzed, 33 group comparators were established. Of these, 12 in total, which represent ≈36.4% of the entire scope of the review, determined no change whatsoever on the BDNF serum concentration levels post-exercise intervention, when compared with the pre-exercise BDNF concentration values and with the controls; a total of 17 comparators, which represent ≈51.5% of the whole scope of studies, concluded an increase in BDNF concentration levels from pre- to post-exercise intervention; lastly, four comparators determined a decrease in BDNF levels, representing ≈12.1% of the total scope of reviewed articles in Table 1.

Table 1.
Summary of studies that have reviewed the relationship between a chronic aerobic exercise intervention and the variation of BDNF blood concentration levels in human subjects.
3.1. Relationship between a Chronic Aerobic Exercise Intervention and the Variation of BDNF Blood Concentration Levels in Human Models
The majority (n = 18) of the studies adopted a protocol of fewer than 12 weeks. An increase in BDNF concentration levels was noted after the intervention in ten studies, whilst 4 studies did not detect any variation as a result of an aerobic exercise program. Some studies (n = 4) reported contradictory results. Concerning the 10 studies with an intervention period ranging from 12 to 36 weeks, 6 of those studies reported no change whatsoever, three an increase, and one contradictory results regarding BDNF concentration levels. From the two studies of more than 36 weeks, one showed an increase and one a decrease in the BDNF concentration levels after the exercise intervention.
From the twelve studies which proposed a PA intervention in healthy people, seven presented no change in BDNF concentration levels, while a total of four studies cited an increase, and one study reported a decrease in BDNF levels. In four studies concerning people with cognitive impairments, one showed an increase in BDNF concentration levels after a PA intervention, two studies presented no change whatsoever, and one displayed contradictory results. Interestingly, the three studies on overweight and obese people reported an increase in BDNF concentration levels after a PA intervention. The results of PA interventions in schizophrenia or related disorders also seem to be positive, with an increase of BDNF concentrations (n = 1). In depressed patients, two studies reported an increase, whilst one study reported no change in BDNF levels after a PA intervention. Studies concerning people with metabolic syndrome (n = 2) or type 2 diabetes (n = 1) recorded no effects or decreases in BDNF concentrations after the intervention.
Training protocols on either a treadmill or free-running increased BDNF concentration levels in six studies. The same number of studies reported no change after the intervention. A decreased level in BDNF concentrations was reported in one study and contradictory results were observed in three studies.
Alternately, on the cycle ergometer, an increase was observed in nine studies, while no effect of training was seen in seven studies which adopted this type of PA intervention. The results in two studies were conflicting. The only study with rowing ergometer training presented no change in BDNF levels. Results are summarized in Table 1.
3.2. Relationship between a Chronic Aerobic Exercise Intervention and the Variation of BDNF Blood Concentration Levels in Animal Models
Concerning the relationship between chronic aerobic exercise interventions and the variation of BDNF blood concentration levels in animal models, 20 studies in total, which met with the exclusion criteria, were extracted (Table 2). The duration between reports was heterogeneous, with the time intervals ranging from 4- to 64-week protocols; the type of exercise varied between swimming and treadmill running; the intensity was considered similar between the articles exhibited in Table 2 (moderate-to-vigorous aerobic intensity). The enzyme-linked immunosorbent assay (ELISA) was the most used analysis technique (n = 14); other BDNF measures used were the Western Blot (n = 5) and the fluorescence-based real-time PCR quantification method (n = 1). The studies (n= 20) displayed in Table 2, regarding animal models, suggest that there seems to be a more consolidated agreement amongst authors concerning the positive relationship between regular exercise training and a consequent increase in BDNF production. Of the 20 articles included in Table 2, 18 denote an upregulation in BDNF concentration levels following an exercise protocol, whereas 2 suggest no change whatsoever.

Table 2.
Summary of studies that have reviewed the relationship between a chronic aerobic exercise intervention and the variation of BDNF blood concentration levels in animal models.
3.3. Relationship between a Chronic Aerobic Exercise Intervention and the Variation of NT-4/5 Blood Concentration Levels in Animal Models
Differently from the studies regarding BDNF and long-term aerobic exercise protocols, which consisted of study populations varying from human to animal models, the NT-4/5 studies concerning the relationship between aerobic exercise and the potential NT-4/5 variation in circulation levels, both centrally and peripherally, are only on animal models. As to the relationship between chronic aerobic exercise interventions and the variation of NT-4/5 blood concentration levels in animal models, two studies met our exclusion criteria (Table 3). The minimum duration of the protocol intervention was, as previously stated, of 4 weeks. The exercise modalities between reports were homologous, with treadmill running being the preferred exercise modality amongst both authors’ studies. Again, the intensity of the exercise between reports was analogous, having been considered as moderate. The conclusions differ between reports; if on the one hand, one report suggests that a long-term aerobic exercise protocol promotes a NT-4/5 expression upregulation, the other appears to indicate that NT-4/5 showed no response whatsoever to exercise (Table 3).

Table 3.
Summary of studies that have reviewed the relationship between a chronic aerobic exercise intervention and the variation of NT-4/5 blood concentration levels in animal models.
4. Discussion
NTs, a family of closely related homodimeric polypeptide growth factors, were originally identified for their role in the survival, development, and function of neurons in both central and peripheral nervous systems [73]. The discovery of nerve growth factor (NGF) by Rita Levi di Montalcini and Viktor Hamburger, in the 1950s, represents a breakthrough in the processes that led to modern-day cell biology [74]. After the discovery of NGF and the understanding of the roles it plays in the central and peripheral nervous systems, other neuron promoting neurotrophic factors belonging to the mammalian family were identified, including BDNF, NT-3, and NT-4/5 [75]. These members appear to share a great amount of structural and chemical similarities, including more than 50% of sequence homologies in the primary structure, relatively similar molecular weights, and three disulfide bonds that form a cysteine knot [8]. All NTs are synthesized as larger precursors known as proNTs, and undergo proteolytic cleavage to become mature, biologically active, NTs [76]. These neurotrophic factors exert their biological effects through the binding action from two highly distinct receptors; the tyrosine kinase receptor (Trk), and a member of the tumor necrosis factor superfamily known as the P75 neurotrophin receptor (p75NTR) [6,75]. Both pro-NTs—the immature neurotrophic factor form—and mature neurotrophins—bind to p75NTR; however, the mature proteins have more affinity with the three members of the tropomyosin-related kinase family of receptor tyrosine kinases [77] when compared to p75NTR. Hence, mature NGF binds to TrkA, BDNF and NT-4/5 bind to TrkB, and NT-3 binds to TrkC [10]. However, there seems to occur a certain overlap, due to some redundancy in the structures of the formerly cited neurotrophic factor cell surface receptors, therefore, NT-3 can also bind to TrkA and TrkB, although with less affinity when compared with the third Trk receptor counterpart, TrkC [10]. According to Phillips (2017) [78], proneurotrophin binding to p75NTR, via activation of a receptor complex composed of p75NTR and sortilin [79], are known to reduce spine complexity and density [80], induce long-term depression [81], promote neuronal cell death [79], and facilitate the resculpting of neuronal circuits [82]. In opposition to the immature neurotrophic binding interaction, mature NTs, which bind to the Trk receptors, induce, according to Phillips (2017) [78], cell survival and differentiation, dendritic spine complexity, long-term potentiation [83,84], synaptic plasticity, and the resculpting of networks [85]. As aforesaid, only mature NTs bind to the Trk receptors, resulting in the activation of three main pathways: Ras, primarily responsible for the control of normal survival and differentiation by activating mitogen-activated protein kinase; phosphatidylinositol 3-kinase, which manages several neuronal functions such as survival and neurite outgrowth through activation of the protein kinase B, commonly known as AKT; and phospholipase C-γ1, which accounts for the control of activity-dependent synaptic plasticity [8]. Similarly to the Trk receptors, p75NTR also activates three major signaling pathways [86]. The first signaling pathway, NF-kappa B, leads to the transcription of multiple genes; the second, through the activation of Jun kinase via p75NTR, leads to neuronal apoptosis; and the last major signaling pathway, Rho activity, controls the growth cone motility [86,87]. Regarding neurotrophic factor secretion, these proteins are known to be produced, released and expressed from both neuronal and non-neuronal cell populations across multiple tissue systems [8,13]. Consequently, although these neurotrophic factors have been acknowledged as molecules responsible for neuronal cell maintenance, it has been well established that they also possess a broad repertoire of functions outside the nervous system [13,15]. Therefore, besides the aforementioned role they exert in neurogenesis regulation, neuronal differentiation and survival, neuronal plasticity, and neuronal conduction [88], they also seem to contribute to the promotion of angiogenesis and survival of adult endothelial cells, vascular smooth muscle cells, and cardiomyocytes; participate in the processes of inflammation and immunity interactions; influence lipid metabolism control; and regulate type 2 diabetes mellitus [89].
4.1. BDNF
In 1982, BDNF, the second member of the neurotrophic family of neurotrophic factors, was discovered through the purification of a homogenized pig brain, where it was recognized for its role in the growth and survival of sensory neurons [90]. Currently, BDNF has been identified as a neurotrophic factor that supports the proliferation, survival and differentiation of neurons in the peripheral and central nervous systems [91].
Although BDNF has been extensively characterized for the essential role it plays in neuronal survival and development, known to serve as a neurotransmitter modulator, and participate in neuronal plasticity [89,92], it also seems to promote actions on cardiac and endothelial cells [93], act as a mediator between airway inflammatory events and neuronal changes (inflammation and immunity) [89], affect energy metabolism (lipid metabolism) [94,95], is also implicated in the cytoprotective action in type 2 diabetes mellitus [96], and, through the ability of this neurotrophic factor to enhance neurogenesis and consequently improve synaptic plasticity, as formerly discussed, evidence suggests that it may be highly linked with multiple neurological conditions such as Alzheimer’s disease, dementia, Huntington’s disease, bipolar disease, and autism [41,49,50].
As previously mentioned, the cellular processing of neurotrophic factors, especially of BDNF, has been significantly reviewed throughout these past years within the literature [97]. Synthesis and maturation of BDNF consists of a multistage process, involving the sequential formation of numerous precursor isoforms [98]. BDNF is initially synthesized as a precursor, identified as a folded pre-pro-BDNF form in the endoplasmatic reticulum [98,99]. Subsequently, the pre-region sequence is removed upon translocation through the Golgi membrane, yielding the 32 kDa isoform, consisting of 129 amino acid proteins of BDNF, also referred to as pro-BDNF [98,99]. Consequently, following the cleavage of the signal peptide, the pro-BDNF isoform is converted to a mature BDNF isoform (m-BDNF) by intracellular (e.g., PC7, furin, and proconvertases), or by extracellular proteases (e.g., metalloproteinases and plasmin), resulting in a 13 kDa polypeptide [30,54,55]. Accumulating evidence has suggested that contrary to what was previously thought, both pro-BDNF and its mature isoform, m-BDNF, are biologically active [100]. Furthermore, the mature form of BDNF is identical between all mammals [101]. Regarding the interactions with different types of receptors according to BDNF’s multiple isoforms, pro-BDNF interacts, preferentially, with the p75NTR through its mature domain, and with the sortilin receptor [98], whereas m-BDNF binds with the TrkB receptor [102]. This neurotrophic factor is found in elevated concentrations in the CNS, predominantly in the brain regions of the hippocampus, cerebral cortex, hypothalamus, and cerebellum [103]. Central BDNF can cross the BBB and, therefore, be found in the bloodstream. However, circulating BDNF can be derived from multiple sources other than the CNS [104,105]. Accordingly, it has been well established that a great number of tissues produce and release this neurotrophic substance, including the lungs, bladder, intestinal tissue, vascular endothelial cells, skeletal and cardiac muscle, peripheral neurons, peripheral blood mononuclear cells, and platelets [106,107,108,109,110,111,112,113,114]. Therefore, it is seemingly difficult to ascertain the peripheric and brain contribution to the seric BDNF levels [104].
The source of BDNF in the peripheral circulation raises several questions and is far from being clear. In 2018, Naeglin et al. [115] searched for the range of BDNF levels that could be considered normal in human blood. They found that BDNF can be measured in human plasma, however, comparisons between populations were not reliable, consequently needing future cohort studies. They also found a correlation between platelet and serum BDNF. Nevertheless, the source of plasma/serum and platelet BDNF was not clarified. On the other hand, a work by Chacón-Fernandéz and colleagues (2016) [116] referred that the possible source of platelet BDNF are megakaryocytes, the progenitors of platelets. Moreover, the same authors suggested that the induced-BDNF release by physical exercise may reflect the changes that happen in megakaryocytes and platelets, including the ability of the latter to retain and release BDNF. In spite of these articles, it is known, as referred to previously, that other organs and tissues, such as the skeletal muscle, are able to produce considerable amounts of BDNF during contraction (aerobic physical exercise); however, the skeletal muscles contribution in the variation levels in plasma and brain BDNF remains unclear.
4.2. NT-4/5
Although the trophic effects on central and peripheral neurons have been well-established both in vivo and in vitro, NT-4, also referred to as NT-4/5, continues to be the least studied member of the mammalian NT family [117]. According to László, et al. (2019) [118], NT-4/5 is responsible for promoting survival and differentiation of hippocampal, noradrenergic and dopaminergic neurons, as well as being involved in the development of chemo-afferent sensory neurons known to innervate the carotid body; furthermore, this neurotrophic factor has a strong survival and proliferative action on NIH 3T3 cells expressing TrkB, however, it seems to have relatively limited activity on 3T3 cells expressing TrkA [119]. Except for being the most recently isolated neurotrophic factor of the mammalian family, as well as being the least well understood, numerous features seem to characterize this protein in a unique manner, when compared with its mammalian neurotrophic family counterparts [120]. Thus, in contrast to the other NTs, its expression is ubiquitous and appears to be less influenced by environmental signals [120]; in addition, it is also expressed at substantially inferior levels when compared with any other neurotrophic factor [121].
NT-4/5 was initially identified in xenopus and viper, having later been discovered in both rats and humans [119]. The mature NT-4/5 sequences between human and rat models are 95% identical at an amino-acid level [122]. Consequently, the immature form of both human and rat NT-4/5 isoforms consists, respectively, of a 210 and a 209 amino-acid sequence; following the removal of the prepropeptide, both human and rat NT-4/5, in its mature form, consists of a 130 amino-acid chain [119], with a molar mass equivalent to 14 kDa [123]. Depending on its isoform—mature or immature—NT-4/5 exerts its biological activities by binding to either TrkB or p75NTR [124,125].
In contrast to BDNF, NGF and NT-3, information concerning the sites of synthesis of NT-4/5 are quite limited, with low concentrations being detected in the whole rat embryo, the whole brain of the adult rat and in some rat and human peripheral tissues [122,126]. Concerning NT-4/5 detection within tissues, results revealed by Timmusk and colleagues (1993) [121] show a developmentally regulated expression of NT-4/5 mRNA in most tissues, with an apparent functional implication for NT-4/5 in the nervous system (e.g., ten brain regions including x and y), as well as in peripheral and non-neuronal tissues (e.g., heart, liver, muscle, skin, lung, kidney, thymus, spleen, submandibular gland, pituitary, thyroid, testis, ovary); however, following conclusions presented by previous authors [122,126], results from Timmusk and colleagues (1993) [121] reveal low levels of NT-4/5 mRNA in a wide range of embryonic and adult rat tissues. Tissue distribution of human NT-4/5 transcripts was identified in a limited number of peripheral tissues, with the highest levels found in the prostate and lower levels in the thymus, placenta, skeletal muscle, and the testis; NT-4/5-hybridizing transcripts were not found in the brain [122]. This neurotrophic factor is also present in human serum [127] and is also capable of crossing the BBB [22]. This is suggestive that these NTs can travel both ways: from the CNS to the periphery and from the periphery into the brain.
4.3. Physical Exercise & Neurotrophic Factors
A kaleidoscope of significant benefits of PA, particularly aerobic exercise [9,128], on human health, have long been correlated and predominantly include the decreased risk of cardiovascular disease, diabetes, cancer, osteoporosis, and CNS diseases [9,129]. Only recently, however, has it been admitted that PA may also stimulate a panoply of molecular and cellular processes [130], known to modulate a cascade of chemical messengers referred to as neurotransmitters and NTs, which act in an activity-dependent manner, resulting in the potentiation of the neural function and in the induction of several events which support the structural and functional plasticity of the brain [5,60,86]; nonetheless, the exact mechanisms through which PA induces an upregulation of neurotrophic factor expression have still not been fully elucidated.
The use of animals for scientific purposes is a relatively antique practice used in biological research and medicine, however, associated with this methodical scientific approach is an array of recurrent ethical concerns [131,132]. There are extraordinary anatomical and physiological similarities between human and animal mammalian models [131] and, as a consequence, an extensive number of reports have been carried out to study and comprehend the relationship between PA and NTs in cellular and animal models [9]; however, it must be taken into consideration that not all of the results can be forthrightly translated to humans [131] and, therefore, must be interpreted with caution.
4.4. BDNF & Aerobic Exercise
BDNF is one of the most extensively reviewed neurotrophic factors belonging to the mammalian family. Therefore, it is not surprising that there is a great number of reports focusing and investigating the effects of PA, in particular aerobic exercise, on BDNF’s concentration variation levels in both human and animal models. One major hallmark related with the neurotrophic factor and physical exercise field of study was the discovery of an active interface known as the BBB, which allows the crosstalk of certain substances (e.g., polypeptides, such as NTs and cytokines) between the periphery and the CNS [133].
4.5. NT-4/5 and Aerobic Exercise
Contrasting the great number of reports and consequential data available regarding BDNF, a reasonably limited sum of studies have assessed the influence of long-term aerobic exercise protocols on the variation levels of NT-4/5 in the cerebral and peripheral circulation [9]. Peculiarly, NT-4/5 studies concerning the relationship between aerobic exercise and the potential NT-4/5 variation in circulation levels, both cerebrally and peripherally, only regard animal models. The conclusions differ markedly between reports; if on one hand, the main outcome from one study [58] suggests that a long-term aerobic exercise protocol promotes a NT-4/5 expression upregulation, the other [18] appears to suggest that NT-4/5 showed no response whatsoever to exercise.
4.6. The Impact of Aerobic Exercise on Circulating Neurotrophin Levels (BDNF and NT-4/5) from Cerebral and Peripheral Circulation: Which Organs Are the Main Contributors?
BDNF, which is present in the blood both at rest and during exercise training is, according to Walsh & Tschakovsky (2018) [105], most likely derived from several tissue sources (lungs, bladder, intestinal tissue, vascular endothelial cells, skeletal and cardiac muscle, peripheral neurons, peripheral blood mononuclear cells, platelets, and the brain) known to produce and release neurotrophic factors into circulation in response to exercise-like stimuli [105,106,107,108,110,113]. As previously mentioned, contrary to BDNF and other members of the neurotrophic factor family, information regarding NT-4/5 is limited. The conclusions presented by Timmusk and colleagues (1993) [121] seem to be in accordance with those also admitted by Ibañez (1996) [120] in the means that this neurotrophic substances expression—NT-4/5—seems to be undoubtedly ubiquitous, found in a broad range of tissues and organs, such as in the heart, liver, muscle, thymus, lung, kidney, testis, ovary, salivary gland, cerebral cortex, brain stem and the hippocampus [121].
As it has been formerly stated, one major hallmark related to the neurotrophic factor and PA field of study was the discovery of the BBB, which has been acknowledged in enabling the crosstalk of NTs—e.g., BDNF and NT-4/5—through saturable transport systems, between the periphery and the CNS [133]. Taken into account that both BDNF and NT-4/5 have been found to be produced and expressed in a variety of tissues and organs, and are correspondingly found in the periphery and CNS, suggesting the existence of a neurotrophic crosstalk loop between systems through the BBB, it is of particular interest to understand up to what extent these multiple cell-populations, known to produce and express neurotrophic factors, contribute in the total amount of BDNF and NT-4/5 found in the blood.
To date, the only study that seems to evaluate the contribution of the human brain to plasma BDNF at rest and during prolonged whole-body exercise was one published by Rasmussen and colleagues (2009) [134]. According to the previously mentioned author, following a four-hour rowing ergometer protocol, completed by eight volunteers, and a two-hour treadmill session, completed by 32 mice, the main outcomes were that in humans, a BDNF release from the brain was detected at rest (p < 0.05), and increased two to threefold during exercise (p < 0.05); concerning the mice study group, exercise provoked a three to fivefold upsurge in BDNF mRNA expression in the hippocampus and cortex, peaking 2 h after the conclusion of the exercise protocol. The results obtained by Rasmussen and colleagues (2009) determined that both at rest and during exercise, the brain contributed for up to 70–80% of the circulating BDNF, consequently suggesting that the brain is, in fact, the main, but not the only contributor, of circulating BDNF. On the other hand, in the case of NT-4/5, to date, no study whatsoever has been conducted in the means to understand up to what extent tissues from the CNS and from the periphery contribute to the NT-4/5 levels found in the blood.
This review has limitations. Firstly, the sample characteristics were not always specified by the authors, making it impossible for an accurate subgroup analysis. Furthermore, the physical fitness levels of the participants that took part in the studies were not always detected, making it difficult to evaluate the conclusions of the included studies. Additionally, especially in the animals’ model studies, the inconsistencies in the duration, type and intensity of the exercise interventions, as well as specific methodological considerations such as blood processing conditions (plasma, serum or platelet), contributed to the subsequent variability of results. Further reports, with both increased sample sizes and longer durations, are needed to evaluate the effect of a chronic aerobic exercise intervention on blood BDNF and NT-4/5 concentrations in a more conclusive manner. Moreover, additional research regarding the relationship between peripheral and cerebral concentrations of both BDNF and NT-4/5 is required to evaluate the effect of long-term (chronic) aerobic exercise on the BDNF and NT-4/5 concentration levels in the blood.
5. Conclusions
This review provides, firstly, an updated overview on the evidence concerning the role that long-term aerobic exercise programs may induce on BDNF and NT-4/5 blood concentration levels and, secondly, attempted to understand up to what extent the literature studied the contribution of cerebral and peripheral BDNF and NT-4/5 secreting cell-populations, responsible for these NTs found in the blood.
Regarding the relationship between BDNF and long-term aerobic exercise protocols, studies performed in human populations appear to demonstrate a certain level of ambiguity on the effects that PA, more specifically aerobic exercise, provokes in the stimulation and enhancement of BDNF blood concentration levels. On the other hand, in animal models, the BDNF and long-term aerobic exercise protocol relationship appears to be relatively unquestionable, in the means that regular exercise training does, indeed, in most cases, lead to an increase in BDNF concentration levels found in the blood.
Concerning the relationship between NT-4/5 and long-term aerobic exercise protocols, evidence is fairly inconsistent due to the lack of studies, consequently making it difficult to reach a final conclusion.
Author Contributions
D.R. and P.T. conceived and designed research. D.R. and P.T. collected the articles, analyzed data and interpreted results and prepared figures and drafted manuscript. F.C.P., L.P., A.B. and A.M. edited and critically revised manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by Fundação para a Ciência e Tecnologia (FCT, Portugal): Speed, crash and run: exersomes boost neuroenergetics and mood in mice on speed project (MOOD EXERsomes, POCI-01-0145-FEDER-030786; PTDC/SAU-DES/30786/2017), Strategic Projects (UID/NEU/04539/2013 and UID/NEU/04539/2019),), Strategic Project UIDB/04539/2020 and UIDP/04539/2020 (CIBB), COMPETE-FEDER (POCI-01-0145-FEDER-007440) and Centro 2020 Regional Operational Programmes (CENTRO-01-0145-FEDER-000012: HealthyAging2020 and CENTRO-01-0145-FEDER- 000008: BrainHealth 2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Dishman, R.K.; Berthoud, H.R.; Booth, F.W.; Cotman, C.W.; Edgerton, V.R.; Fleshner, M.R.; Gandevia, S.C.; Gomez-Pinilla, F.; Greenwood, B.N.; Hillman, C.H.; et al. Neurobiology of exercise. Obesity (Silver Spring) 2006, 14, 345–356. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Pilc, A. The effect of physical activity on the brain derived neurotrophic factor: From animal to human studies. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2010, 61, 533–541. [Google Scholar]
- Zembron-Lacny, A.; Dziubek, W.; Rynkiewicz, M.; Morawin, B.; Woźniewski, M. Peripheral brain-derived neurotrophic factor is related to cardiovascular risk factors in active and inactive elderly men. Braz. J. Med. Biol. Res. 2016, 49. [Google Scholar] [CrossRef] [Green Version]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity–exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Chao, M.V. Downstream Consequences of Exercise Through the Action of BDNF. Brain Plast. 2015, 1, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Friedman, W. Growth Factors; Brady, S., Siegel, G., Albers, W.R., Price, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 546–557. [Google Scholar]
- Al-Qudah, M.A.; Al-Dwairi, A. Mechanisms and regulation of neurotrophin synthesis and secretion. Neurosciences 2016, 21, 306–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippi, G.; Mattiuzzi, C.; Sanchis-Gomar, F. Updated overview on interplay between physical exercise, neurotrophins, and cognitive function in humans. J. Sport Health Sci. 2019, 9, 74–81. [Google Scholar] [CrossRef]
- Kashyap, M.P.; Roberts, C.; Waseem, M.; Tyagi, P. Drug Targets in Neurotrophin Signaling in the Central and Peripheral Nervous System. Mol. Neurobiol. 2018, 55, 6939–6955. [Google Scholar] [CrossRef]
- Chung, J.Y.; Kim, M.W.; Bang, M.S.; Kim, M. Increased expression of neurotrophin 4 following focal cerebral ischemia in adult rat brain with treadmill exercise. PLoS ONE 2013, 8, e52461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hölscher, C. Growth Factors: Neuronal Atrophy. In Reference Module in Neuroscience and Biobehavioral Psychology; Laviola, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–7. [Google Scholar]
- Sakuma, K.; Yamaguchi, A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J. Biomed. Biotechnol. 2011, 2011, 201696. [Google Scholar] [CrossRef] [Green Version]
- Sandrini, L.; Di Minno, A.; Amadio, P.; Ieraci, A.; Tremoli, E.; Barbieri, S.S. Association between Obesity and Circulating Brain-Derived Neurotrophic Factor (BDNF) Levels: Systematic Review of Literature and Meta-Analysis. Int. J. Mol. Sci. 2018, 19, 2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sariola, H. The neurotrophic factors in non-neuronal tissues. Cell. Mol. Life Sci. 2001, 58, 1061–1066. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Ha, C.H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med. 2017, 22, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, C.P.; Kuys, S.S.; Brauer, S.G. The Effect of Aerobic Exercise on Brain-Derived Neurotrophic Factor in People with Neurological Disorders: A Systematic Review and Meta-Analysis. Neural Plast. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maejima, H.; Kanemura, N.; Kokubun, T.; Murata, K.; Takayanagi, K. Exercise enhances cognitive function and neurotrophin expression in the hippocampus accompanied by changes in epigenetic programming in senescence-accelerated mice. Neurosci. Lett. 2018, 665, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Sanchéz, M.A.; Cruz, H.B.; Velasco-Orjuela, G.P.; Quintero, A.P.; Tordecilla-Sanders, A.; Correa-Bautista, J.E.; Triana-Reina, H.R.; García-Hermoso, A.; González-Ruíz, K.; Peña-Guzmán, C.A.; et al. Acute Effects of High Intensity, Resistance, or Combined Protocol on the Increase of Level of Neurotrophic Factors in Physically Inactive Overweight Adults: The BrainFit Study. Front. Physiol. 2018, 9, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareja-Galeano, H.; Alis, R.; Sanchis-Gomar, F.; Cabo, H.; Cortell-Ballester, J.; Gomez-Cabrera, M.C.; Lucia, A.; Viña, J. Methodological considerations to determine the effect of exercise on brain-derived neurotrophic factor levels. Clin. Biochem. 2014, 48, 162–166. [Google Scholar] [CrossRef]
- Ogborn, D.I.; Gardiner, P.F. Effects of exercise and muscle type on BDNF, NT-4/5, and TrKB expression in skeletal muscle. Muscle Nerve 2009, 41, 385–391. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Kastin, A.J. Permeability of the blood–brain barrier to neurotrophins. Brain Res. 1998, 788, 87–94. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Griffin, E.W.; Mullally, S.; Foley, C.; Warmington, S.A.; O’Mara, S.M.; Kelly, A.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011, 104, 934–941. [Google Scholar] [CrossRef]
- Salehi, I.; Hosseini, S.M.; Haghighi, M.; Jahangard, L.; Bajoghli, H.; Gerber, M.; Pühse, U.; Holsboer-Trachsler, E.; Brand, S. Electroconvulsive therapy (ECT) and aerobic exercise training (AET) increased plasma BDNF and ameliorated depressive symptoms in patients suffering from major depressive disorder. J. Psychiatr. Res. 2016, 76, 1–8. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Pilc, A.; Majerczak, J.; Grandys, M.; Zapart-Bukowska, J.; Duda, K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59 (Suppl. 7), 119–132. [Google Scholar]
- Kerling, A.; Kück, M.; Tegtbur, U.; Grams, L.; Weber-Spickschen, S.; Hanke, A.; Stubbs, B.; Kahl, K. Exercise increases serum brain-derived neurotrophic factor in patients with major depressive disorder. J. Affect. Disord. 2017, 215, 152–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babaei, P.; Alamdari, K.A.; Tehrani, B.S.; Damirchi, A. Effect of six weeks of endurance exercise and following detraining on serum brain derived neurotrophic factor and memory performance in middle aged males with metabolic syndrome. J. Sports Med. Phys. Fit. 2013, 53, 437–443. [Google Scholar]
- Damirchi, A.; Tehrani, B.S.; Alamdari, K.A.; Babaei, P. Influence of aerobic training and detraining on serum BDNF, insulin resistance, and metabolic risk factors in middle-aged men diagnosed with metabolic syndrome. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2014, 24, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Herbsleb, M.; de la Cruz, F.; Schumann, A.; Köhler, S.; Puta, C.; Gabriel, H.W.; Reichenbach, J.R.; Bär, K.-J. Changes in fMRI activation in anterior hippocampus and motor cortex during memory retrieval after an intense exercise intervention. Biol. Psychol. 2017, 124, 65–78. [Google Scholar] [CrossRef]
- Castellano, V.; White, L.J. Serum brain-derived neurotrophic factor response to aerobic exercise in multiple sclerosis. J. Neurol. Sci. 2008, 269, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Roh, H.T. Effects of aerobic exercise training on peripheral brain-derived neurotrophic factor and eotaxin-1 levels in obese young men. J. Phys. Ther. Sci. 2016, 28, 1355–1358. [Google Scholar] [CrossRef] [Green Version]
- El-Tamawy, M.S.; Abd-Allah, F.; Ahmed, S.M.; Darwish, M.H.; Khalifa, H.A. Aerobic exercises enhance cognitive functions and brain derived neurotrophic factor in ischemic stroke patients. NeuroRehabilitation 2014, 34, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Goekint, M.; Roelands, B.; De Pauw, K.; Knaepen, K.; Bos, I.; Meeusen, R. Does a period of detraining cause a decrease in serum brain-derived neurotrophic factor? Neurosci. Lett. 2010, 486, 146–149. [Google Scholar] [CrossRef]
- Marusiak, J.; Zeligowska, E.; Mencel, J.; Kisiel-Sajewicz, K.; Majerczak, J.; Zoladz, J.A.; Jaskolski, A.; Jaskolska, A. Interval training-induced alleviation of rigidity and hypertonia in patients with Parkinson’s disease is accompanied by increased basal serum brain-derived neurotrophic factor. J. Rehabil. Med. 2015, 47, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Roh, H.T.; So, W.Y. The effects of aerobic exercise training on oxidant-antioxidant balance, neurotrophic factor levels, and blood-brain barrier function in obese and non-obese men. J. Sport Health Sci. 2017, 6, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, K.-H.; Gold, S.; Witte, J.; Bartsch, K.; Lang, U.E.; Hellweg, R.; Reer, R.; Braumann, K.-M.; Heesen, C. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. J. Neurol. Sci. 2004, 225, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Majerczak, J.; Zeligowska, E.; Mencel, J.; Jaskolski, A.; Jaskolska, A.; Marusiak, J. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2014, 65, 441–448. [Google Scholar]
- Enette, L.; Vogel, T.; Merle, S.; Valard-Guiguet, A.G.; Ozier-Lafontaine, N.; Neviere, R.; Leuly-Joncart, C.; Fanon, J.L.; Lang, P.O. Effect of 9 weeks continuous vs. interval aerobic training on plasma BDNF levels, aerobic fitness, cognitive capacity and quality of life among seniors with mild to moderate Alzheimer’s disease: A randomized controlled trial. Eur. Rev. Aging Phys. Act. Off. J. Eur. Group Res. Elder. Phys. Act. 2020, 17, 2. [Google Scholar] [CrossRef]
- Briken, S.; Rosenkranz, S.C.; Keminer, O.; Patra, S.; Ketels, G.; Heesen, C.; Hellweg, R.; Pless, O.; Schulz, K.-H.; Gold, S. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J. Neuroimmunol. 2016, 299, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Araya, A.V.; Orellana, X.; Godoy, D.; Soto, L.; Fiedler, J. Effect of Exercise on Circulating Levels of Brain-derived Neurotrophic Factor (BDNF) in Overweight and Obese Subjects. Horm. Metab. Res. 2013, 45, 541–544. [Google Scholar] [CrossRef]
- Kimhy, D.; Vakhrusheva, J.; Bartels, M.N.; Armstrong, H.F.; Ballon, J.S.; Khan, S.; Chang, R.W.; Hansen, M.C.; Ayanruoh, L.; Lister, A.; et al. The Impact of Aerobic Exercise on Brain-Derived Neurotrophic Factor and Neurocognition in Individuals With Schizophrenia: A Single-Blind, Randomized Clinical Trial. Schizophr. Bull. 2015, 41, 859–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krogh, J.; Rostrup, E.; Thomsen, C.; Elfving, B.; Videbech, P.; Nordentoft, M. The effect of exercise on hippocampal volume and neurotrophines in patients with major depression–A randomized clinical trial. J. Affect. Disord. 2014, 165, 24–30. [Google Scholar] [CrossRef]
- Maass, A.; Düzel, S.; Brigadski, T.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövdén, M.; Lindenberger, U.; Bäckman, L.; et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage 2016, 131, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Matura, S.; Fleckenstein, J.; Deichmann, R.; Engeroff, T.; Füzéki, E.; Hattingen, E.; Hellweg, R.; Lienerth, B.; Pilatus, U.; Schwarz, S.; et al. Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: Results of the randomised controlled SMART trial. Transl. Psychiatry 2017, 7, e1172. [Google Scholar] [CrossRef] [Green Version]
- Seifert, T.; Brassard, P.; Wissenberg, M.; Rasmussen, P.; Nordby, P.; Stallknecht, B.; Adser, H.; Jakobsen, A.H.; Pilegaard, H.; Nielsen, H.B.; et al. Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R372–R377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffer, T.; Schulte, S.; Hollmann, W.; Bloch, W.; Strüder, H.K. Effects of Strength and Endurance Training on Brain-derived Neurotrophic Factor and Insulin-like Growth Factor 1 in Humans. Horm. Metab. Res. 2008, 41, 250–254. [Google Scholar] [CrossRef]
- Williams, J.S.; Ferris, L.T. Effects of Endurance Exercise Training on Brain- Derived Neurotrophic Factor. J. Exerc. Physiol. Online 2012, 15, 11–17. [Google Scholar]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A.; et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.C.; Kim, J.K.; Lee, N.J.; Kim, S.Y.; Yoon, N.K. Effects of combined exercise on cardiovascular risk factors and serum BDNF level in mid-aged women. J. Exerc. Nutr. Biochem. 2014, 18, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscheweyh, R.; Willemer, C.; Krüger, K.; Duning, T.; Warnecke, T.; Sommer, J.; Völker, K.; Ho, H.; Mooren, F.; Knecht, S.; et al. Physical activity and memory functions: An interventional study. Neurobiol. Aging 2011, 32, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Swift, D.L.; Johannsen, N.M.; Myers, V.H.; Earnest, C.P.; Smits, J.A.J.; Blair, S.N.; Church, T.S. The Effect of Exercise Training Modality on Serum Brain Derived Neurotrophic Factor Levels in Individuals with Type 2 Diabetes. PLoS ONE 2012, 7, e42785. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.H.; Choi, J.H.; Park, J.H.; Kim, I.H.; Cho, J.-H.; Lee, J.-C.; Koo, H.-M.; Hwangbo, G.; Yoo, K.-Y.; Lee, C.H.; et al. Long-Term Exercise Improves Memory Deficits via Restoration of Myelin and Microvessel Damage, and Enhancement of Neurogenesis in the Aged Gerbil Hippocampus After Ischemic Stroke. Neurorehabilit. Neural Repair 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-F.; Chen, H.-I.; Yu, L.; Kuo, Y.-M.; Wu, F.-S.; Chuang, J.-I.; Liao, P.-C.; Jen, C.J. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol. Learn. Mem. 2008, 90, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Chen, H.-I.; Wu, C.-L.; Kuo, Y.-M.; Yu, L.; Huang, A.-M.; Wu, F.-S.; Chuang, J.-I.; Jen, C.J. Differential effects of treadmill running and wheel running on spatial or aversive learning and memory: Roles of amygdalar brain-derived neurotrophic factor and synaptotagmin I. J. Physiol. 2009, 587, 3221–3231. [Google Scholar] [CrossRef] [PubMed]
- Skup, M.; Dwornik, A.; Macias, M.; Sulejczak, D.; Wiater, M.; Czarkowska-Bauch, J. Long-Term Locomotor Training Up-Regulates TrkBFL Receptor-like Proteins, Brain-Derived Neurotrophic Factor, and Neurotrophin 4 with Different Topographies of Expression in Oligodendroglia and Neurons in the Spinal Cord. Exp. Neurol. 2002, 176, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, A.; Baktir, M.A.; Moghadam, S.; Mojabi, F.S.; Sumanth, K.; McNerney, M.W.; Ponnusamy, R.; Salehi, A. Physical exercise induces structural alterations in the hippocampal astrocytes: Exploring the role of BDNF-TrkB signaling. Brain Struct. Funct. 2017, 222, 1797–1808. [Google Scholar] [CrossRef]
- da Silva, S.G.; Unsain, N.; Mascó, D.H.; Toscano-Silva, M.; de Amorim, H.A.; Araujo, B.; Simões, P.S.R.; Mazzacoratti, M.D.G.N.; Mortara, R.; Scorza, F.; et al. Early exercise promotes positive hippocampal plasticity and improves spatial memory in the adult life of rats. Hippocampus 2010, 22, 347–358. [Google Scholar] [CrossRef]
- Alomari, M.A.; Khabour, O.F.; Alzoubi, K.H.; Alzubi, M.A. Forced and voluntary exercises equally improve spatial learning and memory and hippocampal BDNF levels. Behav. Brain Res. 2013, 247, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-J.; Ko, I.-G.; Kim, S.-E.; Hwang, L.; Lee, M.-G.; Kim, D.-Y.; Jung, S.-Y. Age-dependent differences of treadmill exercise on spatial learning ability between young- and adult-age rats. J. Exerc. Rehabil. 2017, 13, 381–386. [Google Scholar] [CrossRef] [Green Version]
- So, J.H.; Huang, C.; Ge, M.; Cai, G.; Zhang, L.; Lu, Y.; Mu, Y. Intense Exercise Promotes Adult Hippocampal Neurogenesis But Not Spatial Discrimination. Front. Cell. Neurosci. 2017, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, N.; Kiray, M.; Sisman, A.R.; Camsari, U.M.; Gencoglu, C.; Baykara, B.; Cetinkaya, C.; Aksu, I. Effects of voluntary and involuntary exercise on cognitive functions, and VEGF and BDNF levels in adolescent rats. Biotech. Histochem. Off. Publ. Biol. Stain. Comm. 2015, 90, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Cassilhas, R.; Lee, K.; Fernandes, J.; Oliveira, M.; Tufik, S.; Meeusen, R.; de Mello, M.T. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 2012, 202, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Ardais, A.; Fioreze, G.; Mioranzza, S.; Botton, P.; Souza, D.; Rocha, J.; Porciúncula, L. The impact of the frequency of moderate exercise on memory and brain-derived neurotrophic factor signaling in young adult and middle-aged rats. Neuroscience 2012, 222, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Etemad, A.; Sheikhzadeh, F.; Ahmadiasl, N. Evaluation of brain-derived neurotrophic factor in diabetic rats. Neurol. Res. 2014, 37, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Maldonado, A.; De Álvarez-Buylla, E.R.; Montero, S.; Melnikov, V.; Castro-Rodríguez, E.; Gamboa-Domínguez, A.; Rodríguez-Hernández, A.; Lemus, M.; Murguía, J.M. Chronic Exercise Increases Plasma Brain-Derived Neurotrophic Factor Levels, Pancreatic Islet Size, and Insulin Tolerance in a TrkB-Dependent Manner. PLoS ONE 2014, 9, e115177. [Google Scholar] [CrossRef]
- Radak, Z.; Toldy, A.; Szabo, Z.; Siamilis, S.; Nyakas, C.; Silye, G.; Jakus, J.; Goto, S. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem. Int. 2006, 49, 387–392. [Google Scholar] [CrossRef]
- Sheikhzadeh, F.; Etemad, A.; Khoshghadam, S.; Asl, N.A.; Zare, P. Hippocampal BDNF content in response to short- and long-term exercise. Neurol. Sci. 2015, 36, 1163–1166. [Google Scholar] [CrossRef]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; Da Silva, S.; Canteiro, P.B.; Casagrande, A.D.S.; Pedroso, G.D.S.; Nesi, R.T.; De Andrade, V.M.; et al. Strength and Aerobic Exercises Improve Spatial Memory in Aging Rats Through Stimulating Distinct Neuroplasticity Mechanisms. Mol. Neurobiol. 2016, 54, 7928–7937. [Google Scholar] [CrossRef]
- Pietrelli, A.; Matković, L.; Vacotto, M.; Lopez-Costa, J.; Basso, N.; Brusco, A. Aerobic exercise upregulates the BDNF-Serotonin systems and improves the cognitive function in rats. Neurobiol. Learn. Mem. 2018, 155, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [Green Version]
- Bekinschtein, P.; Halbach, O.V.B.U. Editorial: Cellular and Molecular Mechanisms of Neurotrophin Function in the Nervous System. Front. Cell. Neurosci. 2020, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Ivanisevic, L.; Saragovi, H.U. Neurotrophins. In Handbook of Biologically Active Peptides; Kastin, A., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1639–1646. [Google Scholar]
- Platholi, J.; Lee, F.S. Neurotrophic Factors. In Handbook of Developmental Neurotoxicology, 2nd ed.; Slikker, W.J., Paule, M.G., Wang, C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 55–64. [Google Scholar]
- Sánchez-Sánchez, J.; Arévalo, J.C. A Review on Ubiquitination of Neurotrophin Receptors: Facts and Perspectives. Int. J. Mol. Sci. 2017, 18, 630. [Google Scholar] [CrossRef] [Green Version]
- Phillips, C. Brain-Derived Neurotrophic Factor, Depression, and Physical Activity: Making the Neuroplastic Connection. Neural Plast. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Teng, H.K.; Teng, K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.; Kermani, P.; Torkin, R.; Chen, Z.-Y.; Lee, F.S.; et al. ProBDNF Induces Neuronal Apoptosis via Activation of a Receptor Complex of p75NTR and Sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Zagrebelsky, M.; Holz, A.; DeChant, G.; Barde, Y.; Bonhoeffer, T.; Korte, M. The p75 Neurotrophin Receptor Negatively Modulates Dendrite Complexity and Spine Density in Hippocampal Neurons. J. Neurosci. 2005, 25, 9989–9999. [Google Scholar] [CrossRef] [Green Version]
- Woo, N.H.; Teng, H.K.; Siao, C.-J.; Chiaruttini, C.; Pang, P.T.; Milner, T.A.; Hempstead, B.L.; Lu, B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005, 8, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, R. Neurobiology of Major Depressive Disorder. Neural Plast. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Christian, K.; Lu, B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008, 89, 312–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messaoudi, E.; Ying, S.-W.; Kanhema, T.; Croll, S.; Bramham, C. Brain-Derived Neurotrophic Factor Triggers Transcription-Dependent, Late Phase Long-Term Potentiation In Vivo. J. Neurosci. 2002, 22, 7453–7461. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of Cell Survival by Secreted Proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef] [Green Version]
- Kojima, M.; Mizui, T. BDNF Propeptide: A Novel Modulator of Synaptic Plasticity. In Vitamins and Hormones; Litwack, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 19–28. [Google Scholar]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [Green Version]
- Prakash, Y.; Thompson, M.A.; Meuchel, L.; Pabelick, C.M.; Mantilla, C.; Zaidi, S.; Martin, R.J. Neurotrophins in lung health and disease. Expert Rev. Respir. Med. 2010, 4, 395–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 6, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Barde, Y.A.; Edgar, D.; Thoenen, H. Purification of a New Neurotrophic Factor from Mammalian Brain. EMBO J. 1982, 1, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Hemmings, S.M.J.; Seedat, S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: Systematic review and meta-regression analysis. Front. Integr. Neurosci. 2013, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Caporali, A.; Emanueli, C. Cardiovascular Actions of Neurotrophins. Physiol. Rev. 2009, 89, 279–308. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, T.; Tsuchida, A.; Itakura, Y.; Nonomura, T.; Ono, M.; Hirota, F.; Inoue, T.; Nakayama, C.; Taiji, M.; Noguchi, H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes 2000, 49, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchida, A.; Nonomura, T.; Nakagawa, T.; Itakura, Y.; Ono-Kishino, M.; Yamanaka, M.; Sugaru, E.; Taiji, M.; Noguchi, H. Brain-derived neurotrophic factor ameliorates lipid metabolism in diabetic mice. Diabetes Obes. Metab. 2002, 4, 262–269. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Lessmann, V.; Brigadski, T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci. Res. 2009, 65, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2017, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Foltran, R.B.; Diaz, S.L. BDNF isoforms: A round trip ticket between neurogenesis and serotonin? J. Neurochem. 2016, 138, 204–221. [Google Scholar] [CrossRef]
- Hashimoto, K. Regulation of brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain by serotonin. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Le Beau, M.M.; Espinosa, R., 3rd; Ip, N.Y.; Belluscio, L.; de la Monte, S.M.; Squinto, S.; Furth, M.E.; Yancopoulos, G.D. Human and rat brain-derived neurotrophic factor and neurotrophin-3: Gene structures, distributions, and chromosomal localizations. Genomics 1991, 10, 558–568. [Google Scholar] [CrossRef]
- Chao, M.V.; Hempstead, B.L. p75 and Trk: A two-receptor system. Trends Neurosci. 1995, 18, 321–326. [Google Scholar] [CrossRef]
- Feter, N.; Alt, R.; Dias, M.; Rombaldi, A. How do different physical exercise parameters modulate brain-derived neurotrophic factor in healthy and non-healthy adults? A systematic review, meta-analysis and meta-regression. Sci. Sports 2019, 34, 293–304. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2014, 60, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.J.; Tschakovsky, M.E. Exercise and circulating BDNF: Mechanisms of release and implications for the design of exercise interventions. Appl. Physiol. Nutr. Metab. 2018, 43, 1095–1104. [Google Scholar] [CrossRef]
- Brunelli, A.; Dimauro, I.; Sgro’, P.G.M.; Emerenziani, G.P.; Magi, F.; Baldari, C.; Guidetti, L.; DI Luigi, L.; Parisi, P.; Caporossi, D. Acute Exercise Modulates BDNF and pro-BDNF Protein Content in Immune Cells. Med. Sci. Sports Exerc. 2012, 44, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, H.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.-I.; Sun, B.; Altar, C.A.; Tandon, N.N. Brain-derived Neurotrophic Factor Is Stored in Human Platelets and Released by Agonist Stimulation. Thromb. Haemost. 2002, 87, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Lommatzsch, M.; Braun, A.; Mannsfeldt, A.; Botchkarev, V.; Botchkareva, N.V.; Paus, R.; Fischer, A.; Lewin, G.R.; Renz, H. Abundant Production of Brain-Derived Neurotrophic Factor by Adult Visceral Epithelia: Implications for Paracrine and Target-Derived Neurotrophic Functions. Am. J. Pathol. 1999, 155, 1183–1193. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2013, 25, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Matthews, V.B.; Astrom, M.B.; Chan, M.H.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerstrom, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagomi, A.; Okada, S.; Yokoyama, M.; Yoshida, Y.; Shimizu, I.; Miki, T.; Kobayashi, Y.; Minamino, T. Role of the central nervous system and adipose tissue BDNF/TrkB axes in metabolic regulation. npj Aging Mech. Dis. 2015, 1, 15009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prigent-Tessier, A.; Quirié, A.; Maguin-Gaté, K.; Szostak, J.; Mossiat, C.; Nappey, M.; Devaux, S.; Marie, C.; Demougeot, C. Physical training and hypertension have opposite effects on endothelial brain-derived neurotrophic factor expression. Cardiovasc. Res. 2013, 100, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Quirie, A.; Hervieu, M.; Garnier, P.; Demougeot, C.; Mossiat, C.; Bertrand, N.; Martin, A.; Marie, C.; Prigent-Tessier, A. Comparative Effect of Treadmill Exercise on Mature BDNF Production in Control versus Stroke Rats. PLoS ONE 2012, 7, e44218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, J.J.; Edgett, B.; Tschakovsky, M.E.; Gurd, B.J. Fasting and exercise differentially regulate BDNF mRNA expression in human skeletal muscle. Appl. Physiol. Nutr. Metab. 2015, 40, 96–98. [Google Scholar] [CrossRef]
- Naegelin, Y.; Dingsdale, H.; Säuberli, K.; Schädelin, S.; Kappos, L.; Barde, Y.-A. Measuring and Validating the Levels of Brain-Derived Neurotrophic Factor in Human Serum. Eneuro 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, P.J.C.; Säuberli, K.; Colzani, M.; Moreau, T.; Ghevaert, C.; Barde, Y.-A. Brain-derived Neurotrophic Factor in Megakaryocytes. J. Biol. Chem. 2016, 291, 9872–9881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zettler, C.; Cupler, E.J.; Hurtado, P.; Wong, K.; Rush, R.A. Neurotrophin 4/5 immunoassay: Identification of sources of errors for the quantification of neurotrophins. J. Neurosci. Methods 2000, 99, 119–127. [Google Scholar] [CrossRef]
- László, A.; Lénárt, L.; Illésy, L.; Fekete, A.; Nemcsik, J. The role of neurotrophins in psychopathology and cardiovascular diseases: Psychosomatic connections. J. Neural. Transm. 2019, 126, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, K.A.; O’Neill, L.A.; Gearing, A.J.; Callard, R.E. NT-4. In The Cytokine FactsBook and Webfacts, 2nd ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 412–414. [Google Scholar]
- Ibanez, C.F. Neurotrophin-4: The odd one out in the neurotrophin family. Neurochem. Res. 1996, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, T.; Belluardo, N.; Metsis, M.; Persson, H. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. Eur. J. Neurosci. 1993, 5, 605–613. [Google Scholar] [CrossRef]
- Ip, N.Y.; Ibanez, C.F.; Nye, S.H.; McClain, J.; Jones, P.F.; Gies, D.R.; Belluscio, L.; Le Beau, M.M.; Espinosa, R., 3rd; Squinto, S.P.; et al. Mammalian neurotrophin-4: Structure, chromosomal localization, tissue distribution, and receptor specificity. Proc. Natl. Acad. Sci. USA 1992, 89, 3060–3064. [Google Scholar] [CrossRef] [Green Version]
- Dąbkowska, M.; Adamczak, M.; Barbasz, J.; Cieśla, M.; Machalinski, B. Adsorption/Desorption Transition of Recombinant Human Neurotrophin 4: Physicochemical Characterization. Langmuir 2017, 33, 9548–9557. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Sarnat, H.B. Development of Olfaction and Taste in the Human Fetus and Neonate. In Fetal and Neonatal Physiology, 5th ed.; Polin, A.R., Abman, S.H., Rowitch, D.H., Benitz, W.E., Fox, W.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1411–1420. [Google Scholar]
- Berkemeier, L.R.; Winslow, J.W.; Kaplan, D.R.; Nikolics, K.; Goeddel, D.V.; Rosenthal, A. Neurotrophin-5: A novel neurotrophic factor that activates trk and trkB. Neuron 1991, 7, 857–866. [Google Scholar] [CrossRef]
- Sorour, N.E.; Elesawy, F.M.; Tabl, H.A.; Ibrahim, M.E.; Akl, E.M. Evaluation of serum levels of neurotrophin 4 and brain-derived nerve growth factor in uremic pruritus patients. Clin. Cosmet. Investig. Dermatol. 2019, 12, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Mrówczyński, W. Health Benefits of Endurance Training: Implications of the Brain-Derived Neurotrophic Factor-A Systematic Review. Neural Plast. 2019, 2019, 5413067. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.G.D.M.; Gobbi, S.; Andreatto, C.A.D.A.; Corazza, D.I.; Pedroso, R.V.; Santos-Galduróz, R.F. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): A systematic review of experimental studies in the elderly. Arch. Gerontol. Geriatr. 2013, 56, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Futur. Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef] [Green Version]
- Kapp, M.B. Ethical and legal issues in research involving human subjects: Do you want a piece of me? J. Clin. Pathol. 2006, 59, 335–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, W.; Kastin, A.J. Penetration of neurotrophins and cytokines across the blood–brain/blood–spinal cord barrier. Adv. Drug Deliv. Rev. 1999, 36, 291–298. [Google Scholar] [CrossRef]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).