Abstract
High-intensity interval training (HIIT) and low-oxygen exposure may inhibit the secretion of appetite-stimulating hormones, suppress appetite, and inhibit dietary intake. Physiological changes affecting appetite are frequent and include appetite hormone (ghrelin, leptin, PYY, and GLP-1) effects and the subjective loss of appetite, resulting in nutritional deficiencies. This paper is a narrative review of the literature to verify the HIIT effect on appetite regulation mechanisms and discusses the possible relationship between appetite effects and the need for high-intensity exercise training in a hypoxic environment. We searched MEDLINE/PubMed and the Web of Science databases, as well as English articles (gray literature by Google Scholar for English articles) through Google Scholar, and the searched studies primarily focused on the acute effects of exercise and hypoxic environmental factors on appetite, related hormones, and energy intake. In a general normoxic environment, regular exercise habits may have accustomed the athlete to intense training and, therefore, no changes occurred in their subjective appetite, but there is a significant effect on the appetite hormones. The higher the exercise intensity and the longer the duration, the more likely exercise is to cause exercise-induced appetite loss and changes in appetite hormones. It has not been clear whether performing HIIT in a hypoxic environment may interfere with the exerciser’s diet or the nutritional supplement intake as it suppresses appetite, which, in turn, affects and interferes with the recovery efficiency after exercise. Although appetite-regulatory hormones, the subjective appetite, and energy intake may be affected by exercise, such as hypoxia or hypoxic exercise, we believe that energy intake should be the main observable indicator in future studies on environmental and exercise interventions.
1. Introduction
Exercise training is an intervention used by athletes to enhance their performance [1,2,3]. In addition, coaches also frequently plan different types of energy system development training (ESD training) according to the athletes’ competition demands, including special training modes for enhancing specific energy systems, such as the ATP–PCr system, the anaerobic glycolytic system, and the aerobic glycolytic system [4,5,6,7]. Currently, high-intensity interval training (HIIT) or high-intensity interval exercise (HIIE) is considered an effective and time-saving exercise strategy to improve endurance sports performance [8]. HIIT is widely used in athletic populations to enhance the efficiency of various energy systems and to promote sports performance [9,10]. On the other hand, sports scientists also use a hypoxic environment, in combination with specific exercise training modes, to promote sports performance. HIIT is increasingly becoming a common practice in the athletic training community. Performing exercise in a hypoxic environment has the benefits of increasing VO2max, reducing post-exercise blood lactate, decreasing the exercise exhaustion time [11], increasing skeletal muscle capillary density, and improving vasodilatation [12]. Therefore, the combination of HIIT in a hypoxic environment is very effective and helpful in enhancing endurance exercise performance for athletes.
However, there are very limited studies on the interval training effects on exercise training, and the benefits. Studies have found that exercise at greater than 60% VO2max may induce a decrease in appetite and suppress the secretion of appetite-promoting hormones [13]. Similarly, low ambient oxygen exposure (FiO2 ≈ 11.5–13.8%) has also been found to suppress appetite and dietary intake [14,15,16]. Current research evidence also suggests that athletes should take appropriate post-exercise nutritional supplementation during the post-exercise recovery period to promote sports performance, stimulate muscle glycogen replenishment, and attenuate exercise-induced muscle damage [17,18,19,20]. After exercise, the muscle state remains, in many ways, similar to the metabolic stresses and challenges experienced during exercise; however, if an appropriate recovery strategy is not performed, such a stress state can further deteriorate and may affect subsequent exercise recoveries and adaptation benefits. Intramuscular high-energy phosphate levels are depleted, glycogen levels are reduced, and exercise-induced cortisol remains at relatively high levels after exercise, which means there is substantial catabolic activity [21,22]. Moreover, other catabolic hormones (i.e., epinephrine and norepinephrine) remain elevated for 30–60 min and gradually return to pre-exercise levels [23]. Furthermore, free radicals produced during exercise are present and result in muscle cell integrity, causing damage for many hours after exercise [24,25]. Therefore, it would be critical for ingesting, with the appropriate nutritional supplementation, better postexercise adaptations. Although the post-exercise dietary supplementation is important to promote recovery, the specific training conditions that combine hypoxia and exercise may affect the willingness of athletes to consume sufficient dietary and nutritional supplements. This may affect and interfere with post-exercise recovery efficiency. On the other hand, according to existing evidence, high-intensity and interval training has been reported to cause negative effects on appetite and also negatively regulate hormonal responses controlling appetite [26,27], but most of the related investigations were conducted in a regular laboratory setting (i.e., sea-level conditions). However, there are only a few studies focusing on the acute impacts of HIIT on appetite regulation under hypoxic conditions.
Therefore, this narrative review is a compilation of the existing literature on the effects of the acute exercise challenge, hypoxic exposure, the combined hypoxic environment, and the acute interval exercise challenge on the subjective appetite and the physiological mechanisms of appetite regulation. A complete compilation of the current knowledge in this field, through a review of the relevant literature, is presented. This review has three main objectives: (1) the effects of high-intensity exercise challenges on appetite regulation; (2) the effects of hypoxic exposure and high-altitude environments on appetite regulation; and (3) the effects of acute hypoxia, combined with interval exercise challenges, on appetite, the related regulatory hormones, and energy intake. Currently, some studies have demonstrated that high-intensity exercise and hypoxia exposure may suppress appetite hormone secretion, suppress appetite, and suppress dietary intake [13,14,28,29,30,31,32,33,34]. However, when athletes need to perform high-intensity exercise training in a hypoxic environment, how one provides effective sports nutrition supplementation is an important sports nutrition issue to be explored. Therefore, we attempt to integrate the current published evidence regarding the potential relationships and appetite-regulatory physiological mechanisms when performing HIIT in a hypoxic environment.
2. Study Type and Search Strategy
We conducted a narrative literature review to examine the potential acute changes in the effects of exercise, as well as hypoxic environmental factors, on appetite and the appetite-regulatory hormones. We searched the scientific literature in MEDLINE/PubMed and the Web of Science (WOS) databases, as well as through Google Scholar for articles published in English from 1980 through to October 2021. Multiple combinations of MeSH terms, entry terms, and keywords (i.e., interval exercise, high-intensity interval exercise, HIIE, high-intensity interval training, HIIT, appetite, hypoxia, and high altitude) were used. All descriptors were searched using the Boolean operators “OR” and “AND” to obtain a comprehensive search. Two hundred and eight articles of potential interest were found. After reading the abstracts, 63 articles were selected for the preparation of this narrative review, and 25 of them were included for the detailed review and for constructing the summary tables (see Figure 1 for the selection flow chart).
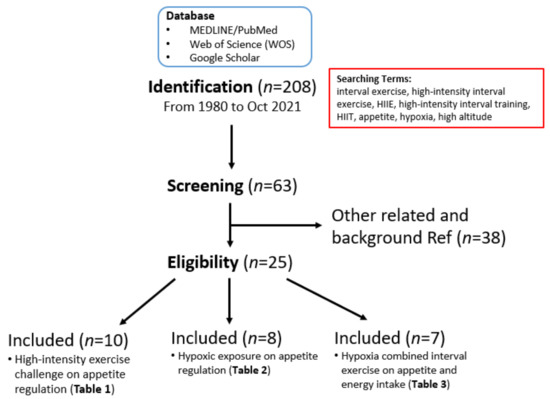
Figure 1.
Search strategy and research article selection process. This flowchart describes the article search for this narrative literature review and the process of selecting articles on HIIT, hypoxia, appetite regulation, other research utilization, evidence-based practice, and knowledge translation for inclusion in the scoping review.
Considering that appetite hormones are decisive factors affecting nutritional intake, we selected articles related to these topics and appetite-related hormonal changes. Furthermore, we selected articles that analyzed the effects of high-intensity exercise in a hypoxic environment on appetite. Although we cannot exclude that there may be other factors (e.g., medical conditions, mental health, dietary patterns, etc.) influencing appetite regulation, the present review focuses on the literature related to the hormonal and subjective appetite regulation responses to exercise and hypoxic stimulation. We used the current findings obtained from both human and animal studies to illustrate the proposed physiological mechanisms and the clinical effects of exercise and hypoxic environmental factors on appetite, the related hormones, and energy intake.
3. The Impact of the High-Intensity Exercise Challenge on Appetite Regulation
Hormones play an important role in the regulation of the body’s internal balance. For example, endurance exercise has been shown to improve body composition, blood pressure, and cardiovascular function more than resistance training [35,36,37]. In addition, exercise also alters hormonal regulation; for example, the concentrations of adrenaline, insulin, cortisol, and growth hormones are increased during exercise in response to physical challenges [38]. In addition, HIIT has also been shown to increase circulating appetite inhibitory hormones (i.e., PYY, GLP-1, etc.) and, thereby, lead to a subjective loss of appetite. This physiological phenomenon is known as exercise-induced anorexia symptoms [26,27]. The relevant literature on the effects of high-intensity exercise challenge on appetite regu-lation is presented in Table 1.
Deighton and Stensel reported that exercise with ≥60% of VO2max strongly suppressed appetite during and shortly after exercise, whether it was running, cycling, swimming, or resistance exercise [13]. In addition, some studies have begun to investigate HIIT, which has become popular in recent years, and found that it also appears to have a suppressive effect on subjective appetite [28]. A comprehensive review of recent studies found that participants with regular exercise habits did not show changes in their subjective appetite. Hormonal effects are seen in appetite. Holliday and Blannin studied 12 participants, aged 21 ± 2 years, with a body mass index (BMI) of 21 ± 1.6 kg/m2, and performed the following four different trials: (1) rest (sedentary control trial); (2) 80% VO2max for 15 min cycling exercise; (3) 80% VO2max for 30 min cycling exercise; and (4) 80% VO2max for 45 min cycling exercise. The results showed no difference in subjective appetite and peptide YY (PYY) among trials. The ghrelin concentration was observed as significantly decreased immediately post-exercise compared to REST and remained below REST at 20 min post-exercise. The glucagon-like peptide-1 (GLP-1) concentration decreased significantly compared to REST. GLP-1 concentrations increased by 36%, 49%, 64%, and 54% immediately after 15 min of continuous exercise, with a 900% reduction in relative energy intake compared to REST [29]. Hazell and colleagues, recruiting 10 males with regular exercise regimes (VO2max: 46.8 ± 4.8 mL/kg/min), completed four different trials: (1) moderate-intensity continuous training (MICT: 65% VO2max for 30 min); (2) high-intensity continuous training (HICT: 85% VO2max for 30 min); (3) sprint interval training (SIT: 6*30 s with 4 min rest); (4) sedentary (control, sedentary trial). The results showed that there was no change in their subjective appetite and GLP-1 among the four trials. However, immediately after exercise, the circulating PYY concentrations were 200% higher in HICT than in the control, and the PYY level of the SIT trials was nearly 400% higher than in the control trial [30]. In the same year, Hazell’s team published another study to compare the gender differences and reported that the subjective appetite was significantly lower by approximately ~87% in SIT and ~83% in MICT, compared to the control in females [27]. In both males and females, the overall circulating PYY and GLP-1 responses were greater in MICT and SIT, compared to the control, and the subjective appetite was lower immediately after MICT and SIT [27]. Moreover, males exhibited greater total PYY concentrations immediately after exercise compared to women. Women had a higher GLP-1 response in MICT and SIT than in the control trial. These results suggest that the total circulating PYY and GLP-1 respond differently to exercise in males and females during the 90 min after exercise, with various intensities [27].
The exercise intensity and the appetite hormonal responses appear to be positively correlated, and subjective appetite may vary by gender and training patterns, especially for participants with regular training who are not acclimated to the exercise intensity and do not perceive any differences when completing visual analog scales (VAS) of appetite. Balaguera-Cortes’ team recruited 10 healthy active men to complete three different trials, including (1) RES (resistance exercise), (2) AER (aerobic exercise), and (3) resting (CON, resting control), and reported that RES showed a 20% decrease in the acylated appetite hormone compared to CON, while PYY and leptin did not change significantly. Moreover, there was no significant difference in energy intake among AER, RES, and CON [39]. Another recent study by Charlot and Chapelot recruited 15 healthy men who exercised regularly to perform HIIE consisting of 13 sets of 90% VO2max exercise for 30 s and 35% VO2max exercise for 60 s (total exercise time: 20 min), moderate-intensity continuous exercises (MIE; 42% VO2 max for 40 min), and the sedentary rest control trial (REST). The results showed that no change occurred in subjective appetite and energy intake, but HIIE delayed their meal by nearly 20 min compared to MIE [40]. The results from the above studies suggest that exercise intensity has a significant effect on appetite hormonal responses and subjective appetite. Taken together, there are several different HIIT exercise modes in the current publications (e.g., high-intensity aerobic interval or sprint interval exercise), and these HIIT exercise modes have been shown to similarly elevate circulating PYY and GLP-1, suggesting acute HIIT might negatively perturb appetite.

Table 1.
Summary of the related studies on the impacts of high-intensity exercise challenge on appetite regulation.
Table 1.
Summary of the related studies on the impacts of high-intensity exercise challenge on appetite regulation.
| Authors (Years) | Subject (Human) | Experimental Design | Experimental Intervention | Dietary Control Methods | Measurement | Major Findings (Δ% Change) |
|---|---|---|---|---|---|---|
| Hazell et al., 2017 [30] |
|
|
| N/A |
|
|
| Holliday & Blannin., 2017 [29] |
|
|
| N/A |
|
Relative energy intake
|
| Hazell et al., 2017 [27] |
BMI: 23.7 ± 2.2 kg/m2 VO2max: 46.8 ± 4.8 mL/kg/min
BMI: 23.5 ± 2.8 kg/m2 VO2max: 40.7 ± 5.4 mL/kg/min |
|
|
|
|
Immediately post-exercise
Immediately post-exercise
|
| Charlot et al., 2019 [40] |
|
|
|
|
|
|
| Douglas et al., 2017 [41] |
BMI: 22.4 ± 1.5kg/m2
BMI: 29.2 ± 2.9 kg/m2 |
|
|
Female: 2820 kJ (71% carbohydrate, 11% protein, 18% fat) |
|
|
| Christ et al., 2006 [42] |
|
|
|
|
|
|
| Holliday & Blannin., 2017 [43] |
|
|
|
|
|
|
| Sim et al., 2014 [44] |
|
|
|
|
|
|
| Poon et al., 2018 [45] |
|
|
| N/A |
|
|
| Matos et al., 2018 [46] |
|
|
|
|
|
|
↑ increase; ↓ decrease; ⇔ no difference. MICT: moderate-intensity continuous training; HICT: high-intensity continuous training; SIT: sprint interval training; CTRL: control; HII ex: high-intensity interval exercises; MIC ex: moderate-intensity continuous exercises; HF: high fat; LF: low fat; EX: exercise; MC: continuous moderate-intensity exercise; HIIT: high-intensity interval training; MICE: moderate-intensity continuous exercise; VICT: vigorous-intensity continuous training; CON: control; HIIE: high-intensity interval exercise; VHI: very-high-intensity intermittent exercise.
4. Low Oxygen Exposure Effects on Appetite Regulation
The relevant literature on the effects of hypoxic challenge on appetite regulation is presented in Table 2. A high altitude is defined as altitudes between 1500 and 3500 m (FiO2 = 17.6–13.8%), very high altitudes are defined as between 3500 and 5500 m (FiO2 = 13.8–10.9%), and extreme altitudes are greater than 5500 m (FiO2 = 10.9%) [47]. In terms of the physiological responses to high altitude environments, atmospheric pressure decreases with increasing vertical altitude from low to high altitude, but the percentage of oxygen remains at 20.9%. The inhaled oxygen pressure (PiO2) decreases in parallel with the atmospheric pressure. This leads to a decrease in alveolar pressure, intra-arterial oxygen pressure (PaO2), and arterial oxygen saturation (SpO2), resulting in a decrease in oxygen delivery to the tissues. When the oxygen concentration in the blood is too low, peripheral chemoreceptors are stimulated in the carotid artery and aortic arch, which are sensitive to the reflex response to hypoxia [48]. Exposure to a high-altitude environment leads to decreased PaO2, increased adrenaline, increased heart rate, increased cardiac output, increased lactate, an altered muscle blood flow, basal metabolic rate, and even decreased VO2max [49].
Hypoxemia may not only affect athletic performance, but it may also lead to altitude sickness, resulting in a loss of appetite, a phenomenon known as high-altitude anorexia. To investigate the high-altitude effect on appetite regulatory hormones, Shukla and colleagues transported 30 participants who had never been to high altitudes before (adaptation group), by helicopter, to an altitude of 3600 m (FiO2 = 13.8%) for 48 h (HA1). The athletes were then transported, by land, to an altitude of 4300 m (FiO2 = 12.5%). After moving to a high altitude for 48 h (HA2a) and 7 days (HA2b), the researchers assessed changes in hunger and blood leptin concentrations for the participants [31]. The results showed that leptin increased by 54.9% and 51% in HA2a and HA2b, compared to normal levels, while hunger decreased by 34.6% and 38.4% in HA1 and HA2a, compared to normal levels. Furthermore, Shukla et al. found that leptin concentrations were about 35% higher in HA2a (7.6 + 0.6 ng/mL in the acclimatized group and 5.6 + 0.5 ng/mL in the control group, p < 0.01, n = 50) and the hunger feeling score was about 30% lower in HA2a compared to the same-age group (the control group) who had lived in the high mountains for more than 6 months [31]. In another study, participants were exposed to a simulated altitude of 4100 m (FiO2 = 13%) for 17 h under normoxic conditions using a hypoxic tent. The results showed a 52% increase in fasting leptin concentrations under hypoxic conditions, but no significant change in postprandial GLP-1 concentrations [50]. These results suggest that when humans are exposed to extreme altitudes, appetite-regulatory hormonal responses can be significantly affected, which, in turn, can have a significant impact on appetite. Not all studies show consistent findings on the leptin elevation due to hypoxic conditions. For example, some studies have reported that exposure of healthy men to hypoxia (FiO2 = 15.0%) for 7 h to 10 days did not significantly alter their subjective appetite or blood leptin concentrations [51,52]. Some studies have reported that hypoxic exposure may lead to a decrease in leptin concentrations [32]. For example, Morishima and Goto subjected eight healthy males (age: 21.0 ± 0.6 years; BMI: 23.4 ± 1.1 kg/m2) to 7 h of rest in a hypoxic chamber in (1) a normoxic rest test (FiO2 = 20.9%) and (2) a hypoxic rest test (FiO2 = 15.0%), with the first meal given at 1 h and at 4 h after entering the laboratory. The first meal given was 3117 kJ (68.4% carbohydrate, 10.1% protein, and 21.5% fat) and the second meal given was 3059 kJ (66.9% carbohydrate, 10.1% protein, and 23.0% fat) for 1 and 4 h, respectively. No significant differences in hormone GLP-1 or leptin blood levels, which are responsible for appetite regulation, were found over a 7-hour period, nor did they specifically alter subjective appetite [52].

Table 2.
Summary of the related studies on low ambient oxygen (hypoxic) exposure effect on appetite regulation.
Table 2.
Summary of the related studies on low ambient oxygen (hypoxic) exposure effect on appetite regulation.
| Authors (Years) | Subject (Human) | Experimental Design | Experimental Intervention | Dietary Control Methods | Measurement | Major Findings (Δ% Change) |
|---|---|---|---|---|---|---|
| Mekjavic et al., 2016 [51] |
|
|
PiO2 = 102.9 mmHg (3000m), 2 days PiO2 = 100.2 mmHg (3200 m), 2 days PiO2 = 97.9 mmHg (3400 m), 4 days
|
|
|
|
| Morishima et al., 2016 [52] |
|
|
|
|
|
|
| Debevec et al., 2014 [53] |
|
|
|
|
|
|
| Matu et al., 2017 [33] |
|
|
|
|
|
|
| Aeberli et al., 2013 [34] |
|
|
|
|
|
|
| Karl et al., 2018 [14] |
Age: 24 ± 7 years BMI: 25.5 ± 3.1 kg/m2
Age: 23 ± 3 years BMI: 27 ± 4 kg/m2 |
|
|
|
| |
| Abu Eid et al., 2018 [54] |
|
|
|
|
|
|
| Zaccaria et al., 2004 [32] |
|
|
| N/A |
|
|
↑ increase; ↓ decrease; ⇔ no difference. NBR: normoxic bed rest; HAMB: hypoxic ambulatory confinement; HBR: hypoxic bed rest.
According to previous literature, the subjective appetite appears to decrease at altitudes above 2500 m (FiO2 = 15.7%) with acute exposure conditions [14,33,34]. In contrast, no differences in subjective appetite appear to be induced at altitudes below 4500 m (FiO2 = 12.3%) or during prolonged acclimation [14,34,51,52,53]. Matu and colleagues recruited 12 British military personnel (eight men, four women; age: 28 ± 4 years; BMI: 23.0 ± 2.1 kg/m2) to spend 14 days climbing from sea level to an altitude of 5140 m (FiO2 = 11.3%), supplementing a fixed daily diet during the climb (49.0 ± 6.6% carbohydrate, 36.3 ± 6.2% fat, and 14.7 ± 2.6% protein) and they investigated the changes in appetite. These soldiers showed approximately a 28% reduction in energy intake compared to sea level, and there was trend of a reduction in hunger (p = 0.07) [33]. In another study [34], ten women and fifteen men (age: 43.8 ± 9.5 years; BMI: 23.8 ± 2.2 kg/m2) climbed to 2980 m (FiO2 = 14.5%) in two days and then to 4599 m (FiO2 = 12.1%) in two days. Breakfast was a fixed daily intake with a total of 400 calories (35% fat, 10% protein, and 54% carbohydrate). A casual diet was prepared for lunch and dinner. At 2980 m altitude, their subjective pre-dinner appetite was found to be approximately 31% lower than that at sea level. The subjective appetite was significantly associated with Lake Louise acute mountain sickness scores (p = 0.043, r = −0.468), but not with gender, age, or BMI [34]. The average energy intake at dinner was reduced by approximately 32%, whereas the appetite and energy intake at 4599 m altitude returned to a comparable level as at sea level. Blood concentrations induced by the appetite-suppressing hormone PYY were not significantly different at 2980 m and 4599 m [34]. Note that at 4600 m (FiO2 = 12.1%) energy intake began to return to levels similar to those at sea level, but during the short climb from sea level to an altitude above 2500 m, appetite and energy intake substantially decreased due to the maladaptation effects to the hypoxic environment. When climbing to higher altitudes, the body needs to adapt to a higher altitude environment again, resulting in the re-emergence of anorexia. These results suggest that when climbing to higher altitudes, the human body needs to adapt to an environment with more stress due to the rapid altitude change, making the hypoxia-induced anorexia reappear.
Although severe hypoxia may affect appetite by suppressing or promoting the secretion of appetite-suppressing hormones, excessive extreme conditions may also cause nausea, vomiting, dizziness, and other acute alpine illnesses, and such symptoms are likely to further generate negative effects on reducing energy intake [33,44,55,56]. Therefore, it is recommended that exposure to moderate hypoxia (a simulated altitude < 3000 m, FiO2 = 14.8%) is more practical for health promotion applications [52]. Although no significant changes in subjective appetite and appetite hormones were found, the study results by Morishima and Goto raise the consideration that exercise in a moderate hypoxic environment could have similar benefits to severe hypoxia [52]. Not only does exercise possibly reduce the risk of acute mountain sickness, it also maintains the post-exercise dietary intake, which could be of great benefit in promoting health and enhancing post-exercise recovery.
5. Acute Hypoxia Effects, Combined with Interval Exercise Challenges on Appetite, Related Regulatory Hormones, and Energy Intake
Exercise may cause exercise-induced anorexia nervosa [26,27], and an exposure to a hypoxic environment may produce high-altitude-induced anorexia nervosa [14,15,16,57]. Therefore, exercise training in a high-altitude environment is likely to have a synergistic, or even additive, effect on the severity of anorexia nervosa, but this phenomenon is still poorly understood. Currently, most studies on hypoxia combined with exercise have focused on the physiological mechanisms and benefits of high-altitude exercise training on athletic performance. Few studies have combined high-altitude environments with exercise challenges to observe appetite and the related regulatory hormonal changes. Furthermore, from the literature review above, it is clear that high-intensity exercise exhibits even greater impacts on appetite regulatory hormone secretion and energy intake compared to continuous exercise [27,30]. We have list the relevant literature on the effects of hypoxic combined interval exercise challenge on appetite regulation and energy intake in Table 3.
HIIT, which consists of high-intensity and low-intensity (or rest) exercise elements, is an effective health promotion and time-saving exercise strategy [8], which is widely used by athletes to enhance the efficiency of energy systems and exercise performance [9,10].
HIIT is not only widely used by athletes to enhance sports performance, but it has also become increasingly popular among the general population in recent years due to its time-saving features [58]. From 2016 to 2020, HIIT was ranked in the top 10 of the American College of Sports Medicine (ACSM) Global Fitness Trends Survey, which shows the high acceptance of this exercise strategy among the general population [59]. However, to date, only a few studies have examined the effects of combining HIIT and hypoxia on appetite and the related hormonal changes [60,61], and most studies regarding the hypoxic environment have focused on continuous exercise [16,55,56,62,63].

Table 3.
Summary of the related studies on acute hypoxia effects combined with interval exercise on appetite and energy intake.
Table 3.
Summary of the related studies on acute hypoxia effects combined with interval exercise on appetite and energy intake.
| Authors (Years) | Subject (Human) | Experimental Design | Experimental Intervention | Dietary Control Methods | Measurement | Major Findings (Δ% Change) |
|---|---|---|---|---|---|---|
| Matu et al., 2017 [56] |
|
|
|
|
|
|
| Bailey et al., 2015 [60] |
|
|
|
|
|
|
| Kojima et al., 2019 [61] |
|
|
| N/A |
|
|
| Debevec et al., 2016 [55] |
|
|
|
|
|
|
| Wasse et al., 2012 [16] |
|
|
|
|
|
|
| Debevec et al., 2014 [62] |
Age: 25.8 ± 2.4 years BMI: 22.9 ± 1.2 kg/m2VO2peak: 42.6 ± 6.1 mL/kg/min
Age: 24.8 ± 3.1 years BMI: 22.3 ± 2.5 kg/m2VO2peak: 42.2 ± 5.0 mL/kg/min |
|
| N/A |
|
|
| Morishima et al., 2014 [63] |
Age: 30 ± 2 years BMI: 25.6 ± 1.2 kg/m2 VO2max: 34.9 ± 2.2 mL/kg/min FiO2: 15%
Age: 32 ± 3 years BMI: 25.4 ± 0.9 kg/m2VO2max: 34.5 ± 2.2 mL/kg/min FiO2: 20.9% |
|
|
|
|
|
↑ increase; ↓ decrease; ⇔ no difference. MIE: continuous moderate-intensity exercise; HIIT: high-intensity interval training; NBR: normoxic bed rest; HAMB: hypoxic ambulatory confinement; HBR: hypoxic bed rest; NOR: normoxic training; HYPO: hypoxic training; NOR: normoxic training; HYPO: hypoxic training.
Acute exposure to high altitude (> 3,500 m) is associated with marked changes in appetite regulation, but the combined effects of the hypoxic environment and HIIT on physiological mechanisms underlying appetite regulation have not been completely studied. Here, we compile recent studies focusing on the acute effects of HIIT on appetite in a hypoxic environment [60,61], in an attempt to understand the effects of low ambient oxygen concentration and HIIT on appetite and its regulatory hormonal responses. Bailey et al. (2015) used a randomized cross-over design to test the effects of four different exercise states on appetite, including: (1) normoxic moderate-intensity exercise (normoxic MIE; intensity: 70% VO2 max; duration: 50 min); (2) hypoxic MIE (FiO2 = 14.5%); (3) normoxic high-intensity interval training (normoxic HIIT; intensity: 90% VO2 max for 3 min plus 50% VO2 max for 3 min, repeated for 6 sets of cycles); and (4) hypoxic HIIT (FiO2 = 14.5%). In this study [60], a fixed breakfast (494 ± 27 calories, with 78% carbohydrate, 16% protein, and 6% fat) was given 2 h before each trial, and a post-exercise meal (741 ± 40 calories, 74.5% carbohydrate, 21% protein and 4.5% fat) was given at the end of the workout. Additionally, venous blood was collected, and the subjective appetite was assessed 2 h before exercise, as well as at the start of exercise, immediately after exercise, 45 min, 60 min, and 105 min after exercise. The authors found that an acute exercise challenge with hypoxia resulted in a rapid decrease in appetite and the suppression of circulating acylated-ghrelin concentrations, and no difference between the conditions of PYY and GLP-1 was observed during exercise. However, the impacts of an acute exercise challenge on appetite appeared to be similar between the two exercise modes [60]. Another study on HIIT in a hypoxic environment was conducted with female collegiate hockey players, who were tested in three different trials, including (1) a normoxic interval sprint exercise (ISE: 8 × 6 s maximal sprints with 30 s intervals for two rounds); (2) hypoxic ISE (FiO2 = 14.5%); and (3) a sedentary controlled trial. Blood was collected and the subjective appetite was evaluated before, immediately after, and 30 min after exercise, and a buffet-style breakfast was given to observe participants’ energy intake [61]. The authors reported that HIIT in both normoxic and hypoxic conditions resulted in comparable degrees of suppression of the subjective appetite, total energy intake (normoxic ISE: −21% vs. hypoxic ISE: −16%), and plasma acylated-ghrelin concentrations (normoxic ISE: −39% vs. hypoxic ISE: −40%), but GLP-1 was not significantly altered by ISE under different environmental oxygen concentrations. Although there was no significant difference in the total caloric intake between hypoxic and normoxic ISE, the reduction in caloric intake in both conditions was primarily due to a significant reduction in the amount of protein and fat intake [61].
However, it is still unclear as to whether a varied level of environmental hypoxia would elicit different degrees of an appetite response during HIIT. Although there is a lack of studies focused on the above issue, the existing studies used continuous exercise under varied hypoxic conditions, which may provide some implications on this aspect [16,55,56,62,63]. For example, Matu and colleagues compared the effects of 60 min of moderate continuous exercise (50% VO2 max) at sea level, 2150 m (FiO2 = 15.8%), and 4300 m (FiO2 = 11.7%) on appetite or appetite-regulating hormonal responses in a continuous exercise regime [56]. The acute effect of moderate continuous exercise (50% VO2 max) on the appetite regulation response was investigated at sea level, at simulated 2150 m (FiO2 = 15.8%) and at simulated 4300 m (FiO2 = 11.7%), and blood appetite regulatory hormones, subjective appetite scores, and post-exercise energy intakes were evaluated. The authors found that the decrease in subjective appetite and the decrease in the circulating ghrelin concentration was more pronounced when the ambient oxygen concentration decreased (i.e., sea level vs. 2150 m vs. 4300 m), but the decrease in total energy intake was equivalent across environmental conditions. Furthermore, there were no significant changes in circulating GLP-1 levels during moderate continuous exercise under hypoxic or normoxic conditions [56].
Although both of the above studies reported similar suppression of subjective appetite and famine by hypoxia or HIIT [60,61], the combination of hypoxia and HIIT did not seem to have additive effects. It is important to emphasize that there are still many experimental design diversities between these two hypoxic HIIT studies, which may affect the practical application of these results. These include gender differences in participants, duration of hypoxia exposure (5 h vs. 1 h), and type of intermittent exercise (longer intervals vs. shorter maximal sprints). Therefore, in practice, because of the paucity of available literature and the wide variation in experimental design, there is no conclusive evidence as to whether hypoxia and HIIT exercise have additive effects on appetite suppression.
6. Concluding Remarks and Suggestions for Future Research
In a normoxic environment, an acute exercise challenge seems to have no effect on subjective appetite, but there is still a significant impact on appetite-regulatory hormones. The possible reason for the above situation is that participants with professional exercise training experience (e.g., recreational sports participants or athletes) are already accustomed to the training intensity and, therefore, do not feel any loss of appetite, but it does not mean that their objective energy intake is not negatively affected. In addition, the higher the intensity of exercise and the longer the duration, the more likely it is to cause an exercise-induced loss of appetite and changes in appetite-regulatory hormones. On the other hand, studies have shown that gender, body fat, and hydration would make a difference in subjective appetite, energy intake, and the related regulation of appetite hormones.
An exposure to a hypoxic environment can lead to a decreased appetite, resulting in a decrease in subjective appetite and a negative effect on appetite-regulatory hormone regulation. However, most of the current research on hypoxia with exercise has investigated the benefits of hypoxic exercise training on exercise performance and has described the physiological mechanisms associated with the performance-enhancing benefits of exercise. On the contrary, there are not sufficient studies to provide details for the possible negative effects of combining hypoxia and HIIE on appetite. Furthermore, the studies involving the hypoxic factor have mostly examined continuous exercise as the mode of exercise. However, to date, only a few studies have examined the effects of combining the popular HIIT with a hypoxic environment on appetite changes. Although appetite-regulatory hormones, subjective appetite, and energy intake may be affected by exercise, hypoxia, or hypoxic exercise, we believe that the energy intake should be the main observable indicator in future studies on environmental and exercise interventions, because energy intake may directly affect the recovery effect after exercise. In addition, the possible effects of appetite regulation hormones and subjective appetite, as a regulatory mechanism, should not be ignored.
Since the specific hypoxic training modes, combined with high-intensity training, were effective in improving sports performance, this training mode, which integrates a hypoxic factor into the high-intensity training, appears to interfere with the desire of athletes’ diets or the desire to take nutritional supplements. This, in turn, affects and perturbs the recovery efficiency after exercise. Therefore, more research evidence may be needed to clarify how to maintain the appetite of trainees and conduct efficient sports nutrition supplementation strategies after hypoxic exercise training.
Author Contributions
Conceptualization, C.-Y.C. and Y.-H.L.; software, K.-X.L. and M.-T.C.; formal analysis, K.-X.L. and S.-C.T.; investigation, C.-C.C., T.M., Y.-H.L. and S.-C.T.; resources, C.-C.C., Y.-H.L., T.M. and S.-C.T.; writing—original draft preparation, C.-Y.C., C.-C.C., Y.-H.L., T.M. and S.-C.T.; writing—review and editing, C.-C.C., T.M., Y.-H.L., T.M. and S.-C.T.; project administration, C.-C.C., M.-T.C., Y.-H.L. and S.-C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Ministry of Science and Technology, Taiwan (Grant number 107-2410-H-845 -018 -MY3 for S.C.T., 109-2628-H-227-002-MY3 for Y.H.L., 108-24-10-H-845-026 for C.Y.C., and 110-2410-H-027-019 for C.C.C.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the National Taipei University of Technology, National Taipei University of Nursing and Health Sciences, and University of Taipei for providing the necessary resources and administrative support throughout the manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matsuo, T.; Saotome, K.; Seino, S.; Shimojo, N.; Matsushita, A.; Iemitsu, M.; Ohshima, H.; Tanaka, K.; Mukai, C. Effects of a low-volume aerobic-type interval exercise on VO2max and cardiac mass. Med. Sci. Sports Exerc. 2014, 46, 42–50. [Google Scholar] [CrossRef]
- Tanaka, H.; Swensen, T. Impact of resistance training on endurance performance. Sports Med. 1998, 25, 191–200. [Google Scholar] [CrossRef] [PubMed]
- García-Pinillos, F.; Cámara-Pérez, J.C.; Soto-Hermoso, V.M.; Latorre-Román, P.Á. A high intensity interval training (HIIT)-based running plan improves athletic performance by improving muscle power. J. Strength Cond. Res. 2017, 31, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, E.; Grzywacz, T.; Luszczyk, M.; Laskowski, R.; Olek, R.A.; Gibson, A.L. Aerobic and anaerobic changes with high-intensity interval training in active college-aged men. J. Strength Cond. Res. 2011, 25, 1104–1112. [Google Scholar] [CrossRef]
- Akgül, M.S. Effect of Wingate-based high intensity interval training on aerobic and anaerobic performance of kick boxers. Phys. Educ. Stud. 2018, 23, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Siahkouhian, M.; Khodadadi, D.; Shahmoradi, K. Effects of high-intensity interval training on aerobic and anaerobic indices: Comparison of physically active and inactive men. Sci. Sports 2013, 28, e119–e125. [Google Scholar] [CrossRef]
- Arazi, H.; Keihaniyan, A.; Eatemadyboroujeni, A.; Oftade, A.; Takhsha, S.; Asadi, A.; Ramirez-Campillo, R. Effects of heart rate vs. speed-based high intensity interval training on aerobic and anaerobic capacity of female soccer players. Sports 2017, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Kilpatrick, M.W.; Jung, M.E.; Little, J.P. High-intensity interval training: A review of physiological and psychological responses. ACSM’s Health Fit. J. 2014, 18, 11–16. [Google Scholar] [CrossRef]
- Engel, F.A.; Ackermann, A.; Chtourou, H.; Sperlich, B. High-intensity interval training performed by young athletes: A systematic review and meta-analysis. Front. Physiol. 2018, 9, 1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquette, M.; Le Blanc, O.; Lucas, S.J.; Thibault, G.; Bailey, D.M.; Brassard, P. Effects of submaximal and supramaximal interval training on determinants of endurance performance in endurance athletes. Scand. J. Med. Sci. Sports 2017, 27, 318–326. [Google Scholar] [CrossRef]
- Czuba, M.; Zając, A.; Maszczyk, A.; Roczniok, R.; Poprzęcki, S.; Garbaciak, W.; Zając, T. The effects of high intensity interval training in normobaric hypoxia on aerobic capacity in basketball players. J. Hum. Kinet. 2013, 39, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Montero, D.; Lundby, C. Effects of exercise training in hypoxia versus normoxia on vascular health. Sports Med. 2016, 46, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Stensel, D.J. Creating an acute energy deficit without stimulating compensatory increases in appetite: Is there an optimal exercise protocol? Proc. Nutr. Soc. 2014, 73, 352–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karl, J.P.; Cole, R.E.; Berryman, C.E.; Finlayson, G.; Radcliffe, P.N.; Kominsky, M.T.; Murphy, N.E.; Carbone, J.W.; Rood, J.C.; Young, A.J.; et al. Appetite suppression and altered food preferences coincide with changes in appetite-mediating hormones during energy deficit at high altitude, but are not affected by protein intake. High Alt. Med. Biol. 2018, 19, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Strasburger, C.J.; Hartmann, G.; Biollaz, J.; Bärtsch, P. Raised leptin concentrations at high altitude associated with loss of appetite. Lancet 1998, 352, 1119–1120. [Google Scholar] [CrossRef]
- Wasse, L.K.; Sunderland, C.; King, J.A.; Batterham, R.L.; Stensel, D.J. Influence of rest and exercise at a simulated altitude of 4000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY. J. Appl. Physiol. 2012, 112, 552–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, R.R. Protein supplements and exercise. Am. J. Clin. Nutr. 2000, 72, 551S–557S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankin, J.W.; Goldman, L.P.; Puglisi, M.J.; Nickols-Richardson, S.M.; Earthman, C.P.; Gwazdauskas, F.C. Effect of post-exercise supplement consumption on adaptations to resistance training. J. Am. Coll. Nutr. 2004, 23, 322–330. [Google Scholar] [CrossRef]
- Caitlin, C.; Diana, P.; Marlia, B.; Elizabeth, A.; Gretchen, A.C. Carbohydrate-supplement form and exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 179–190. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Tipton, K.D.; Miller, S.L.; Wolf, S.E.; Wolfe, R.R. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J. Appl. Physiol. 2000, 88, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; Macdonald, M.J.; McGee, S.L.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Boon, H.; Gijsen, A.P.; Stegen, J.H.; Kuipers, H.; van Loon, L.J. Carbohydrate supplementation during prolonged cycling exercise spares muscle glycogen but does not affect intramyocellular lipid use. Pflug. Arch. 2007, 454, 635–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirwan, J.P.; Cyr-Campbell, D.; Campbell, W.W.; Scheiber, J.; Evans, W.J. Effects of moderate and high glycemic index meals on metabolism and exercise performance. Metab. Clin. Exp. 2001, 50, 849–855. [Google Scholar] [CrossRef]
- Yimcharoen, M.; Kittikunnathum, S.; Suknikorn, C.; Nak-On, W.; Yeethong, P.; Anthony, T.G.; Bunpo, P. Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J. Int. Soc. Sports Nutr. 2019, 16, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ives, S.J.; Bloom, S.; Matias, A.; Morrow, N.; Martins, N.; Roh, Y.; Ebenstein, D.; O'Brien, G.; Escudero, D.; Brito, K.; et al. Effects of a combined protein and antioxidant supplement on recovery of muscle function and soreness following eccentric exercise. J. Int. Soc. Sports Nutr. 2017, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.; Morgan, L.M.; Bloom, S.R.; Robertson, M.D. Effects of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 2007, 193, 251–258. [Google Scholar] [CrossRef]
- Hazell, T.J.; Townsend, L.K.; Hallworth, J.R.; Doan, J.; Copeland, J.L. Sex differences in the response of total PYY and GLP-1 to moderate-intensity continuous and sprint interval cycling exercise. Eur. J. Appl. Physiol. 2017, 117, 431–440. [Google Scholar] [CrossRef]
- Deighton, K.; Barry, R.; Connon, C.E.; Stensel, D.J. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur. J. Appl. Physiol. 2013, 113, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Holliday, A.; Blannin, A. Appetite, food intake and gut hormone responses to intense aerobic exercise of different duration. J. Endocrinol. 2017, 235, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Islam, H.; Hallworth, J.R.; Copeland, J.L. Total PYY and GLP-1 responses to submaximal continuous and supramaximal sprint interval cycling in men. Appetite 2017, 108, 238–244. [Google Scholar] [CrossRef]
- Shukla, V.; Singh, S.N.; Vats, P.; Singh, V.K.; Singh, S.B.; Banerjee, P.K. Ghrelin and leptin levels of sojourners and acclimatized lowlanders at high altitude. Nutr. Neurosci. 2005, 8, 161–165. [Google Scholar] [CrossRef]
- Zaccaria, M.; Ermolao, A.; Bonvicini, P.; Travain, G.; Varnier, M. Decreased serum leptin levels during prolonged high altitude exposure. Eur. J. Appl. Physiol. 2004, 92, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Matu, J.; O’Hara, J.; Hill, N.; Clarke, S.; Boos, C.; Newman, C.; Holdsworth, D.; Ispoglou, T.; Duckworth, L.; Woods, D.; et al. Changes in appetite, energy intake, body composition, and circulating ghrelin constituents during an incremental trekking ascent to high altitude. Eur. J. Appl. Physiol. 2017, 117, 1917–1928. [Google Scholar] [CrossRef]
- Aeberli, I.; Erb, A.; Spliethoff, K.; Meier, D.; Götze, O.; Frühauf, H.; Fox, M.; Finlayson, G.S.; Gassmann, M.; Berneis, K.; et al. Disturbed eating at high altitude: Influence of food preferences, acute mountain sickness and satiation hormones. Eur. J. Nutr. 2013, 52, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Donges, C.E.; Duffield, R. Effects of resistance or aerobic exercise training on total and regional body composition in sedentary overweight middle-aged adults. Appl. Physiol. Nutr. Metab. 2012, 37, 499–509. [Google Scholar] [CrossRef]
- Pal, S.; Radavelli-Bagatini, S.; Ho, S. Potential benefits of exercise on blood pressure and vascular function. J. Am. Soc. Hypertens. 2013, 7, 494–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilmore, J.H.; Knuttgen, H.G. Aerobic exercise and endurance. Phys. Sportsmed. 2003, 31, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kindermann, W.; Schnabel, A.; Schmitt, W.M.; Biro, G.; Cassens, J.; Weber, F. Catecholamines, growth hormone, cortisol, insulin, and sex hormones in anaerobic and aerobic exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 49, 389–399. [Google Scholar] [CrossRef]
- Balaguera-Cortes, L.; Wallman, K.E.; Fairchild, T.J.; Guelfi, K.J. Energy intake and appetite-related hormones following acute aerobic and resistance exercise. Appl. Physiol. Nutr. Metab. 2011, 36, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Charlot, K.; Chapelot, D. Comparison of energy-matched high-intensity interval and moderate-intensity continuous exercise sessions on latency to eat, energy intake, and appetite. Appl. Physiol. Nutr. Metab. 2019, 44, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.A.; King, J.A.; Clayton, D.J.; Jackson, A.P.; Sargeant, J.A.; Thackray, A.E.; Davies, M.J.; Stensel, D.J. Acute effects of exercise on appetite, ad libitum energy intake and appetite-regulatory hormones in lean and overweight/obese men and women. Int. J. Obes. 2017, 41, 1737–1744. [Google Scholar] [CrossRef] [Green Version]
- Christ, E.R.; Zehnder, M.; Boesch, C.; Trepp, R.; Mullis, P.E.; Diem, P.; Décombaz, J. The effect of increased lipid intake on hormonal responses during aerobic exercise in endurance-trained men. Eur. J. Endocrinol. 2006, 154, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, A.; Blannin, A.K. Very low volume sprint interval exercise suppresses subjective appetite, lowers acylated ghrelin, and elevates GLP-1 in overweight individuals: A pilot study. Nutrients 2017, 9, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, A.Y.; Wallman, K.E.; Fairchild, T.J.; Guelfi, K.J. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int. J. Obes. 2014, 38, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Poon, E.T.; Sun, F.H. Post-exercise appetite and ad libitum energy intake in response to high-intensity interval training versus moderate- or vigorous-intensity continuous training among physically inactive middle-aged adults. Nutrients 2018, 10, 1408. [Google Scholar] [CrossRef] [Green Version]
- Matos, V.A.F.; Souza, D.C. Acute effects of high-intensity interval and moderate-intensity continuous exercise on GLP-1, appetite and energy intake in obese men: A crossover trial. Nutrients 2018, 10, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Goto, F.; Kitani, Y. Emergency hyperbaric therapies with the transportable hyperbaric chambers. Jpn. J. Hyperb. Med. 2002, 37, 63–67. [Google Scholar]
- Netzer, N.; Strohl, K.; Faulhaber, M.; Gatterer, H.; Burtscher, M. Hypoxia-related altitude illnesses. J. Travel Med. 2013, 20, 247–255. [Google Scholar] [CrossRef]
- Mazzeo, R.S. Physiological responses to exercise at altitude: An update. Sports Med. 2008, 38, 1–8. [Google Scholar] [CrossRef]
- Snyder, E.M.; Carr, R.D.; Deacon, C.F.; Johnson, B.D. Overnight hypoxic exposure and glucagon-like peptide-1 and leptin levels in humans. Appl. Physiol. Nutr. Metab. 2008, 33, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Mekjavic, I.B.; Amon, M.; Kölegård, R.; Kounalakis, S.N.; Simpson, L.; Eiken, O.; Keramidas, M.E.; Macdonald, I.A. The effect of normobaric hypoxic confinement on metabolism, gut hormones, and body composition. Front. Physiol. 2016, 7, 202. [Google Scholar] [CrossRef] [Green Version]
- Morishima, T.; Goto, K. Ghrelin, GLP-1, and leptin responses during exposure to moderate hypoxia. Appl. Physiol. Nutr. Metab. 2016, 41, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Debevec, T.; Bali, T.C.; Simpson, E.J.; Macdonald, I.A.; Eiken, O.; Mekjavic, I.B. Separate and combined effects of 21-day bed rest and hypoxic confinement on body composition. Eur. J. Appl. Physiol. 2014, 114, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Abu Eid, S.; Hackl, M.T.; Kaplanian, M.; Winter, M.P.; Kaltenecker, D.; Moriggl, R.; Luger, A.; Scherer, T.; Fürnsinn, C. Life Under Hypoxia Lowers Blood Glucose Independently of Effects on Appetite and Body Weight in Mice. Front. Endocrinol. 2018, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Debevec, T.; Simpson, E.J.; Mekjavic, I.B.; Eiken, O.; Macdonald, I.A. Effects of prolonged hypoxia and bed rest on appetite and appetite-related hormones. Appetite 2016, 107, 28–37. [Google Scholar] [CrossRef]
- Matu, J.; Deighton, K.; Ispoglou, T.; Duckworth, L. The effect of moderate versus severe simulated altitude on appetite, gut hormones, energy intake and substrate oxidation in men. Appetite 2017, 113, 284–292. [Google Scholar] [CrossRef]
- Vats, P.; Singh, V.K.; Singh, S.N.; Singh, S.B. High altitude induced anorexia: Effect of changes in leptin and oxidative stress levels. Nutr. Neurosci. 2007, 10, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef]
- Thompson, W.R. Worldwide survey of fitness trends for 2018: The CREP Edition. ACSM’s Health Fit. J. 2017, 21, 10–19. [Google Scholar] [CrossRef]
- Bailey, D.P.; Smith, L.R.; Chrismas, B.C.; Taylor, L.; Stensel, D.J.; Deighton, K.; Douglas, J.A.; Kerr, C.J. Appetite and gut hormone responses to moderate-intensity continuous exercise versus high-intensity interval exercise, in normoxic and hypoxic conditions. Appetite 2015, 89, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Kojima, C.; Kasai, N.; Ishibashi, A.; Murakami, Y.; Ebi, K.; Goto, K. Appetite regulations after sprint exercise under hypoxic condition in female athletes. J. Strength Cond. Res. 2019, 33, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Debevec, T.; Simpson, E.J.; Macdonald, I.A.; Eiken, O.; Mekjavic, I.B. Exercise training during normobaric hypoxic confinement does not alter hormonal appetite regulation. PLoS ONE 2014, 9, e98874. [Google Scholar] [CrossRef] [PubMed]
- Morishima, T.; Kurihara, T.; Hamaoka, T.; Goto, K. Whole body, regional fat accumulation, and appetite-related hormonal response after hypoxic training. Clin. Physiol. Funct. Imaging 2014, 34, 90–97. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).