Abstract
Aedes albopictus is a cosmopolitan mosquito species capable of transmitting arboviruses such as dengue, chikungunya, and Zika. To control this and similar species, public and private entities often rely on pyrethroid insecticides. In this study, we screened Ae. albopictus collected from June to August 2017 in Mecklenburg County, a rapidly growing urban area of North Carolina, for mutations conferring pyrethroid resistance and examined spatiotemporal patterns of specimen size as measured by wing length, hypothesizing that size variation could be closely linked to local abundance, making this easily measured trait a useful surveillance proxy. The genetic screening results indicated that pyrethroid resistance alleles are not present in this population, meaning that this population is likely to be susceptible to this commonly used insecticide class. We detected no significant associations between size and abundance-related factors, indicating that wing-size is not a useful proxy for abundance, and thus not useful to surveillance in this capacity. However, mosquitoes collected in June were significantly larger than July or August, which may result from meteorological conditions, suggesting that short-term weather cues may modulate morphological traits, which could then affect local fecundity and virus transmission dynamics, as previously reported.
1. Introduction
Recent emergences and spread of diseases such as dengue, chikungunya, and Zika have led to an uptick in public interest and concern about vector control for public health in the United States. Greater knowledge of the presence and distribution of disease vectoring Aedes mosquitoes can inform vector control. However, an additional factor has emerged in recent years: insecticide resistance. This study undertook an examination of vector surveillance data for Aedes albopictus mosquitoes in Mecklenburg County, a rapidly growing urban area in the state of North Carolina, to assess potential factors affecting distribution and evidence for the emergence of insecticide resistance.
First identified in the state of Texas in 1985, the invasive Ae. albopictus has dramatically expanded its range in the United States [,]. This expansion is part of a global trend: in the last 50 years, Ae. albopictus has spread to all inhabited continents [] and has become established in both tropical and temperate environments []. This species is a container-breeder and feeds opportunistically, biting a wide range of hosts, although some populations exhibit a preference for mammals, and, more specifically, humans []. While Ae. albopictus is less anthropophilic than Ae. aegypti, it can serve as a vector for the same arboviruses as Ae. aegypti including dengue, chikungunya, Rift Valley fever, yellow fever, and Zika viruses []. Moreover, the opportunistic feeding behavior of Ae. albopictus may allow this species to act as a bridge vector, leading to spillover of zoonotic pathogens into human populations []. Additionally, populations of Ae. albopictus often competitively displace populations of Ae. aegypti [].
Given the vector status of Ae. albopictus, its recent global expansion, and its ability to out-compete other important vector species, it is unsurprising that this species has affected public health and been accordingly targeted by vector control programs. Ae. albopictus has been an important vector of the alphavirus chikungunya in the 2004–2007 epidemic across several Indian Ocean islands [], the 2007 concurrent outbreak with dengue in Gabon [], and the 2007 and 2017 outbreaks in Italy [,]. Additionally, Aedes albopictus has also been implicated as a vector of other groups of arboviruses including the orthobunyavirus La Crosse virus [], which is an enzootic peribunyaviridae involved in neurological disorders in North Carolina, where this study takes place []. Aedes albopictus control often relies on the use of adulticides [], as is the case in North Carolina, where pyrethroid insecticides are commonly used for barrier spraying to control Ae. albopictus []. While insecticide resistance has been documented in Ae. aegypti populations around the world, fewer studies have focused on the resistance status of Ae. albopictus, with most work on this species concentrated in Southeast Asia, where resistance to all four major insecticide classes has been reported []. Previous work in the United States found that Ae. albopictus populations remained broadly susceptible to most insecticide treatments, though low levels of resistance to organophosphates and dichlorodiphenyltrichloroethane (DDT) have been detected in Florida and New Jersey populations []. However, more recently, 30% of Ae. albopictus populations collected throughout Florida were found to be resistant to pyrethroid insecticides [], indicating that resistance may be increasing.
Ae. albopictus abundance varies across space and time, influencing local pathogen transmission potential. Socioeconomic, landscape, and seasonal factors have been associated with Ae. albopictus abundance in many studies. Areas of low socioeconomic status (SES) often have a greater number of discarded containers for Ae. albopictus breeding [], and pupae from Aedes species are more likely to be found in neighborhoods below median income []. This was demonstrated in recent research in Mecklenburg County, North Carolina, where the abundance of gravid Aedes albopictus was significantly higher in low-income neighborhoods []. This work further identified land cover factors associated with Ae. albopictus abundance including the percent of land covered by buildings, tree canopy, grass and shrubs, roads and railroads, and the overall diversity of land cover types in a 30-meter buffered area around sampled sites [,]. Additional studies have indicated that small patches of vegetation in urban areas such as parks, gardens, and playgrounds are often associated with high Ae. albopictus abundance [] and that peaks in abundance often occur in late summer months in temperate climates [,,].
This study aimed to establish a baseline description of the insecticide resistance status and patterns of morphological variation within a population of Ae. albopictus collected from Mecklenburg County, North Carolina. As such, our first objective was to screen adult Ae. albopictus females for genetic mutations indicating resistance to pyrethroids, the most commonly used class of insecticides for barrier spraying in North Carolina []. Additionally, we hypothesized that variation in female Ae. albopictus size would be associated with the socioeconomic, landscape, and seasonal factors that influence Ae. albopictus abundance. Previous studies have found that female Ae. albopictus size is positively correlated with fecundity [,]. We predicted that we would observe larger mean wing lengths in the lower socioeconomic classes, land cover types, and time periods associated with higher Ae. albopictus abundance. Furthermore, vector size influences virus transmission potential, with viral dissemination more likely among smaller individuals, as has been shown for dengue virus in Ae. albopictus [] and La Crosse virus in Ae. triseriatus []. If size can serve as a reliable proxy for abundance or disease transmission potential in a local context, it provides a low-cost means to prioritize and target areas of importance, rather than time-consuming abundance sampling measures.
2. Materials and Methods
2.1. Study Site
We performed our analyses using adult female Aedes albopictus collected from June to August 2017 in Mecklenburg County, North Carolina, which encompasses the city of Charlotte (Figure 1). Mecklenburg County has an average population density of approximately 1900 people per square mile and a median household income of $61,695, with 13.4% of the population classified as persons in poverty in 2017, when these samples were collected [], and the city of Charlotte has been characterized as having pervasive racial segregation and income inequality [,].
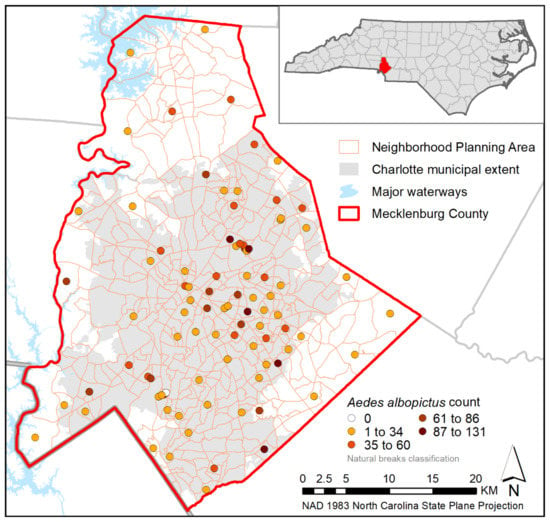
Figure 1.
Collection sites in Mecklenburg County, North Carolina. Sites are colored to represent the total number of Ae. albopictus collected at that location during this study. Inset map shows location of Mecklenburg County in North Carolina.
The Ae. albopictus specimens used in this study were collected from 90 unique sampling sites selected to maximize spatial distribution across the county and to represent the range of values present across a variety of socioeconomic and landscape factors [,]. Briefly, sampling was conducted using Gravid Aedes Traps (GATs) with hay-infused water as an attractant. Traps were emptied on a weekly basis for twelve weeks and specimens were identified morphologically when possible [] or genetically verified to species at the Walter Reed Biosystematic Unit when specimens were degraded. The majority (72%) of collections included Ae. albopictus, with this species representing 86% of the total number of mosquitoes collected. Other identified species included Ae. triseriatus, Ae. vexans, Ae. japonicus, Culex resuans, and Cx. Pipiens [].
2.2. DNA Extraction, Amplification, and Sequencing
We first aimed to determine whether the Ae. albopictus population in Mecklenburg County had any genetic mutations that would indicate resistance to pyrethroid insecticides. We therefore destructively extracted DNA from whole mosquitoes for use in polymerase chain reaction (PCR) using Qiagen DNeasy Isolation Kits (Qiagen Sciences, Germantown, MD, USA). For all samples, we amplified and sequenced two regions of kdr (domain II, 381 bp; domain IV, 280 bp) using the AegSCF20/AegSCR21 and AlbSCF6/AlbSCR8 primer pairs, respectively (Appendix A). Amplification of kdr domain III was unsuccessful. The thermocycler conditions were identical for kdr domains II and IV, an initial denaturing step at 96 °C for 10 min, 40 cycles of 30 s at 96 °C, 30 s at 55 °C, and 45 s at 72 °C, with a final extension step of 10 min at 73 °C (Appendix A, Table A1). All mosquito PCR products were cleaned using exonuclease I and shrimp alkaline phosphatase (Fisher Scientific, Pittsburgh, PA, USA). Primer extension sequencing was performed by Genewiz (South Plainfield, NJ, USA) using Applied Biosystems BigDye version 3.1. The reactions were then run on Applied Biosystem’s 3730xl DNA Analyzer.
We used MegaX [] and BioEdit [] to assemble and form contigs of our forward and reverse reads.
2.3. Wing Length Measurements and Statistical Tests
We aimed to measure the wing length of one Ae. albopictus adult female from each of the 90 collection sites for each month in the collection window. However, because some sites did not yield Ae. albopictus females each month or specimens were in poor condition, we measured 236 wings total (representing 84, 72, and 80 sites in June, July, and August, respectively). We used a camera attached to a dissecting microscope to photograph the mosquito wings and then processed all images with ImageJ [] to measure the length of each wing. Each wing was measured by two of the authors (S.M. and E.S.) independently. Measurements were averaged to determine a consensus length. If there was a difference greater than 2 mm between the independent measurements, a third measurement was taken by a third author (GH) and computed into the average for the month.
To test for statistically significant associations between socioeconomic variables and wing length, we first tested for wing length differences across socioeconomic quintiles based on the 2016 median household income at the neighborhood planning area (NPA) level. The NPA is a unit developed by the Charlotte-Mecklenburg Planning Commission that approximates the census tract, but with improved representation of actual neighborhoods within the county []. Mean wing length measurements across the sampling period per site were tested for normality through visual assessments of plotted distributions and the Shapiro–Wilk test for normality and found not to be normally distributed (p value < 0.004). Since the data remained abnormal even after modification, we conducted a Kruskal–Wallis test and a subsequent pairwise Wilcoxon rank sum test to identify statistically significant differences in wing length across income groups. We tested for associations between mean wing length and the socioeconomic or human demographic variables at the NPA level that have been shown to be related to Ae. albopictus abundance in the study area []. We used Spearman rank correlations due to the non-normal distributions of the explanatory and response variables. These variables included violent crime rate, population density, employment rate, proportion Hispanic population, foreclosure rate, proximity to a park, and proportion African-American population []. All statistical tests were performed using base functions in R v3.5.0 (R Core Team, 2019).
We used the 2012 Mecklenburg County Tree Canopy/Land Cover dataset to test for associations between land cover and wing length. This dataset was developed at a 3.33-foot spatial resolution using object-based image analysis techniques along with 2012 LiDAR data, 2012 National Agriculture Imagery Program imagery, and ancillary spatial datasets []. Land cover types included buildings, roads/railroads, tree canopy, grass/shrubs, water, and other paved surfaces. We generated a 30-meter buffer around each sampling site and calculated the percentage of each land cover type present within each buffer. Previous work has indicated that a 30-meter buffer is the best scale to detect the relationship between high-resolution land cover variables and Aedes abundance []. We tested for correlations between percent of each land cover type present within the buffer and mean wing length at each collection site using Spearman rank correlations. Additionally, we used a Kruskal–Wallis test to identify significant differences in mean wing length at sites classified as rural (n = 5), suburban (n = 63), and urban (n = 20) for each collection month and the total sampling season. These designations were based on percent impervious surface (roads/railroads, other paved surfaces, and buildings) within the 30-meter buffer, based on cut-off values used in similar research []. We tested for statistically significant differences in mean wing length across collection months using a Kruskal–Wallis test and post-hoc Wilcoxon rank sum tests for pairwise differences. We used Global Moran’s I tests to detect spatial autocorrelation in the mean wing lengths across the study area for each month and for the averaged wing lengths for the entire sampling period using the point locations of sampled sites as inputs and inverse distance to conceptualize spatial relationships. All spatial data processing was completed in ArcGIS 10.6 (ESRI, Redlands, CA, USA), a commercial geographic information system.
3. Results
We found no mutations that would infer pyrethroid resistance among our samples from Mecklenburg County, NC. We successfully extracted DNA from 86 mosquitoes, representing 95% of the total 90 collection sites. Amplification and sequencing of kdr domains II and IV were successful for 27 individuals (30% coverage) and 75 individuals (83% coverage), respectively. The resulting sequences for all samples were deposited in GenBank; accession numbers can be found in Appendix B, Table A2.
The mean wing length for the 236 female Ae. albopictus specimens was 2.73 mm (range 1.64 mm to 4.29 mm). The results from the Kruskal–Wallis test to identify differences in wing length across median income quintiles were not statistically significant (Kruskal–Wallis χ2 = 2.645, df = 4, p = 0.619). The Spearman rank correlations between the socioeconomic and human demographic variables identified as being associated with Ae. albopictus abundance and mean wing length did not yield statistically significant associations [].
Tests for associations between land cover and wing length did not yield statistically significant results. This included the Spearman’s rank correlation between the percentage of each land cover type present within the 30-meter buffer around each sampling site and the mean wing length at that site. The Kruskal–Wallis test for differences in mean wing length across rural, suburban, and urban areas based on percent impervious surface did not yield statistically significant results (Kruskal–Wallis χ2 = 1.275, df = 2, p-value = 0.529).
The result from the Kruskal–Wallis test for differences in mean wing length across the three sampling months was statistically significant (Figure 2; Kruskal–Wallis χ2 = 9.950, df = 2, p-value = 0.007). We found a statistically significant difference between wing length measurements of samples collected in June and August (Wilcoxon rank sum test, p-value = 0.008) and between June and July (Wilcoxon rank sum test, p-value = 0.022), but not July and August (Wilcoxon rank sum test, p-value = 0.540). The mean wing lengths of collected mosquitoes was longest in June. The Global Moran’s I tests for spatial autocorrelation in mean wing lengths did not show statistically significant clustering or dispersal when averaged over the entire study period (p-value = 0.668) and for each month individually (June p-value = 0.983; July p-value = 0.279; August p-value = 0.738).
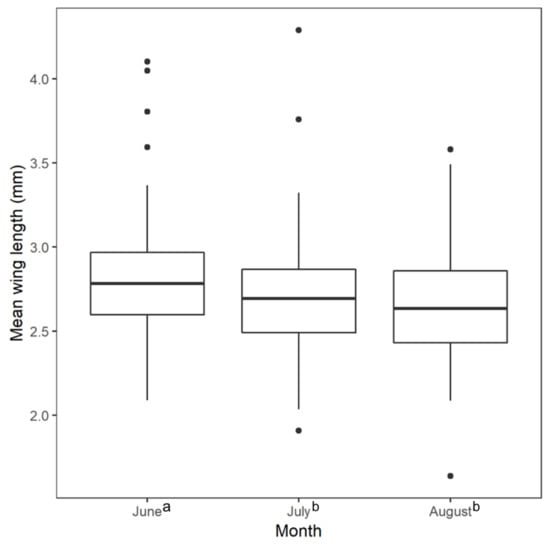
Figure 2.
Mean female Aedes albopictus wing length by collection month. The mean June wing length was longer than the July and August mean wing lengths. There was no significant difference between the July and August mean wing length. Superscript lowercase letters indicate values significantly different from one another in the Kruskal–Wallis tests with a post-hoc Wilcoxon rank sum test at p ≤ 0.05.
4. Discussion
As the range of Ae. albopictus continues to expand, continuous surveillance and study of the species is needed. Regular monitoring of insecticide susceptibility is essential to promptly identify the emergence of resistance and implement appropriate and alternative control measures []. Similarly, having a baseline understanding of the morphology and distribution of vector populations within the context of local socioeconomics, landscape, and temporal influences can inform targeted abatement strategies. In this study, we screened Ae. albopictus collected from Mecklenburg County, North Carolina, for genetic indicators of resistance and examined spatial and temporal patterns of wing length variation among the collected adult female Ae. albopictus specimens.
While the sample size of specimens that we were able to successfully extract and amplify genetic material from was relatively small, which is a limitation, the homogenous lack of voltage-gated sodium channel mutations across the study area strongly suggests that this population is broadly susceptible to pyrethroid insecticides. This matches findings from similar studies in the area. In a 2018 study, researchers found that Ae. albopictus populations from seven North Carolina counties including Mecklenburg County were susceptible to five commonly used pyrethoids in CDC bottle bioassays, with the exception of Pitt County, where developing resistance (93% mortality) to permethrin was documented []. In contrast, resistance to chlorpyrifos and malathion, two commonly used organophosphates, was documented in all seven populations in the same study []. Budgets for mosquito control programs in North Carolina have been dramatically reduced in the past decade [] and a recent survey found that approximately 31% of respondents in North Carolina personally administered insecticides for mosquito control on their property []. This combination of limited resources for oversight and unregulated insecticide applications by private individuals indicates that selection for insecticide resistant mosquitoes will likely continue in this area, although more information is needed to predict whether pyrethroid resistance will develop.
We did not detect significant associations between Ae. albopictus wing length and most of the socioeconomic and landscape factors considered in this study, although these factors were associated with Ae. albopictus abundance in previous research. This means that our hypothesis that larger female Ae. albopictus females with higher fecundity drive increases in local abundance was not supported. While certain areas may produce larger female Ae. albopictus that have more offspring, their impact on local abundance could be countered by high larval densities that result in smaller adults []. Furthermore, the results from the Global Moran’s I tests indicate that wing length did not exhibit spatial autocorrelation, suggesting that female adult size is likely to be the result of multiple random, interacting processes. We did not observe significant differences in wing length between rural, suburban, and urban sites. This is contradictory to recent work conducted in Athens, Georgia, that found that Ae. albopictus emerging from containers placed in urban sites were significantly smaller than those placed in rural sites []. However, this difference was statistically significant only in the fall, and our study was limited to a single summer season of collections. Differences in wing length across land cover types could increase if sampling in Mecklenburg County were to continue into the fall.
We found that Ae. albopictus collected in June had significantly longer wing lengths than Ae. albopictus collected in August and July, while the average number of Ae. albopictus collected in the study area was the lowest in June [], indicating the larger wing spans were not associated with greater abundance. This difference in size could be due to meteorological conditions. The total monthly amounts of precipitation for Charlotte in June, July, and August of 2017 were 4.3 inches, 4.45 inches, and 5.29 inches, respectively []. Observing significantly larger mosquitoes during the month with the least amount of rainfall corresponded with a previous study that found that Ae. albopictus reached their largest size under conditions where their water source was allowed to evaporate completely. In this scenario, increased mortality during the aquatic life stages resulted in fewer adults emerging, but the surviving individuals were larger, possibly due to decreased competition for resources []. Additionally, temperature conditions were lower in June than in July or August during the study period, with an average high of 29.8 ℃ in June compared to 33.1 °C in July and 30.8 °C in August. Higher temperatures have been found to result in the growth of heavier adult Ae. albopictus with shorter wings []. Further work would likely determine the extent to which these meteorological variables interact with each other and other environmental factors to determine Ae. albopictus size.
5. Conclusions
In conclusion, this work served to establish a baseline description of the Ae. albopictus population in Mecklenburg County, North Carolina. While genetic indicators of pyrethroid resistance were not detected, continued surveillance remains critical for early detection of diminished susceptibility. Additionally, while we did not see significant associations between Ae. albopictus wing lengths and several factors that have been linked to abundance in this and similar species, we did observe temporal variation in the wing lengths of this population. Further research will likely illuminate the extent to which spatial and temporal factors influence variation in wing size and other morphological traits in Ae. albopictus and other mosquito species.
Author Contributions
Conceptualization, S.J.M., G.H., A.W., E.D., T.R., M.D., and S.J.R.; Methodology, S.J.M., S.J.R., and G.H.; Software, E.K.S., G.H.; Validation, S.J.R. and G.H.; Formal Analysis, S.J.M., G.H., E.K.S., and S.J.R.; Original Draft Preparation, S.J.M., G.H., and E.K.S.; Writing—Review & Editing, S.J.R., A.W., and E.D.; Visualization, S.J.M. and E.K.S.; Supervision, S.J.R.; Project Administration, S.J.R.; Funding Acquisition, S.J.R. All authors have read and agreed to the published version of the manuscript.
Funding
The contributions of S.J.R., S.J.M., and G.H. were supported by CDC grant 1U01CK000510-01: Southeastern Regional Center of Excellence in Vector-Borne Diseases: The Gateway Program. This publication was supported by the Cooperative Agreement Number above from the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention. ES was supported by the UF Emerging Scholars program. Fieldwork was funded by the Academy for Population Health Innovation, a collaboration between Mecklenburg County Public Health and the University of North Carolina at Charlotte.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors have no conflict of interest to declare.
Appendix A

Table A1.
Primers Used for Amplification of Three Domains of kdr.
Table A1.
Primers Used for Amplification of Three Domains of kdr.
| kdr domain II | Forward | 5’-GACAATGTGGATCGCTTCCC-3’ | Kasai et al., 2011 [] |
| Reverse | 5’-GCAATCTGGCTTGTTAACTTG-3’ | ||
| kdr domain III | Forward | 5’-GAGAACTCGCCGATGAACTT-3’ | Kasai et al., 2011 [] |
| Reverse | 5’-AACAGCAGGATCATGCTCTG-3’ | ||
| kdr domain IV | Forward | 5’-TCGAGAAGTACTTCGTGTCG-3’ | Kasai et al., 2011 [] |
| Reverse | 5’-AACAGCAGGATCATGCTCTG-3’ |
Appendix B

Table A2.
Accession Numbers for kdr Domains II and IV.
Table A2.
Accession Numbers for kdr Domains II and IV.
| Site ID | Neighborhood Classification | kdr Domain II | kdr Domain IV |
|---|---|---|---|
| 2 | 4 | MZ964062 | |
| 3 | 1 | MZ964063 | |
| 4 | 2 | MZ964064 | |
| 5 | 3 | MZ964065 | |
| 6 | 1 | MZ964066 | |
| 7 | 2 | MZ964067 | |
| 8 | 1 | MZ964068 | |
| 9 | 1 | MZ964069 | |
| 10 | 4 | MZ964070 | |
| 11 | 2 | MZ964035 | MZ964071 |
| 12 | 3 | MZ964072 | |
| 13 | 4 | MZ964073 | |
| 14 | 5 | MZ964074 | |
| 16 | 2 | MZ964036 | MZ964075 |
| 17 | 3 | MZ964037 | MZ964076 |
| 18 | 4 | MZ964077 | |
| 19 | 5 | MZ964078 | |
| 20 | 1 | MZ964038 | MZ964079 |
| 22 | 3 | MZ964080 | |
| 23 | 5 | MZ964039 | MZ964081 |
| 24 | 5 | MZ964040 | MZ964082 |
| 25 | 1 | MZ964083 | |
| 26 | 4 | MZ964084 | |
| 27 | 3 | MZ964041 | MZ964085 |
| 28 | 2 | MZ964057 | MZ964086 |
| 29 | 4 | MZ964087 | |
| 30 | 2 | MZ964058 | MZ964088 |
| 31 | 3 | MZ964089 | |
| 33 | 3 | MZ964042 | MZ964090 |
| 34 | 4 | MZ964091 | |
| 35 | 3 | MZ964092 | |
| 37 | 1 | MZ964093 | |
| 38 | 1 | MZ964094 | |
| 40 | 4 | MZ964043 | |
| 41 | 5 | MZ964056 | MZ964095 |
| 42 | 4 | MZ964044 | MZ964096 |
| 43 | 5 | MZ964097 | |
| 44 | 3 | MZ964045 | MZ964098 |
| 45 | 4 | MZ964099 | |
| 47 | 3 | MZ964100 | |
| 48 | 3 | MZ964101 | |
| 50 | 5 | MZ964046 | MZ964102 |
| 51 | 5 | MZ964047 | |
| 52 | 5 | MZ964103 | |
| 53 | 1 | MZ964048 | MZ964104 |
| 54 | 3 | MZ964049 | MZ964105 |
| 55 | 4 | MZ964106 | |
| 56 | 3 | MZ964107 | |
| 57 | 2 | MZ964050 | MZ964108 |
| 58 | 4 | MZ964109 | |
| 59 | 4 | MZ964110 | |
| 61 | 5 | MZ964051 | MZ964111 |
| 62 | 2 | MZ964052 | MZ964112 |
| 63 | 1 | MZ964113 | |
| 64 | 3 | MZ964114 | |
| 65 | 2 | MZ964115 | |
| 66 | 2 | MZ964053 | MZ964116 |
| 67 | 3 | MZ964117 | |
| 68 | 5 | MZ964118 | |
| 69 | 2 | MZ964054 | MZ964119 |
| 71 | 1 | MZ964120 | |
| 72 | 5 | MZ964121 | |
| 73 | 2 | MZ964122 | |
| 75 | 3 | MZ964123 | |
| 76 | 1 | MZ964124 | |
| 77 | 4 | MZ964125 | |
| 78 | 1 | MZ964055 | MZ964126 |
| 79 | 5 | MZ964127 | |
| 80 | 4 | MZ964059 | MZ964128 |
| 81 | 4 | MZ964129 | |
| 82 | 5 | MZ964130 | |
| 83 | 1 | MZ964131 | |
| 85 | 1 | MZ964132 | |
| 86 | 5 | MZ964133 | |
| 87 | 4 | MZ964134 | |
| 88 | 1 | MZ964060 | |
| 89 | 5 | MZ964061 | MZ964135 |
References
- Hahn, M.B.; Eisen, R.J.; Eisen, L.; Boegler, K.A.; Moore, C.G.; McAllister, J.; Savage, H.M.; Mutebi, J. Reported distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus in the United States, 1995–2016 (Diptera: Culicidae). J. Med. Entomol. 2016, 5, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Vanlandingham, D.L.; Higgs, S.; Huang, Y.J.S. Aedes albopictus (Diptera: Culicidae) and mosquito-borne viruses in the United States. J. Med. Entomol. 2016, 5, 1024–1028. [Google Scholar] [CrossRef]
- Rezza, G. Aedes albopictus and the reemergence of Dengue. BMC Public Health 2012, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Delatte, H.; Desvars, A.; Bouétard, A.; Bord, S.; Gimonneau, G.; Vourc’h, G.; Fontenille, D. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Réunion. Vector Borne Zoonotic Dis. 2010, 3, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 7, e0005625. [Google Scholar] [CrossRef]
- Lounibos, L.P.; Bargielowski, I.; Carrasquilla, M.C.; Nishimura, N. Coexistence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Peninsular Florida two decades after competitive displacements. J. Med. Entomol. 2016, 6, 1385–1390. [Google Scholar] [CrossRef]
- Delatte, H.; Paupy, C.; Dehecq, J.S.; Thiria, J.; Failloux, A.B.; Fontenille, D. Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: Biology and control. Parasite 2008, 1, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Nkoghe, D.; Ollomo, B.; Nze-Nkogue, C.; Becquart, P.; Grard, G.; Pourrut, X.; Charrel, R.; Moureau, G.; Ndjoyi-Mbiguino, A.; et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009, 4, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.C.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 9602, 1840–1846. [Google Scholar] [CrossRef]
- Lindh, E.; Argentini, C.; Remoli, M.E.; Fortuna, C.; Faggioni, G.; Benedetti, E. The Italian 2017 outbreak chikungunya virus belongs to an emerging Aedes albopictus-adapted virus cluster introduced from the Indian Subcontinent. Open Forum Infect. Dis. 2018, 1, ofy321. [Google Scholar]
- Gerhardt, R.R.; Gottfried, K.L.; Apperson, C.S.; Davis, B.S.; Erwin, P.C.; Smith, A.B.; Panella, N.A.; Powell, E.E.; Nasci, R.S. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg. Infect. Dis. 2001, 5, 807. [Google Scholar] [CrossRef] [PubMed]
- Byrd, B.D.; Williams, C.J.; Staples, J.E.; Burkhalter, K.L.; Savage, H.M.; Doyle, M.S. Notes from the Field: Spatially associated coincident and noncoincident cases of La Crosse Encephalitis-North Carolina, 2002–2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 39, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microb. Infect. 2009, 14–15, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; White, A.V.; Byrd, B.D.; Reiskind, M.H.; Doyle, M.S. Evaluation of insecticide resistance in Aedes albopictus (Diptera: Culicidae) in North Carolina, 2017. J. Med. Entomol. 2018, 3, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Marcombe, S.; Farajollahi, A.; Healy, S.P.; Clark, G.G.; Fonseca, D.M. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS ONE 2014, 7, e101992. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Ramirez, D.; Thomas, C.; Connelly, C.R. Baseline susceptibility status of Florida populations of Aedes aegypti (Diptera: Culicidae) and Aedes albopictus. J. Med. Entomol. 2020, 5, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Dowling, Z.; Ladeau, S.L.; Armbruster, P.; Biehler, D.; Leisnham, P.T. Socioeconomic status affects mosquito (Diptera: Culicidae) larval habitat type availability and infestation level. J. Med. Entomol. 2013, 4, 764–772. [Google Scholar] [CrossRef]
- LaDeau, S.; Leisnham, P.; Biehler, D.; Bodner, D. Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: Understanding ecological drivers and mosquito-borne disease risk in temperate cities. Int. J. Environ. Res. Public Health 2013, 4, 1505–1526. [Google Scholar] [CrossRef]
- Whiteman, A.; Delmelle, E.; Rapp, T.; Chen, S.; Chen, G.; Dulin, M. A novel sampling method to measure socioeconomic drivers of Aedes albopictus distribution in Mecklenburg County, North Carolina. Int. J. Environ. Res. Public Health 2018, 10, 2179. [Google Scholar] [CrossRef]
- Chen, S.; Whiteman, A.; Li, A.; Rapp, T.; Delmelle, E.; Chen, G.; Brown, C.L.; Robinson, P.; Coffman, M.J.; Janies, D.; et al. An operational machine learning approach to predict mosquito abundance based on socioeconomic and landscape patterns. Landsc. Ecol. 2019, 6, 1295–1311. [Google Scholar] [CrossRef]
- Manica, M.; Filipponi, F.; D’Alessandro, A.; Screti, A.; Neteler, M.; Rosà, R.; Solimini, A.; Della Torre, A.; Caputo, B. Spatial and temporal hot spots of Aedes albopictus abundance inside and outside a south European metropolitan area. PLoS Negl. Trop. Dis. 2016, 6, pe0004758. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, K.S.; Mormann, K.; Juliano, S.A. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2005, 4, 559–570. [Google Scholar] [CrossRef]
- Armbruster, P.; Hutchinson, R.A. Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae). J. Med. Entomol. 2002, 4, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, M.S.; Lord, C.C. The relationship between size and fecundity in Aedes albopictus. J. Vector Ecol. 2000, 2, 212–217. [Google Scholar]
- Alto, B.W.; Reiskind, M.H.; Lounibos, L.P. Size alters susceptibility of vectors to dengue virus infection and dissemination. Am. J. Trop. Med. Hyg. 2008, 79, 688–695. [Google Scholar] [CrossRef]
- Paulson, S.L.; Hawley, W.A. Effect of body size on the vector competence of field and laboratory populations of Aedes triseriatus for La Crosse virus. J. Am. Mosq. Control. Assoc. 1991, 2, 170–175. [Google Scholar]
- American Community Survey 5-Year Estimate. 2017. Available online: https://data.census.gov/cedsci/table?q=&t=Income%20and%20Poverty&g=0500000US37119&y=2017&tid=ACSST5Y2017.S1701 (accessed on 22 August 2021).
- Chetty, R.; Hendren, N.; Kline, P.; Saez, E. Where is the land of opportunity? The geography of intergenerational mobility in the United States. Q. J. Econ. 2014, 4, 1553–1623. [Google Scholar] [CrossRef]
- Garo, L.; Allen-Handy, A.; Lewis, C.W. Race, poverty, and violence exposure: A critical spatial analysis of African American trauma vulnerability and educational outcomes in Charlotte, North Carolina. J. Negro Educ. 2018, 3, 246–269. [Google Scholar] [CrossRef]
- Potter, M. Identification Key to the Genera of Adult Mosquitoes for the World. Walter Reed Biosystematics Unit. 2017. Available online: http://www.wrbu.org/mqID/keysMQZoogeo.html (accessed on 1 September 2018).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 6, 1547–1549. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 7, 36–42. [Google Scholar]
- O’Neil-Dunne, J.; MacFaden, S.; Royar, A. A versatile, production-oriented approach to high-resolution tree-canopy mapping in urban and suburban landscapes using GEOBIA and data fusion. Remote Sens. 2014, 12, 12837–12865. [Google Scholar] [CrossRef]
- Landau, K.I.; Van Leeuwen, W.J.D. Fine scale spatial urban land cover factors associated with adult mosquito abundance and risk in Tucson, Arizona. J. Vector Ecol. 2012, 2, 407–418. [Google Scholar] [CrossRef]
- Murdock, C.C.; Evans, M.V.; McClanahan, T.D.; Miazgowicz, K.L.; Tesla, B. Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLoS Negl. Trop. Dis. 2017, 5, e0005640. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 1, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario, K.L.; Richards, S.L.; Anderson, A.L.; Balanay, J.A.G. Current status of mosquito control programs in North Carolina: The need for cost-effectiveness analysis. J. Environ. Health 2014, 8, 8–15. [Google Scholar]
- Richards, S.L.; Balanay, J.A.G.; Byrd, B.D.; Reiskind, M.H.; Styers, D.M. Regional survey of mosquito control knowledge and usage in North Carolina. J. Am. Mosq. Control. Assoc. 2017, 4, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.K.; Bradley, C.; Apperson, C.S.; Gould, F. An experimental field study of delayed density dependence in natural populations of Aedes albopictus. PLoS ONE 2012, 4, e35959. [Google Scholar] [CrossRef] [PubMed][Green Version]
- United States Climate Data. Available online: https://www.usclimatedata.com/climate/charlotte/north-carolina/united-states/usnc0121 (accessed on 22 August 2021).
- Alto, B.W.; Juliano, S.A. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): Implications for range expansion. J. Med. Entomol. 2001, 5, 646–656. [Google Scholar] [CrossRef]
- Reiskind, M.H.; Zarrabi, A.A. Water surface area and depth determine oviposition choice in Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2012, 1, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Ng, L.C.; Lam-Phua, S.G.; Tang, C.S.; Itokawa, K.; Komagata, O.; Kobayash, M.; Tomita, T. First detection of a putative knockdown resistance gene in major mosquito vector, Aedes albopictus. Jpn. J. Infect. Dis. 2011, 64, 217–221. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).