Abstract
Saliva plays a significant role in oral health and tooth integrity. Salivary components reduce tooth surface exposure to demineralization, protect against teeth wear and aid in enamel remineralization. There is a growing attempt to use salivary markers in diagnosing or predicting caries. However, despite the current information, there has yet to be an agreement among scholars. This study seeks to contribute more evidence on the suitability of salivary biomarkers in dental caries diagnosis. Eligible studies were electronically searched on online databases PubMed, Elsevier’s Scopus, EMBASE and Web of Science, and all the studies that met the inclusion criteria were considered. The PECOS criteria guided the study selection process based on the study question. The risk of bias was assessed using the STROBE checklist. Eighteen articles were included in the analysis. All the studies presented relevant data concerning the study objectives. There was evidence of associations between salivary biomarkers and dental caries, and the correlations were either positive or negative. The studies presented significant heterogeneity; thus, a meta-analysis was not possible. Salivary biomarkers appeared to perform crucial and complementary functions toward tooth integrity and thus may be reliable in predicting or diagnosing dental caries in patients.
Keywords:
caries; dental; oral; biomarkers; dental caries; salivary biomarkers; proteins; systematic review 1. Introduction
Dental caries is a highly prevalent microbial chronic dental disease that affects a greater portion of the world’s population [1]. Besides being multifactorial, dental caries is a sugar drive biofilm-mediated oral disease characterized by the phasic demineralization and demineralization of the hard tissues of the tooth [2]. The saliva surrounding the soft and hard tissues of the oral cavity includes some organic and inorganic components which contain certain factors that play a significant role for caries in the host; hence, their critical role as biomarkers in the diagnosis of caries [3]. In other words, dental caries is the result of the imbalanced cariogenic microorganisms found within the oral biofilm, which triggers the fermentation of available dietary carbohydrates to produce acids [4]. Consequently, the latter causes mineral loss on the dental hard tissues, destroying tooth structure [4]. In accordance, Sandhya et al. stated that the association between the diet, host susceptibility and microorganisms greatly determines the development of dental caries in an individual [5]. Since the teeth are consistently immersed in saliva, the saliva constituents inevitably play a fundamental role in the occurrence and progression of dental caries and are believed to be an important biomarker for caries diagnosis and detection. A biomarker is a molecule characteristic that is objectively measurable and evaluable as an indicator of a given biological or pathological process [6]. The reliable biomarkers are regarded as molecular signatures, which are heavily relied upon in assessing disease risks and in prognosis, diagnosis, or monitoring of the disease.
The proteins within the saliva play a crucial role in maintaining the integrity of the tooth, alongside preventing the incidence of dental caries through various mechanisms [1]; for instance, inhibiting the tooth surfaces exposed to demineralization, generating the acquired enamel pellicle (AEP) to continually protect the teeth from wearing out and enabling enamel remineralization by attracting calcium ions. Nevertheless, the salivary proteins also attribute the defense of the oral cavity glycoproteins such as lactoferrin, proline-rich proteins (PRPs), carbonic anhydrase, immunoglobulins and mucins, which are ideally the most occurring, representing about 20 to 30% of the salivary proteins [7]. The mucins subcategory of MG-1 (MUC5), highly associated with high-intensity dental caries cases, is usually tasked with increasing the salivary velocity, whereas the second category, MG-2 (MUC7), is mostly found in low-intensity cases of caries, triggering the agglutination of the microorganisms within the oral cavity, including the cariogenic bacteria [8,9]. Lactoferrin, on the other hand, inhibits the production of the biofilms and growth of bacteria by attaching iron. Given its impacts on dental erosion and caries formation, the carbonic anhydrase infiltrates the dental plaque and thus neutralizes the bacterial acids bicarbonate buffer system. The cathelicidins that act as salivary antimicrobial peptides have the synergistic impact of high activity against gram-positive or gram-negative bacteria and fungi organisms, which are mainly associated with major risks of dental caries [10].
Given the detailed roles played by the salivary proteins in dental caries pathophysiology, there has been an increased interest in recent decades in using saliva as a diagnostic tool for dental caries. Despite positive findings from the literature, there have been numerous controversies due to the findings’ irreproducibility, primarily due to variance in methodological and statistical approaches, as well as results interpretations. For instance, the study by Piekoszewska-Ziętek et al. [11] established an association between dental caries occurrence and levels of salivary proteins in the oral cavity, contrary to the findings of Martins et al. [12] that disproved any kind of association. The latter conflicting opinions necessitate evaluating available literature evidence to enhance the understanding of this crucial developing discipline. Accordingly, the present systematic review assesses the association between salivary biomarkers or proteins and dental caries occurrence to validate the fundamental role played by the biomarkers in the detection and diagnosis of dental caries.
2. Materials and Methods
2.1. Experimental Approach to the Review Question
The present study was performed per the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [13].
2.2. Focused Question
Is there an established relationship between dental caries occurrence and salivary protein concentrations that substantiates the deployment of salivary biomarkers in detecting and diagnosing dental caries?
2.3. Eligibility Criteria
Studies were included in this study if they met the following inclusion criteria: articles written in English between 2010 and 2022 according to PECOS (Population, Exposure, Comparator, Outcomes, and Study) design.
- Population (P): healthy individuals not under any medication that directly influences saliva composition;
- Exposure (E): salivary components as biomarkers in the dental caries incidence;
- Comparator (C): participants with dental caries (caries-active/high caries) or without caries (caries-free/low caries);
- Outcome (O): concentrations or levels of biomarkers in the study population with regard to increased (high) in the exposed group (caries-free, no caries, fewer caries) versus comparator (caries-active, dental caries patients);
- Study design (S): in vitro studies, cohort studies, cross-sectional, case-control, pilot study and longitudinal studies.
However, studies were excluded based on the following:
- Articles that did not use a predefined index for assessing dental caries in the patient groups;
- Non-English articles published earlier than 2010;
- All irrelevant publications: case reports, reviews, dissertations, unpublished articles, book chapters, reports, etc.
2.4. Literature Search Strategy
A comprehensive literature search was electronically performed in online databases, including PubMed, Elsevier’s Scopus, EMBASE and Web of Science, to identify articles for potential inclusion in the study. The search involved English language records published from January 2010 to September 2022, allowing the inclusion of recently published records yet to be used in other reviews. The search strategy involved the use of search terms and MeSH terms, including “dental caries”, “caries”, “saliva”, “proteins”, “biomarkers”, “stimulated saliva”, “unstimulated saliva”, “whole saliva” and other primary salivary biomarkers discussed in various studies. A search of grey literature was also performed in Google Scholar. Finally, the authors examined the reference lists of the retrieved full-text studies for potential additional articles.
2.5. Study Selection
Two reviewers independently performed the article selection process. All the potential records that met the inclusion criteria were subjected to data screening to select the most relevant studies. After de-duplicating the retrieved records, the titles with keywords were manually screened, followed by abstract screening. The sources deemed relevant were considered for possible inclusion in the study synthesis. Each article was then subjected to full-text screening based on the eligibility criteria, and those that met the criteria were selected. Any discrepancies and differences between the two reviewers were settled through consensus or discussion with the third and fourth authors.
2.6. Data Collection
Data extraction was done by the two reviewers independently and then cross-checked by the third and fourth reviewers for verifying and ascertaining data accuracy. The required information was abstracted into a standardized data sheet. Any disagreements among the reviewers were solved by discussion until a consensus was reached.
The following information was collected from the selected articles: first author’s surname and year of publication, the country where the study was conducted, study design, sample characteristics (i.e., participants’ size and age ranges), type of saliva collected for sampling, time of sample collection, methods of assessing biomarkers in the collected samples, caries index scores in the participating groups, data analysis methods, salivary parameters (biomarkers assessed), concentration levels of biomarkers identified, and main findings and inferences or conclusions.
2.7. Risk of Bias Assessment
The STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) [14] checklist was used to assess the selected articles’ bias risk. The checklist was based on twelve key criteria. Studies that scored eight and above were classified as low risk of bias, scores between four and seven were categorized as moderate bias risk, and those with three and below scores were considered to be a high risk of bias.
3. Results
3.1. Study Search and Selection
A total of 1159 articles were retrieved as potentially relevant records from all the databases searched. Of the total sources, 729 sources were eliminated in the deduplication process. The 430 obtained sources were screened via their titles and abstracts, and 321 articles were excluded due to non-conformity with the study objective. The remaining 109 records were subjected to full-text screening based on the predefined eligibility criteria. Ninety-one studies that did not meet the eligibility standards were excluded from the study. Finally, 18 records were certified to meet the inclusion criteria fully and thus considered for inclusion in this study. Details of the search and selection results are presented in Figure 1.
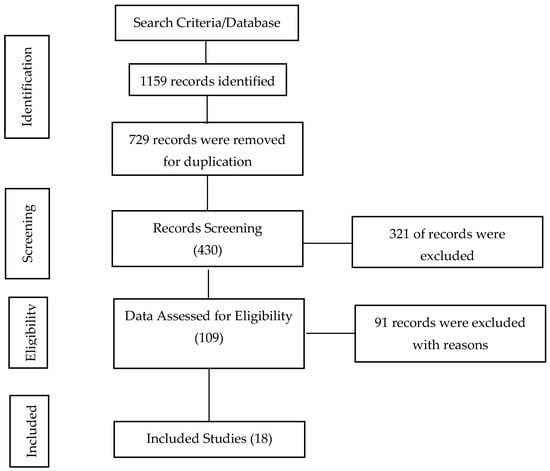
Figure 1.
PRISMA flow diagram of search study selection process.
3.2. Primary Characteristics of Included Studies
A detailed summary of key characteristics and findings of the 18 included studies is presented in Table 1. Four included studies were conducted in India [15,16,17,18], three studies in Poland [19,20,21], two in Brazil [22,23] and one multinational [24]. Each of the remaining seven studies were performed in China [25], Kosovo [26], Chile [27], Romania [28], Colombia [29], Egypt [30], Malaysia [31] and Iraq [32]. All the included studies were conducted within the last 12 years, but the majority were conducted between 2015 and 2021. The articles also presented a significant collective sample size. A total of 1454 participants, of both adults and children of both genders, were recruited in the 18 included studies, with the least sample size reported for a single study being 24 participants [29] and the largest being 188 participants [31]. Nevertheless, of the full sample size, 936 were minors aged between 2 years and 18 years which was reported in 11 of the included sources. Only five records presented data from the 518 adult participants aged between 18 to 50 years old.

Table 1.
Characteristics and Summary of key findings of Included Studies.
Contradictory results were also reported for the salivary total protein evaluated by three studies, among which two articles found a positive correlation of high protein concentration in the caries-active population [17,24] and one study [27] found a negative correlation between high levels in caries-free participants. Two studies also evaluated carbonic anhydrase, but one study found a positive correlation of high CA VI levels in the caries-active group [23], contrary to the other that presented high CA VI concentration levels for the caries-free participants over the caries-active group [30]. Similar to alpha-amylase, two studies [22,28] reported a positive association of higher enzyme concentration in caries-active group. In comparison, one noted a relatively higher concentration in caries-free participants [23], posing that alpha amylase had a protective role. The active caries group were likely to develop more caries with diminishing alpha-amylase levels. In two included studies, contradictory results were also observed for the zinc marker [17,26]. However, for proteinase 3 (PR3) [25], statherin [29,31] and LL-37 [15], a significant negative correlation between the molecules and dental caries was evident since high concentration levels were witnessed in caries-free or low caries risk participants, implying that they significantly reduced caries incidences in the caries-free groups.
With regard to the saliva types sampled from the study population, nine included records evaluated unstimulated saliva [16,18,20,21,25,27,28,29], two studies evaluated the stimulated saliva [31,32], three studies evaluated whole saliva [15,22,23] and the remaining four studies assessed both whole unstimulated and stimulated saliva [17,24,26,30]. The saliva samples were collected mainly in the morning and afternoon hours (between 8.00 a.m. and 1.00 p.m.) for 12 studies; however, six included studies failed to state the time ranges of saliva sample collection from the participants [18,21,22,28,30,32]. The most common dental caries index was the DMF (DMFS/DMFT) used in 15 studies. Of the remaining three, one study used visual detection [28], another ICADS [29] and the last was a combination of ICADS and DMFS [23]. For the salivary biomarkers assessment, the ELISA test was used in six studies [22,23,24,25,27,29], spectrophotometry in four studies [17,26,27,28], radial immunodiffusion using the Diffu-Plate kit in two studies [18,32], the colorimetric test in two studies [16,30], the high sensitivity assays test (USCNK) [19], AGAPPE [15] and USCNK plus ELISA [20], as well as ELISA plus colorimetric kits [31].
3.3. Correlation between Salivary Biomarkers and Dental Caries
These included studies assessed a variety of markers of salivary proteins. Among the markers, mucin, histatin, proline-rich proteins (PRP), lactoperoxidase, C-reactive proteins, cathelicidin (LL-37), immunoglobulin (IgA), albumin, statherin, salivary total protein, SOD, copper, zinc, proteinase 3 (PR3), alpha-amylase and carbonic anhydrase (CA IV) were all assessed. Although there were established correlations between dental caries pathogenicity and concentration levels of each identified biomarker, results in some markers were contradictory from the studies. The IgA was the most prevalent biomarker of all other biomolecules. Of the six studies that evaluated IgA [15,18,20,27,29,32], all except one study found a negative correlation [20], i.e., high concentration was found in the caries-free group compared with the caries-active participants, which attributed to their immense protective role in dental and oral health. Two studies [15,19] evaluated mucin, which established a positive association of high mucin levels in patients with caries prevalence or participants categorized as high caries risk or active caries group. Similar results were observed for histatin 5 [20,21], lactoperoxidase [15,20], C-reactive protein [15], SOD [17] and copper [17].
3.4. Risk of Bias Assessment of Included Studies
All the included studies reported a low risk of bias as shown in Table 2.

Table 2.
STROBE Risk of Bias Assessment of Included Studies. (x: presence of criteria).
4. Discussion
4.1. Summary of Main Findings
The present study aimed to establish the fundamental role of salivary biomarkers with regard to the detection and diagnosis of the prevalence and progression of dental caries across all ages. It was achieved by evaluating for possible evidence of an association between various proteins in the oral cavity or saliva and the prevalence of caries. The articles selected for inclusion satisfied the standard STROBE quality assessment criteria. All the studies showed a correlation between the salivary biochemical markers and dental caries pathogenesis and severity levels. Generally, the study presented various biochemical markers found in saliva samples, which included the antimicrobial peptides (AMPs) molecules (cathelicidin peptide LL-37, beta-defensins, statherin and histatins), major salivary glycoproteins (proline-rich proteins (PRPs) mucins, and immunoglobulins) and minor salivary glycoproteins, which are believed to have a functionally specific contribution to the defense of the oral cavity. However, there were conflicting findings regarding the association of different molecules in this study. For instance, regardless of the statistically significant association between salivary biomarkers of the participants with or without dental caries, a section of studies reported that the concentration of particular biomarkers increased with high caries experience. Insightfully, it is worth noting that other factors, such as the size of samples, age and saliva type, impacted the comparison of results between the included studies, since the other above data varied enormously across the articles. Notwithstanding the above, the association between the salivary biochemical parameters and dental caries is crucial proof that salivary biomarkers are reliable in predicting and diagnosing dental caries.
4.2. Literature Insight into the Correlation Role of Biomarkers on Dental Caries Experience
Dental caries is ideally a multifactorial condition of the teeth; thus, it cannot be rooted in a single biochemical marker to predict or diagnose disease progression or severity. According to scholars, saliva acts as the main natural defense system for the oral cavity, protecting the exposed surfaces of the tooth [33,34]. For instance, saliva can reverse tooth demineralization by its mechanical rinsing capacity or through its buffering capacity, antimicrobial activity, secretion of antimicrobial peptides or the calcium phosphate binding proteins. It can also initiate tooth remineralization with the aid of calcium, fluoride or phosphate to prevent dental caries.
Increased salivary enzymatic biomarkers, including alpha-amylase, proteinase-3 and carbonic anhydrase (CA VI), correlated with increased caries occurrence. For instance, Malawi et al. [30] and Picco et al. [22] noted increased CA VI levels in the caries-free subjects compared with the caries-active subjects. The latter is evidence toward the protective role of CA VI; for example, through the stimulation of oral buffers, pH regulation and accelerated acid neutralization. On the other hand, salivary alpha-amylase was higher in caries-active subjects than in caries-free subjects [28], as opposed to the findings of Borghi et al. [23] who noted higher amylase concentration in the caries-free subjects than in the caries-active subjects, suggesting a negative association with dental caries incidence or progression.
Nonetheless, the literature proposes that amylase binds with the oral bacteria in the dental plaque since it helps in dietary starch hydrolysis that consequently leads to acid formation in dental plaque, accelerating the demineralization of the tooth and even dental caries progression. However, this inference is not ultimate given the conflicting opinions from the literature on amylase concentration and susceptibility of caries.
Other than enzymatic biomarkers, antimicrobial peptides (AMPs) such as cathelicidin peptide LL-37, beta-defensins, statherin and histains also presented significant relationships with dental caries. The AMPs category of biomarkers encompasses peptides with the ability to terminate or inhibit the growth of bacteria in the oral cavity. This study reported upregulation of LL-37 in caries-free individuals [29], as well as increased histatin (HTN-5) in caries-active subjects [20]. With regard to the above extrapolations, salivary levels of AMPs can be useful in dental caries risk assessments.
The evidence presented by the included studies on correlations between dental caries and salivary glycoproteins such as mucins, immunoglobulin and proline-rich proteins is worth noting. With regard to the findings, Gabryel-Porowska et al. [19] found a correlation between increased salivary mucin (MUC1) levels and high caries experience (DMFT above 11), unlike in caries-free subjects. According to proponents, MUC1 is produced from the minor and major salivary glands, including the oral epithelial cells. Hence, it is argued to be a critical element of mucosal protection from the infection since its high concentration leads to bacterial infection of the teeth [35]. As if that was not enough, increased mucins (MUC1) levels in the oral epithelial cells are argued to be triggered by the increased inflammatory cytokines, including the interleukin-1B and interleukin-6, or the Porphyromonas gingivalis infection. Therefore, altered salivary cytokines levels directly influence MUC1 expression in the oral cavity.
Regarding statherin, a study found higher levels in caries-free individuals than in the caries-active group [29]. Similar observations were made in previous studies [36,37], suggesting that statherin had a significant protective role against caries progression. In accordance, statherin depicts a strong hydroxyapatite affinity, binding calcium and maintaining the saliva’s saturation by barring spontaneous phosphate and calcium precipitation. This is what attributes protection to the tooth integrity since it enhances enamel remineralization [38]. Consistent with the above findings on antimicrobial peptides, Mandel et al. [38] agreed in their study, which noted a strong association between PRPs, statherin and histain with the absence of caries. This is attributed to the significant protective role of the phosphopeptides on the tooth structure.
The proline-rich proteins offer an effective mechanism against dental caries progression by attaching to the Streptococcus mutants through the adhesion antigen and exhibiting an immunological reaction that protects the oral cavity from caries. In other words, PRPs minimize dental caries incidence by neutralizing the acidity caused by Streptococcus mutans bacterial functions. Considering the findings, this review also found higher concentration levels of proline-rich proteins in the caries-active group than in the caries-free individuals. The latter was contrary to previous findings that increased salivary PRPs levels were higher in caries-free than in the active study group. However, the PRP, particularly the acidic proline-rich protein, is chemically structured to trigger aminoethylpiperazine (AEP) formation, which is the main host for the bacteria responsible for dental plaque formation. Nevertheless, the acidic PRP inhibits calcium phosphate precipitation and thus boosts calcium homeostasis in the oral cavity, consequently leading to tooth enamel protection.
On a similar account, immunoglobulins constitute humoral immunity and are also responsible for neutralizing the bacteria within the immune system mechanism, a crucial process attached to dental caries. This review established that IgA levels increased for caries-free subjects in the reviewed studies, and the findings agreed with the study by Kuriakose et al. [39]. Another study by Ranadheer et al. [40], performed on children, reported increased IgA concentration levels for the caries-free children compared with the caries-active children, suggesting a significant defensive role of IgA against Streptococcus mutants in a whole saliva sample. However, contrary findings were also presented in this review, noting significantly higher IgA concentration levels in caries-active subjects, similar to other studies [41,42,43]. This study suggested that the caries-free subjects experienced increased IgA levels, implying that a reduction of the IgA concentration increased the susceptibility of dental caries; hence, the reason for low concentration in the caries-active subjects. Despite the equivocal and parallel literature findings, immunoglobulins play a role in controlling dental caries development and progression.
Moreover, this study also assessed salivary total proteins and deduced that the salivary total proteins concentrations showed a statistically critical association with dental caries experience. Again, there were contrary opinions, as reported in the study results. Nevertheless, salivary protein is crucial for protecting the tooth against desiccation. The C-reactive protein, on the other hand, was highly concentrated in caries-active subjects. Like other biomolecules, the C-reactive protein controls the immune response during inflammation development in the oral biofilms and during caries progression [44].
The findings on salivary albumin proteins concluded that salivary albumin controls the severity of dental plaque inflammation and underlying caries disease. It inhibits the progression of dental caries by penetrating the enamel pores to protect it against demineralization. Hence, this study found a significant negative association between salivary albumin and dental caries and a subsequent increase in caries incidence with decreased levels of salivary albumin. The latter findings were echoed by Mungia et al. [45] who reported that albumin is significantly associated with dental caries and other salivary proteins such as mucin and lysozyme. The above was also in agreement with the results of Yoshihara et al. [46] who did a cross-sectional study and reported that serum albumin levels were associated with events of dental caries.
This study also noted increased SOD activity: higher for caries-active than for caries-free participants. Attributing to its antioxidant role, superoxide dismutase (SOD) is regarded as a detoxifying antioxidant enzyme that functions against free radicals and thus catalyzes the dis-mutation of superoxide ions to hydrogen and oxygen peroxide.
4.3. Significance of the Study
There are multiple methods that are used traditionally to detect dental caries such as a visual-tactile examination, radiographs, caries detection dyes and fiber optic transillumination (FOTI) [47]. Recently, technology offered alternative diagnostic tools, including fluorescence, electrical conductance and lasers that provide more accurate information [48]. Dentists routinely combine two diagnostic methods to detect and diagnose dental caries in order to manage it properly. However, there are disagreements on what the most accurate diagnostic method is for detecting dental caries, especially the incipient lesions [49]. Nowadays, interest toward non-invasive and personalized dentistry has been increased. Molecular assays using salivary biomarkers can be an effective tool in detecting the caries in earlier stages and assessing a patient’s caries risk [50]. This will allow a conservative and personalized approach in caries diagnosis and management for each patient. The current study identified different molecular signatures that can be used in caries diagnosis, prognosis, risk assessment and monitoring of the disease.
4.4. Strengths and Limitations
Considering a sample size of 1454 individuals, it’s arguable that this study involved a significantly large sample size which was a core strength. Besides its adherence to the PRISMA statement, this study used predetermined eligibility criteria according to PECOS, which ensured that only highly reliable studies were selected for inclusion. This was a key strength in ensuring that information was synthesized from high-quality studies to boost the reliability of the overall study. However, the study also presented some limitations worth recognizing. The included studies were mostly cohorts and cross-sectional, with one longitudinal and pilot study. In this sense, participant selection bias was inevitable, which would cause a doubly negative effect on the quality of the results. Future research would be more comprehensive by including high-quality studies with sufficient follow-up to establish conclusive information on salivary biomarkers such as dental caries detection or prognosis components.
Nonetheless, the sample size was also a limitation. Some of the studies recruited significantly low sample sizes, which could affect the statistical power when comparing outcomes between the active and caries-free groups. Again, such information might not be of reliable standards to act as evidence for justifying salivary biomarkers as effective diagnosis components for dental caries. Finally, there was high heterogeneity among the study participants with regard to age and geographical exposure. This was an important factor to consider. Another heterogeneity factor was based on the salivary biomarkers evaluated, since some were whole evaluated saliva, unstimulated saliva stimulated or both. This contributed to a wide variation in the data obtained, which could also extend to the overall inferences of the study.
5. Conclusions
This systematic review included 18 studies to produce the study results. Despite conflicting findings on the association between salivary makers and dental caries, each included study provided evidence of a relationship between components of saliva and caries. Given such a consensus, this study concludes that the salivary biomarker component concentration levels could be significant in detecting and diagnosing dental caries for both children and adults.
Author Contributions
Conceptualization, A.A. and R.A.; methodology, A.A., R.A. and Y.G.; software, A.A. and A.M.A.; validation, A.A., R.A. and A.M.A.; formal analysis, A.A. and R.A.; investigation, R.A. and Y.G.; resources, A.A.; data curation, Y.G. and A.M.A.; writing—original draft preparation, A.A.; writing—review and editing, A.A., R.A., Y.G. and A.M.A.; visualization, A.A. and R.A.; supervision, A.A.; project administration, A.A.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hicks, J.; Garcia-Godoy, F.; Flaitz, C. Biological factors in dental caries: Role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J. Clin. Pediatr. Dent. 2003, 28, 47–52. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H. Biomolecules and Biomarkers in Oral Cavity: Bioassays and Immunopathology. J. Immunoass. Immunochem. 2019, 40, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Daliri, E.B.-M.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Subiksha, P.S.; Sandhya, R. Salivary Biomarkers in Diagnosis of Dental Caries—A Review Article. Int. J. Dent. Oral Sci. 2021, 8, 3729–3733. [Google Scholar]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Balsa-Castro, C.; Nibali, L.; Donos, N.; Tomás, I. Accuracy of Single Molecular Biomarkers in Saliva for the Diagnosis of Periodontitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2020, 47, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Belstrøm, D. The Role of Natural Salivary Defences in Maintaining a Healthy Oral Microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef]
- Kesimer, M.; Kiliç, N.; Mehrotra, R.; Thornton, D.J.; Sheehan, J.K. Identification of Salivary Mucin MUC7 Binding Proteins from Streptococcus Gordonii. BMC Microbiol. 2009, 9, 163. [Google Scholar] [CrossRef]
- Thomsson, K.A.; Prakobphol, A.; Leffler, H.; Reddy, M.S.; Levine, M.J.; Fisher, S.J.; Hansson, G.C. The Salivary Mucin MG1 (MUC5B) Carries a Repertoire of Unique Oligosaccharides That Is Large and Diverse. Glycobiology 2002, 12, 1–14. [Google Scholar] [CrossRef]
- Dale, B.A.; Fredericks, L.P. Antimicrobial peptides in the oral environment: Expression and function in health and disease. Curr. Issues Mol. Biol. 2005, 7, 119–133. [Google Scholar] [CrossRef]
- Piekoszewska-Ziętek, P.; Turska-Szybka, A.; Olczak-Kowalczyk, D. Salivary Proteins and Peptides in the Aetiology of Caries in Children: Systematic Literature Review. Oral Dis. 2019, 25, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Buczynski, A.K.; Maia, L.C.; Siqueira, W.L.; de Araujo Castro, G.F.B. Salivary Proteins as a Biomarker for Dental Caries—A Systematic Review. J. Dent. 2013, 41, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 10, 89. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE Guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. S1), S31–S34. [Google Scholar] [CrossRef]
- Nireeksha, N.; Hegde, M.; Kumari, N.S.; Ullal, H.; Kedilaya, V. Salivary Proteins as Biomarkers in Dental Caries: In Vivo Study. Dent. Oral Craniofac. Res. 2017, 3, 4238–4244. [Google Scholar] [CrossRef]
- Khandelwal, A.; Palanivelu, A. Correlation between Dental Caries and Salivary Albumin in Adult Population in Chennai: An in Vivo Study. Braz. Dent. Sci. 2019, 22, 228–233. [Google Scholar] [CrossRef]
- Hegde, M.N.; Hegde, N.D.; Ashok, A.; Shetty, S. Biochemical Indicators of Dental Caries in Saliva: An in Vivo Study. Caries Res. 2014, 48, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Doifode, D.; Damle, S.G. Comparison of Salivary IgA Levels in Caries Free and Caries Active Children. Int. J. Clin. Dent. Sci. 2011, 2, 10–14. [Google Scholar]
- Gabryel-Porowska, H.; Gornowicz, A.; Bielawska, A.; Wójcicka, A.; Maciorkowska, E.; Grabowska, S.Z.; Bielawski, K. Mucin Levels in Saliva of Adolescents with Dental Caries. Med. Sci. Monit. 2014, 20, 72–77. [Google Scholar] [CrossRef]
- Gornowicz, A.; Tokajuk, G.; Bielawska, A.; Maciorkowska, E.; Jabłoński, R.; Wójcicka, A.; Bielawski, K. The Assessment of SIgA, Histatin-5, and Lactoperoxidase Levels in Saliva of Adolescents with Dental Caries. Med. Sci. Monit. 2014, 20, 1095–1100. [Google Scholar] [CrossRef]
- Jurczak, A.; Kościelniak, D.; Papież, M.; Vyhouskaya, P.; Krzyściak, W. A Study on β-Defensin-2 and Histatin-5 as a Diagnostic Marker of Early Childhood Caries Progression. Biol. Res. 2015, 48, 61. [Google Scholar] [CrossRef]
- Picco, D.d.C.R.; Lopes, L.M.; Rocha Marques, M.; Line, S.R.P.; Parisotto, T.M.; Nobre Dos Santos, M. Children with a Higher Activity of Carbonic Anhydrase VI in Saliva Are More Likely to Develop Dental Caries. Caries Res. 2017, 51, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Borghi, G.N.; Rodrigues, L.P.; Lopes, L.M.; Parisotto, T.M.; Steiner-Oliveira, C.; Nobre-dos-Santos, M. Relationship among α Amylase and Carbonic Anhydrase VI in Saliva, Visible Biofilm, and Early Childhood Caries: A Longitudinal Study. Int. J. Paediatr. Dent. 2017, 27, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Murugeshappa, D.G.; Math, S.Y.; Kalra, D.; Ch, P.R.; Pateel, D.G.S. Biochemical & Immunological Assessment of Dental Caries. Ann. Med. Health Sci. Res. 2018, 8, 336–339. [Google Scholar]
- Yang, T.-Y.; Zhou, W.-J.; Du, Y.; Wu, S.-T.; Yuan, W.-W.; Yu, Y.; Su, L.; Luo, Y.; Zhang, J.-H.; Lu, W.-L.; et al. Role of Saliva Proteinase 3 in Dental Caries. Int. J. Oral Sci. 2015, 7, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Sejdini, M.; Begzati, A.; Salihu, S.; Krasniqi, S.; Berisha, N.; Aliu, N. The Role and Impact of Salivary Zn Levels on Dental Caries. Int. J. Dent. 2018, 2018, 8137915. [Google Scholar] [CrossRef]
- Castro, R.J.; Herrera, R.; Giacaman, R.A. Salivary Protein Characteristics from Saliva of Carious Lesionfree and High Caries Adults. Acta Odontol. Latinoam. 2016, 29, 178–185. [Google Scholar] [PubMed]
- Monea, M.; Stoica, A.L. Analysis of Salivary Level of Alpha-Amylase as a Risk Factor for Dental Caries. Acta Med. Transilv. 2018, 23, 93–95. [Google Scholar]
- Angarita-Díaz, M.P.; Simon-Soro, A.; Forero, D.; Balcázar, F.; Sarmiento, L.; Romero, E.; Mira, A. Evaluation of Possible Biomarkers for Caries Risk in Children 6 to 12 Years of Age. J. Oral Microbiol. 2021, 13, 1956219. [Google Scholar] [CrossRef] [PubMed]
- Makawi, Y.; El-Masry, E.; El-Din, H.M. Salivary Carbonic Anhydrase, PH and Phosphate Buffer Concentrations as Potential Biomarkers of Caries Risk in Children. J. Unexplor. Med. Data 2017, 2, 9–15. [Google Scholar] [CrossRef]
- Pateel, D.G.S.; Gunjal, S.; Dutta, S. Association of Salivary Statherin, Calcium, and Proline-Rich Proteins: A Potential Predictive Marker of Dental Caries. Contemp. Clin. Dent. 2022, 13, 84–89. [Google Scholar] [CrossRef]
- Yassin, H.N. Comparison of Immunoglobulin IgA Level in the Stimulated Saliva of Caries-Free and Caries-Active Children Aged 7–10 Years. J. Baghdad Coll. Dent. 2016, 28, 155–158. [Google Scholar] [CrossRef]
- Levine, N. Outfitting Your Practice for Safety and Efficiency. Dent. Prod. Rep. 2020, 54, 41–44. [Google Scholar]
- Hegde, M. Correlation between Dental Caries and Salivary Albumin in Adult Indian Population—An in Vivo Study. Br. J. Med. Med. Res. 2014, 4, 4238–4244. [Google Scholar] [CrossRef]
- McAuley, J.L.; Linden, S.K.; Png, C.W.; King, R.M.; Pennington, H.L.; Gendler, S.J.; Florin, T.H.; Hill, G.R.; Korolik, V.; McGuckin, M.A. MUC1 Cell Surface Mucin Is a Critical Element of the Mucosal Barrier to Infection. J. Clin. Investig. 2007, 117, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Wang, X.; Ren, Q.; Han, S.; Ding, L.; Li, Z.; Zhou, X.; Li, W.; Zhang, L. Comparative Salivary Proteomics Analysis of Children with and without Dental Caries Using the ITRAQ/MRM Approach. J. Transl. Med. 2018, 16, 11. [Google Scholar] [CrossRef]
- Vitorino, R.; Lobo, M.J.C.; Duarte, J.R.; Ferrer-Correia, A.J.; Domingues, P.M.; Amado, F.M.L. The Role of Salivary Peptides in Dental Caries. Biomed. Chromatogr. 2005, 19, 214–222. [Google Scholar] [CrossRef]
- Mandel, I.D.; Zorn, M.; Ruiz, R.; Thompson, R.H., Jr.; Ellison, S.A. The Proteins and Protein-Bound Carbohydrates of Parotid Saliva in Caries-Immune and Caries-Active Adults. Arch. Oral Biol. 1965, 10, 471–475. [Google Scholar] [CrossRef]
- Sundaresan, C.; Mathai, V.; Khosla, E.; Gaffoor, F.M.A.; Kuriakose, S. A Comparative Study of Salivary Buffering Capacity, Flow Rate, Resting PH, and Salivary Immunoglobulin A in Children with Rampant Caries and Caries-Resistant Children. J. Indian Soc. Pedod. Prev. Dent. 2013, 31, 69. [Google Scholar] [CrossRef] [PubMed]
- Ranadheer, E.; Nayak, U.A.; Reddy, N.V.; Rao, V.A.P. The Relationship between Salivary IgA Levels and Dental Caries in Children. J. Indian Soc. Pedod. Prev. Dent. 2011, 29, 106–112. [Google Scholar] [CrossRef]
- Bagherian, A.; Jafarzadeh, A.; Rezaeian, M.; Ahmadi, S.; Rezaity, M.T. Comparison of the Salivary Immunoglobulin Concentration Levels between Children with Early Childhood Caries and Caries-Free Children. Iran. J. Immunol. 2008, 5, 217–221. [Google Scholar]
- Colombo, N.H.; Pereira, J.A.; Da Silva, M.E.R.; Ribas, L.F.F.; Parisotto, T.M.; de Oliveira Mattos-Graner, R.; Smith, D.J.; Duque, C. Relationship between the IgA Antibody Response against Streptococcus Mutans GbpB and Severity of Dental Caries in Childhood. Arch. Oral Biol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Al Amoudi, N.; Al Shukairy, H.; Hanno, A. A Comparative Study of the Secretory IgA Immunoglobulins (s.IgA) in Mothers and Children with SECC versus a Caries Free Group Children and Their Mothers. J. Clin. Pediatr. Dent. 2007, 32, 53–56. [Google Scholar] [CrossRef]
- Gawri, S.; Shukla, P.; Chandrakar, A. A Survey of Micro Flora Present in Dental Caries and It’s Relation to Enviornmental Factors. Recent Res. Sci. Technol. 2012, 16, 9–12. [Google Scholar]
- Mungia, R.; Cano, S.M.; Johnson, D.A.; Dang, H.; Brown, J.P. Interaction of Age and Specific Saliva Component Output on Caries. Aging Clin. Exp. Res. 2008, 20, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, A.; Hanada, N.; Miyazaki, H. Association between Serum Albumin and Root Caries in Community-Dwelling Older Adults. J. Dent. Res. 2003, 82, 218–222. [Google Scholar] [CrossRef]
- Sadasiva, K.; Kumar, K.S.; Rayar, S.; Shamini, S.; Unnikrishnan, M.; Kandaswamy, D. Evaluation of the Efficacy of Visual, Tactile Method, Caries Detector Dye, and Laser Fluorescence in Removal of Dental Caries and Confirmation by Culture and Polymerase Chain Reaction: An in Vivo Study. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. S2), S146–S150. [Google Scholar] [CrossRef]
- Ghodasra, R.; Brizuela, M. Dental Caries Diagnostic Testing; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Foros, P.; Oikonomou, E.; Koletsi, D.; Rahiotis, C. Detection Methods for Early Caries Diagnosis: A Systematic Review and Meta-Analysis. Caries Res. 2021, 55, 247–259. [Google Scholar] [CrossRef]
- Paqué, P.N.; Herz, C.; Wiedemeier, D.B.; Mitsakakis, K.; Attin, T.; Bao, K.; Belibasakis, G.N.; Hays, J.P.; Jenzer, J.S.; Kaman, W.E.; et al. Salivary Biomarkers for Dental Caries Detection and Personalized Monitoring. J. Pers. Med. 2021, 11, 235. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).