Simple Summary
This review explores the impact of cervical microbiome changes on human papillomavirus (HPV) using next-generation sequencing (NGS). HPV poses global health concerns, from benign lesions to cervical cancer. The cervical microbiome, a unique microorganism collection in the cervix, is crucial for cervical health. Recent research suggests that disruptions in the cervical microbiome, marked by reduced Lactobacillus and bacterial overgrowth, may heighten HPV persistence and cervical abnormalities. NGS technology has transformed cervical microbiome studies, revealing insights into microbial diversity and dynamics. Bacterial 16S rRNA gene sequencing proves valuable in understanding the cervical microbiome’s role in HPV infections. NGS-based studies provide personalized insights into individuals’ cervical microbiomes, holding promise for novel diagnostic tools, therapies, and preventive interventions for cervical conditions, including cancer. The research aims to enhance global women’s health through a comprehensive understanding of the cervical-microbiome–HPV relationship.
Abstract
This comprehensive review encompasses studies examining changes in the cervical and cervico-vaginal microbiota (CM and CVM) in relation to human papillomavirus (HPV) using next-generation sequencing (NGS) technology. HPV infection remains a prominent global health concern, with a spectrum of manifestations, from benign lesions to life-threatening cervical cancers. The CM and CVM, a unique collection of microorganisms inhabiting the cervix/vagina, has emerged as a critical player in cervical health. Recent research has indicated that disruptions in the CM and CVM, characterized by a decrease in Lactobacillus and the overgrowth of other bacteria, might increase the risk of HPV persistence and the progression of cervical abnormalities. This alteration in the CM or CVM has been linked to a higher likelihood of HPV infection and cervical dysplasia. NGS technology has revolutionized the study of the cervical microbiome, providing insights into microbial diversity, dynamics, and taxonomic classifications. Bacterial 16S rRNA gene sequencing, has proven invaluable in characterizing the cervical microbiome, shedding light on its role in HPV infections and paving the way for more tailored strategies to combat cervical diseases. NGS-based studies offer personalized insights into an individual’s cervical microbiome. This knowledge holds promise for the development of novel diagnostic tools, targeted therapies, and preventive interventions for cervix-related conditions, including cervical cancer.
1. Introduction
Human papillomaviruses (HPVs) are a group of viruses that may infect the skin and mucous membranes of various body parts, including the cervix []. It is one of the most common sexually transmitted infections worldwide. Among nearly 200 types of HPV, high- and low-risk (hr and lr) types were distinguished for the development of cancerous lesions []. Currently, 14 hrHPV oncogenic virus types, including types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68, are causally associated with cancer development [,]. A persistent infection with oncogenic types may result in cancer of the cervix, anus, vagina, vulva, penis, and the throat. HPV-16, HPV-18, HPV-31, HPV-33, and HPV-58 are most commonly identified in cervical cancer cells, with HPV-16 and HPV-18 being found in over 70% of cervical cancer []. In turn, lrHPV types, including HPV-6, HPV-11, HPV-40, HPV-42, HPV-43, HPV-44, HPV-54, HPV-61, HPV-72, and HPV-82, are responsible for the development of benign papillomatous lesions of the mucous membranes and skin. In practice, these include genital warts and recurrent papillomatosis of the larynx [,]. HPV is primarily transmitted through sexual contact, and factors such as multiple sexual partners, early sexual activity, and a weakened immune system may increase the risk of infection []. Most HPV infections are asymptomatic and transient. However, in some cases, when spontaneous clearance of the virus is not achieved, potentially serious or even life-threatening diseases develop, including cervical cancer. The overwhelming number of infections with various types of HPV resolve spontaneously due to the body’s natural immune response. A persistent HPV infection (i.e., infections lasting >24 months) may lead to oncogenesis in subsequent years [].
2. HPV Infection and Cervical Cancer
Cervical cancer (CC) is a significant global health issue, and the burden of the disease is particularly high in low- and middle-income countries []. CC is the fourth most common cancer in women worldwide []. About 604,000 women develop it annually, of whom about 60% die. The peak incidence is between the ages of 50–60 []. HPV viruses show tropism to the epithelial cells of the mucous membranes and skin. Tropism varies depending on the type of virus. Virions penetrate the basal layer of the epithelium, while the assembly and release of progeny virions take place in the upper layers of the epithelium []. A productive viral replication cycle requires the viral oncoproteins E6 and E7, which create a favorable environment for viral DNA replication in the middle layers of the epithelium, where DNA replication would not normally be possible []. The HPV genome undergoes integration at the reading frame breakpoint for the E2 protein, which controls the expression of E6 and E7. The absence of the E2 protein leads to an increased synthesis of both oncogenic proteins, and their excessive activity, to the neoplastic transformation of the infected cell. In turn, the overexpression and activity of viral oncoproteins E6 and E7 lead to the deregulation of the cell cycle, increased cell division, inhibition of apoptosis, and the accumulation of genetic damage due to inefficient DNA repair, resulting in the development of tumorigenesis []. Regular cervical cancer screening, such as Pap smears and HPV testing, is essential for early detection and timely intervention to prevent cervical cancer and its complications [,,]. HPV vaccination is a highly effective preventive measure against HPV infection and its associated diseases, including cervical cancer. Vaccination can protect against the most common hrHPV types and is recommended for both boys and girls before they become sexually active [].
Lesions referred to as low- or high-grade cervical intraepithelial neoplasia (CIN) are far more common in the cervix []. CIN is a precancerous condition that also results from a persistent infection with HPV within cervical cells []. CIN is classified into three grades, CIN-1, CIN-2, and CIN-3, based on the degree of abnormality in the cervical cells []. CIN-1 represents mild dysplasia and is often associated with low-grade squamous intraepithelial lesions (LSIL) on Pap smears. In young women, CIN-1 lesions commonly regress to normal without treatment due to the body’s intact immune response and the cervical rapid cell turnover []. Approximately 60% of CIN-1 cases regress to normal within one year. In turn, CIN-2 and CIN-3 represent moderate-to-severe dysplasia, and they carry a higher risk of progressing to invasive cervical cancer compared to CIN-1. However, the average time for progression to invasive cancer is still several years [].
3. Relationship between Vaginal and Cervico-Vaginal Microbiota and HPV Infection
Research findings indicate that the microbiota across distinct segments of the female genital tract may share similarities while displaying variations [,,]. These differences are observed as one moves from the vagina to the cervix, endometrium, fallopian tubes, and peritoneal fluid. The prevailing trend in most studies has been to label samples as cervico-vaginal rather than explicitly addressing and distinguishing between cervical and vaginal samples.
The vaginal microbiota (VM), cervical microbiota (CM), and cervico-vaginal microbiota (CVM) describe a collection of microorganisms, including bacteria, viruses, and fungi, that reside in vagina and on the cervix. Various factors may influence the composition of the VM, CM, and CVM, including hormonal changes, sexual activity, hygiene practices, and contraceptive and antibiotic use [,,]. The composition of the CVM may vary among individuals but is generally dominated by Lactobacillus species, which are considered beneficial bacteria []. The most common Lactobacillus species found in the vagina and cervix include Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus jensenii, Lactobacillus acidophilus, and Lactobacillus iners [,,]. Lactic acid, a metabolic byproduct of fermenting sugars produced by lactobacilli, helps maintain a balanced pH, prevents the overgrowth of harmful microorganisms, and supports the local immune system []. The VM and CVM play a crucial role in maintaining the vaginal and cervical condition.
Microbial communities in the vagina and cervico-vaginal environment have been classified into five major community status types (CSTs). However, the current CST classification provides only a partial understanding of the relationship between the microbiota and cervico-vaginal conditions in women. The limitations of the bacterial identification technologies used contribute to that understanding []. Previously, Ravel et al. identified four vaginal different types of community status (CST): I, II, III, and V []. These correspond to the microbiota, showing a predominance of specific Lactobacillus species. CST-I was dominated by L. crispatus, CST-II by L. gasseri, CST-III by L. iners, and CST-V by L. jensenii. In contrast, CST-IV presented a diverse microbial composition. France et al. introduced the VALENCIA (vaginal community state type nearest centroid) classifier tool for consistent assignment of CSTs within the VM of reproductive-age women []. VALENCIA’s applicability was validated on diverse datasets, including reproductive-age women from eastern and southern Africa, adolescent girls, and a diverse group of postmenopausal women. Despite variations in sequencing and bioinformatics, VALENCIA performed well, demonstrating its broad applicability for VM classification. Firstly, of the seven identified CSTs, four were rich in Lactobacillus species. These CSTs were further categorized into thirteen sub-CSTs. Following the naming convention from previous studies, the authors designated them as CST I (L. crispatus-dominated), CST II (L. gasseri-dominated), CST III (L. iners-dominated), and CST V (L. jensenii-dominated). CSTs I and III were more prevalent in the dataset and were subdivided into A and B versions, reflecting variations in the relative abundance of the focal species. Additionally, three CSTs with lower lactobacilli abundance were identified as CST IV-A (high Candidatus Lachnocurva vaginae and moderate G. vaginalis), CST IV-B (high G. vaginalis and low Candidatus L. vaginae), and CST IV-C (low Lactobacillus spp., G. vaginalis, A. vaginae, and Candidatus L. vaginae). CST IV-C was further divided into five sub-CSTs: CST IV-C0 (even community with a moderate amount of Prevotella), CST IV-C1 (Streptococcus-dominated), CST IV-C2 (Enterococcus-dominated), CST IV-C3 (Bifidobacterium-dominated), and CST IV-C4 (Staphylococcus-dominated). Depending on the race, self-identifying black or African American women were less likely to have CST I compared to white or Asian women. Black women showed a higher likelihood of having CST IV-A than white women and CST IV-B compared to white or Asian women. Asian women in the study did not exhibit CST IV-A, and CST IV-B was more common among Hispanic women than white women. Asian women were more likely to have CST III than black or white women, although this association was weaker. No significant associations with race were found for CSTs II, V, or IV-C, potentially due to sample size limitations as these three CSTs are less prevalent [].
Emerging research suggests that the composition of the CVM may influence an individual’s susceptibility to HPV infections and the subsequent development of cervical lesions or cancer []. Disruptions in the normal cervical microbiota, such as a decrease in Lactobacillus species or an overgrowth of other bacteria, were associated with an increased risk of HPV persistence and the progression of cervical abnormalities []. Specifically, a lower abundance of Lactobacillus species and an increased presence of certain types of bacteria, such as Gardnerella vaginalis, were associated with an altered CVM and a higher risk of HPV infection and cervical dysplasia [,]. Additionally, it was suggested that the CVM might affect the local immune response to HPV infections []. Certain bacteria in the microbiome can stimulate immune cells and modulate inflammation, potentially influencing the clearance or persistence of HPV [,]. While HPV vaccination has reduced the cervical cancer burden, non-vaccine-preventable HPV types still pose a risk []. Notably, not all hrHPV-infected women develop cervical cancer, prompting researchers to explore potential protective factors. One hypothesis suggests that beneficial bacteria, like L. acidophilus in the CVM, could contribute to safeguarding against CC development in hrHPV-infected women []. Through detailed microbiome profiling in a Dutch CC screening program found that women with typical cervical smears and a higher L. acidophilus abundance were associated with a lower risk of HSIL, highlighting the role of this bacteria in cervico-vaginal microbial dynamics and continuity [].
Moreover, in another study, Molina et al., in analyzing a longitudinal cohort of 141 women diagnosed with hrHPV infection, found that long-term changes in the CVM composition positively correlate with microbial diversity at two timepoints six-months apart []. Women with an initial high abundance of L. iners tend to have a more stable microbiome composition in subsequent visits compared to those with Lactobacillus-depleted communities at baseline. Additionally, specific species such as L. acidophilus and Megasphaera genomosp type 1 are associated with changes in CSTs between visits. Notably, Gardnerella vaginalis was linked to the stability of Lactobacillus-depleted communities, while L. iners was associated with the instability of Megasphaera genomosp type 1-dominated communities. These findings suggest dynamic CVM patterns during hrHPV infection, offering potential insights for the development of microbiome-based therapies to counter infection progression toward disease [].
The presence and quantity of lactobacilli in the vaginal microbiome vary with age and are influenced by estrogen levels. Lactobacilli play a crucial role in converting glycogen in the mature vaginal epithelium into organic acids, primarily lactate. This acidification of the vaginal environment creates a protective barrier against viral and bacterial pathogens [,]. However, during menopause, when estrogen levels decline, there is a reduction in Lactobacillus populations and an increase in anaerobic bacteria in the vaginal flora []. Nevertheless, several other factors such as ethnicity, sexual activity, hygiene practices, lactation, and dietary habits may also impact the composition of the vaginal microbiota [,]. Changes in the vaginal microbiota may lead to immune regulation and inflammation, which are associated with various gynecological conditions, including bacterial vaginosis [,]. Bacterial vaginosis represents a shift from the predominance of Lactobacillus to a more diverse microbiome with higher levels of anaerobic bacteria like Gardnerella vaginalis, Peptostreptococcus anaerobius, and Porphyromonas uenonis. Importantly, bacterial vaginosis was linked to an increased risk of HPV-related CIN and cervical cancer [,,] (Figure 1). A well-balanced microbiome was linked to a reduced risk of infections, including bacterial vaginosis and urinary tract infections [].
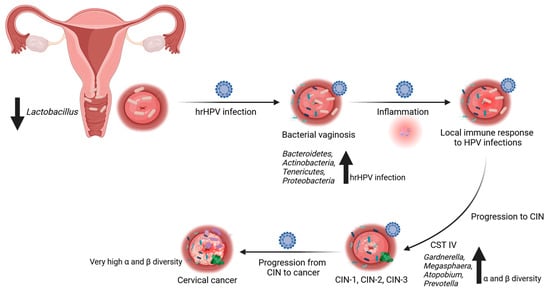
Figure 1.
Relationship between cervical and cervico-vaginal microbiota and high-risk HPV infections. The composition of cervical microbiota can impact vulnerability to high-risk (hr) HPV infections and the subsequent development of cervical lesions or cancer. Disturbances in the normal cervical microbiota, a reduction in Lactobacillus species, or an overgrowth of other bacteria are linked to an elevated risk of persistent HPV infection and the advancement of cervical abnormalities. Bacterial vaginosis (BV) is a shift from the dominance of Lactobacillus to a more diverse microbiome characterized by increased levels of anaerobic bacteria like Gardnerella vaginalis, Peptostreptococcus anaerobius, and Porphyromonas uenoni. BV is associated with an increased susceptibility to HPV-related cervical intraepithelial neoplasia (CIN) and cervical cancer. Decreased abundance of Lactobacillus species and increased presence of Gardnerella vaginalis correlate with an altered cervical and cervico-vaginal microbiome (CM/CVM) and higher risk of HPV infection and cervical dysplasia. Certain bacteria within the CM and CVM can activate immune cells and regulate inflammation, potentially affecting the clearance or persistence of HPV. As the lesions progress, an upward trend in species diversity is noted. Progression from CIN to cancer requires persistent HPV infection. Created with BioRender.com (accessed on 10 December 2023).
Radiation therapy and chemoradiation therapy, whether used for curative or palliative purposes in gynecologic cancers, may impact the composition of the CM and CVM []. In a small-scale study, it was observed that radiation therapy led to a significant decrease in the abundance of cervical bacteria, although there were no discernible changes in the bacterial alpha- or beta-diversity []. Another investigation revealed 13 phylogroups at the genus level that differentiated the cervical microbiota before and after radiation therapy. Furthermore, most of the post-radiation therapy microbiota communities were distinct from those found in a healthy, normal microbiome. Another study indicated a tendency toward lower microbial richness in samples collected from healthy individuals compared to those from patients with gynecological cancer []. In a self-reported study, we showed an increased diversity of the CM associated with cervical cancer []. In healthy premenopausal women, Lactobacillus dominated in the CM, accounting for over 90% of the microbial community. However, in both pre- and postmenopausal cancer patients before treatment, the CM exhibited a heterogeneous composition, with a lower proportion of Lactobacillus, especially in younger patients. At the genus level, we identified taxa that differentiated healthy controls from cancer patients in the pre- and postmenopausal groups, respectively. Furthermore, 31 and 2 genera distinguished pre-radiation from post-radiation samples and pre-radiation from follow-up samples, respectively. Interestingly, microbiome diversity was significantly higher in patients before treatment compared to healthy controls. Such findings highlight significant changes in the CM of cervical cancer patients when compared to healthy controls, with more pronounced alterations occurring after chemoradiation therapy []. However, the exact mechanisms underlying the relationship between the CM and HPV infections are still being investigated, and further research is needed to understand the complexities of this interaction fully.
4. Microbial Influence on Cervical Cancer Development: Immune Responses and Therapeutic Prospects
The comprehensive analysis of the gastrointestinal (GI) microbiota has significantly advanced comprehension of how the human microbiome influences overall host health. Functions supported by the GI microbiome encompass immune system development, digestion, fat metabolism, epithelial homeostasis, and enteric nerve regulation []. Among healthy women, both the gut and vaginal microbiota are shielded from the host by a multi-level barrier system, including a mucus layer, the secretion of soluble immune mediators, and an intact epithelium with tight junctions []. Failure of this multifaceted barrier system can lead to the translocation of pathogenic bacteria across the gut and vaginal epithelia, inducing low-grade chronic inflammation and subsequent diseases, including cancer []. Conversely, cancers in the GI and reproductive tracts can cause inflammation, resulting in dysbiosis and establishing a positive feedback loop that may contribute to disease promotion [].
Wang et al. first revealed significant changes in the diversity and composition of the gut microbiota in CC patients []. Seven genera, including Escherichia–Shigella, Roseburia, Pseudomonas, Lachnoclostridium, Lachnospiraceae_UCG-004, Dorea, and Succinivibrio, exhibited significant differences in relative abundance between CC and controls. Characteristic microbiome features were identified, suggesting a Proteobacteria phylum in CC patients as potential biomarkers [].
In turn, Sims et al. showed a significantly higher alpha diversity in CC patients compared to controls, with this association being more prominent in older women (>50 years) []. Age- and race-adjusted LEfSe analysis revealed multiple taxa differences between the two groups, with Prevotella, Porphyromonas, and Dialister being significantly enriched in CC patients, while Bacteroides, Alistipes, and members of the Lachnospiracea family were significantly enriched in healthy subjects. Importantly, Prevotella-rich environments stimulate dendritic cells (DC) through Toll-like receptor 2 (TLR2), releasing interleukin-1b (IL-1b), IL-6, and IL-23. This facilitates IL-17 production by T helper 17 (Th17) cells, activating neutrophils []. Prevotella’s role in altering host immunity and modulating immunologic pathways may be linked to CC risk and treatment outcomes []. Moreover, in a subsequent study, Sims et al. [] linked gut microbiota diversity and a positive response to chemoradiation in CC patients. The composition variation among patients was associated with both short-term and long-term survival. Short-term survivors exhibited enrichment in Porphyromonas, Porphyromonadaceae, and Dialister, while long-term survivors showed enrichment in Escherichia Shigella, Enterobacteriaceae, and Enterobacteriales. Therefore, modulating the gut microbiota prior to chemoradiation could be a potential avenue to enhance treatment effectiveness and overall outcomes in cervical cancer patients [].
In Kang et al.’s study [], the Prevotella genus was significantly more abundant in the CC group and Clostridium in the HC group. Additionally, a developed machine-learning-based classifier model differentiated CC from controls in terms of seven bacterial genera, i.e., Prevotella, Peptostreptococcus, Finegolida, Ruminococcus, Clostridium, Pseudomonas, and Turibacter. The model exhibited excellent diagnostic performance, providing an effective prediction capability for early invasive CC (ICC). A decrease in butyrate-producing bacteria, including Ruminococcus and Clostridium, was observed in the CC patient group. Butyrate, a vital nutrient in the intestinal tract, is essential for controlling inflammation, preventing leaky gut, and regulating intestinal autophagy and energy metabolism in the human colon []. The reduction in these bacteria may impact overall intestinal health, thereby influencing vaginal health. Chang et al. identified Ruminococcus 2 as a gut flora family closely linked to CC, suggesting its potential as a biomarker for predicting cervical cancer development []. Firmicutes, particularly Ruminococcus, plays a crucial role in polysaccharide degradation and contributes to human metabolism by converting cellulose into host nutrients []. Additionally, Ruminococcus is associated with the intestinal barrier, cellular immunity, inflammation, and metabolism []. In summary, the connection between Prevotella, Ruminococcus, and Clostridium suggests a potential association with an increased risk of early ICC (Figure 2A) [].
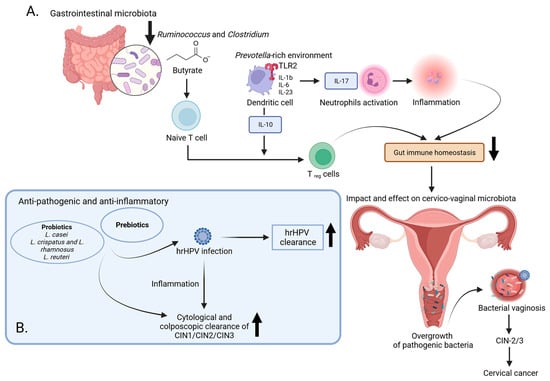
Figure 2.
The impact of microbiota on the progression of cervical cancer: examining immune reactions and potential therapeutic avenues. (A) In healthy women, the gut and vaginal microbiota are protected by a multi-layered barrier system comprising a mucus layer, immune mediators, and an intact epithelium. Failure of this barrier can lead to the translocation of pathogenic bacteria, causing chronic inflammation and cancer. Prevotella-rich environments stimulate dendritic cells via TLR 2, releasing cytokines and promoting immune responses that may be linked to cervical cancer (CC) risk. CC patients show a decrease in butyrate-producing bacteria, essential for controlling inflammation and maintaining intestinal health. Butyrate microbial metabolites also stimulate cells to produce anti-inflammatory compounds, contributing to intestinal homeostasis. The reduction in Ruminococcus and Clostridium may impact overall intestinal health, thereby influencing vaginal health. (B) The use of prebiotics and probiotics has shown promise in preventing HPV-induced cervical malignancy. Results indicated increased rates of HPV clearance, cytological and colposcopic clearance of abnormalities, and improved histological outcomes following treatment. These supplements, known for their positive effects on digestive system function and immune processes, contribute to overall health and may play a role in managing HPV-related cervical lesions. Created with BioRender.com (accessed on 13 January 2024).
The factors influencing the outcome of HPV infection and the mechanisms by which the host immune system safeguards against HPV remain elusive []. TLRs, a class of pattern recognition receptors located in the cytoplasm and on cell membranes, possess the ability to specifically identify pathogen-associated molecular patterns []. As pivotal components of both innate and adaptive immunity, TLRs not only play critical roles in defending against infectious diseases but are also implicated in the initiation and progression of various malignant tumors [,]. Polymorphisms within TLR genes have been linked to CC, though certain inconsistencies exist in the reported results [,,]. However the meta-analysis results indicated that carriers of the +1196T (rs4986791 TLR4), +7764T (rs1927911 TLR4), −1486C (rs187084 TLR9), and +2848A (rs352140 TLR9) alleles, as well as the −2604G/G (rs10759931 TLR4) and −1237C/C (rs5743836 TLR9) genotypes, were associated with an elevated risk of CC []. Bioinformatics analysis unveiled that the −1237T > C (rs5743836) and −1486T > C (rs187084) polymorphisms could impact transcription factor binding sites (RELA, NFKB1, and THAP1) in the TLR9 gene. Additionally, the +2848G > A (rs352140) polymorphism appeared to alter the structure and stability of the TLR4 protein []. These findings suggest that TLR4 and TLR9 gene polymorphisms may influence intracellular signaling pathways, potentially altering immune response patterns and contributing to an increased susceptibility to cervical cancer.
Werner et al. proposed a hypothesis suggesting that the progression of CC might be linked to changes in the expression of innate immune receptors, specifically integrins and TLRs, and that these changes could be induced by infectious agents []. Their investigation involved the analysis of protein expression in cervical biopsy tissues and various cervical-cancer-derived cell lines (HeLa, CaSki, SiHa, C-33 A, and ME180). Immunohistochemistry analysis revealed an upregulation of integrin αv, β3, β4, and β6 expression in the epithelium during the development of cervical cancer. Notably, there was a noticeable increase in integrin β6 expression in cell lines containing HPV genetic material compared to the HPV-negative C-33 A cell line. To investigate the potential effects of bacterial infections on TLRs and integrins, HeLa cells were infected with two pathogens, Escherichia coli and Pseudomonas aeruginosa, while using Lactobacillus reuteri as a control. The results indicated that infection with E. coli or P. aeruginosa, but not with L. reuteri, significantly altered the expression of TLRs and integrins, with a notable impact on TLR4 and integrin β6. Considering the pivotal roles of both integrin β6 and TLR4 in tumorigenesis, these findings suggest that bacterial infections may serve as triggers for cancer development in the HPV-infected cervical epithelium.
Another study by Wang et al. focused on elucidating the expression, distribution, and functional activity of TLR4 in normal cervical tissues, CIN, ICC, and various CC cells infected with HPV []. The findings revealed a correlation between TLR4 expression and histopathological grade, with a higher expression in ICC compared to CIN and a lower expression in normal cervical tissues and malignant cervical stroma. Moreover, TLR4 expression was elevated in SiHa cells (HPV16+) compared to HeLa cells (HPV18+), while no expression was observed in C33A cells (HPV−). Upon treatment with the TLR4 agonist lipopolysaccharide (LPS), SiHa cells exhibited an increased TLR4 expression and developed resistance to apoptosis, a phenomenon not observed in HeLa or C33A cells. Interestingly, LPS treatment did not alter the cell cycle distribution in SiHa cells. The mechanism behind apoptosis resistance seemed to be linked to HPV-16 infection and was not correlated with changes in cell cycle distribution. Targeting TLR4, especially in combination with traditional drug treatments, could represent a novel strategy for more effectively eliminating cancer cells. This approach holds promise for enhancing the efficacy of cancer therapies by addressing specific molecular pathways associated with TLR4 and HPV infection [].
The conventional treatment for CIN involves surgical methods, such as ablative or excisional procedures. However, these approaches primarily address the visible lesion without directly targeting the underlying cause associated with HPV infection. Developing a successful approach to address persistent HPV infection or inflammation could significantly impact global health and have widespread economic implications.
The elimination of genital verrucous lesions with imiquimod is likely facilitated by the stimulation of both innate and cellular immunity [,]. This involves the initiation of antiviral activity through the induction of cytokines, including interferon-α (IFN-α), tumor necrosis factor-α (TNF-α), and ILs []. Imiquimod is known to activate immune cells by interacting with TLR7 on the cell surface, a receptor commonly involved in recognizing pathogens. Activation of cells through imiquimod and TLR7 prompts the secretion of cytokines such as IFN-α, IL-6, and TNF-α, contributing to the antiviral immune response []. Topical imiquimod demonstrates both efficacy and tolerability in treating persistent HPV infection, with or without CIN or vaginal intraepithelial neoplasia (VAIN) []. Moreover, a weekly topical application of 5% imiquimod cream has demonstrated effectiveness in promoting the regression of HSIL in the cervix, as evidenced by histologic response rates []. While imiquimod is considered as a potential treatment for CIN and VAIN, limited attention has been given to investigating the adverse events associated with its vaginal use [,,]. Despite the occurrence of common local and systemic complications, discontinuation of treatment is not a frequent outcome []. Consequently, imiquimod holds promise as a potential alternative to surgical interventions for managing CIN []. A more comprehensive assessment of imiquimod as a therapeutic option for CIN and VAIN is needed, considering both its potential benefits and the associated risks of adverse events.
At present, the management of CC relies on a collaborative approach involving a multidisciplinary team. In the initial phases of the disease, various treatment modalities are available, encompassing surgery, radiation, neoadjuvant chemotherapy, and procedures for fertility preservation []. Concurrent chemoradiation with the use of cisplatin, either alone or in conjunction with other drugs, stands as the conventional therapeutic approach for individuals diagnosed with locally advanced CC []. Various therapeutic options exist for the treatment of metastatic patients dealing with lung metastasis, bone metastasis, single brain metastasis, or multiple brain metastases. The growing focus on human health has led to a rapid rise in the therapeutic and commercial interest in producing supplements, including prebiotics and probiotics, due to their association with the microbiota [,]. Probiotics play a role in multiple aspects of digestive system function, including digestion, metabolism, supporting the innate immunity of epithelial cells, combating pathogens, and facilitating communication between the brain and gut through their adhesion to the human intestines [,]. Additionally, probiotics contribute to immune processes by enhancing antibody responses and suppressing the proliferation of mononuclear cells []. When combined with fermented non-digestible food products, known as prebiotics, they exhibit various beneficial properties, including anti-pathogenic, anti-inflammatory, antidiabetic, and anti-obesity effects [,]. Eleven studies explored the use of prebiotics and probiotics for preventing HPV-induced cervical malignancy []. Among them, six studies utilized commercially available topical vaginal prebiotic-containing preparations, while five studies employed preparations containing probiotics, including strains like Lactobacillus casei, L. crispatus, and L. rhamnosus, both alone and in combination with L. reuteri [,,,,,]. The probiotic studies included oral formulations containing specific strains and one study on a topical vaginal probiotic preparation [,]. The results from the studies on prebiotic preparations were promising, showing increased rates of HPV clearance, cytological and colposcopic clearance of abnormalities, and improved histological outcomes following treatment [,,]. Beyond prebiotic and probiotic preparations, various non-prescription oral and vaginal agents have been explored for potential activity in HPV clearance or the regression of low-grade cervical lesions (Figure 2B). These agents include active hexose-correlated compound, beta-carotene, 3,3′-diindolylmethane, epigallocatechin gallate, indole-3-carbinol, Praneem polyherbal tablets, silicon dioxide with sodium selenite and citric acid, and zinc [,,,,,].
5. Link between Cervical Metabolites and HPV Infection
Interestingly, it is not only the CM that may be a predictor of progressive HPV infection. Another large-scale approach used to determine the impact of HPV infections on the development of CIN or cervical cancer is related to the analysis of metabolite composition.
A rapid metabolite screening method using direct-injection mass spectrometry effectively differentiated between cervical cell samples with different early-stage precancerous changes and between samples where hrHPV either cleared or persisted []. Importantly, such a discrimination was not influenced by a specific strain of hrHPV but it was due to the presence of the virus itself. Furthermore, the metabolite profiling method was successful in distinguishing levels of low-grade cell abnormalities, a task that is challenging with traditional microscopic screening. This capability has the potential to reduce the misclassification of cases and to minimize costly clinic recalls for women, providing a more accurate classification. Additionally, metabolite profiles could unveil new targets for pharmaceutical interventions aimed at influencing the persistence of HPV infections [].
Pappa et al. analyzed the metabolic profiles of four distinct cervical cell lines. Those included normal cervical cells and three types of cervical cancer cell lines []. Among the cancer lines, one was not infected with HPV (C33A), while the other two were HPV-positive (SiHa with HPV16 and HeLa with HPV18). Sophisticated technologies such as ultra-performance liquid chromatography and high-resolution mass spectrometry were used in this investigation. The results revealed significant differences in metabolites among those cell lines, with from 248 to 326 metabolites showing statistically significant variations. Using random forest analysis, unique molecules were identified for each cell line, highlighting distinct metabolic features. Specifically, both HPV-positive cell lines displayed characteristics consistent with the Warburg effect, a metabolic phenomenon commonly associated with cancer. This suggests that the presence of the HPV E6 protein in those cells influenced their metabolism. SiHa and HeLa cells showed signs of increased activity in the purine salvage pathway, while C33A cells exhibited a novel mechanism involving cytidine synthesis. Overall, these findings shed light on the dynamic and HPV-specific rewiring of metabolic pathways in cervical cancer. Such an approach has the potential to offer new insights into the mechanisms underlying cervical carcinogenesis [].
Porcari et al. utilized liquid chromatography-mass spectrometry (LC-MS) to identify specific molecular patterns in cervical cytology samples []. The LC-MS analysis revealed distinct molecular signatures for high-grade SIL (HSIL), including two ceramides and a sphingosine metabolite. Importantly, those molecules were consistently present, regardless of whether the women had an HPV infection, and they might be linked to the precancerous characteristics of the lesions. Statistical models based on these findings could accurately classify and distinguish women with HSIL from those with no cervical lesions. The results suggested that LC-MS had the potential to become an emerging technology for clinical use in cervical cancer screening [].
6. Next-Generation-Sequencing-Based Studies and the Cervical and Cervico-Vaginal Microbiota
Next-generation sequencing (NGS) has revolutionized the study of the CM and CVM. NGS is a high-throughput DNA sequencing technology that allows for the analysis of large amounts of genetic information from diverse microorganisms present in the cervix [,,,,,,,,,,,,,,]. The technology has significantly advanced our understanding of the complexity and diversity of the cervical microbiota and its implications for health and disease. This high-throughput method allows for the analysis of a vast number of microbial species and their relative abundances in a sample. It provides a comprehensive view of the diversity and composition of microbial communities []. NGS data may be used to classify and categorize microbial species based on their genetic sequences. This taxonomic classification helps to understand the prevalence and abundance of specific microbes in the CM and CVM []. NGS captures changes in the CM and CVM over time, allowing researchers to study the dynamic shifts in microbial communities during HPV infection or treatment interventions [,,]. Furthermore, NGS-based studies offer personalized insights into an individual’s CM and CVM, potentially guiding personalized approaches to cervical health management and preventive strategies []. The knowledge gained from NGS studies of the CM and CVM holds promise for developing novel diagnostic tools, targeted therapies, and preventive interventions for cervix-related conditions, including cervical cancer. The 16s rRNA sequencing is a specific application of NGS that focuses on studying the microbial diversity within a sample by targeting the 16S rRNA gene [].
The 16S rRNA gene is present in all bacteria and archaea and contains both highly conserved regions shared among all species and variable regions unique to specific bacterial taxa []. By sequencing and analyzing these variable regions, researchers may identify and classify the bacteria present in a sample, even if the organisms cannot be cultured in a laboratory [].
7. Evidence from NGS-Based Studies
In this article, we presented a review of the literature on the relationship between HPV infection and changes among the CM and CVM from studies conducted in the period 2012–2023 using NGS technology (Table 1).

Table 1.
Data extracted from studies using next-generation sequencing technology to investigate the relationships between cervical and cervico-vaginal microbiota and HPV infections.
HrHPV infection is the most important factor responsible for cervical cancer development. Nevertheless, the relationship between HPV infection and cancer development is complex and not fully elucidated. For instance, the development of premalignant lesions is associated with a persistent HPV infection. However, to date, the reasons for HPV persistence in some women and clearance in others have not been fully understood. Although the persistence of hrHPV infection is crucial for cervical cancer development, the risk for cancer progression is diverse among women with persistent HPV infection. It was hypothesized that a certain profile or disturbances of the CM and CVM might be associated with HPV-related cervical cancer development. Knowledge of the microbiome promoting a persistent HPV infection or, on the contrary, having a protective effect against HPV could prove extremely useful in adopting strategies for primary prevention. This review paper summarizes the studies on the association of the CM and CVM with HPV infection, precancerous cervical lesions, and cervical cancer.
Based on the analyzed literature (Table 1), significant differences were observed in the cervicovaginal microbiome between the HPV-positive and HPV-negative women. Generally, a wider variety of bacterial species was observed in the HPV-positive women, primarily due to the abundance of species other than Lactobacillus and a shift towards anaerobic bacteria [,,,,,,,,,,,]. The majority of the studies indicated that HPV-negative women had a normal cervical microbiota characterized by the abundance of Lactobacillus. In addition, several authors suggested that the diversity of CM and CVM correlated with the severity of CIN lesions [,,,,,,,,,,] and that bacterial diversity was negatively associated with HPV clearance []. These results suggest that the cervical microbiota may have a significant influence on cervical cancer development.
Besides CM and CVM diversity, the studies revealed specific microbes related to persistent HPV infections and CIN development. The majority of the studies indicated Gardnerella, Prevotella, and Megasphaera as species associated with an HPV infection and CIN [,,,,,,,,,,]. The mechanisms through which certain microbiota interfere with HPV infections demonstrated in in vitro experiments include the disruption of the cervical epithelial barrier by regulating adherence junction proteins, cervical immune responses, and miRNA expression []. The abundance of the bacterial species in the cervical microbiota makes it challenging to indicate one or a few species responsible for a persistent HPV infection and CIN development, and it seems that interactions between cervical microbiota and HPV may be more complex. For instance, Huang et al. [] found that Oribacterium, Lachnobacterium, and Thermus in the cervicovaginal microbiota were more likely to be associated with HPV-16, while Motilibacter was related to HPV-52. Moreover, they reported that the composition of Litorilinea and Paludibaculum with a concomitant paucity of Lactobacillus iners was more likely to be associated with HPV-58. The results indicated that certain bacterial species were more likely to coexist with particular HPV types in the cervical epithelium.
Numerous studies demonstrated that the abundance of Lactobacillus in the cervicovaginal flora was correlated with HPV clearance [,]. However, the roles of various Lactobacillus species may be different. Contradictory results were reported on Lactobacillus iners—it was reported to be correlated with HPV clearance by some authors [,], while others found its association with HPV persistence [,] and CIN-2+ lesions []. Such discrepancies may be due to the different study populations, the site of sample collection (cervical vs. vaginal swab), and the detection method used. Furthermore, in the study by Arokiyaraj et al., Lactobacillus johnsonii was related to HPV persistence, while Lactobacillus crispatus was predominant in the subjects with HPV clearance [].
Several studies investigated the influence of the CM and CVM on the progression of HPV-related cervical lesions. Overall, the paucity of Lactobacillus spp. and increased microbiota diversity were associated with CIN occurrence and progression to cervical cancer [,,], with Gardnerella vaginalis, Prevotella, Megasphaera, and Atopobium vaginae being particularly indicated [,,,,]. According to Zhang et al. [], the cervical microbiota may affect cervical cancerogenesis directly and indirectly by affecting the natural history of cervical HPV infection. In the above-mentioned study, the authors observed indirect effects of Pseudomonas stutzeri, Bacteroides fragilis, Lactobacillus delbrueckii, Atopobium vaginae, and Streptococcus agalactiae mediated by an HPV infection on CIN status. However, direct effects (association with CIN-2+ development) were related to a decrease in the abundance of Pseudomonas stutzeri and Atopobium vaginae. In addition, the authors observed that in the case of Pseudomonas stutzeri, the direct and indirect actions were opposite. This suggests that the interplay between the cervical microbiota, HPV infection and cervical cancer development is complex.
The composition of the CM and CVM is dynamic and alters with time. This was reflected in several studies that compared the microbiome of patients before and after the treatment of cervical lesions with a loop electrosurgical excision procedure. A tendency towards an increase in Lactobacillus spp. and a less diverse bacterial environment was observed after the surgical treatment of CIN lesions [,,,]. These findings bring further questions as to whether the disruption of the CM and CVM is the cause or the effect of HPV infections. In light of the available evidence on the significant differences in the CM and CVM between women with the clearance of HPV infection and those with a persistent infection [,,,,], it seems that certain microbiota impact the course of a previously acquired HPV infection rather than impact the probability of HPV acquisition. In other words, the clinical implications of an HPV infection may be determined by the cervical microbiota. Furthermore, a study by Ravilla et al. suggested that the response to the HPV vaccine might be related to the content of the CM []. Therefore, the knowledge of a specific microbial environment promoting the progression of CIN lesions could help to identify women at the highest risk of cervical cancer. Furthermore, modifications to the CM could be used as a therapeutic measure to boost HPV clearance.
As regards the limitations of this review article, the retrospective nature and a limited number of samples in most of the studies have to be mentioned. Furthermore, ethnicity might be another confounding factor. Vikramdeo et al. observed differences in the microbiota among women with cervical preneoplasia originating from various racial groups, and the authors suggested that this might explain why certain racial groups differed in terms of HPV incidence and the risk of progression [].
8. Conclusions
Several studies indicated that abnormal CV and CVM might participate in HPV-related cervical cancer development. This implies several clinical issues. For instance, the investigation of the CM and CVM may be used as a part of screening for cervical cancer. However, one of the limitations of using microbiomes for screening purposes is that they are dynamic and subject to constant change. Even if a population at a low risk of cervical cancer could be identified, their cervicovaginal environment could alter towards a high-risk pattern over time. In addition, the treatment of abnormal cervical microbiota may be useful for the management of HPV infection and CIN. However, this requires further prospective trials to evaluate the impact of the above-mentioned intervention. Finally, it is important to evaluate whether the alterations in the microbiome could be a consequence of HPV infection and/or precancerous cervical lesions rather than a factor influencing the course of HPV infection (persistence/clearance). Further large-scale investigations are needed to verify the role of the microbiome in HPV infection and HPV-related cervical lesions.
Author Contributions
Conceptualization, N.Z.-L.; writing—original draft preparation, M.G.-S., K.B.-D., M.D., E.E.H., N.Z.-L., S.S., A.H. and M.M.; writing—review and editing, M.G.-S., K.B.-D., M.D., E.E.H., N.Z.-L., S.S., A.H., M.M. and M.C.; visualization, E.E.H. and N.Z.-L.; supervision, N.Z.-L.; funding acquisition, E.E.H. and N.Z.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Centre of Postgraduate Medical Education (grant numbers: 501-1-009-12-23 and 501-1-009-12-21/MG2 (N.Z.-L.)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haręża, D.A.; Wilczyński, J.R.; Paradowska, E. Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins. Int. J. Mol. Sci. 2022, 23, 1818. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chua, B.W.B.; Ma, V.Y.; Alcántar-Fernández, J.; Wee, H.L. Is It Time to Genotype Beyond HPV16 and HPV18 for Cervical Cancer Screening? Int. J. Public. Health 2022, 67, 1604621. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Pedretti, F.; Mauramati, S.; Filauro, M.; Vallin, A.; Mora, F.; Crosetti, E.; Succo, G.; Peretti, G.; Benazzo, M. Recurrent Laryngeal Papillomatosis: Multimodal Therapeutic Strategies. Literature Review and Multicentre Retrospective Study. Acta Otorhinolaryngol. Ital. 2023, 43, S111–S122. [Google Scholar] [CrossRef]
- Leslie, S.W.; Sajjad, H.; Kumar, S. Genital Warts. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Okunade, K.S. Human Papillomavirus and Cervical Cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global Estimates of Incidence and Mortality of Cervical Cancer in 2020: A Baseline Analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; De Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Cervical Cancer Statistics I World Cancer Research Fund International. Available online: https://www.wcrf.org/cancer-trends/cervical-cancer-statistics/ (accessed on 23 October 2023).
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef]
- Moody, C. Mechanisms by Which HPV Induces a Replication Competent Environment in Differentiating Keratinocytes. Viruses 2017, 9, 261. [Google Scholar] [CrossRef]
- Von Witzleben, A.; Wang, C.; Laban, S.; Savelyeva, N.; Ottensmeier, C.H. HNSCC: Tumour Antigens and Their Targeting by Immunotherapy. Cells 2020, 9, 2103. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.; Arbyn, M.; Turkot, M.H.; Wieszczy, P.; Miłosz, K.; Kamiński, M.F.; Didkowska, J.; Bidziński, M.; Olszewski, W.; Wielgoś, M.; et al. A Roadmap for a Comprehensive Control of Cervical Cancer in Poland: Integration of Available Solutions into Current Practice in Primary and Secondary Prevention. Eur. J. Cancer Prev. 2020, 29, 157–164. [Google Scholar] [CrossRef]
- Nowakowski, A.; Jach, R.; Szenborn, L.; Bidzinski, M.; Jackowska, T.; Kotarski, J.; Mastalerz-Migas, A.; Nitsch-Osuch, A.; Pinkas, J.; Sawicki, W.; et al. Recommendations of the Polish Society of Gynaecologists and Obstetricians, Polish Paediatric Society, Polish Society of Family Medicine, Polish Society of Vaccinology, Polish Society of Oncological Gynaecology and Polish Society of Colposcopy and Pathophysiology of the Uterine Cervix on Prophylactic Vaccinations against Infections with Human Papillomaviruses in Poland. Ginekol. Pol. 2023, 94, 759–767. [Google Scholar] [CrossRef]
- Basoya, S.; Anjankar, A. Cervical Cancer: Early Detection and Prevention in Reproductive Age Group. Cureus 2022, 14, e31312. [Google Scholar] [CrossRef]
- Charde, S.H.; Warbhe, R.A. Human Papillomavirus Prevention by Vaccination: A Review Article. Cureus 2022, 14, e30037. [Google Scholar] [CrossRef] [PubMed]
- Mello, V.; Sundstrom, R.K. Cervical Intraepithelial Neoplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kornovski, Y.; Slavchev, S.; Kostov, S.; Ivanova, Y.; Yordanov, A. Precancerous Lesions of the Cervix—Aetiology, Classification, Diagnosis, Prevention. Oncol. Clin. Pract. 2021, 17, 271–276. [Google Scholar] [CrossRef]
- Tierney, K.E.; Roman, L.D.; Matsuo, K. Management of Cervical Dysplasia. In Handbook of Gynecology; Shoupe, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–11. ISBN 978-3-319-17002-2. [Google Scholar]
- Gholiof, M.; Adamson-De Luca, E.; Wessels, J.M. The Female Reproductive Tract Microbiotas, Inflammation, and Gynecological Conditions. Front. Reprod. Health 2022, 4, 963752. [Google Scholar] [CrossRef]
- Kumar, L.; Dwivedi, M.; Jain, N.; Shete, P.; Solanki, S.; Gupta, R.; Jain, A. The Female Reproductive Tract Microbiota: Friends and Foe. Life 2023, 13, 1313. [Google Scholar] [CrossRef]
- Plesniarski, A.; Siddik, A.B.; Su, R.-C. The Microbiome as a Key Regulator of Female Genital Tract Barrier Function. Front. Cell. Infect. Microbiol. 2021, 11, 790627. [Google Scholar] [CrossRef]
- Moosa, Y.; Kwon, D.; De Oliveira, T.; Wong, E.B. Determinants of Vaginal Microbiota Composition. Front. Cell. Infect. Microbiol. 2020, 10, 467. [Google Scholar] [CrossRef]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal Microbiota, Women’s Health, and Reproductive Outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Cascardi, E.; Cazzato, G.; Daniele, A.; Silvestris, E.; Cormio, G.; Di Vagno, G.; Malvasi, A.; Loizzi, V.; Scacco, S.; Pinto, V.; et al. Association between Cervical Microbiota and HPV: Could This Be the Key to Complete Cervical Cancer Eradication? Biology 2022, 11, 1114. [Google Scholar] [CrossRef]
- Molina, M.A.; Melchers, W.J.G.; Núñez-Samudio, V.; Landires, I. The Emerging Role of Lactobacillus Acidophilus in the Cervicovaginal Microenvironment. Lancet Microbe 2023, S2666524723003154. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus Iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-P.; Chen, W.-C.; Cheng, C.-M.; Shen, C.-J. Vaginal PH Value for Clinical Diagnosis and Treatment of Common Vaginitis. Diagnostics 2021, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.; Andralojc, K.M.; Huynen, M.A.; Leenders, W.P.J.; Melchers, W.J.G. In-Depth Insights into Cervicovaginal Microbial Communities and HrHPV Infections Using High-Resolution Microbiome Profiling. NPJ Biofilms Microbiomes 2022, 8, 75. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- France, M.T.; Ma, B.; Gajer, P.; Brown, S.; Humphrys, M.S.; Holm, J.B.; Waetjen, L.E.; Brotman, R.M.; Ravel, J. VALENCIA: A Nearest Centroid Classification Method for Vaginal Microbial Communities Based on Composition. Microbiome 2020, 8, 166. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The Vaginal Microbiota, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia: What Do We Know and Where Are We Going Next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef]
- Stoian, I.L.; Botezatu, A.; Fudulu, A.; Ilea, C.G.; Socolov, D.G. Exploring Microbiota Diversity in Cervical Lesion Progression and HPV Infection through 16S RRNA Gene Metagenomic Sequencing. J. Clin. Med. 2023, 12, 4979. [Google Scholar] [CrossRef] [PubMed]
- Santella, B.; Schettino, M.T.; Franci, G.; De Franciscis, P.; Colacurci, N.; Schiattarella, A.; Galdiero, M. Microbiota and HPV: The Role of Viral Infection on Vaginal Microbiota. J. Med. Virol. 2022, 94, 4478–4484. [Google Scholar] [CrossRef]
- Ntuli, L.; Mtshali, A.; Mzobe, G.; Liebenberg, L.J.; Ngcapu, S. Role of Immunity and Vaginal Microbiome in Clearance and Persistence of Human Papillomavirus Infection. Front. Cell. Infect. Microbiol. 2022, 12, 927131. [Google Scholar] [CrossRef]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.A.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and Cervical Cancer. Pathobiology 2021, 88, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-W.; Long, H.-Z.; Cheng, Y.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. From Microbiome to Inflammation: The Key Drivers of Cervical Cancer. Front. Microbiol. 2021, 12, 767931. [Google Scholar] [CrossRef]
- Choi, S.; Ismail, A.; Pappas-Gogos, G.; Boussios, S. HPV and Cervical Cancer: A Review of Epidemiology and Screening Uptake in the UK. Pathogens 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Andralojc, K.M.; Molina, M.A.; Qiu, M.; Spruijtenburg, B.; Rasing, M.; Pater, B.; Huynen, M.A.; Dutilh, B.E.; Ederveen, T.H.A.; Elmelik, D.; et al. Novel High-Resolution Targeted Sequencing of the Cervicovaginal Microbiome. BMC Biol. 2021, 19, 267. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.; Melchers, W.J.G.; Andralojc, K.M.; Leenders, W.P.J.; Huynen, M.A. Longitudinal Analysis on the Ecological Dynamics of the Cervicovaginal Microbiome in HrHPV Infection. Comput. Struct. Biotechnol. J. 2023, 21, 4424–4431. [Google Scholar] [CrossRef]
- Alizadehmohajer, N.; Shojaeifar, S.; Nedaeinia, R.; Esparvarinha, M.; Mohammadi, F.; Ferns, G.A.; Ghayour-Mobarhan, M.; Manian, M.; Balouchi, A. Association between the Microbiota and Women’s Cancers—Cause or Consequences? Biomed. Pharmacother. 2020, 127, 110203. [Google Scholar] [CrossRef]
- Tsakmaklis, A.; Vehreschild, M.; Farowski, F.; Trommer, M.; Kohler, C.; Herter, J.; Marnitz, S. Changes in the Cervical Microbiota of Cervical Cancer Patients after Primary Radio-Chemotherapy. Int. J. Gynecol. Cancer 2020, 30, 1326–1330. [Google Scholar] [CrossRef]
- Hay, P.E.; Ugwumadu, A.; Chowns, J. Sex, Thrush and Bacterial Vaginosis. Int. J. STD AIDS 1997, 8, 603–608. [Google Scholar] [CrossRef]
- Dabee, S.; Passmore, J.-A.S.; Heffron, R.; Jaspan, H.B. The Complex Link between the Female Genital Microbiota, Genital Infections, and Inflammation. Infect. Immun. 2021, 89, e00487-20. [Google Scholar] [CrossRef]
- Brusselaers, N.; Shrestha, S.; Van De Wijgert, J.; Verstraelen, H. Vaginal Dysbiosis and the Risk of Human Papillomavirus and Cervical Cancer: Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2019, 221, 9–18.e8. [Google Scholar] [CrossRef]
- King, C.C.; Jamieson, D.J.; Wiener, J.; Cu-Uvin, S.; Klein, R.S.; Rompalo, A.M.; Shah, K.V.; Sobel, J.D. Bacterial Vaginosis and the Natural History of Human Papillomavirus. Infect. Dis. Obstet. Gynecol. 2011, 2011, 319460. [Google Scholar] [CrossRef] [PubMed]
- Holdcroft, A.M.; Ireland, D.J.; Payne, M.S. The Vaginal Microbiome in Health and Disease—What Role Do Common Intimate Hygiene Practices Play? Microorganisms 2023, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Zeber-Lubecka, N.; Kulecka, M.; Lindner, B.; Krynicki, R.; Paziewska, A.; Nowakowski, A.; Bidzinski, M.; Ostrowski, J. Increased Diversity of a Cervical Microbiome Associates with Cervical Cancer. Front. Oncol. 2022, 12, 1005537. [Google Scholar] [CrossRef] [PubMed]
- Tsementzi, D.; Pena-Gonzalez, A.; Bai, J.; Hu, Y.; Patel, P.; Shelton, J.; Dolan, M.; Arluck, J.; Khanna, N.; Conrad, L.; et al. Comparison of Vaginal Microbiota in Gynecologic Cancer Patients Pre- and Post-radiation Therapy and Healthy Women. Cancer Med. 2020, 9, 3714–3724. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Han, M.; Wang, N.; Han, W.; Ban, M.; Sun, T.; Xu, J. Gut Microbes in Gynecologic Cancers: Causes or Biomarkers and Therapeutic Potential. Front. Oncol. 2022, 12, 902695. [Google Scholar] [CrossRef]
- Muls, A.; Andreyev, J.; Lalondrelle, S.; Taylor, A.; Norton, C.; Hart, A. Systematic Review: The Impact of Cancer Treatment on the Gut and Vaginal Microbiome in Women With a Gynecological Malignancy. Int. J. Gynecol. Cancer 2017, 27, 1550–1559. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Zhao, J.; Gong, L.; Zhang, Y.; Wang, X.; Yuan, Z. Altered Diversity and Composition of the Gut Microbiome in Patients with Cervical Cancer. AMB Expr. 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.T.; Colbert, L.E.; Zheng, J.; Delgado Medrano, A.Y.; Hoffman, K.L.; Ramondetta, L.; Jazaeri, A.; Jhingran, A.; Schmeler, K.M.; Daniel, C.R.; et al. Gut Microbial Diversity and Genus-Level Differences Identified in Cervical Cancer Patients versus Healthy Controls. Gynecol. Oncol. 2019, 155, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The Immune Response to Prevotella Bacteria in Chronic Inflammatory Disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.T.; El Alam, M.B.; Karpinets, T.V.; Dorta-Estremera, S.; Hegde, V.L.; Nookala, S.; Yoshida-Court, K.; Wu, X.; Biegert, G.W.G.; Delgado Medrano, A.Y.; et al. Gut Microbiome Diversity Is an Independent Predictor of Survival in Cervical Cancer Patients Receiving Chemoradiation. Commun. Biol. 2021, 4, 237. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-U.; Jung, D.-R.; Lee, Y.H.; Jeon, S.Y.; Han, H.S.; Chong, G.O.; Shin, J.-H. Dynamics of Fecal Microbiota with and without Invasive Cervical Cancer and Its Application in Early Diagnosis. Cancers 2020, 12, 3800. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s Role in Human Health and the Current Progress towards Its Clinical Application to Treat Gastrointestinal Disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Chang, L.; Qiu, L.; Lei, N.; Zhou, J.; Guo, R.; Gao, F.; Dong, S.; Chen, M.; Wu, F.; Qin, B. Characterization of Fecal Microbiota in Cervical Cancer Patients Associated with Tumor Stage and Prognosis. Front. Cell. Infect. Microbiol. 2023, 13, 1145950. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: History, Chemistry, and Formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The Gut Microbiota Is Associated with Immune Cell Dynamics in Humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, Y.; Li, C. The Role of TLRs in Cervical Cancer with HPV Infection: A Review. Signal Transduct. Target. Ther. 2017, 2, 17055. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Regulation of Adaptive Immunity by the Innate Immune System. Science 2010, 327, 291–295. [Google Scholar] [CrossRef]
- Takeda, K. Toll-like Receptors in Innate Immunity. Int. Immunol. 2004, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Chauhan, A.; Raithatha, N.; Patel, P.; Desai, A.; Jain, N. Absence of Association between TLR4 Thr399Ile Polymorphism and Cervical Cancer Susceptibility. Meta Gene 2018, 17, 249–255. [Google Scholar] [CrossRef]
- Pandey, S.; Mittal, R.D.; Srivastava, M.; Srivastava, K.; Singh, S.; Srivastava, S.; Mittal, B. Impact of Toll-like Receptors [TLR] 2 (−196 to −174 Del) and TLR 4 (Asp299Gly, Thr399Ile) in Cervical Cancer Susceptibility in North Indian Women. Gynecol. Oncol. 2009, 114, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Zidi, S.; Sghaier, I.; Gazouani, E.; Mezlini, A.; Yacoubi-Loueslati, B. Evaluation of Toll-Like Receptors 2/3/4/9 Gene Polymorphisms in Cervical Cancer Evolution. Pathol. Oncol. Res. 2016, 22, 323–330. [Google Scholar] [CrossRef]
- de Moura, E.L.; dos Santos, I.F.; de Freitas, P.P.; da Silva, D.M.; dos Santos, A.C.M.; Lira Neto, A.B.; e Silva, A.C.P.; Barbosa, N.R.; Nascimento, C.A.; Balliano, T.L.; et al. Polymorphisms in Toll-like Receptors Genes Changes the Host’s Immune Response and Is Associated with Cervical Cancer. Immunobiology 2022, 227, 152187. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; DeCarlo, C.A.; Escott, N.; Zehbe, I.; Ulanova, M. Expression of Integrins and Toll-like Receptors in Cervical Cancer: Effect of Infectious Agents. Innate Immun. 2012, 18, 55–69. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, Y.; Shi, Y.; Xia, X.; Wang, S.; Duan, H. Expression and Functional Analysis of Toll-like Receptor 4 in Human Cervical Carcinoma. J. Membr. Biol. 2014, 247, 591–599. [Google Scholar] [CrossRef]
- Arany, I.; Tyring, S.K.; Stanley, M.A.; Tomai, M.A.; Miller, R.L.; Smith, M.H.; McDermott, D.J.; Slade, H.B. Enhancement of the Innate and Cellular Immune Response in Patients with Genital Warts Treated with Topical Imiquimod Cream 5%. Antivir. Res. 1999, 43, 55–63. [Google Scholar] [CrossRef]
- Borella, F.; Gallio, N.; Mangherini, L.; Cassoni, P.; Bertero, L.; Benedetto, C.; Preti, M. Recent Advances in Treating Female Genital Human Papillomavirus Related Neoplasms with Topical Imiquimod. J. Med. Virol. 2023, 95, e29238. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Sohn, K.-C.; Choi, D.-K.; Shi, G.; Hong, D.; Lee, H.-E.; Whang, K.U.; Lee, Y.H.; Im, M.; Lee, Y.; et al. Roles of TLR7 in Activation of NF-ΚB Signaling of Keratinocytes by Imiquimod. PLoS ONE 2013, 8, e77159. [Google Scholar] [CrossRef]
- Lin, C.-T.; Qiu, J.-T.; Wang, C.-J.; Chang, S.-D.; Tang, Y.-H.; Wu, P.-J.; Jung, S.-M.; Huang, C.-C.; Chou, H.-H.; Jao, M.-S.; et al. Topical Imiquimod Treatment for Human Papillomavirus Infection in Patients with and without Cervical/Vaginal Intraepithelial Neoplasia. Taiwan. J. Obstet. Gynecol. 2012, 51, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.O.; Possati-Resende, J.C.; Salcedo, M.P.; Schmeler, K.M.; Accorsi, G.S.; Fregnani, J.H.T.G.; Antoniazzi, M.; Pantano, N.P.; Santana, I.V.V.; Matsushita, G.M.; et al. Topical Imiquimod for the Treatment of High-Grade Squamous Intraepithelial Lesions of the Cervix: A Randomized Controlled Trial. Obstet. Gynecol. 2021, 137, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Polterauer, S.; Natter, C.; Rahhal, J.; Hefler, L.; Tempfer, C.B.; Heinze, G.; Stary, G.; Reinthaller, A.; Speiser, P. Treatment of Cervical Intraepithelial Neoplasia With Topical Imiquimod: A Randomized Controlled Trial. Obstet. Gynecol. 2012, 120, 152–159. [Google Scholar] [CrossRef]
- Inayama, Y.; Yamanishi, Y.; Nakatani, E.; Aratake, J.; Sasagasako, N.; Yamada, K.; Gou, R.; Kawamura, A.; Yamanishi, M.; Kosaka, K. Imiquimod for Vaginal Intraepithelial Neoplasia 2–3: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2021, 160, 140–147. [Google Scholar] [CrossRef]
- Inayama, Y.; Takamatsu, S.; Hamanishi, J.; Mizuno, K.; Horinouchi, N.; Yamanoi, K.; Taki, M.; Murakami, R.; Yamaguchi, K.; Kosaka, K.; et al. Imiquimod for Cervical and Vaginal Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2023, 142, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Harish, P.; Malik, P.S.; Khurana, S. Chemotherapy and Targeted Therapy in the Management of Cervical Cancer. Curr. Probl. Cancer 2018, 42, 120–128. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in Fecal Microbiota Composition by Probiotic Supplementation in Healthy Adults: A Systematic Review of Randomized Controlled Trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef]
- Mitra, A.; Gultekin, M.; Burney Ellis, L.; Bizzarri, N.; Bowden, S.; Taumberger, N.; Bracic, T.; Vieira-Baptista, P.; Sehouli, J.; Kyrgiou, M. Genital Tract Microbiota Composition Profiles and Use of Prebiotics and Probiotics in Gynaecological Cancer Prevention: Review of the Current Evidence, the European Society of Gynaecological Oncology Prevention Committee Statement. Lancet Microbe 2023, 2023, S2666524723002574. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, F.; Tajadadi-Ebrahimi, M.; Kolahdooz, F.; Mazouchi, M.; Hadaegh, H.; Jamal, A.-S.; Mazroii, N.; Asemi, S.; Asemi, Z. The Consumption of Synbiotic Bread Containing Lactobacillus Sporogenes and Inulin Affects Nitric Oxide and Malondialdehyde in Patients with Type 2 Diabetes Mellitus: Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 2016, 35, 506–513. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Criscuolo, A.A.; Sesti, F.; Piccione, E.; Mancino, P.; Belloni, E.; Gullo, C.; Ciotti, M. Therapeutic Efficacy of a Coriolus Versicolor-Based Vaginal Gel in Women with Cervical Uterine High-Risk HPV Infection: A Retrospective Observational Study. Adv. Ther. 2021, 38, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Lavitola, G.; Della Corte, L.; De Rosa, N.; Nappi, C.; Bifulco, G. Effects on Vaginal Microbiota Restoration and Cervical Epithelialization in Positive HPV Patients Undergoing Vaginal Treatment with Carboxy-Methyl-Beta-Glucan. BioMed Res. Int. 2020, 2020, 5476389. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.; López, A.C.; González, S.P.; Palacios, S.; Dexeus, D.; Centeno-Mediavilla, C.; Coronado, P.; de la Fuente, J.; López, J.A.; Vanrell, C.; et al. Efficacy of a Coriolus Versicolor–Based Vaginal Gel in Women With Human Papillomavirus–Dependent Cervical Lesions: The PALOMA Study. J. Low. Genit. Tract. Dis. 2021, 25, 130–136. [Google Scholar] [CrossRef]
- Laccetta, G.; Carrone, A.; Burratti, M.; Mancino, P. Effect of the Treatment with β-Glucan in Women with Cervical Cytologic Report of Atypical Squamous Cells of Undetermined Significance (ASCUS) and Low-Grade Squamous Intraepithelial Lesions (L-SIL). Minerva Ginecol. 2015, 67, 113–120. [Google Scholar]
- Stentella, P.; Biamonti, A.; Carraro, C.; Inghirami, P.; Mancino, P.; Pietrangeli, D.; Votano, S.; Lazzari, P.; De Medici, C. Efficacy of Carboxymethyl Beta-Glucan in Cervical Intraepithelial Neoplasia: A Retrospective, Case-Control Study. Minerva Obs. Gynecol. 2017, 69, 425–430. [Google Scholar] [CrossRef]
- Gil-Antuñano, S.P.; Serrano Cogollor, L.; López Díaz, A.C.; González Rodríguez, S.P.; Dexeus Carter, D.; Centeno Mediavilla, C.; Coronado Martín, P.; de la Fuente Valero, J.; López Fernández, J.A.; Vanrell Barbat, C.; et al. Efficacy of a Coriolusversicolor-Based Vaginal Gel in Human Papillomavirus-Positive Women Older Than 40 Years: A Sub-Analysis of PALOMA Study. J. Pers. Med. 2022, 12, 1559. [Google Scholar] [CrossRef]
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus Crispatus M247 Oral Administration: Is It Really an Effective Strategy in the Management of Papillomavirus-Infected Women? Infect. Agents Cancer 2022, 17, 53. [Google Scholar] [CrossRef]
- Di Pierro, F.; Criscuolo, A.A.; Dei Giudici, A.; Senatori, R.; Sesti, F.; Ciotti, M.; Piccione, E. Oral Administration of Lactobacillus Crispatus M247 to Papillomavirus-Infected Women: Results of a Preliminary, Uncontrolled, Open Trial. Minerva Obs. Gynecol. 2021, 73, 621–631. [Google Scholar] [CrossRef]
- Smith, J.A.; Gaikwad, A.A.; Mathew, L.; Rech, B.; Faro, J.P.; Lucci, J.A.; Bai, Y.; Olsen, R.J.; Byrd, T.T. AHCC® Supplementation to Support Immune Function to Clear Persistent Human Papillomavirus Infections. Front. Oncol. 2022, 12, 881902. [Google Scholar] [CrossRef] [PubMed]
- dE Vet, H.C.W.; Knipschild, P.G.; Willebrand, D.; Schouten, H.J.A.; Sturmans, F. The Effect of Beta-Carotene on the Regression and Progression of Cervical Dysplasia: A Clinical Experiment. J. Clin. Epidemiol. 1991, 44, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, L.; Sukhikh, G.; Kiselev, V.; Paltsev, M.; Drukh, V.; Kuznetsov, I.; Muyzhnek, E.; Apolikhina, I.; Andrianova, E. Double-Blind Randomized Placebo-Controlled Multicenter Clinical Trial (Phase IIa) on Diindolylmethane’s Efficacy and Safety in the Treatment of CIN: Implications for Cervical Cancer Prevention. EPMA J. 2015, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.-S.; Yoo, J.; Huh, S.-W.; Kim, C.-K.; Lee, J.-M.; Namkoong, S.-E.; Bae, S.-M.; Lee, I.P. Protective Effects of Green Tea Extracts (Polyphenon E and EGCG) on Human Cervical Lesions. Eur. J. Cancer Prev. 2003, 12, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.C.; Crowley-Nowick, P.; Bradlow, H.L.; Sepkovic, D.W.; Schmidt-Grimminger, D.; Howell, P.; Mayeaux, E.J.; Tucker, A.; Turbat-Herrera, E.A.; Mathis, J.M. Placebo-Controlled Trial of Indole-3-Carbinol in the Treatment of CIN. Gynecol. Oncol. 2000, 78, 123–129. [Google Scholar] [CrossRef]
- Ayatollahi, H.; Rajabi, E.; Yekta, Z.; Jalali, Z. Efficacy of Oral Zinc Sulfate Supplementation on Clearance of Cervical Human Papillomavirus (HPV); A Randomized Controlled Clinical Trial. Asian Pac. J. Cancer Prev. 2022, 23, 1285–1290. [Google Scholar] [CrossRef]
- Walker, H.; Burrell, M.; Flatley, J.; Powers, H. A Metabolite Profiling Method for Diagnosis of Precancerous Cervical Lesions and HPV Persistence. Bioanalysis 2017, 9, 601–608. [Google Scholar] [CrossRef]
- Pappa, K.I.; Daskalakis, G.; Anagnou, N.P. Metabolic Rewiring Is Associated with HPV-Specific Profiles in Cervical Cancer Cell Lines. Sci. Rep. 2021, 11, 17718. [Google Scholar] [CrossRef]
- Porcari, A.M.; Negrão, F.; Tripodi, G.L.; Pitta, D.R.; Campos, E.A.; Montis, D.M.; Martins, A.M.A.; Eberlin, M.N.; Derchain, S.F.M. Molecular Signatures of High-Grade Cervical Lesions. Front. Oncol. 2018, 8, 99. [Google Scholar] [CrossRef]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Román, M.; Téllez-Sosa, J.; Martínez-Barnetche, J.; Cortina-Ceballos, B.; López-Estrada, G.; Delgado-Romero, K.; Burguete-García, A.I.; Cantú, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef]
- Fang, B.; Li, Q.; Wan, Z.; OuYang, Z.; Zhang, Q. Exploring the Association Between Cervical Microbiota and HR-HPV Infection Based on 16S RRNA Gene and Metagenomic Sequencing. Front. Cell Infect. Microbiol. 2022, 12, 922554. [Google Scholar] [CrossRef]
- Kawahara, R.; Fujii, T.; Kukimoto, I.; Nomura, H.; Kawasaki, R.; Nishio, E.; Ichikawa, R.; Tsukamoto, T.; Iwata, A. Changes to the Cervicovaginal Microbiota and Cervical Cytokine Profile Following Surgery for Cervical Intraepithelial Neoplasia. Sci. Rep. 2021, 11, 2156. [Google Scholar] [CrossRef]
- Kaelin, E.A.; Skidmore, P.T.; Łaniewski, P.; Holland, L.A.; Chase, D.M.; Herbst-Kralovetz, M.M.; Lim, E.S. Cervicovaginal DNA Virome Alterations Are Associated with Genital Inflammation and Microbiota Composition. mSystems 2022, 7, e00064-22. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Yang, Y.; Liao, H. Changes in the Cervicovaginal Microbiota Composition of HPV16-infected Patients after Clinical Treatment. Cancer Med. 2022, 11, 5037–5049. [Google Scholar] [CrossRef]
- Oh, H.Y.; Kim, B.-S.; Seo, S.-S.; Kong, J.-S.; Lee, J.-K.; Park, S.-Y.; Hong, K.-M.; Kim, H.-K.; Kim, M.K. The Association of Uterine Cervical Microbiota with an Increased Risk for Cervical Intraepithelial Neoplasia in Korea. Clin. Microbiol. Infect. 2015, 21, 674.e1–674.e9. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Ollberding, N.J.; Kumar, R.; Macaluso, M.; Alvarez, R.D.; Morrow, C.D. Cervical Microbiota Associated with Higher Grade Cervical Intraepithelial Neoplasia in Women Infected with High-Risk Human Papillomaviruses. Cancer Prev. Res. 2016, 9, 357–366. [Google Scholar] [CrossRef]
- Ritu, W.; Enqi, W.; Zheng, S.; Wang, J.; Ling, Y.; Wang, Y. Evaluation of the Associations Between Cervical Microbiota and HPV Infection, Clearance, and Persistence in Cytologically Normal Women. Cancer Prev. Res. 2019, 12, 43–56. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Wiik, J.; Sengpiel, V.; Kyrgiou, M.; Nilsson, S.; Mitra, A.; Tanbo, T.; Monceyron Jonassen, C.; Møller Tannæs, T.; Sjøborg, K. Cervical Microbiota in Women with Cervical Intra-Epithelial Neoplasia, Prior to and after Local Excisional Treatment, a Norwegian Cohort Study. BMC Women’s Health 2019, 19, 30. [Google Scholar] [CrossRef]
- Wu, S.; Ding, X.; Kong, Y.; Acharya, S.; Wu, H.; Huang, C.; Liang, Y.; Nong, X.; Chen, H. The Feature of Cervical Microbiota Associated with the Progression of Cervical Cancer among Reproductive Females. Gynecol. Oncol. 2021, 163, 348–357. [Google Scholar] [CrossRef]
- Zhai, Q.; Zhang, W.; Zhang, Z.; Fu, Y.; Li, Y.; Wang, X.; Li, L.; Meng, Y. Characteristics of the Cervicovaginal Microenvironment in Childbearing-Age Women with Different Degrees of Cervical Lesions and HR-HPV Positivity. Pol. J. Microbiol. 2021, 70, 489–500. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Gao, W.; Pan, Y.; Gao, Y.; Shen, J.; Xiong, H. The Direct and Indirect Association of Cervical Microbiota with the Risk of Cervical Intraepithelial Neoplasia. Cancer Med. 2018, 7, 2172–2179. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, J.; Lu, Y.; Cai, Q.; Liu, H.; Xu, C. Cervical Microbiome Is Altered in Cervical Intraepithelial Neoplasia after Loop Electrosurgical Excision Procedure in China. Sci. Rep. 2018, 8, 4923. [Google Scholar] [CrossRef]
- Wei, L.Q.; Cheong, I.H.; Yang, G.H.; Li, X.G.; Kozlakidis, Z.; Ding, L.; Liu, N.N.; Wang, H. The Application of High-Throughput Technologies for the Study of Microbiome and Cancer. Front. Genet. 2021, 12, 699793. [Google Scholar] [CrossRef]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.-M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef]
- Winand, R.; Bogaerts, B.; Hoffman, S.; Lefevre, L.; Delvoye, M.; Van Braekel, J.; Fu, Q.; Roosens, N.H.; De Keersmaecker, S.C.; Vanneste, K. Targeting the 16S RRNA Gene for Bacterial Identification in Complex Mixed Samples: Comparative Evaluation of Second (Illumina) and Third (Oxford Nanopore Technologies) Generation Sequencing Technologies. Int. J. Mol. Sci. 2019, 21, 298. [Google Scholar] [CrossRef]
- Clarridge, J.E. Impact of 16S RRNA Gene Sequence Analysis for Identification of Bacteria on Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef]
- Smith, B.C.; McAndrew, T.; Chen, Z.; Harari, A.; Barris, D.M.; Viswanathan, S.; Rodriguez, A.C.; Castle, P.; Herrero, R.; Schiffman, M.; et al. The Cervical Microbiome over 7 Years and a Comparison of Methodologies for Its Characterization. PLoS ONE 2012, 7, e40425. [Google Scholar] [CrossRef]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of Cervico-Vaginal Microbiota in Women Developing Persistent High-Risk Human Papillomavirus Infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef]
- Curty, G.; Costa, R.L.; Siqueira, J.D.; Meyrelles, A.I.; Machado, E.S.; Soares, E.A.; Soares, M.A. Analysis of the Cervical Microbiome and Potential Biomarkers from Postpartum HIV-Positive Women Displaying Cervical Intraepithelial Lesions. Sci. Rep. 2017, 7, 17364. [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Li, F.; Zhao, J.; Wan, X.; Wang, K. Cervicovaginal Microbiota Composition Correlates with the Acquisition of High-Risk Human Papillomavirus Types. Int. J. Cancer 2018, 143, 621–634. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Seo, S.S.; Kwon, M.; Lee, J.K.; Kim, M.K. Association of Cervical Microbial Community with Persistence, Clearance and Negativity of Human Papillomavirus in Korean Women: A Longitudinal Study. Sci. Rep. 2018, 8, 15479. [Google Scholar] [CrossRef]
- Ravilla, R.; Coleman, H.N.; Chow, C.-E.; Chan, L.; Fuhrman, B.J.; Greenfield, W.W.; Robeson, M.S.; Iverson, K.; Spencer, H.; Nakagawa, M. Cervical Microbiome and Response to a Human Papillomavirus Therapeutic Vaccine for Treating High-Grade Cervical Squamous Intraepithelial Lesion. Integr. Cancer Ther. 2019, 18, 153473541989306. [Google Scholar] [CrossRef]
- Onywera, H.; Williamson, A.-L.; Mbulawa, Z.Z.A.; Coetzee, D.; Meiring, T.L. The Cervical Microbiota in Reproductive-Age South African Women with and without Human Papillomavirus Infection. Papillomavirus Res. 2019, 7, 154–163. [Google Scholar] [CrossRef]
- Usyk, M.; Zolnik, C.P.; Castle, P.E.; Porras, C.; Herrero, R.; Gradissimo, A.; Gonzalez, P.; Safaeian, M.; Schiffman, M.; Burk, R.D.; et al. Cervicovaginal Microbiome and Natural History of HPV in a Longitudinal Study. PLoS Pathog. 2020, 16, e1008376. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, T.; Zhang, D.; Zong, X.; Bai, H.; Bi, H.; Liu, Z. Distinction between Vaginal and Cervical Microbiota in High-Risk Human Papilloma Virus-Infected Women in China. BMC Microbiol. 2021, 21, 90. [Google Scholar] [CrossRef]
- Sasivimolrattana, T.; Chantratita, W.; Sensorn, I.; Chaiwongkot, A.; Oranratanaphan, S.; Bhattarakosol, P.; Bhattarakosol, P. Cervical Microbiome in Women Infected with HPV16 and High-Risk HPVs. Int. J. Environ. Res. Public. Health 2022, 19, 14716. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, H.; Yuan, L.; Chen, X.; Huang, X.; Wang, K.; Li, Z. Vaginal Microbiota and HPV Clearance: A Longitudinal Study. Front. Oncol. 2022, 12, 955150. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Xiao, W.-Y.; Fang, J.-F.; Dong, Y.-H.; Ye, K.-F.; He, M.-P.; Wang, Y.-S.; Li, X.; Zhao, Z.-M.; Yuan, T.; et al. Genital Microbiota of Women From Six Ethnic Groups With and Without Human Papillomavirus Infection in Shangri-La, China. Front. Cell. Infect. Microbiol. 2022, 12, 935068. [Google Scholar] [CrossRef]
- Hu, J.; Wu, Y.; Quan, L.; Yang, W.; Lang, J.; Tian, G.; Meng, B. Research of Cervical Microbiota Alterations with Human Papillomavirus Infection Status and Women Age in Sanmenxia Area of China. Front. Microbiol. 2022, 13, 1004664. [Google Scholar] [CrossRef]
- Guo, C.; Dai, W.; Zhou, Q.; Gui, L.; Cai, H.; Wu, D.; Hou, J.; Li, C.; Li, S.; Du, H.; et al. Cervicovaginal Microbiota Significantly Changed for HPV-Positive Women with High-Grade Squamous Intraepithelial Lesion. Front. Cell. Infect. Microbiol. 2022, 12, 973875. [Google Scholar] [CrossRef]
- Vikramdeo, K.S.; Anand, S.; Pierce, J.Y.; Singh, A.P.; Singh, S.; Dasgupta, S. Distribution of Microbiota in Cervical Preneoplasia of Racially Disparate Populations. BMC Cancer 2022, 22, 1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.; Liang, Y.; Wei, L.; Zhang, W.; Li, L. Observation of the Cervical Microbiome in the Progression of Cervical Intraepithelial Neoplasia. BMC Cancer 2022, 22, 362. [Google Scholar] [CrossRef]
- Liu, H.; Liang, H.; Li, D.; Wang, M.; Li, Y. Association of Cervical Dysbacteriosis, HPV Oncogene Expression, and Cervical Lesion Progression. Microbiol. Spectr. 2023, 10, e00151-22. [Google Scholar] [CrossRef]
- Vargas-Robles, D.; Romaguera, J.; Alvarado-Velez, I.; Tosado-Rodríguez, E.; Dominicci-Maura, A.; Sanchez, M.; Wiggin, K.J.; Martinez-Ferrer, M.; Gilbert, J.A.; Forney, L.J.; et al. The Cervical Microbiota of Hispanics Living in Puerto Rico Is Nonoptimal Regardless of HPV Status. mSystems 2023, 8, e00357-23. [Google Scholar] [CrossRef]
- Teka, B.; Yoshida-Court, K.; Firdawoke, E.; Chanyalew, Z.; Gizaw, M.; Addissie, A.; Mihret, A.; Colbert, L.E.; Napravnik, T.C.; El Alam, M.B.; et al. Cervicovaginal Microbiota Profiles in Precancerous Lesions and Cervical Cancer among Ethiopian Women. Microorganisms 2023, 11, 833. [Google Scholar] [CrossRef]
- Rokos, T.; Holubekova, V.; Kolkova, Z.; Hornakova, A.; Pribulova, T.; Kozubik, E.; Biringer, K.; Kudela, E. Is the Physiological Composition of the Vaginal Microbiome Altered in High-Risk HPV Infection of the Uterine Cervix? Viruses 2022, 14, 2130. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical Intraepithelial Neoplasia Disease Progression Is Associated with Increased Vaginal Microbiome Diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef] [PubMed]
- Qingqing, B.; Jie, Z.; Songben, Q.; Juan, C.; Lei, Z.; Mu, X. Cervicovaginal Microbiota Dysbiosis Correlates with HPV Persistent Infection. Microb. Pathog. 2021, 152, 104617. [Google Scholar] [CrossRef]
- Chorna, N.; Romaguera, J.; Godoy-Vitorino, F. Cervicovaginal Microbiome and Urine Metabolome Paired Analysis Reveals Niche Partitioning of the Microbiota in Patients with Human Papilloma Virus Infections. Metabolites 2020, 10, 36. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Ntritsos, G.; Smith, A.; Tsilidis, K.K.; Marchesi, J.R.; Bennett, P.R.; Moscicki, A.-B.; Kyrgiou, M. The Vaginal Microbiota Associates with the Regression of Untreated Cervical Intraepithelial Neoplasia 2 Lesions. Nat. Commun. 2020, 11, 1999. [Google Scholar] [CrossRef]
- Berggrund, M.; Gustavsson, I.; Aarnio, R.; Lindberg, J.H.; Sanner, K.; Wikström, I.; Enroth, S.; Bunikis, I.; Olovsson, M.; Gyllensten, U. Temporal Changes in the Vaginal Microbiota in Self-Samples and Its Association with Persistent HPV16 Infection and CIN2+. Virol. J. 2020, 17, 147. [Google Scholar] [CrossRef]
- Liu, J.; Luo, M.; Zhang, Y.; Cao, G.; Wang, S. Association of High-Risk Human Papillomavirus Infection Duration and Cervical Lesions with Vaginal Microbiota Composition. Ann. Transl. Med. 2020, 8, 1161. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).