Simple Summary
It is unclear what the risk of negative health outcomes is after cancer during adolescence and young adulthood. We conducted a review to understand the risk of second cancers, chronic conditions, and death in adolescent and young adult (AYA) cancer survivors and found factors that increase the risk. In total, 652 studies were identified, of which 106 were included in the review: 23 for second cancers, 34 for chronic conditions, and 54 for deaths. The number of included studies increased over time, from four studies in 2010 to 17 in 2020. The studies found that AYA cancer survivors are at an increased risk of second cancers, chronic conditions, and deaths. In particular, the following factors increased risk: radiation exposure for second cancers; younger attained age and earlier calendar period of diagnosis for chronic conditions; and non-Hispanic Black or Hispanic, low socioeconomic status, and earlier calendar period of diagnosis for deaths.
Abstract
Risk factors associated with late effects in survivors of adolescent and young adult (AYA) cancer are poorly understood. We conducted a systematic scoping review to identify cohort studies published in English from 2010–2020 that included: (1) cancer survivors who were AYAs (age 15–39 years) at diagnosis and (2) outcomes of subsequent malignant neoplasms (SMNs), chronic conditions, and/or late mortality (>5 years postdiagnosis). There were 652 abstracts identified and, ultimately, 106 unique studies were included, of which 23, 34, and 54 studies related to the risk of SMNs, chronic conditions, and mortality, respectively. Studies investigating late effects among survivors of any primary cancer reported that AYA cancer survivors were at higher risk of SMN, chronic conditions, and all-cause mortality compared to controls. There was an indication that the following factors increased risk: radiation exposure (n = 3) for SMNs; younger attained age (n = 4) and earlier calendar period of diagnosis (n = 3) for chronic conditions; and non-Hispanic Black or Hispanic (n = 5), low socioeconomic status (n = 3), and earlier calendar period of diagnosis (n = 4) for late mortality. More studies including the full AYA age spectrum, treatment data, and results stratified by age, sex, and cancer type are needed to advance knowledge about late effects in AYA cancer survivors.
1. Introduction
An estimated 1.2 million adolescents and young adults (AYAs), aged 15–39 years, were diagnosed with cancer in 2020 globally [,]. Although the incidence of AYA cancer is increasing or stable in many countries [], screening, diagnosis, and treatment continue to improve, leading to an overall decrease in all-cause and cancer-related mortality []. As a result, there is a growing population of AYA cancer survivors who will spend the majority of their lives at risk of late effects due to their cancer and its treatment. However, the burden of late effects and their associated risk factors in AYA cancer survivors are not well understood.
Although several systematic reviews investigating late effects in survivors of childhood [,,,] and adult [,,] cancers have been published recently, to our knowledge, only one systematic literature review investigating late effects specifically among AYA cancer survivors has been published in the last decade, and that focused on the 16–29 year age range []. Reviews that examine late effects in both childhood and AYA cancers [,,] discuss a striking lack of studies specifically investigating late effects in AYA cancer survivors relative to a robust body of literature for childhood cancer survivors []. Given important social and biological differences of AYA cancer, a review of the current evidence on the relationship between tumor-, treatment-, and patient-related risk factors and common late effects in AYA cancer survivors is warranted.
We thus sought to conduct a systematic scoping review to examine the current state of the literature studying late effects among AYA cancer survivors. Specifically, we aimed to describe the burden of subsequent malignant neoplasms (SMNs), chronic conditions (including hospitalizations and medication prescriptions as surrogates), and late mortality—key late effects, which lead to substantial years of life lost or living with a disability—in AYA cancer survivors and identify risk factors for each late effect. Finally, by summarizing the available literature, we aimed to identify knowledge gaps that will inform future research.
2. Methods
2.1. Eligibility Criteria
The three outcomes of interest in this study were (1) SMNs; (2) chronic conditions, which were physical or psychological in nature and diagnosed by a medical professional or for which a hospitalization or pharmacological prescription acted as a surrogate for a diagnosis (e.g., diagnosis of, hospitalization from, or prescription for a disease); and (3) late mortality, defined as a death occurring more than 5 years after the original cancer diagnosis. Studies that presented results for SMNs or chronic conditions were eligible if these conditions developed at any point after diagnosis. Studies were deemed eligible for inclusion in the scoping review if they met the following criteria defined a priori: (1) The study participants included individuals with a history of cancer who were AYAs (age 15–39 years) at the time of diagnosis, including studies not exclusively focused on AYAs (e.g., 0–19 year olds) if age-stratified results, which captured the AYA subgroup, were reported; (2) the study included one or more of the three outcomes defined above; (3) the research was a prospective or retrospective cohort study to allow the examination of the relationship between the AYA cancer exposure and the late effect; and (4) the study was original research published in English in the years 2010–2020. Conference abstracts, theses, reviews, and sources of grey literature were excluded. Case-control studies were not included because they are less suitable for producing evidence of causality and are more prone to bias than cohort studies.
2.2. Study Selection
We searched PubMed using three separate searches that aligned with each of the three outcomes of interest (Supplementary Table S1). Identified titles and abstracts published between 1 January 2010 and 31 December 2020 were screened independently by two reviewers (CRB and RLD). Disagreements were resolved by discussion until consensus was reached. If consensus could not be reached, a third expert reviewer (MMFB) was consulted. This process was also used to screen the full text of articles. The reference lists of all included articles were then examined independently by CRB and RLD, and additional articles were included in the scoping review until a consensus among the research team was reached. The following information was then abstracted for each included study: primary author, date of publication, country of origin, purpose of the study, data source, outcome ascertainment (medical records, registries, self-report, etc.), study design, type(s) of cancer studied, diagnosis period, age at diagnosis, survival entry date, overall study size or AYA cancer population size within a larger cohort, length of follow-up, key findings, and identified risk factors. Patient- (e.g., demographic, social, and lifestyle), treatment- (e.g., type and year of treatment), and tumor-related (e.g., type and histology) risk factors were all eligible based on their potential to influence the occurrence of late effects. As per the methodology of scoping reviews, we did not appraise the quality or risk of bias of the included studies []. Where possible, we abstracted the most-adjusted risk estimates from the results.
3. Results
The three unique searches in PubMed yielded a total of 652 records (SMNs: 199, chronic conditions: 187, late mortality: 266) (Figure 1). Hand-searching of included studies identified an additional 46 eligible studies. Following title and abstract screening, 225 records were deemed potentially relevant to the scoping review and eligible for full-text screening. After full-text screening, a total of 111 studies (SMNs: 23, chronic conditions: 34, late mortality: 54), of which 106 were unique.
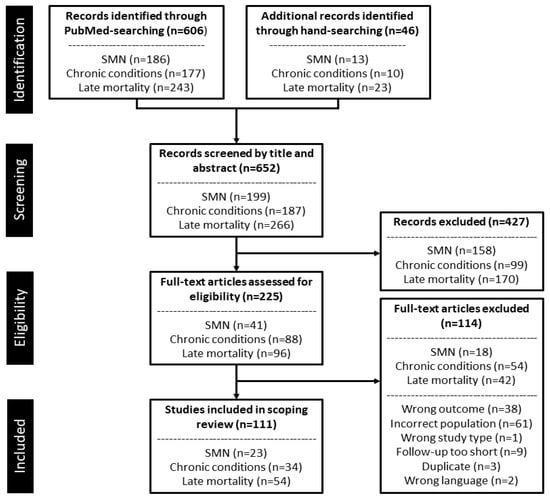
Figure 1.
PRISMA flow diagram for the study selection process. Abbreviations: SMN, subsequent malignant neoplasm.
All studies in this review had retrospective cohort designs. The number of included studies increased over time, with 44% being published from 2018–2020 (Figure 2). The studies were conducted predominantly in North America (63%) and Europe (29%). Only 45% captured the entire 15–39 years age range, while a further 17% focused on a childhood cohort, which overlapped with the youngest AYAs. Half of the studies made within-group comparisons, while the remainder used an external comparison group, primarily the general population (30%), childhood cancer survivors (12%), or siblings (5%). Sixty (54%) studies included participants from a mixed-cancer population, while the remaining studies examined specific tumors or tumor groups. In equal proportions, participants entered the study at the time of diagnosis (38%) or as 5-year survivors (38%). Finally, the median sample size across the three outcomes was 3053 AYA participants, ranging from 7 to 401,287.
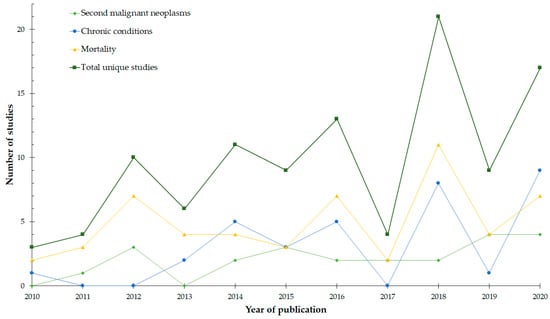
Figure 2.
The number of studies included in the scoping review for each outcome of subsequent malignant neoplasms, chronic conditions, and mortality according to year of publication. Duplicates (studies containing information on more than one outcome) were removed from the line indicating the total number of studies.
3.1. Subsequent Malignant Neoplasms
Studies investigating the development of any kind of SMN among a mixed-cancer population and reporting a single overall estimate for the standardized incidence ratio (SIR) found that AYA cancer survivors were 1.6 (95% confidence interval [95% CI]:1.6–1.6) to 4.3 (95% CI:3.6–5.1) times more likely to experience an SMN relative to that of a primary malignant neoplasm expected in the general population (Table 1) [,,,]. Absolute excess risk (AER) for developing an SMN in these same cohorts ranged between 15.9 (95% CI:12.1–19.8) and 25.9 (95% CI not reported) per 10,000 person-years at risk. The risk of developing specific types of SMNs ranged widely, with SIRs ranging from 1.1 (95% CI:1.1–1.2) for breast cancer to 2.0 (95% CI:1.9–2.0) for lung cancer and 3.0 (95% CI:2.6–3.5) for meningeal cancer.

Table 1.
The burden of subsequent malignant neoplasms (SMNs) among adolescent and young adult cancer survivors by tumor group (n = 23).
For the development of any SMN after a specific first primary cancer diagnosis, SIRs ranged from 1.2 (95% CI:1.1–1.4) after a bladder cancer diagnosis in men [] to 9.0 (95% CI:3.4–16.5) after a diagnosis of a bone cancer [,]. In particular, Hodgkin lymphoma (HL) survivors experienced the highest risk of SMN, which was 3 to 8 times that of the general population, [,] with an excess of 0.1 (95% CI not reported) to 111 (95% CI not reported) SMNs per 10,000 person-years [,].
Risk Factors Associated with Subsequent Malignant Neoplasms
The influence of sex on SMN risk was inconsistent (Figure 3; Supplementary Table S2). When mixed cancer populations were assessed, Chao et al. found female sex to be a risk factor (incidence rate ratio [IRR]:1.3, 95% CI:1.1–1.6), [] while Aben et al. found it to be protective (SIRmale:3.1, 95% CI:2.7–3.6; SIRfemale:2.0, 95% CI:1.8–2.3) []. Investigations of specific primary tumor groups suggested that female sex was a risk factor for SMN in HL survivors (HR:1.8, 95% CI: 1.0–1.3), [] but protective in melanoma (HR:0.7, 95% CI:0.6–0.8) and thyroid (OR:0.6, 95% CI:0.5–0.7) cancer survivor populations [,]. Similarly, older age at diagnosis was found to increase the risk of developing an SMN overall and for melanoma and thyroid cancer survivors, [,,] while other studies have associated younger age at diagnosis or treatment with increased risk of SMNs in HL survivors [,,].
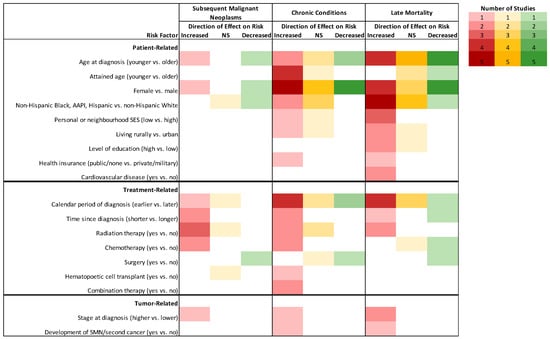
Figure 3.
Descriptive representation of the direction of effect for patient-, tumor-, or treatment-related factors described in three or more studies on the risk for subsequent malignant neoplasms, chronic conditions and hospitalizations, and late mortality among AYA cancer survivors in cohorts with combined cancer types. Deeper colors represent more studies (n = 5) and white cells represent that no study investigated that risk factor (n = 0). Reference categories appear in parentheses. Abbreviations: AAPI, Asian-American and Pacific Islander; NS, non-significant; SES, socioeconomic status; SMN, subsequent malignant neoplasm.
Possible treatment-related risk factors were reported in eight studies. Four studies reported that having radiotherapy elevated the risk of SMNs up to 2.7 times (95% CI:1.0–7.7) that expected, among AYA cancer survivors overall and among specific tumor groups [,,,], while two studies reported no significant effect of radiotherapy on the risk of SMNs in HL survivors [,]. Conversely, a study noted more than a 4-fold (95% CI:3.3–5.4) increase in the risk of SMNs in survivors of HL who received radiotherapy in addition to chemotherapy, relative to those who received chemotherapy only []. Treatment with chemotherapy for any primary malignancy increased the risk of SMN (SIR:6.3, 95% CI:4.1–9.4) []. Finally, the cumulative incidence of developing an SMN was higher for survivors of acute lymphoblastic leukemia (ALL) or non-HL (NHL) who received hematopoietic transplant compared to those who did not [,].
3.2. Chronic Conditions
Compared to control groups, AYAs with any primary malignancy had a 38–90% increased risk of first hospitalization (Table 2) [,]. In particular, AYA survivors of leukemia, NHL, HL, and central nervous system (CNS), head and neck, and bone tumors were about 4 times (95% CI:3.7–4.8) [] more likely to develop chronic conditions, as well as more severe chronic conditions that led to hospitalization, relative to the general population or a matched population [,,,,,,,,]. Similarly, a cohort of AYA survivors of leukemia, CNS malignancy, HL, NHL, Wilms tumor, neuroblastoma, soft-tissue sarcoma, and bone cancer had a 4.2-fold (95% CI:3.7–4.8) increased risk for developing one or more severe or disabling health conditions compared to siblings [].

Table 2.
The burden of chronic conditions among adolescent and young adult cancer survivors by tumor group (n = 34).
When specific conditions were assessed, AYA cancer survivors were at an increased risk of nearly all diseases assessed, with a 2- to 3-fold risk of cardiomyopathy, stroke, premature ovarian failure, chronic liver disease, and renal failure reported compared to a non-cancer population []. Studies also identified that circulatory and endocrine systems, and mental health, were impacted negatively by cancer and its treatment among AYAs. Specifically, AYA cancer survivors experience a 2.4-fold (95% CI:1.9–2.9) risk of developing cardiovascular disease (CVD) [] and a 1.3-fold (95% CI:1.3–1.3) risk of hospitalization with CVD relative to the general population []. Five-year survivors of cancer diagnosed between 20–34 have a significantly higher risk of cardiomyopathy or heart failure (HR:3.6, 95% CI:2.8–4.6), atherosclerosis, or brain vascular thrombosis (HR:1.7, 95% CI:1.4–2.0), myocardial infarction or cardiac ischemia (HR:1.8, 95% CI:1.5–2.1), and cardiac arrhythmia (HR:1.4, 95% CI:1.2–1.7) []. When results were stratified by tumor groups, the 10-year cumulative incidence of CVD was reported to be the highest among survivors of CNS tumors (7.3%, 95% CI:1.6–8.3), ALL (6.9%, 95% CI:5.2–8.7), and acute myeloid leukemia (AML) (6.8%, 95% CI:5.3–8.7) []. 95% Hospitalizations for endocrine disorders were also elevated relative to the general population []. Finally, one study reported an 80% increased risk of hospitalizations for mental health and psychiatric disorders among AYA survivors of any cancer, [] which corresponded to 4.5 times (95% CI:3.9–5.3) more purchases of antidepressants compared to siblings [] and 1.9 times (95% CI:1.0–3.5) the odds of purchases compared to childhood cancer survivors [].
Risk Factors Associated with Chronic Conditions
Patient-related risk factors for the development of a chronic condition included age at diagnosis and treatment, sex, race/ethnicity, and neighborhood socioeconomic status (SES) (Figure 3; Supplementary Table S3). Younger age (vs. older age) at any cancer diagnosis or treatment was associated with a greater risk of hospitalization or hospital contact related to cerebrovascular events (SIR15–19:3.6, 95% CI:3.0–4.2 vs. SIR35–39:1.2, 95% CI:1.2–1.3), [] and incidence of endocrine disorders (RR15–19:4.0, 95% CI:3.4–4.8 vs. RR30–34:1.6, 95% CI1.5–1.8), [] as well as CVD after HL (coronary heart disease: SIR:8.8, 95% CI:6.3–12.3; heart failure: SIR:8.9, 95% CI:25.2–57.4) []. Conversely, older age (vs. younger age) at diagnosis was associated with a higher risk of CVD (HR:3.6, 95% CI:2.1–3.2) [] and diabetes (IRR:2.4, 95% CI:1.7–3.5) [] after any cancer, and endocrine disorders after cutaneous melanoma (HR:1.4, 95% CI:1.1–1.7) []. Compared to their counterparts in the general population, AYA cancer survivors of a younger attained age were hospitalized at higher rates for cardiovascular [], endocrine [], some cerebrovascular [], and for somatic diseases [], whereas AYA survivors of an older attained age were hospitalized at similar rates to the comparison group. There was no consistent trend in the effect of sex on hospitalizations or the development of chronic conditions. Among survivors of any type of AYA cancer, female sex was associated with a higher risk of any morbidity leading to hospitalization (RR:1.5, 95% CI:1.2–1.9) [], anxiety or depression (HR:1.6, 95% CI:1.2–2.1) [,], endocrine disorders (HR = 1.9, 95% CI:1.6–2.1) [], and thyroid disorders (IRR:2.1, 95% CI:1.4–3.0) []. In other studies, male sex was associated with a higher risk of any hospitalization (HR:1.8, 95% CI:1.6–1.9) [], cerebrovascular events (SIRmale:1.5, 95% CI:1.5–1.6) [], and CVD (HR:1.4, 95% CI:1.2–1.7) [].
All of the studies that reported on the relationship between chronic conditions and social determinants of health were conducted in the USA. Compared to Whites, identifying as Black (IRR:2.3, 95% CI:1.4–3.5), Hispanic (IRR:2.2, 95% CI:1.6–3.0), or Asian/Pacific Islander (IRR:1.8, 95% CI:1.1–2.8) was associated with an elevated risk of diabetes for all AYA cancer survivors [] and hospitalization from any cause for HL survivors []. Similarly, residing in a low-SES neighborhood (vs. high-SES) was associated with a higher risk of most chronic conditions among survivors of HL [] and cutaneous melanoma [], and a 1.6-fold (95% CI:1.3–1.8) risk of CVD among survivors of any type of cancer [].
Various cancer therapies, including chemotherapy, radiotherapy, and stem cell transplants, were investigated for their role in the development of chronic conditions. In a mixed-cancer cohort, the combination of chemotherapy, radiotherapy, and surgery was associated with an 80% increase in chronic conditions compared to chemotherapy alone (95% CI:1.1–3.1), but no other treatment combinations were associated with significantly more conditions []. When assessed individually, receipt of radiotherapy increased the risk of stroke (IRR:3.5, 95% CI:5.9–37.2), diabetes (IRR:1.9, 95% CI:1.3–2.9), and thyroid disorders (IRR:3.1, 95% CI:2.2–4.4) for AYA survivors of any type of cancer [], as well as diseases of the nervous system for survivors of brain tumors (HR:3.3, 95% CI:1.8–6.2) []. Radiotherapy was linked to a 3.4-fold risk of endocrine (total body or chest vs. none) and cardiac conditions (≥15 Gy chest radiation vs. none) for survivors of bone cancers, leukemia, CNS malignancies, HL, NHL, Wilms tumor, neuroblastoma, and soft tissue sarcoma []. However, radiotherapy was not associated with the overall risk of health conditions among ALL survivors []. Exposure to cytotoxic drugs was associated with an increased risk of CVD among 2-year survivors of any type of cancer (3.5% vs. 2.0% for radiotherapy only) [] but was not associated with an overall increase in hospital-related morbidity among survivors of HL []. Finally, among survivors of NHL, HL, and ALL, stem cell transplants were also associated with an increased overall risk of more than 20 conditions of various organ systems [,,].
3.3. Late Mortality
Studies that captured populations of survivors of all types of AYA cancer reported standardized mortality ratios (SMRs) for all-cause mortality ranging from 4.2 (95% CI:4.0–4.3) to 5.9 (95% CI:4.9–6.9; AER:5.3) (Table 3) [,]. SMRs for cause-specific deaths were highest for neoplastic causes (i.e., progression or recurrence of primary cancer and second cancers) (SMR:10.9, 95% CI:10.4–11.2 and SMR:7.8, 95% CI:7.0–8.7), infections (SMR:4.0, 95% CI:2.1–5.8), and pulmonary diseases (SMR:7.4, 95% CI:5.7–9.5) [,]. Primary diagnoses of ALL (SMR:14.2, 95% CI:7.4–20.9) and cancers of the CNS (SMR:12.3, 95% CI:11.2–13.4 and SMR:7.8, 95% CI:6.6–9.2) were reported as having some of the highest risks of late, all-cause mortality [,,].

Table 3.
The burden of late mortality among adolescent and young adult cancer survivors by tumor group (n = 54).
Risk Factors Associated with Late Mortality
Among AYAs diagnosed with any type of primary cancer, females experienced significantly less excess mortality compared to males when adjusted for years of follow-up, calendar period, and comorbidities (HR:0.7; 95% CI:0.6–0.7) (Figure 3; Supplementary Table S4) []. One study provided evidence that the conditional survival advantages, specifically for circulatory and respiratory disease deaths, experienced by females for many cancers were most pronounced in the years closer to diagnosis []. Females were also reported to have an overall survival advantage relative to males in the following tumor-specific populations: NHL (HR:0.6, 95% CI:0.6–0.8) [], HL (HR:0.8, 95% CI:0.7–0.9) [,], melanoma (HR:0.7, 95% CI:0.6–0.8) [], AML (HR:0.8, 95% CI:0.7–0.9) [] and thyroid cancer (HR:0.4, 95% CI:0.3–0.5) []. When specific causes of death were assessed, two studies found that males surviving any type of cancer were more at risk of dying from respiratory conditions [], whereas female survivors of HL and NHL were more at risk of dying from CVD []. There was a lack of consensus as to how age at diagnosis affects the risk of late mortality (Figure 3). One study reported significantly more excess mortality (HR:1.4, 95% CI:1.2–1.6) in AYAs diagnosed between the ages of 30–39 compared to AYAs diagnosed between the ages of 15–19 []. Conversely, Prasad et al. reported a significantly higher SMR for all causes for AYAs diagnosed between 15–19 (SMR:9.2, 95% CI:7.8–10.6) compared to 20–34 (SMR:5.8, 95% CI:5.4–6.2) []. When assessed for specific tumor types, AYAs diagnosed at an older age compared to a younger age also had poorer survival outcomes after NHL [], HL [], testicular cancer [], Ewing sarcoma [], thyroid cancer [], and melanoma [].
When socioeconomic risk factors were investigated, Black AYAs with any type of cancer had a higher risk of death from any cause (HR:1.9, 95% CI:1.8–2.0) [] overall and specifically after HL [,] and testicular cancer [] compared to non-Hispanic Whites. Non-Hispanic Black (HR:1.7; 95% CI:1.1–2.8) [] AYA cancer survivors also experienced a greater risk of death from non-cancer causes. Likewise, in Australia, individuals who identified as Aboriginal had a statistically significant 47% increase in excess mortality compared to non-Aboriginals []. Similarly, several studies reported higher risks of late mortality for individuals with lower SES or living in lower SES neighborhoods (vs. higher SES) who survived any type of cancer (HR:1.1, 95% CI:1.0–1.3 and HR:1.4, 95% CI:1.3–1.5) [,], a brain tumor, HL, leukemia, NHL, thyroid cancer or sarcoma [], as well as thyroid [] or testicular cancer [] and HL [].
Regarding tumor-related risk factors, having more extensive disease or a higher stage of cancer at diagnosis [,,,,] was associated with increased risks in late mortality for tumor-specific and mixed-cancer cohorts. Excluding one study [], studies reported that excess mortality from AYA cancer decreased over calendar time, with people diagnosed, treated, or enrolled in studies in later years experiencing better survival outcomes [,,,,,,,]. For treatment-related risk factors, the evidence associating radiotherapy, chemotherapy, and surgery with late mortality was mixed among the included studies. Two studies conducted in a combined cancer population associated radiotherapy with a 2.0- (95% CI:1.3–3.1) and 1.5- (95% CI:1.0–2.1) fold increase in death from any cause and SMNs, respectively [,], while tumor type-specific studies reported non-significant associations [,] or decreased risks []. Conversely, among survivors of any type of AYA cancer, the risk of late mortality from any cause was not statistically different based on receipt of chemotherapy [], though specific tumor types, such as bone and soft tissue sarcomas (SMR:3.2, 95% CI:2.3–4.3) [], were found to have increased risk of late mortality if exposed to chemotherapy. Finally, not having surgery was associated with an increase in cancer-specific death among survivors of head and neck squamous cell carcinoma (HR:1.6; 95% CI:1.2–2.1) [] but not in combined cancer [] or bone and soft tissue sarcoma cohorts [].
4. Discussion
For AYA cancer survivors, the development of late effects after cancer can exacerbate the challenges of being a young person, such as balancing social relationships, new careers, and education with limited practical knowledge and financial resources [,]. Recognizing the poor understanding of late effects in AYA cancer survivors, this scoping review was conducted to identify published peer-reviewed research that reported on SMNs, chronic conditions, and late mortality risks in this under-researched population. Our findings emphasize the high burden of negative health outcomes experienced by AYA cancer survivors later in life. What remains less clear, based on the evidence from this review, is which factors contribute most to the increased risk of developing late effects, in part due to the lack of detailed treatment exposure investigations and the high level of heterogeneity among the studies. Genetic and lifestyle factors may also modify the mechanisms of established relationships between cancer therapies and late effects [,], yet references to factors like obesity, smoking, and physical activity were scarce within the reviewed literature [].
It is understood generally that cancer treatments, namely, radiotherapy and chemotherapy, are associated with morbidity and premature mortality, and multimodal therapies intensify these risks [,,,]. Studies included in this review reported that AYAs in mixed-cancer cohorts experience an estimated 2.6-, 1.9-, and 10.4-fold increase in the risk for SMNs, hospitalization from any health condition, and 25-year all-cause mortality, respectively, compared to control groups [,,]. Studies of childhood cancer survivors have shown a dose-response relationship between radiotherapy and the development of secondary sarcomas and breast, thyroid, CNS, and gastrointestinal cancers [,,,]. Similarly, specific chemotherapeutic drugs have been identified as risk factors for the late effects investigated in this review, with specific thresholds (e.g., high-dose intravenous methotrexate defined as any single dose ≥1000 mg/m2) noted by the Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers []. Based on the literature identified in this review, detailed treatment-related risk factors cannot be identified, as few studies include the necessary treatment exposure data to make such assertions. While entities such as the International Late Effects of Childhood Cancer Guideline Harmonization Group are developing guidelines for long-term follow-up for AYA cancer survivors, the evidence used is drawn primarily from studies of childhood cancer survivors [].
Treatment type is an important predictor of late morbidity, but unmodifiable risk factors may also play a role []. Sex-related differences in survival may be related to biological factors such as sex hormones and immune response, behavioral factors such as females’ increased self-awareness of their bodies [] or tendency towards health-seeking behaviors compared to men [], and clinical factors such as differences in screening practices (e.g., colorectal cancer) []. Age-related differences in the development of late effects may be fundamentally different between younger and older AYAs, as 15–20 year-olds may still be undergoing growth associated with puberty and the rapid proliferation of tissues brought on by sex hormones []. These tissues are particularly vulnerable to damage caused by radiation, potentially affecting the maturation of organs and systems associated with teenaged growth spurts (e.g., gonads and musculoskeletal system) [,]. In contrast, AYAs aged 20–39 may be exposed to a different set of carcinogenic exposures at work (e.g., asbestos) or in life (e.g., alcohol and smoking) that might alter their risk profile for late effects.
Variable background risks for mortality and chronic conditions also contextualize the findings in this review. Among studies that examined the risk of chronic conditions by attained age, most found that compared to their counterparts in the general population, AYAs of a younger attained age had a heightened risk of developing these late effects than AYAs of an older attained age. This finding may be explained by the background risk of chronic conditions increasing with age, leading to less excess morbidity among older AYA cancer survivors.
4.1. Opportunities for Future Research
The identification of effective cancer treatments with fewer short- and long-term side effects is a research priority for AYA cancer survivors []. Before interventions can be improved for long-term safety, the risks associated with current treatments must be better understood. Through this scoping review, we can propose several avenues for future research to help elucidate existing knowledge gaps. Broadly, there is a paucity of evidence generated from large, well-characterized cohort studies with lengthy follow-up, which include persons aged 15–39 and report results according to tumor type, sex, and age at diagnosis []. The existence of a body of such evidence would allow health professionals to stratify the risk of their patients for late effects and refer high-risk patients for suitable interventions []. Detailed treatment information, such as chemotherapy and radiation dosing, is also needed. Unlike childhood cancer survivors, for whom clinical cohorts such as the North American Childhood Cancer Survivor Study [] and the St. Jude Lifetime Cohort Study [] exist, few cohorts of AYA cancer survivors include this level of granularity due to the substantially greater number of AYA cancer survivors and the fact that many cohorts are established using population-based cancer registries in which only crude treatment information is recorded [].
Another area of future investigation relates to SMNs, as it was the least investigated late effect that we explored. SMNs are a well-studied outcome after childhood cancer and a study included in this scoping review found that AYAs surviving any type of cancer have a higher absolute risk of developing an SMN compared to children or older adult survivors []. Lung cancer is reported to account for a large proportion of these excess cancers in AYA cancer survivors, highlighting the need to consider the effect of modifiable lifestyle factors, like smoking, as risk factors for late effects []. Some estimates suggest that 35% of second cancers developed by adult cancer survivors can be attributed to the adverse effects of alcohol and smoking [].
Indeed, we identified only one study [] that studied lifestyle factors such as physical activity, overweight and obesity, alcohol use, or smoking as possible risk factors for late effects in AYA cancer survivors, despite the potential for poor health behaviors to modify the risk of therapy-related complications, with or without interaction with genetics [,]. This area is one of active research within adult oncology that has led to changes in clinical practice [,] as positive health behaviors have shown some promise for shielding cancer survivors from SMNs, chronic conditions, and premature mortality, both directly and indirectly. Given that AYA cancer survivors have a significantly higher prevalence of unhealthy lifestyle behaviors relative to individuals with no history of cancer [,,], this knowledge gap presents another important opportunity for future work [].
Finally, there is a dearth of evidence generated from populations in low- and middle-income countries (LMICs), despite these countries bearing the vast majority of cancer deaths []. Compared to high-income countries, survival rates in LMICs are estimated to be 11% to 81% lower, meaning that many people will not survive their cancer long enough to develop late effects []. Underdiagnoses, misdiagnoses, delayed presentation, and unavailability of treatment or abandonment of therapy contribute to lower rates of survival []. Some of these challenges may be corrected by rectifying the general shortage of healthcare professionals who manage patient loads that are often significantly higher than in most high-income countries []. Resource constraints further limit access to cancer drugs deemed essential by the World Health Organization [,] as well as the capacity for population-based cancer registries []. Without adequate cancer-related surveillance, it is difficult to develop effective policy priorities for improving cancer outcomes and health equity in LMICs. AYA oncology should be a priority matter due to the significant social and economic implications of loss of life due to cancer in this age group [], which accounts for 40% of the world’s population [].
4.2. Limitations
Despite the comprehensive nature of our scoping review, we must recognize several limitations. First, we found substantial heterogeneity across the included studies in terms of cancer types, subgroups, data sources, reference groups, methodology, and analysis. Additionally, we have not examined the quality of the evidence generated from each of the studies because grading of evidence was beyond the scope of this review. As a result, the findings of this review should be interpreted with caution as the included studies have not been assessed according to their individual clinical and methodological contexts. Another limitation of our review is that the search strategy was limited to one language, one database, and 11 years of evidence, which may contribute to selection bias and the lack of studies conducted in LMICs. Generally, English-language restrictions have not been shown to impact the conclusions of systematic reviews []. Additionally, we decided a priori to not include “childhood” or “adult” in our search terms, even though the AYA age group can overlap with each of these more general definitions. However, we are confident that through hand-searching of reference lists, we identified most or all childhood and adult survivor cohorts with results that are relevant to this review.
5. Conclusions
AYA cancer survivors experience a high level of morbidity many years into their survival. In this comprehensive scoping review, we summarized peer-reviewed literature published since 2010, describing the late-effect burden and examining patient-, tumor-, and treatment-related risk factors associated with late effects after AYA cancer. In doing so, this review highlighted substantial gaps in knowledge about the experience of AYA cancer survivors compared to what is known about children and middle-aged and older adults. Large, observational cohort studies including the full AYA age spectrum, long follow-up, detailed treatment exposure data, and results stratified by tumor type, age at diagnosis, and sex are still needed to help reduce the disease burden and increase quality of life in this unique cancer survivor population.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13194870/s1. Table S1: Examples of keywords used in PubMed literature searches. Keywords were appropriately combined using Boolean operators and searches were limited to the year 2010 to current. Table S2: Risk factors associated with subsequent malignant neoplasms (SMNs) among adolescent and young adult cancer survivors by tumor group (n = 23). Table S3: Risk factors associated with chronic conditions among adolescent and young adult cancer survivors by tumor group (n = 34). Table S4: Risk factors associated with late mortality among adolescent and young adult cancer survivors by tumor group (n = 54).
Author Contributions
Conceptualization, M.M.F.-B.; literature search, data abstraction, and data analysis, C.R.-B. and R.L.D.; writing—original draft preparation, C.R.-B. and R.L.D.; writing—review and editing, all authors (C.R.-B., R.L.D., R.D.B., S.G., P.C.N., S.J.M., M.M.F.-B.). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in this article and supplementary material.
Acknowledgments
The authors have no acknowledgements to make.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018; Available online: https://gco.iarc.fr/today (accessed on 2 June 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Harper, A.; Ruan, Y.; Barr, R.; Frazier, A.L.; Ferlay, J.; Steliarova-Foucher, E.; Fidler-Benaoudia, M.M. International Trends in the Incidence of Cancer Among Adolescents and Young Adults. J. Natl. Cancer Inst. 2020, 112, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawade, P.; Hudson, M.; Kaste, S.; Neglia, J.; Masker, K.W.; Constine, L.; Robison, L.; Ness, K. A Systematic Review of Selected Musculoskeletal Late Effects in Survivors of Childhood Cancer. Curr. Pediatr. Rev. 2015, 10, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Pierson, C.; Waite, E.; Pyykkonen, B. A meta-analysis of the neuropsychological effects of chemotherapy in the treatment of childhood cancer. Pediatr. Blood Cancer 2016, 63, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, J.; Higham, C.; Langer, T.; Denzer, C.; Brabant, G. Long-Term Endocrine and Metabolic Consequences of Cancer Treatment: A Systematic Review. Endocr. Rev. 2018, 40, 711–767. [Google Scholar] [CrossRef] [Green Version]
- Scholz-Kreisel, P.; Spix, C.; Blettner, M.; Eckerle, S.; Faber, J.; Wild, P.; Merzenich, H.; Hennewig, U. Prevalence of cardiovascular late sequelae in long-term survivors of childhood cancer: A systematic review and meta-analysis. Pediatr. Blood Cancer 2017, 64, e26428. [Google Scholar] [CrossRef]
- Duijts, S.F.A.; Van Egmond, M.P.; Spelten, E.; Van Muijen, P.; Anema, J.R.; Van Der Beek, A.J. Physical and psychosocial problems in cancer survivors beyond return to work: A systematic review. Psycho-Oncology 2013, 23, 481–492. [Google Scholar] [CrossRef]
- Bandak, M.; Jørgensen, N.; Juul, A.; Vogelius, I.R.; Lauritsen, J.; Kier, M.G.; Mortensen, M.S.; Glovinski, P.; Daugaard, G.; Vester-Glowinski, P.V. Testosterone deficiency in testicular cancer survivors—A systematic review and meta-analysis. Andrology 2016, 4, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Gerstl, B.; Sullivan, E.; Ives, A.D.; Saunders, C.; Wand, H.; Anazodo, A. Pregnancy Outcomes After a Breast Cancer Diagnosis: A Systematic Review and Meta-analysis. Clin. Breast Cancer 2017, 18, e79–e88. [Google Scholar] [CrossRef]
- Woodward, E.; Jessop, M.; Glaser, A.; Stark, D. Late effects in survivors of teenage and young adult cancer: Does age matter? Ann. Oncol. 2011, 22, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, A.; van den Berg, M.H.; van Leeuwen, F.E.; Kaspers, G.J.L.; Lambalk, C.B.; van Dulmen-den Broeder, E. Chemo-therapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: A systematic review. Cancer Treat. Rev. 2017, 53, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Friend, A.J.; Feltbower, R.G.; Hughes, E.J.; Dye, K.P.; Glaser, A.W. Mental health of long-term survivors of childhood and young adult cancer: A systematic review. Int. J. Cancer 2018, 143, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Gerstl, B.; Sullivan, E.; Chong, S.; Chia, D.; Wand, H.; Anazodo, A. Reproductive Outcomes After a Childhood and Adolescent Young Adult Cancer Diagnosis in Female Cancer Survivors: A Systematic Review and Meta-Analysis. J. Adolesc. Young-Adult Oncol. 2018, 7, 627–642. [Google Scholar] [CrossRef]
- Peters, M.; Godfrey, C.; McInerney, P.; Munn, Z.; Trico, A.; Khalil, H. Chapter 11: Scoping Reviews. In JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Lee, J.S.; DuBois, S.G.; Coccia, P.F.; Bleyer, A.; Olin, R.L.; Goldsby, R.E. Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer 2015, 122, 116–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Goddard, K.; Spinelli, J.J.; Gotay, C.; McBride, M.L. Risk of Late Mortality and Second Malignant Neoplasms among 5-Year Survivors of Young Adult Cancer: A Report of the Childhood, Adolescent, and Young Adult Cancer Survivors Research Program. J. Cancer Epidemiol. 2012, 2012, 103032. [Google Scholar] [CrossRef]
- Teepen, J.C.; Van Leeuwen, F.E.; Tissing, W.J.; Broeder, E.V.D.-D.; Heuvel-Eibrink, M.M.V.D.; Van Der Pal, H.J.; Loonen, J.; Bresters, D.; Versluys, B.; Neggers, S.J.C.M.M.; et al. Long-Term Risk of Subsequent Malignant Neoplasms After Treatment of Childhood Cancer in the DCOG LATER Study Cohort: Role of Chemotherapy. J. Clin. Oncol. 2017, 35, 2288–2298. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, D.; Shaw, A.; Xie, L. Risk of a second cancer in Canadians diagnosed with a first cancer in childhood or adolescence. EClinicalMedicine 2019, 16, 107–120. [Google Scholar] [CrossRef]
- Aben, K.K.; Van Gaal, C.; Van Gils, N.A.; Van Der Graaf, W.T.; Zielhuis, G.A. Cancer in adolescents and young adults (15–29 years): A population-based study in the Netherlands 1989–2009. Acta Oncol. 2012, 51, 922–933. [Google Scholar] [CrossRef]
- Henderson, T.O.; Oeffinger, K.C.; Whitton, J.; Leisenring, W.; Neglia, J.; Meadows, A.; Crotty, C.; Rubin, D.T.; Diller, L.; Inskip, P.; et al. Secondary gastrointestinal malignancies in childhood cancer survivors: A cohort study. Ann. Intern. Med. 2012, 156, 757. [Google Scholar] [CrossRef]
- Hayek, S.; Dichtiar, R.; Shohat, T.; Silverman, B.; Ifrah, A.; Boker, L.K. Risk of second primary neoplasm and mortality in childhood cancer survivors based on a national registry database. Cancer Epidemiol. 2018, 57, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Bright, C.J.; Reulen, R.C.; Winter, D.L.; Stark, D.P.; McCabe, M.G.; Edgar, A.B.; Frobisher, C.; Hawkins, M.M. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): A population-based, cohort study. Lancet Oncol. 2019, 20, 531–545. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.; Bhatia, S.; Xu, L.; Cannavale, K.L.; Wong, F.L.; Huang, P.-Y.S.; Cooper, R.; Armenian, S.H. Incidence, Risk Factors, and Mortality Associated with Second Malignant Neoplasms Among Survivors of Adolescent and Young Adult Cancer. JAMA Netw. Open 2019, 2, e195536. [Google Scholar] [CrossRef] [PubMed]
- Fidler-Benaoudia, M.; Reulen, R.; Winter, D.L.; Allodji, R.; Bagnasco, F.; Bárdi, E.; Bautz, A.; Bright, C.J.; Byrne, J.; Feijen, E.A.M.; et al. Risk of Subsequent Bone Cancers Among 69 460 Five-Year Survivors of Childhood and Adolescent Cancer in Europe. J. Natl. Cancer Inst. 2017, 110, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reulen, R.C.; Wong, K.F.; Bright, C.J.; Winter, D.L.; Alessi, D.; Allodji, R.M.; Bagnasco, F.; Bárdi, E.; Bautz, A.; Byrne, J.; et al. Risk of digestive cancers in a cohort of 69 460 five-year survivors of childhood cancer in Europe: The PanCareSurFup study. Gut 2020, 70, 1520–1528. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Higgins, C.D.; Smith, P.; Cunningham, D.; Hancock, B.W.; Horwich, A.; Hoskin, P.; Lister, T.A.; Radford, J.; Rohatiner, A.Z.; et al. Second Cancer Risk after Chemotherapy for Hodgkin’s Lymphoma: A Collaborative British Cohort Study. J. Clin. Oncol. 2011, 29, 4096–4104. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Cooke, R.; Bates, A.; Cunningham, D.; Falk, S.J.; Gilson, D.; Hancock, B.W.; Harris, S.J.; Horwich, A.; Hoskin, P.; et al. Breast Cancer Risk After Supradiaphragmatic Radiotherapy for Hodgkin’s Lymphoma in England and Wales: A National Cohort Study. J. Clin. Oncol. 2012, 30, 2745–2752. [Google Scholar] [CrossRef]
- Schaapveld, M.; Aleman, B.M.P.; Van Eggermond, A.M.; Janus, C.P.M.; Krol, S.; Van Der Maazen, R.W.M.; Roesink, J.M.; Raemaekers, J.M.M.; De Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef]
- Xavier, A.C.; Costa, L.J. Changes in the use of radiation therapy for early classical Hodgkin lymphoma in adolescents and young adults: Implications for survival and second malignancies. Leuk. Lymphoma 2015, 56, 2339–2343. [Google Scholar] [CrossRef]
- Bhuller, K.S.; Zhang, Y.; Li, D.; Sehn, L.H.; Goddard, K.; McBride, M.L.; Rogers, P.C. Late mortality, secondary malignancy and hospitalisation in teenage and young adult survivors of Hodgkin lymphoma: Report of the Childhood/Adolescent/Young Adult Cancer Survivors Research Program and the BC Cancer Agency Centre for Lymphoid Cancer. Br. J. Haematol. 2016, 172, 757–768. [Google Scholar] [CrossRef]
- Van Eggermond, A.M.; Schaapveld, M.; Janus, C.P.; De Boer, J.P.; Krol, S.; Zijlstra, J.M.; Van Der Maazen, R.W.; Kremer, L.C.; Van Leerdam, M.E.; Louwman, M.; et al. Infradiaphragmatic irradiation and high procarbazine doses increase colorectal cancer risk in Hodgkin lymphoma survivors. Br. J. Cancer 2017, 117, 306–314. [Google Scholar] [CrossRef]
- Goldfarb, M.; Freyer, D.R. Comparison of secondary and primary thyroid cancer in adolescents and young adults. Cancer 2014, 120, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; DuBois, S.G.; Boscardin, W.J.; Wustrack, R.L.; Goldsby, R.E. Secondary malignant neoplasms among children, adolescents, and young adults with osteosarcoma. Cancer 2014, 120, 3987–3993. [Google Scholar] [CrossRef]
- Sultan, I.; Rihani, R.; Hazin, R.; Rodriguez-Galindo, C. Second malignancies in patients with Ewing Sarcoma Family of Tumors: A population-based study. Acta Oncol. 2010, 49, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, R.; Li, Q.W.; Malogolowkin, M.H.; Alvarez, E.M.; Ribeiro, R.C.; Wun, T.; Keegan, T.H.M. Chronic medical conditions and late effects following non-Hodgkin lymphoma in HIV-uninfected and HIV-infected adolescents and young adults: A population-based study. Br. J. Haematol. 2020, 190, 371–384. [Google Scholar] [CrossRef]
- Gingrich, A.A.; Sauder, C.A.; Goldfarb, M.; Li, Q.; Wun, T.; Keegan, T.H. Disparities in the Occurrence of Late Effects following Treatment among Adolescent and Young Adult Melanoma Survivors. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Muffly, L.; Maguire, F.B.; Li, Q.; Kennedy, V.; Keegan, T.H. Late Effects in Survivors of Adolescent and Young Adult Acute Lymphoblastic Leukemia. JNCI Cancer Spectr. 2020, 4, pkaa025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugbjerg, K.; Olsen, J.H. Long-term Risk of Hospitalization for Somatic Diseases in Survivors of Adolescent or Young Adult Cancer. JAMA Oncol. 2016, 2, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.; Kaddas, H.K.; Ou, J.Y.; Ramsay, J.M.; Trogdon, J.G.; Kirchhoff, A.C.; Nichols, H.B. Hospitalization after Adolescent and Young Adult (AYA) Cancer: A Population-Based Study in Utah. Cancer Epidemiol. Biomark. Prev. 2020, 29, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Suh, E.; Stratton, K.L.; Leisenring, W.M.; Nathan, P.C.; Ford, J.S.; Freyer, D.R.; McNeer, J.L.; Stock, W.; Stovall, M.; Krull, K.R.; et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: A retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020, 21, 421–435. [Google Scholar] [CrossRef]
- Smith, L.; Glaser, A.W.; Peckham, D.; Greenwood, D.C.; Feltbower, R.G. Respiratory morbidity in young people surviving cancer: Population-based study of hospital admissions, treatment-related risk factors and subsequent mortality. Int. J. Cancer 2018, 145, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Licht, S.D.F.; Maraldo, M.V.; Specht, L.; Nielsen, T.T.; Winther, J.F.; Rugbjerg, K. Risk factors for cardiovascular disease in 5-year survivors of adolescent and young adult cancer: A Danish population-based cohort study. Cancer 2019, 126, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Bright, C.J.; Hawkins, M.M.; Guha, J.; Henson, K.E.; Winter, D.L.; Kelly, J.S.; Feltbower, R.G.; Hall, M.; Cutter, D.J.; Edgar, A.B.; et al. Risk of Cerebrovascular Events in 178 962 Five-Year Survivors of Cancer Diagnosed at 15 to 39 Years of Age. Circulation 2017, 135, 1194–1210. [Google Scholar] [CrossRef] [PubMed]
- Keegan, T.H.M.; Kushi, L.H.; Li, Q.; Brunson, A.; Chawla, X.; Chew, H.K.; Malogolowkin, M.; Wun, T. Cardiovascular disease incidence in adolescent and young adult cancer survivors: A retrospective cohort study. J. Cancer Surviv. 2018, 12, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lorenzi, M.F.; Goddard, K.; Spinelli, J.J.; Gotay, C.; McBride, M.L. Late morbidity leading to hospitalization among 5-year survivors of young adult cancer: A report of the childhood, adolescent and young adult cancer survivors research program. Int. J. Cancer 2013, 134, 1174–1182. [Google Scholar] [CrossRef]
- Rugbjerg, K.; Mellemkjaer, L.; Boice, J.D.; Køber, L.; Ewertz, M.; Olsen, J.H. Cardiovascular Disease in Survivors of Adolescent and Young Adult Cancer: A Danish Cohort Study, 1943–2009. J. Natl. Cancer Inst. 2014, 106, dju110. [Google Scholar] [CrossRef] [Green Version]
- Kero, A.; Järvelä, L.; Arola, M.; Malila, N.; Madanat-Harjuoja, L.; Matomäki, J.; Lähteenmäki, P. Cardiovascular morbidity in long-term survivors of early-onset cancer: A population-based study. Int. J. Cancer 2013, 134, 664–673. [Google Scholar] [CrossRef]
- Bradley, N.M.; Lorenzi, M.F.; Abanto, Z.; Sheps, S.; Broemeling, A.M.; Spinelli, J.J.; Goddard, K.; Pritchard, S.; Rogers, P.; McBride, M.L. Hospitalisations 1998–2000 in a British Columbia population-based cohort of young cancer survivors: Report of the Childhood/Adolescent/Young Adult Cancer Survivors (CAYACS) Research Program. Eur. J. Cancer 2010, 46, 2441–2448. [Google Scholar] [CrossRef]
- Deyell, R.J.; Lorenzi, M.; Ma, S.; Rassekh, S.R.; Collet, J.-P.; Spinelli, J.J.; McBride, M.L. Antidepressant use among survivors of childhood, adolescent and young adult cancer: A report of the childhood, adolescent and young adult cancer survivor (CAYACS) research program. Pediatr. Blood Cancer 2012, 60, 816–822. [Google Scholar] [CrossRef]
- Brewster, D.H.; Clark, D.; Hopkins, L.; Bauer, J.; Wild, S.H.; Edgar, A.B.; Wallace, W.H. Subsequent hospitalisation experience of 5-year survivors of childhood, adolescent, and young adult cancer in Scotland: A population based, retrospective cohort study. Br. J. Cancer 2013, 110, 1342–1350. [Google Scholar] [CrossRef]
- Kirchhoff, A.C.; Fluchel, M.N.; Wright, J.; Ying, J.; Sweeney, C.; Bodson, J.; Stroup, A.M.; Smith, K.R.; Fraser, A.; Kinney, A. Risk of Hospitalization for Survivors of Childhood and Adolescent Cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1280–1289. [Google Scholar] [CrossRef] [Green Version]
- Van Laar, M.; Feltbower, R.G.; Gale, C.P.; Bowen, D.T.; Oliver, S.E.; Glaser, A. Cardiovascular sequelae in long-term survivors of young peoples’ cancer: A linked cohort study. Br. J. Cancer 2014, 110, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Ahomäki, R.; Gunn, M.E.; Madanat-Harjuoja, L.M.; Matomäki, J.; Malila, N.; Lähteenmäki, P.M. Late psychiatric morbidity in survivors of cancer at a young age: A nationwide registry-based study. Int. J. Cancer 2014, 137, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Asdahl, P.H.; Winther, J.F.; Bonnesen, T.G.; Licht, S.D.F.; Gudmundsdottir, T.; Holmqvist, A.S.; Malila, N.; Tryggvadottir, L.; Wesenberg, F.; Dahlerup, J.F.; et al. Gastrointestinal and liver disease in Adult Life After Childhood Cancer in Scandinavia: A population-based cohort study. Int. J. Cancer 2016, 139, 1501–1511. [Google Scholar] [CrossRef]
- Kero, A.; Madanat-Harjuoja, L.; Järvelä, L.; Malila, N.; Matomäki, J.; Lähteenmäki, P. Health conditions associated with metabolic syndrome after cancer at a young age: A nationwide register-based study. Cancer Epidemiol. 2016, 41, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.; Xu, L.; Bhatia, S.; Cooper, R.; Brar, S.; Wong, F.L.; Armenian, S.H. Cardiovascular Disease Risk Profiles in Survivors of Adolescent and Young Adult (AYA) Cancer: The Kaiser Permanente AYA Cancer Survivors Study. J. Clin. Oncol. 2016, 34, 1626–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, M.V.; Rugbjerg, K.; Licht, S.D.F.; Johansen, C.; Schmiegelow, K.; Andersen, K.K.; Winther, J.F. Endocrine Late Effects in Survivors of Cancer in Adolescence and Young Adulthood. JAMA Netw. Open 2018, 1, e180349. [Google Scholar] [CrossRef]
- Krawczuk-Rybak, M.; Panasiuk, A.; Stachowicz-Stencel, T.; Zubowska, M.; Skalska-Sadowska, J.; Sęga-Pondel, D.; Czajńska-Deptuła, A.; Sławińska, D.; Badowska, W.; Kamieńska, E.; et al. Health status of Polish children and adolescents after cancer treatment. Eur. J. Nucl. Med. Mol. Imaging 2017, 177, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Nathan, P.C.; Nachman, A.; Sutradhar, R.; Kurdyak, P.; Pole, J.; Lau, C.; Gupta, S. Adverse mental health outcomes in a population-based cohort of survivors of childhood cancer. Cancer 2018, 124, 2045–2057. [Google Scholar] [CrossRef] [PubMed]
- Ahomäki, R.; Kero, A.; Koivisto, M.; Madanat-Harjuoja, L.; Malila, N.; Lähteenmäki, P. Purchases of antidepressants after cancer at a young age in Finland. Int. J. Cancer 2018, 144, 1227–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, R.; Scott, E.; Yeh, M.Y.; Wong, K.; Rushing, T.; Huh, W.; Orgel, E. Association of body mass index with toxicity and survival in pediatric patients treated with cisplatin-containing regimens. Pediatr. Hematol. Oncol. 2020, 38, 239–250. [Google Scholar] [CrossRef]
- Chao, C.; Bhatia, S.; Xu, L.; Cannavale, K.L.; Wong, F.L.; Huang, P.-Y.S.; Cooper, R.; Armenian, S.H. Chronic Comorbidities Among Survivors of Adolescent and Young Adult Cancer. J. Clin. Oncol. 2020, 38, 3161–3174. [Google Scholar] [CrossRef]
- Yu, B.; Fritz, R.; Vega, M.; Merino, M. Dissociation of Pubertal Development Abnormality and Gonadal Dysfunction in Childhood Cancer Survivors. J. Adolesc. Young-Adult Oncol. 2020, 9, 490–495. [Google Scholar] [CrossRef]
- Van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.M.; Krol, S.; Petersen, E.J.; Raemaekers, J.M.M.; Kok, W.E.M.; Aleman, B.M.P.; Van Leeuwen, F.E. Cardiovascular Disease After Hodgkin Lymphoma Treatment. JAMA Intern. Med. 2015, 175, 1007–1017. [Google Scholar] [CrossRef]
- Keegan, T.H.M.; Li, Q.; Steele, A.; Alvarez, E.M.; Brunson, A.; Flowers, C.R.; Glaser, S.L.; Wun, T. Sociodemographic disparities in the occurrence of medical conditions among adolescent and young adult Hodgkin lymphoma survivors. Cancer Causes Control. 2018, 29, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Gunn, M.E.; Malila, N.; Lähdesmäki, T.; Arola, M.; Grönroos, M.; Matomäki, J.; Lähteenmäki, P. Late new morbidity in survivors of adolescent and young-adulthood brain tumors in Finland: A registry-based study. Neuro-Oncology 2015, 17, 1412–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perisa, M.P.; Stanek, J.; Setty, B.A.; Nicol, K.; Yeager, N. Evaluating Age-related Disparity of Outcomes in Ewing Sarcoma Patients Treated at a Pediatric Academic Medical Center. J. Pediatr. Hematol. 2020, 43, e702–e706. [Google Scholar] [CrossRef] [PubMed]
- Haggar, F.A.; Pereira, G.; Preen, D.; Holman, C.D.J.; Einarsdottir, K. Cancer Survival and Excess Mortality Estimates among Adolescents and Young Adults in Western Australia, 1982–2004: A Population-Based Study. PLoS ONE 2013, 8, e55630. [Google Scholar] [CrossRef] [Green Version]
- Garwicz, S.; Anderson, H.; Olsen, J.H.; Winther, J.F.; Sankila, R.; Langmark, F.; Tryggvadóttir, L.; Möller, T.R. Nordic Society for Pediatric Hematology for the Association of the Nordic Cancer Registries (ANCR) and the Nordic Society for Pediatric Hematology Oncology (NOPHO) Late and very late mortality in 5-year survivors of childhood cancer: Changing pattern over four decades-Experience from the Nordic countries. Int. J. Cancer 2011, 131, 1659–1666. [Google Scholar] [CrossRef]
- Prasad, P.K.; Signorello, L.B.; Friedman, D.L.; Boice, J.D.; Pukkala, E. Long-term non-cancer mortality in pediatric and young adult cancer survivors in Finland. Pediatr. Blood Cancer 2011, 58, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Henrique, L.A.; Back, I.D.C. Cancer mortality among adolescents and young adults: A historical cohort in a reference institution for cancer treatment in Santa Catarina/South of Brazil 2002–2013. Cancer Epidemiol. 2016, 45, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Henson, K.E.; Reulen, R.C.; Winter, D.L.; Bright, C.J.; Fidler, M.M.; Frobisher, C.; Guha, J.; Wong, K.F.; Kelly, J.; Edgar, A.B.; et al. Cardiac Mortality Among 200 000 Five-Year Survivors of Cancer Diagnosed at 15 to 39 Years of Age. Circulation 2016, 134, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Berkman, A.M.; Brewster, A.M.; Jones, L.W.; Yu, J.; Lee, J.J.; Peng, S.A.; Crocker, A.; Ater, J.L.; Gilchrist, S.C. Racial Differences in 20-Year Cardiovascular Mortality Risk Among Childhood and Young Adult Cancer Survivors. J. Adolesc. Young-Adult Oncol. 2017, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Smitherman, A.; Nichols, H.B. Conditional relative survival among long-term survivors of adolescent and young adult cancers. Cancer 2018, 124, 3037–3043. [Google Scholar] [CrossRef] [Green Version]
- Fidler, M.M.; Reulen, R.; Bright, C.J.; Henson, K.E.; Kelly, J.S.; Jenney, M.; Ng, A.; Whelan, J.; Winter, D.L.; Frobisher, C.; et al. Respiratory mortality of childhood, adolescent and young adult cancer survivors. Thorax 2018, 73, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.; Lund, J.L.; Weaver, M.A.; Wood, W.A.; Olshan, A.F.; Nichols, H.B. Disparities in Mortality from Noncancer Causes among Adolescents and Young Adults with Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1417–1426. [Google Scholar] [CrossRef] [Green Version]
- Bagnasco, F.; Caruso, S.; Andreano, A.; Valsecchi, M.G.; Jankovic, M.; Biondi, A.; Miligi, L.; Casella, C.; Terenziani, M.; Massimino, M.; et al. Late mortality and causes of death among 5-year survivors of childhood cancer diagnosed in the period 1960–1999 and registered in the Italian Off-Therapy Registry. Eur. J. Cancer 2019, 110, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Moke, D.J.; Tsai, K.; Hamilton, A.S.; Hwang, A.; Liu, L.; Freyer, D.R.; Deapen, D. Emerging Cancer Survival Trends, Disparities, and Priorities in Adolescents and Young Adults: A California Cancer Registry-Based Study. JNCI Cancer Spectr. 2019, 3. [Google Scholar] [CrossRef]
- Armenian, S.H.; Xu, L.; Mph, K.L.C.; Wong, F.L.; Bhatia, S.; Chao, C. Cause-specific mortality in survivors of adolescent and young adult cancer. Cancer 2020, 126, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Cuglievan, B.; Berkman, A.; Dibaj, S.; Wang, J.; Andersen, C.R.; Livingston, J.A.; Gill, J.; Bleyer, A.; Roth, M. Impact of Lagtime, Health Insurance Type, and Income Status at Diagnosis on the Long-Term Survival of Adolescent and Young Adult Cancer Patients. J. Adolesc. Young-Adult Oncol. 2021, 10, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Kent, E.E.; Morris, R.A.; Largent, J.A.; Ziogas, A.; Sender, L.S.; Anton-Culver, H. Socioeconomic Impacts on Survival Differ by Race/Ethnicity among Adolescents and Young Adults with Non-Hodgkin’s Lymphoma. J. Cancer Epidemiol. 2010, 2010, 824691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellino, S.M.; Geiger, A.M.; Mertens, A.C.; Leisenring, W.M.; Tooze, J.A.; Goodman, P.; Stovall, M.; Robison, L.L.; Hudson, M.M. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood 2011, 117, 1806–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, J.; Xie, L. Sex disparity in childhood and young adult acute myeloid leukemia (AML) survival: Evidence from US population data. Cancer Epidemiol. 2015, 39, 892–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keegan, T.H.; DeRouen, M.; Parsons, H.M.; Clarke, C.A.; Goldberg, D.; Flowers, C.R.; Glaser, S.L. Impact of Treatment and Insurance on Socioeconomic Disparities in Survival after Adolescent and Young Adult Hodgkin Lymphoma: A Population-Based Study. Cancer Epidemiol. Biomark. Prev. 2016, 25, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Patel, C.G.; Michaelson, E.; Chen, Y.H.; Silver, B.; Marcus, K.J.; Stevenson, M.A.; Mauch, P.M.; Ng, A.K. Reduced Mortality Risk in the Recent Era in Early-Stage Hodgkin Lymphoma Patients Treated with Radiation Therapy with or without Chemo-therapy. Int. J. Radiat. Oncol. Biol. Physics. 2018, 100, 498–506. [Google Scholar] [CrossRef]
- Goldman, J.M.; Majhail, N.S.; Klein, J.P.; Wang, Z.; Sobocinski, K.A.; Arora, M.; Horowitz, M.M.; Rizzo, J.D. Relapse and Late Mortality in 5-Year Survivors of Myeloablative Allogeneic Hematopoietic Cell Transplantation for Chronic Myeloid Leukemia in First Chronic Phase. J. Clin. Oncol. 2010, 28, 1888–1895. [Google Scholar] [CrossRef]
- Chen, Y.; Kantarjian, H.; Wang, H.; Cortes, J.; Ravandi, F. Acute promyelocytic leukemia: A population-based study on incidence and survival in the United States, 1975–2008. Cancer 2012, 118, 5811–5818. [Google Scholar] [CrossRef] [Green Version]
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved Survival for Children and Adolescents with Acute Lymphoblastic Leukemia between 1990 and 2005: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef]
- Canner, J.; Alonzo, T.A.; Franklin, J.; Freyer, D.R.; Gamis, A.S.; Ma, R.B.G.; Lange, B.J.; Meshinchi, S.; Woods, W.G.; Perentesis, J.; et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients. Cancer 2013, 119, 4162–4169. [Google Scholar] [CrossRef] [Green Version]
- Woods, W.G.; Franklin, A.R.K.; Alonzo, T.A.; Ma, R.B.G.; Donohue, K.A.; Othus, M.; Horan, J.; Appelbaum, F.R.; Estey, E.H.; Bloomfield, C.D.; et al. Outcome of adolescents and young adults with acute myeloid leukemia treated on COG trials compared to CALGB and SWOG trials. Cancer 2013, 119, 4170–4179. [Google Scholar] [CrossRef] [Green Version]
- Wolfson, J.; Sun, C.-L.; Wyatt, L.; Stock, W.; Bhatia, S. Adolescents and Young Adults with Acute Lymphoblastic Leukemia and Acute Myeloid Leukemia: Impact of Care at Specialized Cancer Centers on Survival Outcome. Cancer Epidemiol. Biomark. Prev. 2017, 26, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Zhu, X.; Tang, B.; Zhang, X.; Zhang, L.; Geng, L.; Liu, H.; Sun, Z. Transplants of unrelated cord blood or sibling allogeneic peripheral blood stem cells/bone marrow in adolescent and young adults with chronic myeloid leukemia: Comparable outcomes but better chronic GVHD-free and relapse-free survival among survivors with cord blood. Oncotarget 2017, 9, 2848–2857. [Google Scholar] [CrossRef] [Green Version]
- Baron, F.; Efficace, F.; Cannella, L.; Muus, P.; Trisolini, S.; Halkes, C.J.M.; Fazi, P.; Vignetti, M.; Marie, J.; Chiusolo, P.; et al. Impact of the type of anthracycline and of stem cell transplantation in younger patients with acute myeloid leukaemia: Long-term follow up of a phase III study. Am. J. Hematol. 2020, 95, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Venkitachalam, R.; Szabo, A.; Murthy, G.S.G. Population-Level Outcomes of Pediatric Acute Promyelocytic Leukemia in the United States. J. Pediatr. 2020, 223, 114–119.e5. [Google Scholar] [CrossRef] [PubMed]
- Fosså, S.D.; Cvancarova, M.; Chen, L.; Allan, A.L.; Oldenburg, J.; Peterson, D.R.; Travis, L.B. Adverse Prognostic Factors for Testicular Cancer–Specific Survival: A Population-Based Study of 27,948 Patients. J. Clin. Oncol. 2011, 29, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Pollack, L.A.; Li, J.; Berkowitz, Z.; Weir, H.K.; Wu, X.-C.; Ajani, U.A.; Ekwueme, D.U.; Li, C.; Pollack, B.P. Melanoma survival in the United States, 1992 to 2005. J. Am. Acad. Dermatol. 2011, 65, S78.e1–S78.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, A.C.; Baade, P.; Coory, M.; Aitken, J.F.; Smithers, M. Population-Based 20-Year Survival Among People Diagnosed with Thin Melanomas in Queensland, Australia. J. Clin. Oncol. 2012, 30, 1462–1467. [Google Scholar] [CrossRef] [Green Version]
- Reed, K.B.; Brewer, J.D.; Lohse, C.M.; Bringe, K.E.; Pruitt, C.N.; Gibson, L.E. Increasing Incidence of Melanoma Among Young Adults: An Epidemiological Study in Olmsted County, Minnesota. Mayo Clin. Proc. 2012, 87, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Gamba, C.S.; Clarke, C.A.; Keegan, T.H.M.; Tao, L.; Swetter, S.M. Melanoma Survival Disadvantage in Young, Non-Hispanic White Males Compared with Females. JAMA Dermatol. 2013, 149, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Plym, A.; Ullenhag, G.J.; Breivald, M.; Lambe, M.; Berglund, A. Clinical characteristics, management and survival in young adults diagnosed with malignant melanoma: A population-based cohort study. Acta Oncol. 2013, 53, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Smoll, N.R.; Gautschi, O.P.; Radovanovic, I.; Schaller, K.; Weber, D.C. Incidence and relative survival of chordomas. Cancer 2013, 119, 2029–2037. [Google Scholar] [CrossRef]
- Youn, P.; Milano, M.T.; Constine, L.S.; Travis, L.B. Long-term cause-specific mortality in survivors of adolescent and young adult bone and soft tissue sarcoma: A population-based study of 28,844 patients. Cancer 2014, 120, 2334–2342. [Google Scholar] [CrossRef]
- Keegan, T.H.; Grogan, R.H.; Parsons, H.M.; Tao, L.; White, M.; Onel, K.; Horn-Ross, P.L. Sociodemographic Disparities in Differentiated Thyroid Cancer Survival Among Adolescents and Young Adults in California. Thyroid 2015, 25, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeRouen, M.C.; Mujahid, M.; Srinivas, S.; Keegan, T.H. Disparities in Adolescent and Young Adult Survival after Testicular Cancer Vary by Histologic Subtype: A Population-Based Study in California 1988–2010. J. Adolesc. Young-Adult Oncol. 2016, 5, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, B.J.; Goldfarb, M. Age at Primary Malignancy Determines Survival in Adolescent and Young Adults That Develop a Secondary Thyroid Cancer. J. Adolesc. Young-Adult Oncol. 2016, 5, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.N.; Chastain, K.; Chou, J.F.; Moskowitz, C.S.; Adsuar, R.; Wexler, L.H.; Chou, A.J.; DeRosa, A.; Candela, J.; Magnan, H.; et al. Morbidity and mortality after treatment of Ewing sarcoma: A single-institution experience. Pediatr. Blood Cancer 2017, 64, e26562. [Google Scholar] [CrossRef]
- Bownes, L.V.; Stafman, L.L.; Maizlin, I.I.; Dellinger, M.; Gow, K.W.; Goldin, A.B.; Goldfarb, M.; Langer, M.; Raval, M.V.; Doski, J.J.; et al. Socioeconomic disparities affect survival in malignant ovarian germ cell tumors in AYA population. J. Surg. Res. 2017, 222, 180–186.e3. [Google Scholar] [CrossRef]
- Challapalli, S.D.; Simpson, M.C.; Boakye, E.A.; Pannu, J.S.; Costa, D.J.; Osazuwa-Peters, N. Head and Neck Squamous Cell Carcinoma in Adolescents and Young Adults: Survivorship Patterns and Disparities. J. Adolesc. Young-Adult Oncol. 2018, 7, 472–479. [Google Scholar] [CrossRef]
- Chen, I.; Pasalic, D.; Fischer-Valuck, B.; Frangoul, H.; DeWees, T.; Shinohara, E.T.; Perkins, S.M. Disparity in Outcomes for Adolescent and Young Adult Patients Diagnosed with Pediatric Solid Tumors Across 4 Decades. Am. J. Clin. Oncol. 2018, 41, 471–475. [Google Scholar] [CrossRef]
- Chu, Q.D.; Hsieh, M.-C.; Chu, Y.; Lyons, J.; Kandil, E.; Corsetti, R.; White, R.K.; Gnerlich, J.L.; Wu, X.-C. Do rural patients with operable breast cancer fare worse than urban patients in Louisiana? Results of the Louisiana cancer consortium. Surgery 2020, 168, 653–661. [Google Scholar] [CrossRef]
- Baker, K.S.; Syrjala, K.L. Long-term complications in adolescent and young adult leukemia survivors. Hematology 2018, 2018, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Barr, R.D.; Ferrari, A.; Ries, L.; Whelan, J.; Bleyer, W.A. Cancer in Adolescents and Young Adults. JAMA Pediatr. 2016, 170, 495–501. [Google Scholar] [CrossRef]
- Travis, L.B.; Wahnefried, W.D.; Allan, J.; Wood, M.E.; Ng, A.K. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat. Rev. Clin. Oncol. 2013, 10, 289–301. [Google Scholar] [CrossRef]
- Armenian, S.H.; Robison, L.L. Childhood cancer survivorship. Curr. Opin. Pediatr. 2013, 25, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, J.D.; Hall, M.D.; Mahajan, A.; Paulino, A.C.; Wolden, S.; Constine, L.S. Radiotherapy and Late Effects. Pediatr. Clin. N. Am. 2020, 67, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.; Bhatia, S.; Henderson, T.O.; Nathan, P.C.; Yan, A.; Teepen, J.C.; Morton, L.M. Subsequent Primary Neoplasms: Risks, Risk Factors, Surveillance, and Future Research. Pediatr. Clin. N. Am. 2020, 67, 1135–1154. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.C.; Herman, J.; Lega, I.C.; Mitchell, L.; Hodgson, D.; Edelstein, K.; Travis, L.B.; Sabiston, C.M.; Thavendiranathan, P.; Gupta, A.A. Young Adult Cancer Survivorship: Recommendations for Patient Follow-up, Exercise Therapy, and Research. JNCI Cancer Spectr. 2020, 5, pkaa099. [Google Scholar] [CrossRef]
- Anderson, C.; Nichols, H.B. Trends in Late Mortality among Adolescent and Young Adult Cancer Survivors. J. Natl. Cancer Inst. 2020, 112, 994–1002. [Google Scholar] [CrossRef]
- Veiga, L.H.; Curtis, R.E.; Morton, L.M.; Withrow, D.R.; Howell, R.M.; Smith, S.A.; Weathers, R.E.; Oeffinger, K.C.; Moskowitz, C.S.; Henderson, T.O.; et al. Association of Breast Cancer Risk After Childhood Cancer with Radiation Dose to the Breast and Anthracycline Use. JAMA Pediatr. 2019, 173, 1171–1179. [Google Scholar] [CrossRef]
- Henderson, T.O.; Rajaraman, P.; Stovall, M.; Constine, L.S.; Olive, A.; Smith, S.A.; Mertens, A.; Meadows, A.; Neglia, J.; Hammond, S.; et al. Risk Factors Associated with Secondary Sarcomas in Childhood Cancer Survivors: A Report From the Childhood Cancer Survivor Study. Int. J. Radiat. Oncol. 2012, 84, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, G.T.; Stovall, M.; Robison, L.L. Long-Term Effects of Radiation Exposure among Adult Survivors of Childhood Cancer: Results from the Childhood Cancer Survivor Study. Radiat. Res. 2010, 174, 840–850. [Google Scholar] [CrossRef] [Green Version]
- Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers; Version 3.0; Children’s Oncology Group: Monrovia, CA, USA, 2008. [Google Scholar]
- Kremer, L.C.; Mulder, R.L.; Oeffinger, K.C.; Bhatia, S.; Landier, W.; Levitt, G.; Constine, L.S.; Wallace, W.H.; Caron, H.N.; Armenian, S.H.; et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the international late effects of Childhood Cancer Guideline Harmonization Group. Pediatr. Blood Cancer 2012, 60, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Institute of Health and Welfare. Young Australians: Their Health and Wellbeing; Australian Institute of Health and Welfare: Canberra, Australia, 2011. [Google Scholar]
- Rana, R.H.; Alam, F.; Alam, K.; Gow, J. Gender-specific differences in care-seeking behaviour among lung cancer patients: A systematic review. J. Cancer Res. Clin. Oncol. 2020, 146, 1169–1196. [Google Scholar] [CrossRef] [PubMed]
- Rubin, P.; Williams, J.P.; Devesa, S.S.; Travis, L.B.; Constine, L.S. Cancer Genesis Across the Age Spectrum: Associations with Tissue Development, Maintenance, and Senescence. Semin. Radiat. Oncol. 2010, 20, 3–11. [Google Scholar] [CrossRef]
- National Cancer Research Institute. Top 10 Research Priorities for Teenage and Young Adult Cancer Identified. Available online: https://www.ncri.org.uk/top-10-research-priorities-for-teenage-and-young-adult-cancer-identified/ (accessed on 2 May 2021).
- Fidler, M.M.; Frobisher, C.; Hawkins, M.M.; Nathan, P.C. Challenges and opportunities in the care of survivors of adolescent and young adult cancers. Pediatr. Blood Cancer 2019, 66, e27668. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.L.; Mertens, A.C.; Boice, J.D.; Breslow, N.E.; Donaldson, S.S.; Green, D.M.; Li, F.P.; Meadows, A.T.; Mulvihill, J.J.; Neglia, J.; et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi-institutional collaborative project. Med. Pediatr. Oncol. 2002, 38, 229–239. [Google Scholar] [CrossRef]
- Hudson, M.M.; Ness, K.K.; Nolan, V.G.; Armstrong, G.T.; Green, D.M.; Morris, E.B.; Spunt, S.L.; Metzger, M.L.; Krull, K.R.; Klosky, J.L.; et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr. Blood Cancer 2010, 56, 825–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, R.E.; Freedman, D.M.; Ron, E.; Ries, L.A.G.; Hacker, D.G.; Edwards, B.K.; Tucker, M.A.; Fraumeni, J.F., Jr. (Eds.) New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973–2000; National Cancer Institute: Bethesda, MD, USA, 2006.
- Pugh, G.; Gravestock, H.L.; Hough, R.E.; King, W.M.; Wardle, J.; Fisher, A. Health Behavior Change Interventions for Teenage and Young Adult Cancer Survivors: A Systematic Review. J. Adolesc. Young-Adult Oncol. 2016, 5, 91–105. [Google Scholar] [CrossRef]
- Vijayvergia, N.; Denlinger, C.S. Lifestyle Factors in Cancer Survivorship: Where We Are and Where We Are Headed. J. Pers. Med. 2015, 5, 243–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, E.; Buchanan, N.; Ms, J.T.; Fairley, T.; Moore, A.; Richardson, L.C. Health status of adolescent and young adult cancer survivors. Cancer 2012, 118, 4884–4891. [Google Scholar] [CrossRef] [Green Version]
- Warner, E.L.; Nam, G.E.; Zhang, Y.; McFadden, M.; Wright, J.; Spraker-Perlman, H.; Kinney, A.; Oeffinger, K.C.; Kirchhoff, A.C. Health behaviors, quality of life, and psychosocial health among survivors of adolescent and young adult cancers. J. Cancer Surviv. 2015, 10, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Kaul, S.; Veeranki, S.P.; Rodriguez, A.M.; Kuo, Y.-F. Cigarette smoking, comorbidity, and general health among survivors of adolescent and young adult cancer. Cancer 2016, 122, 2895–2905. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2015, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Howard, S.C.; Hunger, S.P.; Antillon, F.G.; Metzger, M.L.; Israels, T.; Harif, M.; Rodriguez-Galindo, C. Treating Childhood Cancer in Low- and Middle-Income Countries. Cancer Wash. DC World Bank Group 2015, 3, 121–146. [Google Scholar] [CrossRef]
- Bleyer, A.; Ferrari, A.; Whelan, J.; Barr, R.D. Global assessment of cancer incidence and survival in adolescents and young adults. Pediatr. Blood Cancer 2017, 64, e26497. [Google Scholar] [CrossRef]
- Magrath, I.; Steliarova-Foucher, E.; Epelman, S.; Ribeiro, R.C.; Harif, M.; Li, C.-K.; Kebudi, R.; Macfarlane, S.D.; Howard, S.C. Paediatric cancer in low-income and middle-income countries. Lancet Oncol. 2013, 14, e104–e116. [Google Scholar] [CrossRef]
- Barr, R.; Robertson, J. Access to Cytotoxic Medicines by Children with Cancer: A Focus on Low and Middle Income Countries. Pediatr. Blood Cancer 2015, 63, 287–291. [Google Scholar] [CrossRef] [Green Version]
- List, J.M.; O’Connor, J.M. How Should Low- and Middle-Income Countries Motivate Equity in Cancer Prevention and Control? AMA J. Ethics 2020, 22, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Ginsburg, O. Breast and cervical cancer control in low and middle-income countries: Human rights meet sound health policy. J. Cancer Policy 2013, 1, e35–e41. [Google Scholar] [CrossRef] [Green Version]
- Dobrescu, A.; Nussbaumer-Streit, B.; Klerings, I.; Wagner, G.; Persad, E.; Sommer, I.; Herkner, H.; Gartlehner, G. Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: A systematic review. J. Clin. Epidemiol. 2021, 137, 209–217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).