Abstract
(1) Background: Cephalosporins (CA) are the first-line antibiotic prophylaxis recommended to prevent surgical site infection (SSI) after cardiac surgery. The combination of vancomycin/gentamicin (VGA) might represent a good alternative, but few studies have evaluated its efficacy in SSI prevention. (2) Methods: A single-centre retrospective study was conducted over a 13-year period in all consecutive adult patients undergoing elective cardiac surgery. Patients were stratified according to the type of antibiotic prophylaxis. CA served as the first-line prophylaxis, and VGA was used as the second-line prophylaxis. The primary endpoint was SSI occurrence at 90 days, which was defined as the need for reoperation due to SSI. (3) Results: In total, 14,960 adult patients treated consecutively from 2006 to 2019 were included in this study, of whom 1774 (12%) received VGA and 540 (3.7%) developed SSI. VGA patients had higher severity with increased 90-day mortality. Nevertheless, the frequency of SSI was similar between CA and VGA patients. However, the microbiological aetiologies were different, with more Gram-negative bacteria noted in the VGA group. (4) Conclusions: VGA seems to be as effective as CA in preventing SSI.
1. Introduction
Since the advent of antibiotics in the 1940s [1,2], antibiotic prophylaxis has played an important role in the prevention of infections, including cardiac surgery [3]. However, 80 years later, despite international guidelines [4], the modalities of the administration of antibiotic prophylaxis remain heterogeneous [5]. In cardiac surgery, the reference prophylaxis policy directs the administration of cephalosporins before skin incision and up to 24–48 h after, and the main alternative is the administration of vancomycin [4,6]. Indeed, in many cardiac surgery studies, vancomycin appears less effective for preventing surgical site infections (SSIs) than anti-staphylococcal penicillins or first/second-generation cephalosporins, especially those infections caused by methicillin-susceptible staphylococci (MSS) [7,8,9,10]. This finding might be explained by the reduced bactericidal effect of vancomycin [11,12] and less optimal penetration into mediastinal tissues during cardiac surgery under cardiopulmonary bypass (CPB) [13]. However, several studies did not identify major differences between these two antibiotic regimens, particularly when the duration of administration was considered [14,15]. In addition, some studies have found a significant effect of the timing of vancomycin injection, with an increased risk of SSI in cases of delayed administration [15,16]. Finally, in our centre, vancomycin is combined with gentamicin to enlarge the spectrum of prophylaxis and target Gram-negative bacterial infections [17]. Interestingly, few studies have compared the efficacy of the combination of vancomycin and gentamicin to that of cephalosporins.
Thus, the aim of our retrospective, single-centre study was to assess whether the combination of vancomycin and gentamicin (VGA) is a valuable alternative to cephalosporins (CA) to prevent SSI in cardiac surgery with CPB.
2. Materials and Methods
2.1. Study Design
This single-center retrospective study was performed in a tertiary care teaching hospital performing more than 1000 annual cardiac surgical procedures. Between 2006 and 2019, all consecutive patients who underwent cardiac surgery with cardiopulmonary bypass (CPB) were eligible. The exclusion criteria were a previous history of cardiac surgery in the past year and missing clinical information (including antibiotic prophylaxis).
2.2. Definitions
SSI was defined as the need for reoperation within 90 days after index surgery for local or systemic infection involving the sternotomy scar [18,19]. The sternal scar was assessed daily after surgery, and the date of SSI was the date of return to the operating room for SSI. Only the first reoperation for suspected SSI was considered. The validation of SSI was made during multidisciplinary regular meetings including a cardiac surgeon, an intensive-care physician, and a physician of the infection control unit. Deep SSI (dSSI) was defined as the need for sternal reopening with deep purulent discharge or destruction of the sternal bone/deep sternal osteomyelitis. Otherwise, the SSI was defined as a superficial SSI (sSSI) requiring reoperation. Obesity was defined as a body mass index >30 kg/m2. Acute infective endocarditis was defined as endocarditis under antibiotics at the time of surgery. Cardiac insufficiency corresponds to patients with a left ventricular ejection fraction of less than 30%.
2.3. Selection of Antibiotic Prophylaxis and Protocol of Administration
During the study period, first- and second-generation cephalosporins were used the first-line intraoperative antibiotic prophylaxis. Cefamandole was administered from 2006 to 2015, and cefazolin was administered from 2016 to 2019. Cephalosporins were administered according to the following protocol: 30 mg/kg at induction with a reinjection every 4 h during the operation and then a dose of 1 g per 6 h for 24 h. Moreover, 1 g was administered into the priming of the CB. The combination of vancomycin/gentamicin was the second-line antibiotic prophylaxis selected in cases of allergy to beta-lactam, prior antibiotherapy ≥ 72 h, prior hospitalization in a unit with a high prevalence of methicillin-resistant Staphylococcus aureus (MRSA) and/or in patients known to be colonized by MRSA. VGA was administered after the induction and from 5 to 30 min before the surgical incision through a central line according to the following protocol: vancomycin was perfused over 1 to 2 h at a dose of 30 mg/kg, and 500 mg was added to the priming of CPB. Then, 1 g was administered 12 h after the end of surgery. Gentamicin was perfused over 30 min as a single dose of 5–6 mg/kg. All patients underwent preoperative nasal screening for S. aureus carriage and preoperative nasal decontamination with mupirocin for 5 days, most frequently started on the day before surgery [20].
2.4. Objectives
The primary outcome was SSI occurrence at D-90 after index surgery. Secondary outcomes were the type of SSI, microbiological features, death at D-90, and occurrence of SSI based on propensity score-matching.
2.5. Data Collection and Ethics
Data were extracted from three databases. The first registry, Cardiobase, prospectively includes all adult patients who underwent cardiac surgery with CPB. This database has been approved by the Paris-Nord Biomedical Research Project Evaluation Committee (CERB, IRB No. 00006477), the Advisory Committee on Information Processing in Health Research (Paris, France) and the Commission Nationale Informatique et Liberté (CNIL, Paris, France). It is registered on ClinicalTrials.gov (NCT03393169). The following data are collected in this registry: demographic data, medical history, type of surgery and perioperative data. The Cardiobase information on SSIs was combined with a second database collected by the Infection Control Unit [18,20]. Finally, survival data were extracted from public French data of Institut National de la Statistique et des Etudes Economiques (INSEE) from 2006 to 2021, where the date of death was recorded.
2.6. Patient Management
Chronic therapies were administered on the day of surgery except for angiotensin-converting enzyme inhibitors/angiotensin receptor blockers. CA was administered at the time of anaesthesia induction, whereas VGA was infused after induction when the central venous catheter was inserted and before the surgical incision. Otherwise, patient intraoperative management was performed as previously described [21]. In our centre, coronary artery bypass grafting (CABG) is performed only with the internal mammary arteries (IMA), even for multivessel disease. For this purpose, the two IMA are harvested using the “skeletonization technique” (without collateral veins or endothoracic fascia). Then, the right IMA is implanted as a free graft on the left IMA using the so-called Y or T techniques. Finally, the use of sequential distal lateral anastomoses allows for multiple coronary branches to be targeting while respecting a harmonious bypass flow path. Secondary angioplasty is reserved for rare cases where the downstream bed of the right coronary is not accessible to bypass. After surgery, patients were admitted to the cardiac surgical intensive care unit for at least 48 h.
2.7. Statistical Analysis
Continuous variables were expressed as medians with interquartile ranges (IQRs) and were compared with the Mann–Whitney U test or the Kruskal–Wallis test as appropriate. Categorical variables were expressed as absolute numbers and proportions and were compared with Fisher’s exact test or the chi-square test, as appropriate.
Time-to-event analyses were estimated with Kaplan–Meier analyses. To assess whether SSI was an independent factor for death, multivariate analyses were performed using a Cox model. For all models, variables with nominal two-tailed p values less than 0.05 were entered into the multivariate model, except for variables with obvious collinearity. The final models were selected using backwards stepwise regression and evaluated using the AIC and Tjur’s R2 coefficient of discrimination.
To address confounding by indication of VGA and other sources of bias arising from the use of observational data, we estimated full propensity score matching without replacement [22] using the Matchit package [23], which calls functions from the optimatch package [24]. The propensity score was estimated using logistic regression of the likelihood of total VGA administration (see Figure S1), and covariate balance was assessed using the cobalt package [25]. All statistical analyses were performed using R software (R Core Team, 2014, R Foundation for Statistical Computing, Vienna, Austria). Figures were produced using the ‘ggplot2 package’, and statistics were produced using the ‘stat package’. Missing values were not imputed. A p value < 0.05 was considered statistically significant. Reports of statistical analyses respected the STROBE recommendations for cohort studies [26] (checklist available in Appendix A).
3. Results
3.1. Homogeneity of the Cohort
From 2006 to 2019, 14,770 (99%) patients out of 14,960 fulfilled the inclusion criteria (Figure 1). The annual number of patients included was stable throughout the study period (Supplementary Figure S2A). Among these patients, 540 (3.6%) developed a postoperative SSI, 151 (1%) a dSSI, and 389 (2.6%) an sSSI requiring reoperation. Whereas SSI occurrence remained stable across time (Supplementary Figure S2B), a reduction in VGA use was observed from 16% in 2010–2011 to 7% in 2018–2019 (p < 0.001) (Supplementary Figure S2C).
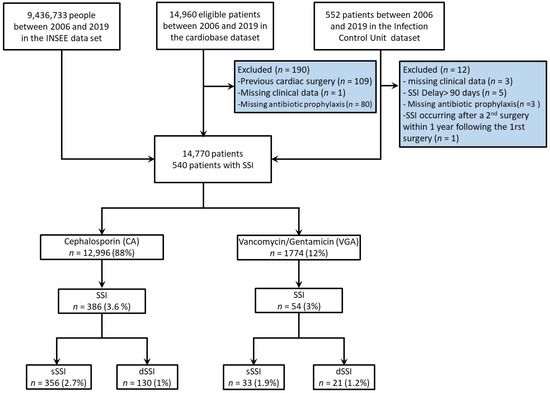
Figure 1.
Flow chart of the study. INSEE: Institut National de la Statistique et des Etudes Economiques; SSI, surgical site infection. dSSI, deep SSI; sSSI: superficial SSI.
3.2. Patient Characteristics Based on the Type of Antibiotic Prophylaxis
Compared to patients who received CA, those who received VGA were younger and had a lower BMI, fewer cardiovascular risk factors (arterial hypertension, diabetes mellitus, and dyslipidaemia) and a lower proportion of ischaemic heart disease (52% vs. 32%, p < 0.001) (Table 1). However, these patients had more comorbidities, including cardiac insufficiency (4.3 vs. 6.5%, p < 0.001), chronic obstructive pulmonary disease (COPD), and cirrhosis. Moreover, VGA was strongly associated with surgical emergency, preoperative critical state and acute infective endocarditis. Overall, 62% (474/759) of patients with endocarditis received VGA. Similarly, the EuroSCORE II and the CPB duration were higher in patients who received VGA (4% (2–9) vs. 2% (1–4) and 71 min (51–104) vs. 58 min (45–83), respectively, both p < 0.001). VGA was more frequently used in valve surgery than in CABG (17% (993/5794) vs. 5.7% (328/5715), p < 0.001). Finally, VGA, compared to CA, was associated with a more complicated postoperative course with more catecholamine requirements, higher blood loss, longer ICU length of stay and higher 90-day (D90) mortality (10% vs. 4.8%, p < 0.001, Supplementary Figure S3).

Table 1.
Perioperative characteristics of patients according to the type of prophylaxis.
3.3. Relationship between Antibiotic Prophylaxis and SSI in the General Population
The type of antibiotic prophylaxis was not associated with SSI occurrence (Table 1 and Figure 2), and the time to SSI was similar between VGA and CA (15 (12–26) vs. 18 (12–27) days, p = 0.77). The main independent parameters associated with SSI (Table 2 and Table S1) included older age, female sex, obesity, diabetes mellitus, cardiac insufficiency, ischaemic heart disease, and end-stage renal disease. Furthermore, SSIs were associated with CABG, short CPB duration and catecholamine requirements after CB. In multivariate analysis, VGA was not associated with SSI (Table 2).
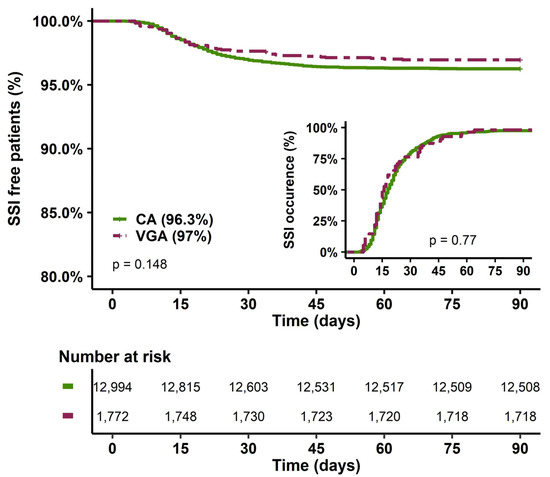
Figure 2.
SSI-free patients at 90 days and delay of SSI occurrence according to the type of prophylaxis. CA, cephalosporin antibiotic prophylaxis; VGA, vancomycin/gentamicin antibiotic prophylaxis; SSI, surgical site infection.

Table 2.
Multivariate analysis of risk factors for SSI.
Of note, in univariate analysis, the occurrence of sSSI appeared to be lower in the VGA group than in the CA group (Table 1 and Figure 3A), without a difference in the timing of sSSI occurrence. However, this association did not persist in multivariate analysis (Supplementary Table S2). No association was found between dSSI occurrence and type of prophylaxis.
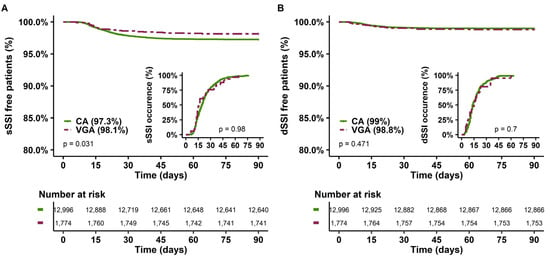
Figure 3.
sSSI-free and dSSI-free patients at 90 days and delay of SSI occurrence according to the type of prophylaxis. (A) Superficial SSI; (B) Deep SSI. CA: cephalosporin antibiotic prophylaxis, VGA vancomycin/gentamicin antibiotic prophylaxis. SSI, surgical site infection.
3.4. Microbiological Characteristics of Patients According to the Type of SSI
The microbiological nature of the SSI was different according to the type of prophylaxis (Table 3). Gram-negative bacteria were more frequently noted in VGA than in CA (52% vs. 35%, p = 0.013). In contrast, Gram-positive bacteria were more frequently noted in CA (69% vs. 50%, p = 0.006), especially enterococci, representing 13% of SSI in the CA group. In contrast, only one enterococcal infection was observed in the VGA group.

Table 3.
Bacterial characteristics of SSI according to antibiotic prophylaxis.
3.5. Relationship between Antibiotic Prophylaxis and SSI in Propensisty Score Analsysis
Given the heterogeneity of the study population, three sensitivity analyses were performed. The first was a propensity score using the full matching method.
In this analysis, the main risk factors associated with SSI (Table 4), sSSI and dSSI (Supplementary Table S3) remained the same. However, acute infective endocarditis became a risk factor for dSSI, and cardiac transplantation was only associated with dSSI. Of note, in these analyses, end-stage renal disease (ESRD) was the risk factor with the highest OR. In all analyses, VGA was not associated with SSI.

Table 4.
Multivariate analysis of risk factors for SSI in a propensity score analysis.
3.6. Relationships between Antibiotic Prophylaxis and SSI in a Sensitivity Analysis Excluding Active Infective Endocarditis
The second sensitivity analysis excluded patients with active infective endocarditis. Indeed, these patients may have a lower risk of SSI due to concomitant antibiotic therapy. In addition, VGA was over-represented among these patients, with 62% receiving VGA, while they represented only 12% of the overall population. In this analysis, VGA patients still presented more comorbidities and severity risk factors with higher EuroSCORE II compared to CA patients (3 (2–7) vs. 2 (1–4), p < 0.001) (Supplementary Table S4). No difference was noted in the occurrence of SSI, sSSI and dSSI (Figure 4). In multivariate analysis, VGA was not a risk factor of SSI (Table 5), sSSI or dSSI (Supplementary Table S5).
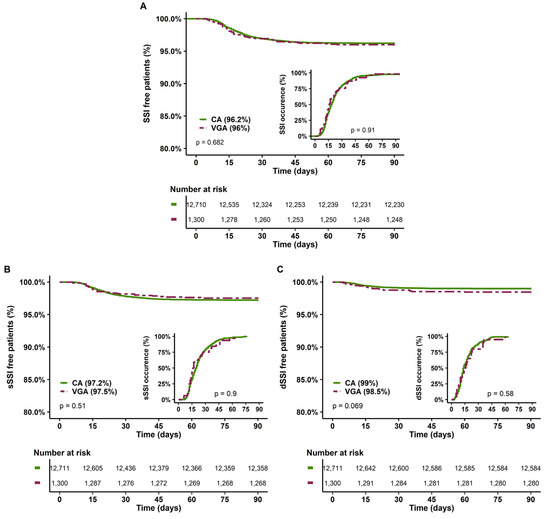
Figure 4.
SSI-free patients at 90 days and delay of SSI occurrence according to the type of prophylaxis in the sensitivity analysis, excluding infective active endocarditis. (A) All SSI, (B) Superficial SSI; (C) Deep SSI. CA, cephalosporin antibiotic prophylaxis; VGA, vancomycin/gentamicin antibiotic prophylaxis; SSI, surgical site infection.

Table 5.
Multivariate analysis of risk factors for SSI in a sensitivity analysis, excluding endocarditis.
3.7. Relationships between Antibiotic Prophylaxis and SSI in a Sensitivity Analysis Restricted to CABG
Finally, a sensitivity analysis was performed in the homogeneous subgroup of patients undergoing coronary artery bypass grafting, a population at high risk of developing SSI. In this subgroup, VGA was less frequently associated with patient severity (Supplementary Table S6). In the VGA group, patients were more often female, with a higher frequency of insulin-dependent diabetes, end-stage renal failure and chronic obstructive pulmonary disease, and slightly greater EuroSCORE II (2 (1–3) vs. 1 (1,2), p < 0.001) (Supplementary Table S6).
In this analysis, SSI were more frequent in the VGA group compared to the CA group (9.8% vs. 6.3, p = 0.012, Figure 5A), and especially superficial SSI (7.6% vs. 5%, p = 0.038, Figure 5B) but without a difference in the delay of occurrence (16 days (12–24) vs. 19 (13–27), p = 0.18, respectively). However, in multivariate analysis, VGA was not associated with SSI occurrence (OR = 1.27 [0.84–1.92], p = 0.254, Table 6), with sSSI or dSSI (Supplementary Table S7).

Figure 5.
SSI-free patients at 90 days and delay of SSI occurrence according to the type of prophylaxis in the sensitivity analysis restricted to CABG. (A) All SSI, (B) Superficial SSI; (C) Deep SSI. CA, cephalosporin antibiotic prophylaxis; CABG, coronary artery bypass graft, VGA, vancomycin/gentamicin antibiotic prophylaxis; SSI, surgical site infection.

Table 6.
Multivariate analysis of risk factors for SSI in a sensitivity analysis restricted to CABG.
4. Discussion
4.1. Summary of the Main Results
In our long-term cohort, VGA was prescribed more frequently in the most severe patients, including those with acute infective endocarditis. In univariate analysis, VGA was associated with a lower risk of sSSI, but this association did not persist in multivariate analysis. In addition, no association was found with sSSI and dSSI, even when performing three sensitivity analyses. Therefore, our study suggests that VGA could represent a good alternative to cephalosporins. However, our study also underlines the microbiological differences according to the type of prophylaxis. Specifically, VGA was associated with more Gram-negative bacteria and fewer Gram-positive bacteria in SSI.
4.2. Comparison with Other Studies and Physiopathological Hypotheses
4.2.1. SSI Occurrence According to the Type of Antibiotic Prophylaxis
The similar efficacy of CA and VGA to prevent SSI is consistent with the large multicentre retrospective study on 21,396 patients [27] and the 2012 meta-analysis, which included nine studies, regardless of the duration of antibiotic prophylaxis, five of which had a similar duration of administration of vancomycin and cephalosporins [14].
In the overall analysis of the meta-analysis that included 6022 patients, vancomycin administration was associated with a slight increase in the frequency of SSIs (OR = 1.33 [1.08–1.64]), regardless of the duration of antibiotic prophylaxis, but this association disappeared in the subgroup analysis that included 2278 patients with a similar duration of antibiotic prophylaxis (OR = 0.81 [0.57–1.15]).
4.2.2. Prevention of Gram-Negative SSI by Gentamicin
In addition, our study suggests an increased incidence of Gram-negative bacteria in the VGA group. This association was not found in the 2012 meta-analysis, although this analysis found more postoperative Gram-negative pneumonia in the vancomycin group. This lack of association in this meta-analysis may be due to a lack of power, as our population was more than twice as large [14]. The increase in Gram-negative SSI is surprising and deceptive, given that gentamicin was associated with vancomycin to reduce Gram-negative bacteria infection. This finding is in contrast to that noted in the meta-analysis, where vancomycin was used alone [14]. From this perspective, gentamicin seems to have a minimal impact on the prevention of SSI. A first explanation for this could be the high prevalence of gentamicin resistance in a nosocomial environment [28,29]. However, active endocarditis patients, who are at high risk of bacterial resistance due to long term antibiotherapy, had a low prevalence of SSI. In addition, the better coverage of Gram-positive bacteria, such as enterococci, offered by vancomycin could favour Gram-negative infections. Indeed, cephalosporins are not effective against this pathogen [30,31], which may explain why, out of the 63 enterococcal SSIs in our study, 62 occurred in the CA group, compared with one in the vancomycin group. Therefore, for patients at high risk for SSI who are not allergic to beta-lactams, our results suggest that the combination of vancomycin and cefazolin could be tested to provide better coverage of Gram-negative and Gram-positive bacteria, as used by some teams [16]. A prospective multicentre study evaluating this combination would provide answers [32]. Otherwise, broad-spectrum cephalosporins, such as ceftaroline [33,34], could represent another option, providing a more protective effect for patients allergic to beta-lactam. This antibiotic offers two advantages for our combination of VGA. First, in the absence of veinotoxicity, it could be administered before central-line insertion. Second, it is less nephrotoxic, and the prophylactic administration of vancomycin and, especially, the combination of vancomycin/aminoglycoside has been associated with an increased risk of acute kidney injury [27]. However, no data are available for this indication. The results from 2014, assessing ceftaroline for this indication, are not available (clinical trial number = NCT02307006) [35], and the risk of selecting new resistance and the alteration in gut microbiota may limit its use.
4.2.3. Timing of Antibiotic Prophylaxis and Role of Gentamicin
Nevertheless, for patients allergic to beta-lactams, gentamicin may reinforce the administration of vancomycin. Indeed, in our study, the exact start date of antibiotic prophylaxis was not collected. However, in our protocol, VGA started before the surgical incision and after the insertion of the central line. Therefore, our protocol is not in accordance with French and international recommendations [6], which suggest that the vancomycin infusion should end before the surgical incision. Indeed, some studies have shown an increased risk of SSI when vancomycin is delayed [15,16]. Garey et al. [15] reported that starting vancomycin 0–15 min before the surgery incision was associated with an increased risk of SSI with an OR of 11.6 (2.6–52.4) compared to patients where vancomycin was started 16–60 min before incision. Three hypotheses could explain the discrepancy with our study. First, the population of the Garey et al. study was small (15 patients in the 0- to 15-min group and 176 in the 16- to 60-min group) and may lead to an overestimation of the risk. Second, some patients began receiving vancomycin from 15 to 30 min before incision, which is the optimal time according to this study. Third, gentamicin was not used in this study. This antibiotic offered a fast bactericidal effect [36], which is increased by vancomycin [37]. Therefore, gentamicin may offer some advantages.
4.3. Limits and Strengths
This study is one of the largest studies on SSI in cardiac surgery and, more specifically, on the combination of vancomycin/gentamicin. The inclusion of patients and their surgical management were stable over time. Thus, the SSI rate was stable throughout the study. However, the use of VGA therapy decreased over time, which was possibly related to a decrease in the MRSA rate in our population.
Other limitations are present. First, this is a retrospective study, which may lead to collection bias. This bias is limited by the fact that much of the information was collected prospectively. Second, it is a monocentric study, which may limit generalization. This study found SSI rates and risk factors for SSI in line with previously published studies [38,39,40,41], and the results are consistent with previously published articles [14,27]. Thus, we assume that this bias is limited. Third, the exact timing of the injection of the antibiotic was not collected, which does not allow for us to take this factor into account in the occurrence of SSI. Fifth, the patients in the VGA group had a higher mortality rate than those in the CA group. Given this heterogeneity, we performed a propensity score analysis, which showed similar results. We used the “full matching” method, which has the advantage of retaining the whole population by assigning a weight to each patient. Then, we performed two additional subgroup analyses, first by excluding active endocarditis and then by selecting a homogeneous population of patients undergoing isolated CABG. The fact that the results of all three sensitivity analyses concurred with the full population analysis limits this severity bias.
5. Conclusions
In this prolonged cohort of consecutive patients, the incidence and risk factors for SSI were comparable to those reported in the literature. VGA was more commonly used in the most severe patients than in CA. However, our results did not find any significant increase in risk of SSI. Differences in the microbiological cultures of SSI were observed according to the type of antibiotic prophylaxis. Therefore, VGA represents a valuable alternative for SSI prevention. Further studies are needed to confirm these results.
Supplementary Materials
The following supporting information can be downloaded: https://www.mdpi.com/article/10.3390/antibiotics12010085/s1, Figure S1: Covariate balance in the propensity matched samples; Figure S2: Characteristics of the patients during the inclusion period; Figure S3: 90-day mortality according to the type of prophylaxis; Table S1: Perioperative characteristics of patients according to SSI subtype occurrence; Table S2: Multivariate analysis of risk factors for superficial and deep SSI; Table S3: Multivariate analysis of risk factors for superficial and deep SSI in the propensity score analysis; Table S4: Perioperative characteristics of patients according to the type of prophylaxis in the sensibility analysis excluding patients with active endocarditis; Table S5: Multivariate analysis of risk factors for superficial and deep SSI in the sensi-tivity analysis excluding active endocarditis; Table S6: Perioperative characteristics of patients according to the type of prophylaxis in the sensibility analysis restricted to patients with isolated CABG; Table S7: Multivariate analysis of risk factors for superficial and deep SSI in the sensi-tivity analysis restricted to patients undergoing isolated CABG.
Author Contributions
Conceptualization, C.d.T., S.P., J.-C.L. and P.M. (Philippe Montravers); Data curation, C.d.T., T.S., S.P. and N.D.; Formal analysis, C.d.T. and P.M. (Pierre Mutuon); Investigation, C.d.T., X.D. and B.I.; Methodology, C.d.T. and P.M. (Philippe Montravers); Project administration, C.d.T.; Software, C.d.T. and P.M. (Pierre Mutuon); Supervision, J.-C.L.; Validation, C.d.T.; Visualization, M.P., X.D., N.G., B.I. and S.K.; Writing—original draft, C.d.T.; Writing—review and editing, C.d.T., J.-C.L. and P.M. (Philippe Montravers). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Comité d’Evaluation de l’Ethique des projets deRecherche Biomédicale (CEERB) Paris Nord (Institutional Review Board—IRB 00006477, 19 March 2015.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study, and the analysis used anonymous clinical data.
Data Availability Statement
Data are available upon request.
Acknowledgments
Jonathan Lehacaut for his work in collecting Cardiobase data.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
STROBE Statement—Checklist of items that should be included in reports of cohort studies.
| Item No | Recommendation | Page | |
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 2 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 2 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 2, 3 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 2 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 2 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | 2 |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | - | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 2, 3 |
| Data sources/ measurement | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 3, 4 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 3, 4 |
| Study size | 10 | Explain how the study size was arrived at | 3, 4 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 3, 4 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 3, 4 |
| (b) Describe any methods used to examine subgroups and interactions | 3, 4 | ||
| (c) Explain how missing data were addressed | 3, 4 | ||
| (d) If applicable, explain how loss to follow-up was addressed | - | ||
| (e) Describe any sensitivity analyses | 3, 4 | ||
| Results | |||
| Participants | 13 | (a) Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 4 and Figure 1 |
| (b) Give reasons for non-participation at each stage | Figure 1 | ||
| (c) Consider use of a flow diagram | Figure 1 | ||
| Descriptive data | 14 | (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders | Table 1 |
| (b) Indicate number of participants with missing data for each variable of interest | Table 1 | ||
| (c) Summarise follow-up time (e.g., average and total amount) | Supplementary Figure S1 | ||
| Outcome data | 15 | Report numbers of outcome events or summary measures over time | Figure 2 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included | Table 2 |
| (b) Report category boundaries when continuous variables were categorized | yes | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | No | ||
| Other analyses | 17 | Report other analyses done—e.g., analyses of subgroups and interactions, and sensitivity analyses | Table 4 and Table S2 |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 12 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 13 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 12, 13 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 14 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 14 |
References
- Aminov, R.I. A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Front. Microbio. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Firor, W.M.; Jonas, A.F. The Use of Sulfanilylguanidine in Surgical Patients. Ann. Surg. 1941, 114, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Altemeier, W.A.; Culbertson, W.R.; Veto, M. Prophylactic Antibiotic Therapy. AMA Arch. Surg. 1955, 71, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Uva, M.; Head, S.J.; Milojevic, M.; Collet, J.-P.; Landoni, G.; Castella, M.; Dunning, J.; Gudbjartsson, T.; Linker, N.J.; Sandoval, E.; et al. 2017 EACTS Guidelines on Perioperative Medication in Adult Cardiac Surgery. Eur. J. Cardio-Thorac. Surg. 2018, 53, 5–33. [Google Scholar] [CrossRef]
- Ackah, J.K.; Neal, L.; Marshall, N.R.; Panahi, P.; Lloyd, C.; Rogers, L.J. Antimicrobial Prophylaxis in Adult Cardiac Surgery in the United Kingdom and Republic of Ireland. J. Infect. Prev. 2021, 22, 83–90. [Google Scholar] [CrossRef]
- Antibioprophylaxie en chirurgie et médecine interventionnelle (patients adultes)—La SFAR. Société Française d’Anesthésie et de Réanimation . 2018. Available online: https://sfar.org/mise-a-jour-de-la-rfe-antibioprophylaxie-2017/ (accessed on 8 December 2022).
- Finkelstein, R.; Rabino, G.; Mashiah, T.; Bar-El, Y.; Adler, Z.; Kertzman, V.; Cohen, O.; Milo, S. Surgical Site Infection Rates Following Cardiac Surgery: The Impact of a 6-Year Infection Control Program. Am. J. Infect. Control. 2005, 33, 450–454. [Google Scholar] [CrossRef]
- Bolon, M.K.; Morlote, M.; Weber, S.G.; Koplan, B.; Carmeli, Y.; Wright, S.B. Glycopeptides Are No More Effective than B-Lactam Agents for Prevention of Surgical Site Infection after Cardiac Surgery: A Meta-Analysis. Clin. Infect. Dis. 2004, 38, 1357–1363. [Google Scholar] [CrossRef]
- Kaye, K.S.; Devine, S.T.; Ford, K.D.; Anderson, D.J. Surgical Site Infection Prophylaxis Strategies for Cardiothoracic Surgery: A Decision-Analytic Model. Scand. J. Infect. Dis. 2012, 44, 948–955. [Google Scholar] [CrossRef][Green Version]
- Bull, A.L.; Worth, L.J.; Richards, M.J. Impact of Vancomycin Surgical Antibiotic Prophylaxis on the Development of Methicillin-Sensitive Staphylococcus Aureus Surgical Site Infections: Report From Australian Surveillance Data (VICNISS). Ann. Surg. 2012, 256, 1089–1092. [Google Scholar] [CrossRef]
- Apellaniz, G.; Valdés, M.; Perez, R.; Martin, F.; Soria, F.; Garcia, A.; Gòmez, J.; Vicente, T. Comparison of the Effectiveness of Various Antibiotics in the Treatment of Methicillin-Susceptible Staphylococcus Aureus Experimental Infective Endocarditis. J. Chemother. 1991, 3, 91–97. [Google Scholar] [CrossRef]
- LaPlante, K.L.; Rybak, M.J. Impact of High-Inoculum Staphylococcus Aureus on the Activities of Nafcillin, Vancomycin, Linezolid, and Daptomycin, Alone and in Combination with Gentamicin, in an In Vitro Pharmacodynamic Model. Antimicrob. Agents Chemother. 2004, 48, 4665–4672. [Google Scholar] [CrossRef]
- Martin, C.; Alaya, M.; Mallet, M.N.; Viviand, X.; Ennabli, K.; Said, R.; De Micco, P. Penetration of Vancomycin into Mediastinal and Cardiac Tissues in Humans. Antimicrob. Agents Chemother. 1994, 38, 396–399. [Google Scholar] [CrossRef][Green Version]
- Lador, A.; Nasir, H.; Mansur, N.; Sharoni, E.; Biderman, P.; Leibovici, L.; Paul, M. Antibiotic Prophylaxis in Cardiac Surgery: Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2012, 67, 541–550. [Google Scholar] [CrossRef]
- Garey, K.W. Timing of Vancomycin Prophylaxis for Cardiac Surgery Patients and the Risk of Surgical Site Infections. J. Antimicrob. Chemother. 2006, 58, 645–650. [Google Scholar] [CrossRef]
- Cotogni, P.; Barbero, C.; Passera, R.; Fossati, L.; Olivero, G.; Rinaldi, M. Violation of Prophylactic Vancomycin Administration Timing Is a Potential Risk Factor for Rate of Surgical Site Infections in Cardiac Surgery Patients: A Prospective Cohort Study. BMC Cardiovasc. Disord. 2017, 17, 73. [Google Scholar] [CrossRef][Green Version]
- Saginur, R.; Croteau, D.; Bergeron, M.G. Comparative Efficacy of Teicoplanin and Cefazolin for Cardiac Operation Prophylaxis in 3027 Patients. J. Thorac. Cardiovasc. Surg. 2000, 120, 1120–1130. [Google Scholar] [CrossRef]
- Lemaignen, A.; Birgand, G.; Ghodhbane, W.; Alkhoder, S.; Lolom, I.; Belorgey, S.; Lescure, F.-X.; Armand-Lefevre, L.; Raffoul, R.; Dilly, M.-P.; et al. Sternal Wound Infection after Cardiac Surgery: Incidence and Risk Factors According to Clinical Presentation. Clin. Microbiol. Infect. 2015, 21, 674.e11–674.e18. [Google Scholar] [CrossRef]
- Centers for disease control and Prevention CDC/NHSN Surveillance Definitions for Specific Types of Infections. Natl. Healthc. Saf. Netw. 2022, 30.
- Lemaignen, A.; Armand-Lefevre, L.; Birgand, G.; Mabileau, G.; Lolom, I.; Ghodbane, W.; Dilly, M.-P.; Nataf, P.; Lucet, J.-C. Thirteen-Year Experience with Universal Staphylococcus Aureus Nasal Decolonization Prior to Cardiac Surgery: A Quasi-Experimental Study. J. Hosp. Infect. 2018, 100, 322–328. [Google Scholar] [CrossRef]
- Couffignal, C.; Amour, J.; Ait-Hamou, N.; Cholley, B.; Fellahi, J.-L.; Duval, X.; Costa De Beauregard, Y.; Nataf, P.; Dilly, M.-P.; Provenchère, S.; et al. Timing of β-Blocker Reintroduction and the Occurrence of Postoperative Atrial Fibrillation after Cardiac Surgery. Anesthesiology 2020, 132, 267–279. [Google Scholar] [CrossRef]
- Stuart, E.A.; Cole, S.R.; Bradshaw, C.P.; Leaf, P.J. The Use of Propensity Scores to Assess the Generalizability of Results from Randomized Trials: Use of Propensity Scores to Assess Generalizability. J. R. Stat. Soc. Ser. A 2011, 174, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, J.S. Multivariate and Propensity Score Matching Software with Automated Balance Optimization: The Matching Package for R. J. Stat. Soft. 2011, 42, 49. [Google Scholar] [CrossRef]
- Hansen, B.B.; Klopfer, S.O. Optimal Full Matching and Related Designs via Network Flows. J. Comput. Graph. Stat. 2006, 15, 609–627. [Google Scholar] [CrossRef]
- Greifer, N.; Stuart, E.A. Matching Methods for Confounder Adjustment: An Addition to the Epidemiologist’s Toolbox. Epidemiol Rev. 2022, 43, 118–129. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Branch-Elliman, W.; O’Brien, W.; Strymish, J.; Itani, K.; Wyatt, C.; Gupta, K. Association of Duration and Type of Surgical Prophylaxis With Antimicrobial-Associated Adverse Events. JAMA Surg. 2019, 154, 590. [Google Scholar] [CrossRef]
- Solomon, S.; Akeju, O.; Odumade, O.A.; Ambachew, R.; Gebreyohannes, Z.; Van Wickle, K.; Abayneh, M.; Metaferia, G.; Carvalho, M.J.; Thomson, K.; et al. Prevalence and Risk Factors for Antimicrobial Resistance among Newborns with Gram-Negative Sepsis. PLoS ONE 2021, 16, e0255410. [Google Scholar] [CrossRef]
- Fleischmann, W.A.; Greenwood-Quaintance, K.E.; Patel, R. In Vitro Activity of Plazomicin Compared to Amikacin, Gentamicin, and Tobramycin against Multidrug-Resistant Aerobic Gram-Negative Bacilli. Antimicrob. Agents Chemother. 2020, 64, e01711-19. [Google Scholar] [CrossRef]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. Enterococcal Infection—Treatment and Antibiotic Resistance. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Bourque, M.; Quintiliani, R.; Tilton, R.C. Synergism of Cefazolin-Gentamicin Against Enterococci. Antimicrob. Agents Chemother. 1976, 10, 157–163. [Google Scholar] [CrossRef]
- Peel, T.; Astbury, S.; Cheng, A.C.; Paterson, D.; Buising, K.; Spelman, T.; Tran-Duy, A.; de Steiger, R.S. Multicentre Randomised Double-Blind Placebo Controlled Trial of Combination Vancomycin and Cefazolin Surgical Antibiotic Prophylaxis: The Australian Surgical Antibiotic Prophylaxis (ASAP) Trial. BMJ Open 2019, 9, e033718. [Google Scholar] [CrossRef]
- Steed, M.E.; Rybak, M.J. Ceftaroline: A New Cephalosporin with Activity Against Resistant Gram-Positive Pathogens. Pharmacotherapy 2010, 30, 375–389. [Google Scholar] [CrossRef]
- Ceftaroline: A New Cephalosporin with Activity Against Methicillin-Resistant Staphylococcus Aureus (MRSA). Clin. Med. Rev. Ther. 2011, 3, 1–17. [CrossRef]
- The Use of Ceftaroline as Surgical Prophylaxis in Surgery With Risk of MRSA Infection (PREVTAROLINE). 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT02307006 (accessed on 28 December 2022).
- McGrath, B.J.; Kang, S.L.; Kaatz, G.W.; Rybak, M.J. Bactericidal Activities of Teicoplanin, Vancomycin, and Gentamicin Alone and in Combination against Staphylococcus Aureus in an in Vitro Pharmacodynamic Model of Endocarditis. Antimicrob. Agents Chemother. 1994, 38, 2034–2040. [Google Scholar] [CrossRef][Green Version]
- Cottagnoud, P.; Cottagnoud, M.; Täuber, M.G. Vancomycin Acts Synergistically with Gentamicin against Penicillin-Resistant Pneumococci by Increasing the Intracellular Penetration of Gentamicin. Antimicrob. Agents Chemother. 2003, 47, 144–147. [Google Scholar] [CrossRef]
- Sharma, M.; Berriel-Cass, D.; Baran, J. Sternal Surgical-Site Infection Following Coronary Artery Bypass Graft Prevalence, Microbiology, and Complications During a 42-Month Period. Infect. Control Hosp. Epidemiol. 2004, 25, 468–471. [Google Scholar] [CrossRef]
- Heilmann, C.; Stahl, R.; Schneider, C.; Sukhodolya, T.; Siepe, M.; Olschewski, M.; Beyersdorf, F. Wound Complications after Median Sternotomy: A Single-Centre Study. Interact. CardioVascular Thorac. Surg. 2013, 16, 643–648. [Google Scholar] [CrossRef]
- Raja, S.G.; Rochon, M.; Jarman, J.W.E. Brompton Harefield Infection Score (BHIS): Development and Validation of a Stratification Tool for Predicting Risk of Surgical Site Infection after Coronary Artery Bypass Grafting. Int. J. Surg. 2015, 16, 69–73. [Google Scholar] [CrossRef]
- Brunet, A.; N’Guyen, Y.; Lefebvre, A.; Poncet, A.; Robbins, A.; Bajolet, O.; Saade, Y.; Ruggieri, V.G.; Rubin, S. Obesity and Preoperative Anaemia as Independent Risk Factors for Sternal Wound Infection After Coronary Artery Bypass Graft Surgery with Pedicled (Non-Skeletonized) Internal Mammary Arteries: The Role of Thoracic Wall Ischemia? VHRM 2020, 16, 553–559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).