Simple Summary
Coronaviruses are worldwide distributed RNA-viruses affecting several species, causing a broad spectrum of diseases with a zoonotic potential and the ability to jump from one host species to a different one, including humans. In the perspective of ‘One Health’ and the well-known recent Coronavirus-associated epidemics and pandemic, the aim of this review is to list all the animal species affected by Coronaviruses and to describe the lesions and the target organs. Information is given on the pathogenesis and the gross and histological lesions of pets, ferrets, bovines, sheep, goats, equine, swine, wild animals, non-human primates, marine mammals, laboratory animals, fish, reptiles, amphibian, and, briefly, humans.
Abstract
Coronaviruses (CoVs) are worldwide distributed RNA-viruses affecting several species, including humans, and causing a broad spectrum of diseases. Historically, they have not been considered a severe threat to public health until two outbreaks of COVs-related atypical human pneumonia derived from animal hosts appeared in 2002 and in 2012. The concern related to CoVs infection dramatically rose after the COVID-19 global outbreak, for which a spill-over from wild animals is also most likely. In light of this CoV zoonotic risk, and their ability to adapt to new species and dramatically spread, it appears pivotal to understand the pathophysiology and mechanisms of tissue injury of known CoVs within the “One-Health” concept. This review specifically describes all CoVs diseases in animals, schematically representing the tissue damage and summarizing the major lesions in an attempt to compare and put them in relation, also with human infections. Some information on pathogenesis and genetic diversity is also included. Investigating the lesions and distribution of CoVs can be crucial to understand and monitor the evolution of these viruses as well as of other pathogens and to further deepen the pathogenesis and transmission of this disease to help public health preventive measures and therapies.
1. Introduction
In December 2019, numerous cases of viral interstitial pneumonia started to be diagnosed in people in China in Wuhan, Hubei province [], after which the infection spread in many countries. On 30 January 2020, the World Health Organization (WHO) classified the global outbreak as a “public health emergency of international concern” [], forcing all affected countries to take preventive measures in order to limit the spread of the infection. In the perspective of the One-Health concept, human lives are in a constant relationship with animals including pets, production animals, and wildlife. The interface humans–animals and the different environments shared are indeed a source of diseases that could impact strongly on public health as well as on social and economic levels, as we are currently experiencing during the recent pandemic event, even if not only restricted to the latter. Historically, the use of antibiotics and the introduction of vaccine campaigns seemed to control recurrent infectious disease outbreaks. Nevertheless, as a concomitant effect, not only has antibiotic resistance increased but there has been a substantial emergence of diseases, mainly of viral origin, from wildlife to humans, occasionally causing fatal outbreaks and pandemics [,,]. For this reason, much effort has been put in, since then, by the scientific community in order to better understand, during the current pandemic, the specific etiological agent involved, the pathophysiology of the infection, the therapeutic responses and the best measures to confine the outbreaks. The agent isolated from pneumonia cases was classified as belonging to the Coronaviridae family, initially classified as 2019 novel coronavirus (nCoV) and subsequently renamed as Severe Acute Respiratory Syndrome (SARS)-CoV-2. As of 8 October 2020, the WHO reported that 235 countries were affected by SARC-CoV-2-associated disease (COVID-19), with 35,897,739 confirmed cases and a total of 1,048,781 confirmed deaths [].
Historically, CoVs were not considered a severe threat to public health until two outbreaks of atypical pneumonia appeared in the recent past. The first, in 2002—later renamed SARS and caused by SARS-CoV—associated with high rates of fatalities, reaching up to 10% []. The second, ten years later, named Middle East Respiratory Syndrome (MERS), from the geographical area of the first isolation and caused by another pathogenic CoV (MERS-CoV), had a fatality rate up to 37% [].
CoVs are well-known by the scientific community and particularly by veterinarians, as they can cause a wide range of diseases, mainly affecting the respiratory, gastro-intestinal, and central nervous systems [], in a large number of host species, from birds to mammals, including humans [].
CoVs are positive single-strand enveloped RNA viruses (+ssRNA) [] with the largest genome (27–32 Kb) among all RNA viruses []. The first isolation dates back to 1968, when a group of virologists described the structure of a new group of viruses—isolated from humans, mice (mouse hepatitis virus), and avian species (avian infectious bronchitis)—and sent their conclusion to Nature []. They highlighted a characteristic common fringe of 200 Å long rounded to petal-like projections from the viral membrane, having the appearance of the “solar corona”, subsequently also identified as a “crown”, hence Coronavirus from the solar corona-like shape and the Latin word corona that means crown []. These projections constitute the typical “Spike” glycoproteins, which characterize all CoV membranes.
All CoVs belong to order Nidovirales, suborder Cornidovirineae, family Coronaviridae, subfamily Coronavirinae []. The members of this subfamily can be divided into four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. Alpha- and Beta-coronaviruses affect mammals, Gammacoronaviruses cause diseases in avian species, whereas Deltacoronaviruses rarely infect mammals with a more specific tropism for birds [,]. Further division of genera into subgroups/subgenera is also described [].
Not only do CoVs affect several species, causing a broad spectrum of disease, but some of them also have a zoonotic potential and the ability to jump from one reservoir species to a different species, including humans, usually through a bridging species. Regarding the two most relevant examples, the dromedary camel (Camelus dromedarius) has been identified as a bridging species of MERS-CoV that most likely spilled over from bats, whereas SARS-CoVs jumped to humans from civets (Paguma larvata) infected by maintenance bat hosts such as Rhinolophus sinicus and R. ferrumequinum [,]. SARS-CoV-2 has been analyzed throughout genome sequencing, showing 96.2% overall genome sequence identity with Bat CoV RaTG13, suggesting that Bat CoV and human SARS-CoV-2 might share a common ancestor []. As an example, one SARS-CoV-2-related coronavirus isolated from a Malayan pangolin showed 100%, 98.6%, 97.8%, and 90.7% amino acid identity with SARS-CoV-2 envelope (E), membrane (M), nucleocapsid (N), and spike (S) genes respectively, and therefore, whether SARS-CoV-2 has other reservoirs and/or intermediate hosts still remains a question to be addressed in the current scenario [,].
In light of the CoVs’ zoonotic risk and their ability to adapt to new species, it appears pivotal to understand the pathophysiology and mechanisms of tissue injury of known CoVs within the “One-Health” concept [].
Even though there are still many doubts regarding the pathophysiological mechanisms in animals, this paper provides brief and general information on the CoV pathogenesis, together with a review of old and new CoV-associated animal diseases. Because the disease in bats is generally asymptomatic and very little information is available on associated lesions, these animals are not included in this review. The main aim of this review is to focus on gross and microscopic lesions associated to CoVs in different species, including pathogenesis when available.
2. Pathogenesis
2.1. Viral Life Cycle
The viral life cycle has common steps for all studied CoVs, both in humans and animals (Scheme 1). CoV infection begins with the interplay of the virion (Scheme 2) with host cells. Various specific host cell receptors mainly mediate viral entry strategies. These strategies and the receptors’ tissue distribution influence the viral tropism and pathogenicity [,]. Among some of the recognized CoV receptors there are the well-known angiotensin-converting enzyme 2 (ACE2) for SARS-CoV and SARS-CoV-2, the dipeptidyl peptidase 4 (DPP4, also known as CD26) for MERS [], the aminopeptidase N—mainly used by Alphadoronavirus, and 5-N-acetyl-9-O-acetyl neuraminic acid (Neu5,9Ac2) and Carcinoembryonic antigen-related cell adhesion molecule 1 (biliary glycoprotein) (CEACAM1)—mainly for Betacoronavirus [].
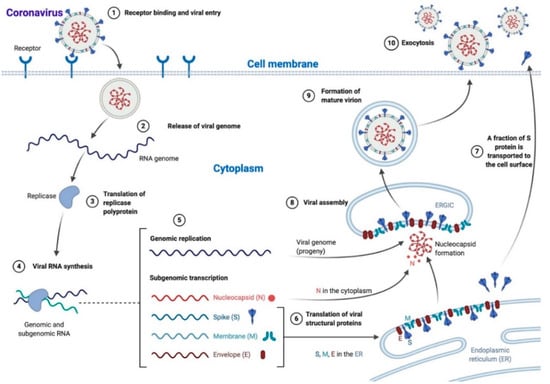
Scheme 1.
Coronavirus life cycle. Most Coronaviruses enter the cell after S protein–receptor interaction (1), the viral genome is released in the cytoplasm (2) and translated into the replicase polyprotein (3) to synthetize viral RNA (4). Genomic (progeny) and subgenomic RNAs are produced (5) and the latter are translated to structural and accessory proteins that can be inserted in the endoplasmic reticulum (ER; 6) and moved to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC), while a fraction of S protein is transported directly to the cell surface where it mediates cell–cell fusion (7). In the ERGIC, the viral assembly and the encapsidation of genomic RNA by N protein take place, leading to nucleocapsid formation (8). Mature virions are then transported in smooth-walled vesicles (9) and released via exocytosis (10). N: nucleocapsid, S: spike, M: membrane, E: envelope. The scheme has been created with BioRender.com.
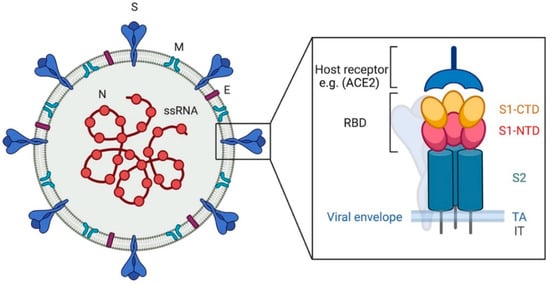
Scheme 2.
Coronavirus, viral particle. The image shows, on the left, a graphic representation of a viral particle. The black box shows the structure of the spike protein. N: nucleocapsid, S: spike, M: membrane, E: envelope. RBD: receptor binding domain, S1-CTD: subunit 1 C-terminal domain, S1-NTD: subunit 1 N-terminal domain, S2: subunit 2, TA: transmembrane anchor, IT: intracellular tail. The scheme has been created with BioRender.com.
Viral attachment is predominantly mediated by the S protein–receptor interaction. Receptor binding causes major structural changes in the S protein (Scheme 2, see below), leading to viral fusion with the host cell membrane and access to the cytoplasm, where the viral genome is released [] (Scheme 1). Depending on viral strain and on host cell type, this fusion process can occur directly at the cell surface or can be mediated by endocytosis []. Neurotropic CoV strains (i.e., mouse hepatitis virus, JHM strain) can mediate receptor-independent virus entry into host cells as a unique ability among viruses [].
Once within the cytoplasm, the viral genome translates into the replicase polyprotein, which then uses the genome as a template for viral RNA synthesis. Viral RNA synthesis produces, through negative-strand intermediates, both genomic and subgenomic RNAs, the latter being translated into structural and accessory proteins [] (Scheme 1). Viral structural S, E, and M proteins are inserted into the endoplasmic reticulum (ER) and then move to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) where viral assembly takes place (Scheme 1). Encapsidation of genomic RNA by N protein leads to nucleocapsid formation, followed by the production of mature virions through budding and association with ERGIC membranes containing viral structural proteins []. This process is mainly directed by M protein, which controls the interactions between structural proteins during virion assembly []. Mature virions are then transported to the plasma membrane in smooth-walled vesicles and released via exocytosis (Scheme 1). In some CoV strains, a fraction of S protein is not assembled into mature virions but gets transported to the cell surface (Scheme 1) where it mediates cell-to-cell fusion with the formation of multinucleated syncytial cells. As such, it facilitates the infection in adjacent cells without the need for extracellular viruses and the consequent escape of the host immune surveillance [,]. The site of virion release can differ between CoVs. For instance, transmissible gastroenteritis virus (TGEV) in swine is preferentially released at the infected host cells’ apical membrane, while the mouse hepatitis virus (MHV) favors the basolateral cellular surface. This difference in the release site can influence viral pathogenicity, as in this case, TGEV usually causes a localized enteric infection with an intraluminal release, while MHV can cause systemic disease [].
2.2. Viral Spike Protein
The S protein plays a major role in CoV pathogenesis, host and tissue tropism, and host immune response. The CoV S protein is a large class I viral membrane fusion (transmembrane) glycoprotein composed of three segments: a large ectodomain, a transmembrane anchor, and an intracellular tail (Scheme 2). The ectodomain consists of a receptor-binding subunit S1 (N-terminal) and a membrane-fusion subunit S2 (C-terminal) [,]. S1 subunit, in turn, has two major subdomains, an N-terminal domain and a C-terminal domain: one or both these subdomains can function as a receptor-binding domain (RBD), binding sugars or recognizing protein receptors, respectively []. The S2 subunit comprises the fusion peptide and is responsible for membrane fusion [,,].
After receptor binding, CoV S protein goes through major conformational changes. These changes are necessary for virus–cell fusion and entry. They consist of proteolytic processing of S protein itself by host proteases. Host proteases are, therefore, crucial for membrane fusion and entry. The most important source of these proteolytic enzymes is represented by the lysosomal proteases found in virtually all cell types. Additional tissue-specific availability of these enzymes can most likely influence tissue tropism of CoVs []. Similarly, the spillover potential of CoVs is also influenced both by the RBD–receptor interaction and by this proteolytic processing of S protein [,].
CoV S protein’s role is not only limited to viral fusion and entry. S protein is one of the major immunogens in CoVs and the main target of neutralizing antibodies in natural infections []. Moreover, S protein is also thought to have a key role in altering innate antiviral immune response through translational repression of mRNA transcripts, thus inhibiting interferon (IFN) and cytokine production, favoring viral infection and spreading []. However, several other immunogenic CoV proteins are investigated, particularly in human diseases, to understand deeper specific immune responses better and in order to develop efficacious vaccination campaigns.
2.3. Viral Mutation and Recombination
One of the striking features of CoVs is surely represented by their genetic plasticity. There is a high frequency of genetic changes for many CoVs, which forms the basis of their zoonotic potential []. For this, CoVs exploit two major mechanisms: mutation and recombination.
2.4. Mutation
Like in other single-stranded RNA viruses, genomic mutations do occur in CoVs [] and are mainly due to their viral replicase, which does not possess good proofreading, but is efficient enough in maintaining large genomes without accumulating catastrophic errors and leading to progressive differentiation of viral progeny [].
Mutational events can affect the CoV pathogenicity and host range. A striking example of how mutations influence tissue tropism and pathogenesis can be found in TGEV and porcine respiratory coronavirus (PRCoV). TGEV can infect both intestinal and respiratory cells, while PRCoV, an attenuated variant of TGEV, can only infect the respiratory tract, even if it binds to the same receptor as TGEV (porcine amino-peptidase N). This difference in tissue tropism might derive from the lack of hemagglutinating activity of PRCoV as a consequence of a deletion in the S1 domain compared to TGEV. Therefore, PRCoV is incapable of infecting intestinal cells, and its pathogenicity is consequently reduced []. Another example of mutational events that causes tissue tropism change can be found in the different pathogenicity between the widespread feline enteric coronavirus (FECV) and the lethal feline infectious peritonitis virus (FIPV). FIPV develops in individual cats persistently infected with FECV. Mutations in accessory and S genes could enable the virus to efficiently replicate in monocytes and macrophages, leading to the diffuse and lethal disease caused by FIPV, rather than the mild enteric form induced by the enterotropic FECV [,].
Mutations do not only influence CoV tissue tropism and pathogenicity but are also a key event for virus spillover. While the mutational events that have led to SARS-CoV-2 spillover are still not clear, the genetic rearrangements that caused SARS-CoV to host jump and outbreak have been extensively described. SARS-CoV is thought to have passed from bats, considered the natural reservoir for most animal CoVs, to humans using palm civets []. Both human and palm civet CoVs bind to the ACE2 receptor, but studies have shown that human SARS-CoV can bind both human and palm civet ACE2, while palm civet virus cannot bind human ACE2. This difference in receptor selectivity has been related to two-point mutations in the RBD of the human virus, showing its adaptation to the new host []. More recently, Korber and collaborators described an amino acid change in the SARS-CoV-2 spike protein caused by an A-to-G nucleotide mutation that led to a new variant, namely G614 []. The authors hypothesized that this new variant may have a fitness advantage over the original D614 form. Most importantly, they reported that the G614 variant was associated with potentially higher viral loads in COVID-19 patients, even though there was no significant association with increased disease severity [].
2.5. Recombination
The marked tendency of CoVs to recombine with other CoVs (homologous recombination) and with RNAs of different viruses and other organisms (heterologous recombination) is related to their particular replicating machinery, as thoroughly described in a recent review []. Moreover, their exceptionally large RNA genome increases the probability of both mutational and recombination events. Genetic recombination has been extensively documented in both animal (i.e., MHV, TGEV, feline CoV, canine CoV) and human (i.e., OC43, NL63, HKU1, SARS-CoV, MERS-CoV) CoVs []. One of the best examples of genetic recombination in CoVs can be found in serotype II feline CoV (FCoV). This virus originates from the recombination event between FCoV and canine CoV (CCoV). Through this homologous recombination, serotype I FCoV acquires the CCoV S gene, including its neighboring regions, resulting in a change in the receptor-binding domain with critical biological consequences []. Serotype II FCoV can uses the feline aminopeptidase N (fAPN), a metalloprotease expressed in many host tissues, whereas it has been proven that serotype I FCoV uses different host cell receptors [].
4. Discussion
The tremendous amount of animal diseases caused worldwide by a variety of CoV strains in several species indicate the vast spreading of CoVs in the ecosystem and their ability to change, adapt, and progressively cause new animal diseases over time. The adaptation during cross-species jumps in different species including domestic and wild mammals, as well as birds, may play a role in enabling viral spillover from natural hosts to humans. Moreover, infection of domestic species from human CoVs has also been partially documented and recently demonstrated for SARS-CoV-2 [], indicating a potential mutual role in the transmission of the infection.
Extensive genomic studies of both human and animal CoVs allow for a better understanding of the origin and evolution of pathogenic CoVs, pivotal for disease control and treatment, and hopefully helping in avoiding the new wide spreading of life-threatening diseases. In the One-Health age, an increased animal-to-human transmission is already evident of viral pathogens such as Ebola, influenza viruses, Hendra, Nipah, and CoVs. Ecosystem changes, including climate changes, urbanization with increased human population, and cultural and social changes, as well as secular traditions [], account for this new spreading of zoonotic epidemics of which we should regrettably expect more in the future.
For these reasons, animals, humans, and the environment should be considered as part of the same scenario and a better understanding of the interaction between the different components could help in preventing and controlling any future spill-over towards the human sphere. A proper management of environmental factors by increasing attention to land usages aimed to preserve biodiversity, to prevent a wild/domestic interaction, and to avoid stressful conditions to wild species reservoirs seems to be a good approach in reducing spill-over risks. This stresses the urgent need of multidisciplinary approaches and a constant monitoring of the wild animal sphere. A proper surveillance program including constant reporting and investigations on dead wild and domestic species could help to anticipate the spread of a similar epidemic.
As previously discussed, CoVs, like other positive-strand RNA viruses, have the ability to manifest and acquire genetic diversity due to some typical features, such as the infidelity of the RNA-dependent RNA polymerase, the high frequency of homologous and heterologous RNA recombination, and the large genomes []. This genetic variability confers to CoVs the high potential of evolution that occasionally allows them to overcome species barriers and host specificity [,,].
Some studies indicate that possibly all CoVs are genetically derived from common ancestors residing in bats, which are usually naturally infected and asymptomatic long-lasting reservoir (Alpha and Betacoronavirus), and in birds (Delta and Gammacoronavirus) [,,,]. The different behavior of coronaviruses in bats and birds could also be related to the unique properties of these two groups of animals. The diversity of bats and birds themselves is huge, their flying capacity has allowed them to spread worldwide, and their habits provide frequent opportunities of aggregation [].
The genomic diversity of CoVs accounts for their variation in species adaptation related to receptor binding ability and, consequently, tissue tropism, producing localized versus systemic diseases affecting different organ systems []. As an example, SARS-CoV uses ACE2 as a receptor and primarily infects ciliated bronchial epithelial cells and type II pneumocytes, whereas MERS-CoV uses DPP4 and infects non-ciliated bronchial epithelial cells and type II pneumocytes [].
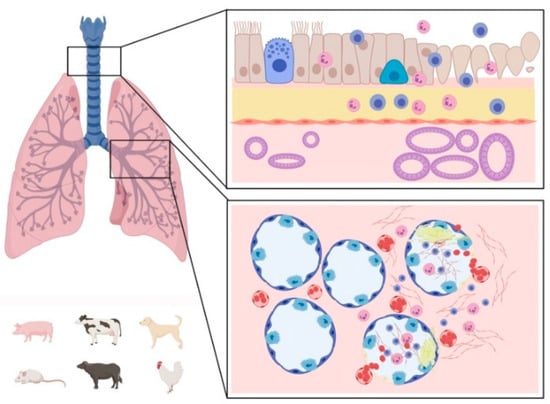
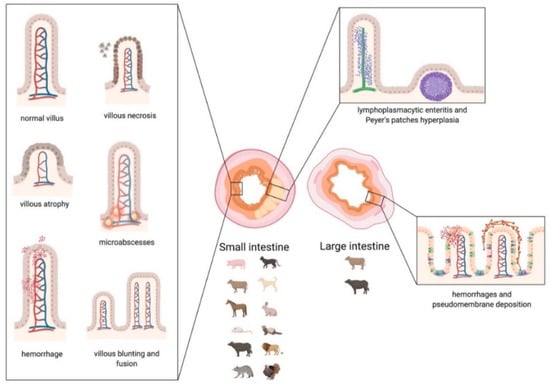
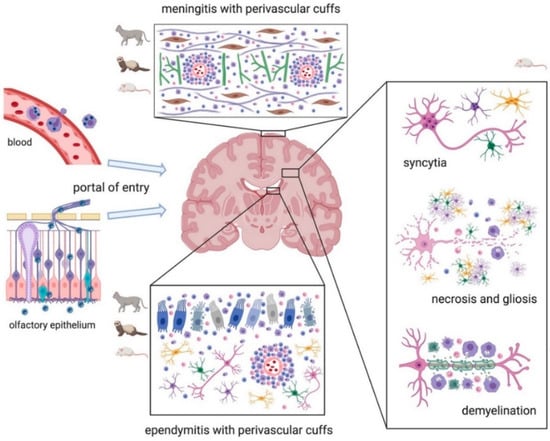
As described in the text and summarized in Table S1, in CoV infections, three major organ systems appear to be involved: the respiratory, the alimentary, and the nervous system (Scheme 3, Scheme 4 and Scheme 5). Usually, human CoVs cause mainly respiratory diseases (Alpha- and Beta-coronavirus), whereas other mammals manifest predominantly gastroenteritis and a less frequent, but typical for some diseases/species, nervous involvement (Alpha- and Beta-coronavirus). All Alpha- and Beta-coronaviruses seem to have originated in bats, whereas a separate origin is postulated for avian Delta- and Gamma-coronavirus, rarely affecting mammals (i.e., pigs) and causing mainly respiratory pathologies [,,].
Interestingly, genomic diversity can not only modify CoVs among species but also within the same species, conferring new ability to spread within the organism. A typical example is the case of FIP, for which risk factors for host and environmental spreading, maintenance, and genetic change associated with increased disease severity are ascribed to animal-to-animal contact and poor infection controls. Additionally, the importance of the genetic background of the host is also relevant, as demonstrated by the similar disease evolution in domestic and wild felids.
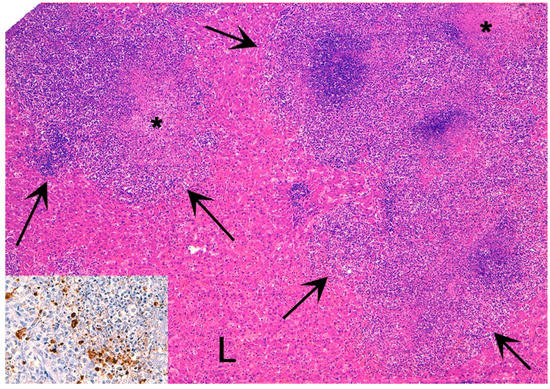
Notably, histopathological evaluation of CoVs in animals has underlined similarities with human CoVs, such as the typical alveolar damage and the vascular thrombosis with fibrinous exudation, occasional syncytia formation, depletion of lymphoid organs, and the direct intestinal epithelial damage. As for many other pathological processes (e.g., tumors), animals could therefore not only benefit from human medicine but also represent a model as well as an important ring in epidemiological chains that need to be studied and monitored.
Investigating the lesions and distribution of CoVs can therefore be crucial to understand and monitor the evolution of these viruses as well as of other pathogens in light of the One-Health approach. Unfortunately, mainly in animals, accurate postmortem examinations and histopathological investigations are infrequently performed. However, histopathological characterization, especially in cases with fatal COVID-19, is considered critical to further understand the pathogenesis and transmission of this disease in order to help public health-preventive measures and therapies.
5. Conclusions
In conclusion, we believe that further work is absolutely needed in order to better characterize the transmission barrier(s) between CoVs and different animal species, along with the virus- and the host-related factors underlying cross-species jumping within different environments as well as at the level of the various ecological interfaces. Strengthening public health surveillance systems, including veterinary services and wildlife monitoring, could provide early warnings and predict possible future emergencies [].
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/10/12/2377/s1, Table S1: Coronavirus-associated diseases in animals and list of lesions in the main affected tissues.
Author Contributions
V.Z., S.F. and M.C. have given major contribution to conceptualization and assembling of the review. F.B., G.B., A.C., L.C., C.C., G.C., S.D.V., M.E.G., S.M., V.M., N.R., A.S., F.T. and R.V. have equally contributed to specific paragraphs and realization of the schemes, they are listed in alphabetical order. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Sammarco is supported by an American-Italian Cancer Foundation Post-Doctoral Research Fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef]
- Wu, D.; Wu, T.; Liu, Q.; Yang, Z. The SARS-CoV-2 outbreak: What we know. Int. J. Infect. Dis. 2020, 94, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.A.; Daszak, P.; Wood, J.L.N. One Health, emerging infectious diseases and wildlife: Two decades of progress? Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160167. [Google Scholar] [CrossRef] [PubMed]
- Kreuder Johnson, C.; Hitchens, P.L.; Smiley Evans, T.; Goldstein, T.; Thomas, K.; Clements, A.; Joly, D.O.; Wolfe, N.D.; Daszak, P.; Karesh, W.B.; et al. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci. Rep. 2015, 5, 14830. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, J.M.; DeLiberto, T.; Nguyen, N. Optimization of human, animal, and environmental health by using the One Health approach. J. Vet. Sci. 2017, 18, 263. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 2 December 2020).
- Shi, Z.L.; Guo, D.; Rottier, P.J.M. Coronavirus: Epidemiology, genome replication and the interactions with their hosts. Virol. Sin. 2016, 31, 1–2. [Google Scholar] [CrossRef]
- Sun, J.; He, W.; Wang, L.; Lai, A.; Ji, X.; Zhai, X.; Li, G.; Suchard, M.A.; Tian, J.; Zhou, J.; et al. COVID-19: Epidemiology, Evolution, and Cross-Disciplinary Perspectives. Trends Mol. Med. 2020, 26, 483–495. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Almeida, J.D.; Berry, D.M.; Cunningham, C.H.; Hamre, D.; Hofstad, M.S.; Mallucci, L.; McIntosh, K.; Tyrrell, D.A.J. Virology: Coronaviruses. Nature 1968, 220, 650. [Google Scholar]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses ICTV. Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 1 October 2020).
- Luk, H.K.H.; Li, X.; Fung, J.; Lau, S.K.P.; Woo, P.C.Y. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect. Genet. Evol. 2019, 71, 21–30. [Google Scholar] [CrossRef] [PubMed]
- De Wit, E.; Van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [CrossRef]
- Lam, T.T.-Y.; Jia, N.; Zhang, Y.-W.; Shum, M.H.-H.; Jiang, J.-F.; Zhu, H.-C.; Tong, Y.-G.; Shi, Y.-X.; Ni, X.-B.; Liao, Y.; et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, X.; Tan, Y.; Li, Q.; Xu, C.; Xu, J.; Hao, L.; Zeng, Z.; Luo, X.; Liu, F.; et al. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 2020, 127, 110195. [Google Scholar] [CrossRef]
- Weiss, S.R.; Leibowitz, J.L. Coronavirus Pathogenesis. In Advances in Virus Research; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 85–164. [Google Scholar]
- Masters, P.S. The Molecular Biology of Coronaviruses. Adv. Virus Res. 2006, 65, 193–292. [Google Scholar]
- Fehr, A.R.; Perlman, S. Coronaviruses: An Overview of Their Replication and Pathogenesis. In Coronaviruses; Maier, H., Bickerton, E., Britton, P., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1282, pp. 1–23. [Google Scholar]
- Weiss, S.R.; Navas-Martin, S. Coronavirus Pathogenesis and the Emerging Pathogen Severe Acute Respiratory Syndrome Coronavirus. Microbiol. Mol. Biol. Rev. 2005, 69, 635–664. [Google Scholar] [CrossRef]
- Hulswit, R.J.G.; de Haan, C.A.M.; Bosch, B.-J. Coronavirus spike protein and tropism changes. In Advances in Virus Research; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 29–57. [Google Scholar]
- Tortorici, M.A.; Veesler, D. Structural insights into coronavirus entry. In Advances in Virus Research; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 93–116. [Google Scholar]
- Wong, L.Y.R.; Lui, P.Y.; Jin, D.Y. A molecular arms race between host innate antiviral response and emerging human coronaviruses. Virol. Sin. 2016, 31, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. An update on feline infectious peritonitis: Virology and immunopathogenesis. Vet. J. 2014, 201, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Tekes, G.; Thiel, H.J. Feline Coronaviruses: Pathogenesis of Feline Infectious Peritonitis, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 96. [Google Scholar]
- Vijaykrishna, D.; Smith, G.J.D.; Zhang, J.X.; Peiris, J.S.M.; Chen, H.; Guan, Y. Evolutionary Insights into the Ecology of Coronaviruses. J. Virol. 2007, 81, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C. An update on canine coronaviruses: Viral evolution and pathobiology. Vet. Microbiol. 2008, 132, 221–234. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C. Canine Coronavirus: Not Only an Enteric Pathogen. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1121–1132. [Google Scholar] [CrossRef]
- Erles, K.; Toomey, C.; Brooks, H.W.; Brownlie, J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 2003, 310, 216–223. [Google Scholar] [CrossRef]
- Decaro, N.; Mari, V.; von Reitzenstein, M.; Lucente, M.S.; Cirone, F.; Elia, G.; Martella, V.; King, V.L.; Di Bello, A.; Varello, K.; et al. A pantropic canine coronavirus genetically related to the prototype isolate CB/05. Vet. Microbiol. 2012, 159, 239–244. [Google Scholar] [CrossRef]
- Buonavoglia, C.; Decaro, N.; Martella, V.; Elia, G.; Campolo, M.; Desario, C.; Castagnaro, M.; Tempesta, M. Canine Coronavirus Highly Pathogenic for Dogs. Emerg. Infect. Dis. 2006, 12, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Kipar, A.; Meli, M.L. Feline Infectious Peritonitis: Still an Enigma? Vet. Pathol. 2014, 51, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, Y.; Alcaraz, A.; Bossong, F.J.; Collisson, E.W.; Diniz, P.P.V.P. Feline Coronavirus in Multicat Environments. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1133–1169. [Google Scholar] [CrossRef] [PubMed]
- Kipar, A.; May, H.; Menger, S.; Weber, M.; Leukert, W.; Reinacher, M. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet. Pathol. 2005, 42, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sigurardóttir, Ó.G.; Kolbjornsen, O.; Lutz, H. Orchitis in a cat associated with coronavirus infection. J. Comp. Pathol. 2001, 124, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Rota, A.; Paltrinieri, S.; Jussich, S.; Ubertalli, G.; Appino, S. Priapism in a castrated cat associated with feline infectious peritonitis. J. Feline Med. Surg. 2008, 10, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Osumi, T.; Mitsui, I.; Leutenegger, C.M.; Okabe, R.; Ide, K.; Nishifuji, K. First identification of a single amino acid change in the spike protein region of feline coronavirus detected from a coronavirus-associated cutaneous nodule in a cat. J. Feline Med. Surg. Open Rep. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Ernandes, M.A.; Cantoni, A.M.; Armando, F.; Corradi, A.; Ressel, L.; Tamborini, A. Feline coronavirus-associated myocarditis in a domestic longhair cat. J. Feline Med. Surg. Open Rep. 2019, 5. [Google Scholar] [CrossRef]
- Foley, J.E.; Leutenegger, C. A Review of Coronavirus Infection in the Central Nervous System of Cats and Mice. J. Vet. Intern. Med. 2001, 15, 438–444. [Google Scholar] [CrossRef]
- Zappulli, V.; Caliari, D.; Cavicchioli, L.; Tinelli, A.; Castagnaro, M. Systemic fatal type II coronavirus infection in a dog: Pathological findings and immunohistochemistry. Res. Vet. Sci. 2008, 84, 278–282. [Google Scholar] [CrossRef]
- Evermann, J.F.; Abbott, J.R.; Han, S. Canine coronavirus-associated puppy mortality without evidence of concurrent canine parvovirus infection. J. Vet. Diagn. Investig. 2005, 17, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Erles, K.; Brownlie, J. Investigation into the causes of canine infectious respiratory disease: Antibody responses to canine respiratory coronavirus and canine herpesvirus in two kennelled dog populations. Arch. Virol. 2005, 150, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Erles, K.; Brownlie, J. Canine Respiratory Coronavirus: An Emerging Pathogen in the Canine Infectious Respiratory Disease Complex. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Brooks, H.W.; Szladovits, B.; Erles, K.; Gibbons, R.; Shields, S.; Brownlie, J. Tropism and pathological findings associated with canine respiratory coronavirus (CRCoV). Vet. Microbiol. 2013, 162, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Kipar, A.; Köhler, K.; Leukert, W.; Reinacher, M. A comparison of lymphatic tissues from cats with spontaneous feline infectious peritonitis (FIP), cats with FIP virus infection but no FIP, and cats with no infection. J. Comp. Pathol. 2001, 125, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.D. Coronavirus infection in cats. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 1–16. [Google Scholar] [CrossRef]
- Kipar, A.; Kremendahl, J.; Addie, D.D.; Leukert, W.; Grant, C.K.; Reinacher, M. Fatal enteritis associated with coronavirus infection in cats. J. Comp. Pathol. 1998, 119, 1–14. [Google Scholar] [CrossRef]
- Licitra, B.N.; Whittaker, G.R.; Dubovi, E.J.; Duhamel, G.E. Genotypic characterization of canine coronaviruses associated with fatal canine neonatal enteritis in the United States. J. Clin. Microbiol. 2014, 52, 4230–4238. [Google Scholar] [CrossRef]
- Priestnall, S.L.; Mitchell, J.A.; Walker, C.A.; Erles, K.; Brownlie, J. New and Emerging Pathogens in Canine Infectious Respiratory Disease. Vet. Pathol. 2014, 51, 492–504. [Google Scholar] [CrossRef]
- Tilocca, B.; Soggiu, A.; Musella, V.; Britti, D.; Sanguinetti, M.; Urbani, A.; Roncada, P. Molecular basis of COVID-19 relationships in different species: A One Health perspective. Microbes Infect. 2020, 22, 218–220. [Google Scholar] [CrossRef]
- Priestnall, S.L. Canine Respiratory Coronavirus: A Naturally Occurring Model of COVID-19? Vet. Pathol. 2020, 57, 467–471. [Google Scholar] [CrossRef]
- Xia, X. Extreme Genomic CpG Deficiency in SARS-CoV-2 and Evasion of Host Antiviral Defense. Mol. Biol. Evol. 2020, 37, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Hatta, M.; Chiba, S.; Maemura, T.; Fan, S.; Takeda, M.; Kinoshita, N.; Hattori, S.I.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; et al. Transmission of SARS-CoV-2 in Domestic Cats. N. Engl. J. Med. 2020, 383, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.H.; Kiupel, M.; West, K.H.; Raymond, J.T.; Grant, C.K.; Glickman, L.T. Coronavirus-associated epizootic catarrhal enteritis in ferrets. J. Am. Vet. Med. Assoc. 2000, 217, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Kiupel, M.; Maes, R.K. Ferret coronavirus-associated diseases. Vet. Clin. N. Am. Exot. Anim. Pract. 2010, 13, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.G.; Kiupel, M.; Maes, R.K. Molecular characterization of a novel coronavirus associated with epizootic catarrhal enteritis (ECE) in ferrets. Virology 2006, 349, 164–174. [Google Scholar] [CrossRef]
- Autieri, C.R.; Miller, C.L.; Scott, K.E.; Kilgore, A.; Papscoe, V.A.; Garner, M.M.; Haupt, J.L.; Bakthavatchalu, V.; Muthupalani, S.; Fox, J.G. Systemic Coronaviral Disease in 5 Ferrets. Comp. Med. 2015, 65, 508–516. [Google Scholar]
- Lescano, J.; Quevedo, M.; Gonzales-Viera, O.; Luna, L.; Keel, M.K.; Gregori, F. First Case of Systemic Coronavirus Infection in a Domestic Ferret (Mustela putorius furo) in Peru. Transbound. Emerg. Dis. 2015, 62, 581–585. [Google Scholar] [CrossRef]
- Doria-Torra, G.; Vidaña, B.; Ramis, A.; Amarilla, S.P.; Martínez, J. Coronavirus Infection in Ferrets: Antigen Distribution and Inflammatory Response. Vet. Pathol. 2016, 53, 1180–1186. [Google Scholar] [CrossRef]
- Fujii, Y.; Tochitani, T.; Kouchi, M.; Matsumoto, I.; Yamada, T.; Funabashi, H. Glomerulonephritis in a ferret with feline coronavirus infection. J. Vet. Diagn. Investig. 2015, 27, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Wills, S.E.; Beaufrère, H.H.; Brisson, B.A.; Fraser, R.S.; Smith, D.A. Pancreatitis and systemic coronavirus infection in a ferret (Mustela putorius furo). Comp. Med. 2018, 68, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Schlottau, K.; Rissmann, M.; Graaf, A.; Schön, J.; Sehl, J.; Wylezich, C.; Höper, D.; Mettenleiter, T.C.; Balkema-Buschmann, A.; Harder, T.; et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: An experimental transmission study. Lancet Microbe 2020, 1, e218–e225. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, S.G.; Kim, S.M.; Kim, E.H.; Park, S.J.; Yu, K.M.; Chang, J.H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020, 27, 704–709.e2. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.; Kok, A.; de Meulder, D.; Bestebroer, T.M.; Lamers, M.M.; Okba, N.M.A.; Fentener van Vlissingen, M.; Rockx, B.; Haagmans, B.L.; Koopmans, M.P.G.; et al. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat. Commun. 2020, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Boileau, M.J.; Kapil, S. Bovine Coronavirus Associated Syndromes. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 123–146. [Google Scholar] [CrossRef]
- Decaro, N.; Campolo, M.; Desario, C.; Cirone, F.; D’Abramo, M.; Lorusso, E.; Greco, G.; Mari, V.; Colaianni, M.L.; Elia, G.; et al. Respiratory disease associated with bovine coronavirus infection in cattle herds in Southern Italy. J. Vet. Diagn. Investig. 2008, 20, 28–32. [Google Scholar] [CrossRef]
- Decaro, N.; Mari, V.; Desario, C.; Campolo, M.; Elia, G.; Martella, V.; Greco, G.; Cirone, F.; Colaianni, M.L.; Cordioli, P.; et al. Severe outbreak of bovine coronavirus infection in dairy cattle during the warmer season. Vet. Microbiol. 2008, 126, 30–39. [Google Scholar] [CrossRef]
- Lin, X.Q.; O’Reilly, K.L.; Storz, J. Antibody responses of cattle with respiratory coronavirus infections during pathogenesis of shipping fever pneumonia are lower with antigens of enteric strains than with those of a respiratory strain. Clin. Diagn. Lab. Immunol. 2002, 9, 1010–1013. [Google Scholar] [CrossRef]
- Saif, L.J. Bovine respiratory coronavirus. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 349–364. [Google Scholar] [CrossRef]
- McNulty, M.S.; Bryson, D.G.; Allan, G.M.; Logan, E.F. Coronavirus infection of the bovine respiratory tract. Vet. Microbiol. 1984, 9, 425–434. [Google Scholar] [CrossRef]
- Hick, P.; Read, A.; Lugton, I.; Busfield, F.; Dawood, K.; Gabor, L.; Hornitzky, M.; Kirkland, P. Coronavirus infection in intensively managed cattle with respiratory disease. Aust. Vet. J. 2012, 90, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.; Lin, X.; Purdy, C.W.; Chouljenko, V.N.; Kousoulas, K.G.; Enright, F.M.; Gilmore, W.C.; Briggs, R.E.; Loan, R.W. Coronavirus and Pasteurella infections in bovine shipping fever pneumonia and Evans’ criteria for causation. J. Clin. Microbiol. 2000, 38, 3291–3298. [Google Scholar] [CrossRef] [PubMed]
- Kapil, S.; Trent, A.M.; Goyal, S.M. Excretion and persistence of bovine coronavirus in neonatal calves. Arch. Virol. 1990, 115, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W.; Herd, H.R.; Sorensen, N.J.; Confer, A.W.; Ritchey, J.W.; Ridpath, J.F.; Burge, L.J. Enteric disease in postweaned beef calves associated with Bovine coronavirus clade 2. J. Vet. Diagn. Investig. 2015, 27, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-O.; Halbur, P.G.; Bruna, J.D.; Sorden, S.D.; Yoon, K.-J.; Janke, B.H.; Chang, K.-O.; Saif, L.J. Detection and isolation of coronavirus from feces of three herds of feedlot cattle during outbreaks of winter dysentery-like disease. J. Am. Vet. Med. Assoc. 2000, 217, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, R.; Singh, K.P.; Singh, V.; Malik, Y.P.S.; Kamdi, B.; Singh, R.; Kashyap, G. Immunohistochemical and molecular detection of natural cases of bovine rotavirus and coronavirus infection causing enteritis in dairy calves. Microb. Pathog. 2020, 138, 103814. [Google Scholar] [CrossRef] [PubMed]
- Saif, L.J.; Redman, D.R.; Moorhead, P.D.; Theil, K.W. Experimentally induced coronavirus infections in calves: Viral replication in the respiratory and intestinal tracts. Am. J. Vet. Res. 1986, 47, 1426–1432. [Google Scholar] [PubMed]
- Ellis, J. What is the evidence that bovine coronavirus is a biologically significant respiratory pathogen in cattle? Can. Vet. J. 2019, 60, 147–152. [Google Scholar] [PubMed]
- Park, S.J.; Kim, G.Y.; Choy, H.E.; Hong, Y.J.; Saif, L.J.; Jeong, J.H.; Park, S.I.; Kim, H.H.; Kim, S.K.; Shin, S.S.; et al. Dual enteric and respiratory tropisms of winter dysentery bovine coronavirus in calves. Arch. Virol. 2007, 152, 1885–1900. [Google Scholar] [CrossRef] [PubMed]
- Valarcher, J.-F.; Taylor, G. Bovine respiratory syncytial virus infection. Vet. Res. 2007, 38, 153–180. [Google Scholar] [CrossRef] [PubMed]
- Amer, H.M. Bovine-like coronaviruses in domestic and wild ruminants. Anim. Heal. Res. Rev. 2018, 19, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Gomaa, M.; Shehata, M.; El-Taweel, A.; Kayed, A.E.; Abiadh, A.; Jrijer, J.; Moatasim, Y.; Kutkat, O.; Bagato, O.; et al. Middle East respiratory syndrome coronavirus infection in non-camelid domestic mammals. Emerg. Microbes Infect. 2019, 8, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Kanno, T.; Bannai, H.; Tsujimura, K.; Yamanaka, T.; Kokado, H. Antibody response to equine coronavirus in horses inoculated with a bovine coronavirus vaccine. J. Vet. Med. Sci. 2017, 79, 1889–1891. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.S.; Breslin, J.J.; Breuhaus, B.; Vivrette, S.; Smith, L.G. Characterization of a coronavirus isolated from a diarrheic foal. J. Clin. Microbiol. 2000, 38, 4523–4526. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Mapes, S.; Wademan, C.; White, A.; Ball, R.; Sapp, K.; Burns, P.; Ormond, C.; Butterworth, K.; Bartol, J.; et al. Emerging outbreaks associated with equine coronavirus in adult horses. Vet. Microbiol. 2013, 162, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Vin, R.; Leutenegger, C.; Mittel, L.D.; Divers, T.J. Equine coronavirus: An emerging enteric virus of adult horses. Equine Vet. Educ. 2016, 28, 216–223. [Google Scholar] [CrossRef]
- Fielding, C.L.; Higgins, J.K.; Higgins, J.C.; Mcintosh, S.; Scott, E.; Giannitti, F.; Mete, A.; Pusterla, N. Disease Associated with Equine Coronavirus Infection and High Case Fatality Rate. J. Vet. Intern. Med. 2015, 29, 307–310. [Google Scholar] [CrossRef]
- Giannitti, F.; Diab, S.; Mete, A.; Stanton, J.B.; Fielding, L.; Crossley, B.; Sverlow, K.; Fish, S.; Mapes, S.; Scott, L.; et al. Necrotizing Enteritis and Hyperammonemic Encephalopathy Associated With Equine Coronavirus Infection in Equids. Vet. Pathol. 2015, 52, 1148–1156. [Google Scholar] [CrossRef]
- Narita, M.; Nobumoto, K.; Takeda, H.; Moriyama, T.; Morita, Y.; Nakaoka, Y. Prevalence of Disease with Inference of Equine Coronavirus Infection Among Horses Stabled in a Draft-Horse Racecourse. J. Jpn. Vet. Med. Assoc. 2011, 64, 535–539. [Google Scholar] [CrossRef][Green Version]
- Oue, Y.; Ishihara, R.; Edamatsu, H.; Morita, Y.; Yoshida, M.; Yoshima, M.; Hatama, S.; Murakami, K.; Kanno, T. Isolation of an equine coronavirus from adult horses with pyrogenic and enteric disease and its antigenic and genomic characterization in comparison with the NC99 strain. Vet. Microbiol. 2011, 150, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Oue, Y.; Morita, Y.; Kondo, T.; Nemoto, M. Epidemic of equine coronavirus at obihiro racecourse, Hokkaido, Japan in 2012. J. Vet. Med. Sci. 2013, 75, 1261–1265. [Google Scholar] [CrossRef]
- Miszczak, F.; Tesson, V.; Kin, N.; Dina, J.; Balasuriya, U.B.R.; Pronost, S.; Vabret, A. First detection of equine coronavirus (ECoV) in Europe. Vet. Microbiol. 2014, 171, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.; Rush, B.R.; Cox, J.; DeBey, B.; Kapil, S. Neonatal enterocolitis associated with coronavirus infection in a foal: A case report. J. Vet. Diagn. Investig. 2000, 12, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Vin, R.; Leutenegger, C.M.; Mittel, L.D.; Divers, T.J. Enteric coronavirus infection in adult horses. Vet. J. 2018, 231, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gonzales Arguedas, M. Coronavirus infections. In Equine Infectious Diseases; Sellon, D., Long, M., Eds.; Saunders Elsevier: Amsterdam, The Netherlands, 2007; pp. 184–185. [Google Scholar]
- Moon, H.W.; Norman, J.O.; Lambert, G. Age dependent resistance to transmissible gastroenteritis of swine (TGE). I. Clinical signs and some mucosal dimensions in small intestine. Can. J. Comp. Med. Rev. Can. Med. Comp. 1973, 37, 157–166. [Google Scholar]
- Kemeny, L.; Wiltsey, V.; Riley, J. Upper respiratory infection of lactating sows with transmissible gastroenteritis virus following contact exposure to infected piglets. Cornell Vet. 1975, 65, 352–362. [Google Scholar] [PubMed]
- Saif, L.J. Animal coronavirus vaccines: Lessons for SARS. Dev. Biol. (Basel) 2004, 119, 129–140. [Google Scholar]
- Saif, L.J. Animal coronaviruses: What can they teach us about the severe acute respiratory syndrome? Rev. Sci. Tech. Off. Int. Epiz. 2004, 23, 643–660. [Google Scholar] [CrossRef]
- Shoup, D.I.; Swayne, D.E.; Jackwood, D.J.; Saif, L.J. Immunohistochemistry of transmissible gastroenteritis virus antigens in fixed paraffin-embedded tissues. J. Vet. Diagn. Investig. 1996, 8, 161–167. [Google Scholar] [CrossRef]
- Thake, D.C. Jejunal epithelium in transmissible gastroenteritis of swine. An electron microscopic and histochemical study. Am. J. Pathol. 1968, 53, 149–168. [Google Scholar] [PubMed]
- Pensaert, M.B.; Debouck, P.; Reynolds, D.J. An immunoelectron microscopic and immunofluorescent study on the antigenic relationship between the coronavirus-like agent, CV 777, and several coronaviruses. Arch. Virol. 1981, 68, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Investig. 2013, 25, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Wang, Q.; Scheuer, K.A.; Lu, Z.; Zhang, Y.; Saif, L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014, 20, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Madson, D.M.; Magstadt, D.R.; Arruda, P.H.E.; Hoang, H.; Sun, D.; Bower, L.P.; Bhandari, M.; Burrough, E.R.; Gauger, P.C.; Pillatzki, A.E.; et al. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet. Microbiol. 2014, 174, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Niederwerder, M.C.; Hesse, R.A. Swine enteric coronavirus disease: A review of 4 years with porcine epidemic diarrhoea virus and porcine deltacoronavirus in the United States and Canada. Transbound. Emerg. Dis. 2018, 65, 660–675. [Google Scholar] [CrossRef]
- Saif, L.; Pensaert, M.; Sestak, K.; Yeo, S.; Jung, K. Coronaviruses. In Diseases of Swine; Zimmerman, J., Ed.; Elsevier Science BV: Amsterdam, The Netherlands, 2012; pp. 501–524. [Google Scholar]
- Wang, L.; Hayes, J.; Sarver, C.; Byrum, B.; Zhang, Y. Porcine deltacoronavirus: Histological lesions and genetic characterization. Arch. Virol. 2016, 161, 171–175. [Google Scholar] [CrossRef]
- Park, J.; Shin, H. Porcine epidemic diarrhea virus infects and replicates in porcine alveolar macrophages. Virus Res. 2014, 191, 143–152. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Horie, M.; Onoue, K.; Noguchi, M.; Akioka, K.; Masatani, T.; Miura, N.; Ozawa, M.; Tanimoto, A. Development of a Model of Porcine Epidemic Diarrhea in Microminipigs. Vet. Pathol. 2019, 56, 711–714. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Liang, X.; Lou, F.; Oglesbee, M.; Krakowka, S.; Li, J. Origin, Evolution, and Virulence of Porcine Deltacoronaviruses in the United States. MBio 2015, 6, 1472–1474. [Google Scholar] [CrossRef]
- Jung, K.; Hu, H.; Eyerly, B.; Lu, Z.; Chepngeno, J.; Saif, L.J. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg. Infect. Dis. 2015, 21, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Vitosh-Sillman, S.; Loy, J.D.; Brodersen, B.; Kelling, C.; Doster, A.; Topliff, C.; Nelson, E.; Bai, J.; Schirtzinger, E.; Poulsen, E.; et al. Experimental infection of conventional nursing pigs and their dams with Porcine deltacoronavirus. J. Vet. Diagn. Investig. 2016, 28, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gauger, P.; Stafne, M.; Thomas, J.; Arruda, P.; Burrough, E.; Madson, D.; Brodie, J.; Magstadt, D.; Derscheid, R.; et al. Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5-day-old neonatal piglets. Virology 2015, 482, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Fan, H.; Lan, T.; Yang, X.L.; Shi, W.F.; Zhang, W.; Zhu, Y.; Zhang, Y.W.; Xie, Q.M.; Mani, S.; et al. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 2018, 556, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Vlasova, A.N.; Kenney, S.P.; Saif, L.J. Emerging and re-emerging coronaviruses in pigs. Curr. Opin. Virol. 2019, 34, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, Y.; Shi, H.; Chen, J.; Zhang, X.; Wang, X.; Zhou, L.; Liu, J.; Zhang, J.; Ji, Z.; et al. Swine acute diarrhea syndrome coronavirus-induced apoptosis is caspase- and cyclophilin D- dependent. Emerg. Microbes Infect. 2020, 9, 439–456. [Google Scholar] [CrossRef]
- Mora-Díaz, J.C.; Piñeyro, P.E.; Houston, E.; Zimmerman, J.; Giménez-Lirola, L.G. Porcine hemagglutinating encephalomyelitis virus: A review. Front. Vet. Sci. 2019, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Lu, H.; Zhao, K.; Liu, W.; Gao, W.; Lan, Y.; Zhao, J.; Tang, B.; Song, D.; He, W.; et al. Identification and genetic characterization of porcine hemagglutinating encephalomyelitis virus from domestic piglets in China. Arch. Virol. 2014, 159, 2329–2337. [Google Scholar] [CrossRef]
- Quiroga, M.A.; Cappuccio, J.; Piñeyro, P.; Basso, W.; Moré, G.; Kienast, M.; Schonfeld, S.; Cáncer, J.L.; Arauz, S.; Pintos, M.E.; et al. Hemagglutinating encephalomyelitis coronavirus infection in pigs, Argentina. Emerg. Infect. Dis. 2008, 14, 484–486. [Google Scholar] [CrossRef]
- Cox, E.; Hooyberghs, J.; Pensaert, M.B. Sites of replication of a porcine respiratory coronavirus related to transmissible gastroenteritis virus. Res. Vet. Sci. 1990, 48, 165–169. [Google Scholar] [CrossRef]
- Opriessnig, T.; Giménez-Lirola, L.G.; Halbur, P.G. Polymicrobial respiratory disease in pigs. Anim. Health Res. Rev. 2011, 12, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Evermann, J.F.; Foreyt, W.; Maag-Miller, L.; Leathers, C.W.; McKeirnan, A.J.; LeaMaster, B. Acute hemorrhagic enteritis associated with canine coronavirus and parvovirus infections in a captive coyote population. J. Am. Vet. Med. Assoc. 1980, 177, 784–786. [Google Scholar] [PubMed]
- Foreyt, W.J.; Evermann, J.F. Serologic survey of canine coronavirus in wild coyotes in the western United States, 1972–1982. J. Wildl. Dis. 1985, 21, 428–430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsunemitsu, H.; El-Kanawati, Z.R.; Smith, D.R.; Reed, H.H.; Saif, L.J. Isolation of coronaviruses antigenically indistinguishable from bovine coronavirus from wild ruminants with diarrhea. J. Clin. Microbiol. 1995, 33, 3264–3269. [Google Scholar] [CrossRef] [PubMed]
- Evermann, J.F.; Heeney, J.L.; Roelke, M.E.; McKeirnan, A.J.; O’Brien, S.J. Biological and pathological consequences of feline infectious peritonitis virus infection in the cheetah. Arch. Virol. 1988, 102, 155–171. [Google Scholar] [CrossRef]
- Heeney, J.L.; Evermann, J.F.; McKeirnan, A.J.; Marker-Kraus, L.; Roelke, M.E.; Bush, M.; Wildt, D.E.; Meltzer, D.G.; Colly, L.; Lukas, J. Prevalence and implications of feline coronavirus infections of captive and free-ranging cheetahs (Acinonyx jubatus). J. Virol. 1990, 64, 1964–1972. [Google Scholar] [CrossRef]
- Van Rensburg, I.B.; Silkstone, M.A. Concomitant feline infectious peritonitis and toxoplasmosis in a cheetah (Acinonyx jubatus). J. S. Afr. Vet. Assoc. 1984, 55, 205–207. [Google Scholar]
- Evermann, J.; Roelke, M.; Briggs, M. Feline coronavirus infections of cheetahs. Feline Pract. 1986, 16, 21–28. [Google Scholar]
- Stephenson, N.; Swift, P.; Moeller, R.B.; Worth, S.J.; Foley, J. Feline infectious peritonitis in a mountain lion (Puma concolor), California, USA. J. Wildl. Dis. 2013, 49, 408–412. [Google Scholar] [CrossRef]
- Paul-Murphy, J.; Work, T.; Hunter, D.; McFie, E.; Fjelline, D. Serologic survey and serum biochemical reference ranges of the free-ranging mountail lion (Felis Concolor) in California. J. Wildl. Dis. 1994, 30, 205–215. [Google Scholar] [CrossRef][Green Version]
- Mwase, M.; Shimada, K.; Mumba, C.; Yabe, J.; Squarre, D.; Madarame, H. Positive Immunolabelling for Feline Infectious Peritonitis in an African Lion (Panthera leo) with Bilateral Panuveitis. J. Comp. Pathol. 2015, 152, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Watt, N.J.; MacIntyre, N.J.; McOrist, S. An extended outbreak of infectious peritonitis in a closed colony of european wildcats (Felis silvestris). J. Comp. Pathol. 1993, 108, 73–79. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, J.H.; Yoon, S.W.; Noh, J.Y.; Ahn, M.J.; Kim, Y.; Jeong, D.G.; Kim, H.K. Detection of bovine coronavirus in nasal swab of non-captive wild water deer, Korea. Transbound. Emerg. Dis. 2018, 65, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Kim, H.R.; Bae, Y.C.; Lee, O.S.; Oem, J.K. Detection and characterization of bovine-like coronaviruses from four species of zoo ruminants. Vet. Microbiol. 2011, 148, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Hasoksuz, M.; Alekseev, K.; Vlasova, A.; Zhang, X.; Spiro, D.; Halpin, R.; Wang, S.; Ghedin, E.; Saif, L.J. Biologic, Antigenic, and Full-Length Genomic Characterization of a Bovine-Like Coronavirus Isolated from a Giraffe. J. Virol. 2007, 81, 4981–4990. [Google Scholar] [CrossRef] [PubMed]
- Cebra, C.K.; Mattson, D.E.; Baker, R.J.; Sonn, R.J.; Dearing, P.L. Potential pathogens in feces from unweaned llamas and alpacas with diarrhea. J. Am. Vet. Med. Assoc. 2003, 223, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Cebra, C.K.; Baker, R.J.; Mattson, D.E.; Cohen, S.A.; Alvarado, D.E.; Rohrmann, G.F. Analysis of the genome sequence of an alpaca coronavirus. Virology 2007, 365, 198–203. [Google Scholar] [CrossRef]
- Vergara-Alert, J.; van den Brand, J.M.A.; Widagdo, W.; Muñoz, M.; Raj, V.S.; Schipper, D.; Solanes, D.; Cordón, I.; Bensaid, A.; Haagmans, B.L.; et al. Livestock susceptibility to infection with middle east respiratory syndrome coronavirus. Emerg. Infect. Dis. 2017, 23, 232–240. [Google Scholar] [CrossRef]
- Crossley, B.M.; Mock, R.E.; Callison, S.A.; Hietala, S.K. Identification and characterization of a novel alpaca respiratory coronavirus most closely related to the human coronavirus 229E. Viruses 2012, 4, 3689–3700. [Google Scholar] [CrossRef]
- Killerby, M.E.; Biggs, H.M.; Midgley, C.M.; Gerber, S.I.; Watson, J.T. Middle east respiratory syndrome coronavirus transmission. Emerg. Infect. Dis. 2020, 26, 191–198. [Google Scholar] [CrossRef]
- Haagmans, B.L.; Al Dhahiry, S.H.S.; Reusken, C.B.E.M.; Raj, V.S.; Galiano, M.; Myers, R.; Godeke, G.J.; Jonges, M.; Farag, E.; Diab, A.; et al. Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect. Dis. 2014, 14, 140–145. [Google Scholar] [CrossRef]
- Hemida, M.G.; Elmoslemany, A.; Al-Hizab, F.; Alnaeem, A.; Almathen, F.; Faye, B.; Chu, D.K.W.; Perera, R.A.P.M.; Peiris, M. Dromedary Camels and the Transmission of Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Transbound. Emerg. Dis. 2017, 64, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Farag, E.; Sikkema, R.S.; Mohamedani, A.A.; De Bruin, E.; Oude Munnink, B.B.; Chandler, F.; Kohl, R.; Van Der Linden, A.; Okba, N.M.A.; Haagmans, B.L.; et al. MERS-CoV in camels but not camel handlers, Sudan, 2015 and 2017. Emerg. Infect. Dis. 2019, 25, 2333–2335. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Wernery, U.; Wong, E.Y.M.; Tsang, A.K.L.; Johnson, B.; Yip, C.C.Y.; Lau, C.C.Y.; Sivakumar, S.; Cai, J.P.; et al. Novel betacoronavirus in dromedaries of the Middle East, 2013. Emerg. Infect. Dis. 2014, 20, 560–572. [Google Scholar] [CrossRef]
- Decaro, N.; Martella, V.; Elia, G.; Campolo, M.; Mari, V.; Desario, C.; Lucente, M.S.; Lorusso, A.; Greco, G.; Corrente, M.; et al. Biological and genetic analysis of a bovine-like coronavirus isolated from water buffalo (Bubalus bubalis) calves. Virology 2008, 370, 213–222. [Google Scholar] [CrossRef]
- Decaro, N.; Cirone, F.; Mari, V.; Nava, D.; Tinelli, A.; Elia, G.; Di Sarno, A.; Martella, V.; Colaianni, M.L.; Aprea, G.; et al. Characterisation of bubaline coronavirus strains associated with gastroenteritis in water buffalo (Bubalus bubalis) calves. Vet. Microbiol. 2010, 145, 245–251. [Google Scholar] [CrossRef]
- Majhdi, F.; Minocha, H.C.; Kapil, S. Isolation and characterization of a coronavirus from elk calves with diarrhea. J. Clin. Microbiol. 1997, 35, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Chasey, D.; Reynolds, D.J.; Bridger, J.C.; Debney, T.G.; Scott, A.C. Identification of coronaviruses in exotic species of Bovidae. Vet. Rec. 1984, 115, 602–603. [Google Scholar] [CrossRef]
- Larsen, A.E.; Gorham, J.R. A new mink enteritis: An initial report. Vet. Med. Small Anim. Clin. 1975, 70, 291–292. [Google Scholar]
- Vlasova, A.N.; Halpin, R.; Wang, S.; Ghedin, E.; Spiro, D.J.; Saif, L.J. Molecular characterization of a new species in the genus Alphacoronavirus associated with mink epizootic catarrhal gastroenteritis. J. Gen. Virol. 2011, 92, 1369–1379. [Google Scholar] [CrossRef]
- Oreshkova, N.; Molenaar, R.J.; Vreman, S.; Harders, F.; Oude Munnink, B.B.; Hakze-van der Honing, R.W.; Gerhards, N.; Tolsma, P.; Bouwstra, R.; Sikkema, R.S.; et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance 2020, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Molnar, B.; Duchamp, C.; Möstl, K.; Diehl, P.A.; Betschart, B. Comparative survey of canine parvovirus, canine distemper virus and canine enteric coronavirus infection in free-ranging wolves of central Italy and south-eastern France. Eur. J. Wildl. Res. 2014, 60, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Alfano, F.; Dowgier, G.; Valentino, M.P.; Galiero, G.; Tinelli, A.; Decaro, N.; Fusco, G. Identification of pantropic canine coronavirus in a wolf (Canis lupus italicus) in Italy. J. Wildl. Dis. 2019, 55, 504–508. [Google Scholar] [PubMed]
- Rosa, G.M.; Santos, N.; Grøndahl-Rosado, R.; Fonseca, F.P.; Tavares, L.; Neto, I.; Cartaxeiro, C.; Duarte, A. Unveiling patterns of viral pathogen infection in free-ranging carnivores of northern Portugal using a complementary methodological approach. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101432. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jing, H.; Xu, H.; Jiang, X.; Kan, B.; Liu, Q.; Wan, K.; Cui, B.; Zheng, H.; Cui, Z.; et al. Surveillance on severe acute respiratory syndrome associated coronavirus in animals at a live animal market of Guangzhou in 2004. Zhonghua Liu Xing Bing Xue Za Zhi 2005, 26, 84–87. [Google Scholar] [PubMed]
- Guan, Y.; Zheng, B.J.; He, Y.Q.; Liu, X.L.; Zhuang, Z.X.; Cheung, C.L.; Luo, S.W.; Li, P.H.; Zhang, L.J.; Guan, Y.J.; et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science 2003, 302, 276–278. [Google Scholar] [CrossRef]
- Martin, H.D.; Zeidner, N.S. Concomitant Cryptosporidia, Coronavirus and Parvovirus Infection in a Raccoon (Procyon lotor). J. Wildl. Dis. 1992, 28, 113–115. [Google Scholar] [CrossRef]
- Dong, B.Q.; Liu, W.; Fan, X.H.; Vijaykrishna, D.; Tang, X.C.; Gao, F.; Li, L.F.; Li, G.J.; Zhang, J.X.; Yang, L.Q.; et al. Detection of a Novel and Highly Divergent Coronavirus from Asian Leopard Cats and Chinese Ferret Badgers in Southern China. J. Virol. 2007, 81, 6920–6926. [Google Scholar] [CrossRef]
- Song, H.D.; Tu, C.C.; Zhang, G.W.; Wang, S.Y.; Zheng, K.; Lei, L.C.; Chen, Q.X.; Gao, Y.W.; Zhou, H.Q.; Xiang, H.; et al. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl. Acad. Sci. USA 2005, 102, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- East, M.L.; Moestl, K.; Benetka, V.; Pitra, C.; Höner, O.P.; Wachter, B.; Hofer, H. Coronavirus infection of spotted hyenas in the Serengeti ecosystem. Vet. Microbiol. 2004, 102, 1–9. [Google Scholar] [CrossRef]
- Goller, K.V.; Fickel, J.; Hofer, H.; Beier, S.; East, M.L. Coronavirus genotype diversity and prevalence of infection in wild carnivores in the Serengeti National Park, Tanzania. Arch. Virol. 2013, 158, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Kallies, R.; Philipps, H.; Gopner, G.; Muller, M.A.; Eckerle, I.; Brunink, S.; Drosten, C.; Drexler, J.F. Characterization of a Novel Betacoronavirus Related to Middle East Respiratory Syndrome Coronavirus in European Hedgehogs. J. Virol. 2014, 88, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Monchatre-Leroy, E.; Boué, F.; Boucher, J.-M.; Renault, C.; Moutou, F.; Ar Gouilh, M.; Umhang, G. Identification of Alpha and Beta Coronavirus in Wildlife Species in France: Bats, Rodents, Rabbits, and Hedgehogs. Viruses 2017, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Delogu, M.; Cotti, C.; Lelli, D.; Sozzi, E.; Trogu, T.; Lavazza, A.; Garuti, G.; Castrucci, M.R.; Vaccari, G.; De Marco, M.A.; et al. Eco-Virological Preliminary Study of Potentially Emerging Pathogens in Hedgehogs (Erinaceus europaeus) Recovered at a Wildlife Treatment and Rehabilitation Center in Northern Italy. Animals 2020, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, X.-D.; Liao, Y.; Guan, X.-Q.; Guo, W.-P.; Xing, J.-G.; Holmes, E.C.; Zhang, Y.-Z. Discovery of a Highly Divergent Coronavirus in the Asian House Shrew from China Illuminates the Origin of the Alphacoronaviruses. J. Virol. 2017, 91, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Patrono, L.V.; Samuni, L.; Corman, V.M.; Nourifar, L.; Röthemeier, C.; Wittig, R.M.; Drosten, C.; Calvignac-Spencer, S.; Leendertz, F.H. Human coronavirus OC43 outbreak in wild chimpanzees, Côte d’Ivoire, 2016 correspondence. Emerg. Microbes Infect. 2018, 7, 2–5. [Google Scholar] [CrossRef]
- Mihindukulasuriya, K.A.; Wu, G.; St. Leger, J.; Nordhausen, R.W.; Wang, D. Identification of a Novel Coronavirus from a Beluga Whale by Using a Panviral Microarray. J. Virol. 2008, 82, 5084–5088. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Tsang, A.K.L.; Hui, S.-W.; Fan, R.Y.Y.; Martelli, P.; Yuen, K.-Y. Discovery of a Novel Bottlenose Dolphin Coronavirus Reveals a Distinct Species of Marine Mammal Coronavirus in Gammacoronavirus. J. Virol. 2014, 88, 1318–1331. [Google Scholar] [CrossRef]
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar]
- Wang, W.; Lin, X.; Guo, W.; Zhou, R.; Wang, M.; Wang, C.-Q.; Ge, S.; Mei, S.-H.; Li, M.-H.; Shi, M.; et al. Discovery, diversity and evolution of novel coronaviruses sampled from rodents in China. Virology 2015, 474, 19–27. [Google Scholar] [CrossRef]
- Ge, X.Y.; Yang, W.H.; Zhou, J.H.; Li, B.; Zhang, W.; Shi, Z.L.; Zhang, Y.Z. Detection of alpha- and betacoronaviruses in rodents from Yunnan, China. Virol. J. 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Woo, P.C.Y.; Li, K.S.M.; Tsang, A.K.L.; Fan, R.Y.Y.; Luk, H.K.H.; Cai, J.-P.; Chan, K.-H.; Zheng, B.-J.; Wang, M.; et al. Discovery of a Novel Coronavirus, China Rattus Coronavirus HKU24, from Norway Rats Supports the Murine Origin of Betacoronavirus 1 and Has Implications for the Ancestor of Betacoronavirus Lineage A. J. Virol. 2015, 89, 3076–3092. [Google Scholar] [CrossRef] [PubMed]
- Tsoleridis, T.; Onianwa, O.; Horncastle, E.; Dayman, E.; Zhu, M.; Danjittrong, T.; Wachtl, M.; Behnke, J.M.; Chapman, S.; Strong, V.; et al. Discovery of novel alphacoronaviruses in European rodents and shrews. Viruses 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; Li, F.; Dragomir, I.; Chua, M.M.W.; Gregory, B.D.; Weiss, S.R. Analysis of the Host Transcriptome from Demyelinating Spinal Cord of Murine Coronavirus-Infected Mice. PLoS ONE 2013, 8, e75346c. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, J.L.; Peña, C.; Retegui, L.A. Autoimmune hepatitis-like disease in C57BL/6 mice infected with mouse hepatitis virus A59. Int. Immunopharmacol. 2011, 11, 1591–1598. [Google Scholar] [CrossRef]
- Homberger, F.R. Enterotropic mouse hepatitis virus. Lab. Anim. 1997, 31, 97–115. [Google Scholar] [CrossRef]
- Compton, S.R.; Ball-Goodrich, L.J.; Johnson, L.K.; Johnson, E.A.; Paturzo, F.X.; Macy, J.D. Pathogenesis of enterotropic mouse hepatitis virus in immunocompetent and immunodeficient mice. Comp. Med. 2004, 54, 681–689. [Google Scholar]
- Percy, D.H.; Barthold, S.W. Pathology of Laboratory Rodents and Rabbits, 4th ed.; Barthold, S.W., Griffey, S.M., Percy, D.H., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2016; ISBN 9781118924051. [Google Scholar]
- Blau, D.M.; Turbide, C.; Tremblay, M.; Olson, M.; Létourneau, S.; Michaliszyn, E.; Jothy, S.; Holmes, K.V.; Beauchemin, N. Targeted Disruption of the Ceacam1(MHVR) Gene Leads to Reduced Susceptibility of Mice to Mouse Hepatitis Virus Infection. J. Virol. 2001, 75, 8173–8186. [Google Scholar] [CrossRef]
- Taguchi, F.; Hirai-Yuki, A. Mouse hepatitis virus receptor as a determinant of the mouse susceptibility to MHV infection. Front. Microbiol. 2012, 3, 1999–2002. [Google Scholar] [CrossRef]
- Leibowitz, J.L.; Srinivasa, R.; Williamson, S.T.; Chua, M.M.; Liu, M.; Wu, S.; Kang, H.; Ma, X.-Z.; Zhang, J.; Shalev, I.; et al. Genetic Determinants of Mouse Hepatitis Virus Strain 1 Pneumovirulence. J. Virol. 2010, 84, 9278–9291. [Google Scholar] [CrossRef][Green Version]
- Barthold, S.W.; Smith, A.L. Mouse hepatitis virus S in weanling Swiss mice following intranasal inoculation. Lab. Anim. Sci. 1983, 33, 355–360. [Google Scholar] [PubMed]
- Skinner, D.; Marro, B.S.; Lane, T.E. Chemokine CXCL10 and Coronavirus-Induced Neurologic Disease. Viral Immunol. 2019, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Chatterjee, D.; Addya, S.; Khan, R.S.; Kenyon, L.C.; Choe, A.; Cohrs, R.J.; Shindler, K.S.; Das Sarma, J. Demyelinating strain of mouse hepatitis virus infection bridging innate and adaptive immune response in the induction of demyelination. Clin. Immunol. 2016, 170, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Shindler, K.S.; Chatterjee, D.; Biswas, K.; Goyal, A.; Dutt, M.; Nassrallah, M.; Khan, R.S.; Das Sarma, J. Macrophage-Mediated Optic Neuritis Induced by Retrograde Axonal Transport of Spike Gene Recombinant Mouse Hepatitis Virus. J. Neuropathol. Exp. Neurol. 2011, 70, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Khan, R.S.; Dine, K.; Das Sarma, J.; Shindler, K.S. Intracranial inoculation is more potent than intranasal inoculation for inducing optic neuritis in the mouse hepatitis virus-induced model of multiple sclerosis. Front. Cell. Infect. Microbiol. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Singh, M.; Kishore, A.; Maity, D.; Sunanda, P.; Krishnarjuna, B.; Vappala, S.; Raghothama, S.; Kenyon, L.C.; Pal, D.; Sarma, J. Das A proline insertion-deletion in the spike glycoprotein fusion peptide of mouse hepatitis virus strongly alters neuropathology. J. Biol. Chem. 2019, 294, 8064–8087. [Google Scholar] [CrossRef]
- Kakizaki, M.; Kashiwazaki, H.; Watanabe, R. Mutant murine hepatitis virus-induced apoptosis in the hippocampus. Jpn. J. Infect. Dis. 2014, 67, 9–16. [Google Scholar] [CrossRef][Green Version]
- Kashiwazaki, H.; Nomura, R.; Matsuyama, S.; Taguchi, F.; Watanabe, R. Spongiform degeneration induced by neuropathogenic murine coronavirus infection. Pathol. Int. 2011, 61, 184–191. [Google Scholar] [CrossRef]
- Hooks, J.J.; Percopo, C.; Wang, Y.; Detrick, B. Retina and retinal pigment epithelial cell autoantibodies are produced during murine coronavirus retinopathy. J. Immunol. 1993, 151, 3381–3389. [Google Scholar]
- Bender, S.J.; Weiss, S.R. Pathogenesis of murine coronavirus in the central nervous system. J. Neuroimmune Pharmacol. 2010, 5, 336–354. [Google Scholar] [CrossRef]
- Tardieu, M.; Goffinet, A.; Harmant-van Rijckevorsel, G.; Lyon, G. Ependymitis, leukoencephalitis, hydrocephalus, and thrombotic vasculitis following chronic infection by mouse hepatitis virus 3 (MHV 3). Acta Neuropathol. 1982, 58, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Virelizier, J.L.; Dayan, A.D.; Allison, A.C. Neuropathological effect of persistent infection of mice by mouse hepatitis virus. Infect. Immun. 1975, 12, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Compton, S.R.; Ball-Goodrich, L.J.; Zeiss, C.J.; Johnson, L.K.; Johnson, E.A.; Macy, J.D. Pathogenesis of mouse hepatitis virus infection in gamma interferon-deficient mice is modulated by co-infection with Helicobacter hepaticus. Comp. Med. 2003, 53, 197–206. [Google Scholar] [PubMed]
- Compton, S.R.; Ball-Goodrich, L.J.; Paturzo, F.X.; Macy, J.D. Transmission of enterotropic mouse hepatitis virus from immunocompetent and immunodeficient mice. Comp. Med. 2004, 54, 29–35. [Google Scholar] [PubMed]
- Manjunath, S.; Kulkarni, P.G.; Nagavelu, K.; Samuel, R.J.; Srinivasan, S.; Ramasamy, N.; Hegde, N.R.; Gudde, R.S. Sero-prevalence of rodent pathogens in India. PLoS ONE 2015, 10, e0131706. [Google Scholar] [CrossRef]
- Percy, D.H.; Lynch, J.A.; Descôteaux, J.P. Central Nervous System Lesions in Suckling Mice and Rats Inoculated Intranasally with Sialodacryoadenitis Virus. Vet. Pathol. 1986, 23, 42–49. [Google Scholar] [CrossRef]
- Compton, S.R.; Vivas-Gonzalez, B.E.; Macy, J.D. Reverse transcriptase polymerase chain reaction-based diagnosis and molecular characterization of a new rat coronavirus strain. Lab. Anim. Sci. 1999, 49, 506–513. [Google Scholar] [PubMed]
- Liang, C.T.; Shih, A.; Chang, Y.H.; Liu, C.W.; Lee, Y.T.; Hsieh, W.C.; Huang, Y.L.; Huang, W.T.; Kuang, C.H.; Lee, K.H.; et al. Microbial contaminations of laboratory mice and rats in Taiwan from 2004 to 2007. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 381–386. [Google Scholar] [PubMed]
- Yoo, D.; Pei, Y.; Christie, N.; Cooper, M. Primary structure of the sialodacryoadenitis virus genome: Sequence of the structural-protein region and its application for differential diagnosis. Clin. Diagn. Lab. Immunol. 2000, 7, 568–573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kunita, S.; Mori, M.; Terada, E. Sequence Analysis of the Nucleocapsid Protein Gene of Rat Coronavirus SDAV-681. Virology 1993, 193, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.N.; Percy, D.H.; Jonas, A.M. Characterization of the virus of sialodacryoadenitis of rats: A member of the coronavirus group. J. Infect. Dis. 1972, 126, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.J.; Manzer, R.; Miura, T.A.; Groshong, S.D.; Ito, Y.; Travanty, E.A.; Leete, J.; Holmes, K.V.; Mason, R.J. Rat respiratory coronavirus infection: Replication in airway and alveolar epithelial cells and the innate immune response. J. Gen. Virol. 2009, 90, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.A.; Wang, J.; Holmes, K.V.; Mason, R.J. Rat coronaviruses infect rat alveolar type I epithelial cells and induce expression of CXC chemokines. Virology 2007, 369, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Schoeb, T.R.; Davis, J.K.; Simecka, J.W.; Cassell, G.H.; Lindsey, J.R. Comparative severity of respiratory lesions of sialodacryoadenitis virus and Sendai virus infections in LEW and F344 rats. Vet. Pathol. 1995, 32, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Bihun, C.G.; Percy, D.H. Morphologic changes in the nasal cavity associated with sialodacryoadenitis virus infection in the Wistar rat. Vet. Pathol. 1995, 32, 1–10. [Google Scholar] [CrossRef]
- Wojcinski, Z.W.; Percy, D.H. Sialodacryoadenitis Virus-associated Lesions in the Lower Respiratory Tract of Rats. Vet. Pathol. 1986, 23, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Schoeb, T.R. Respiratory Diseases of Rodents. Vet. Clin. N. Am. Exot. Anim. Pract. 2000, 3, 481–496. [Google Scholar] [CrossRef]
- Wickham, L.A.; Huang, Z.; Lambert, R.W.; Sullivan, D.A. Effect of sialodacryoadenitis virus exposure on acinar epithelial cells from the rat lacrimal gland. Ocul. Immunol. Inflamm. 1997, 5, 181–195. [Google Scholar] [CrossRef]
- Jaax, G.P.; Jaax, N.K.; Petrali, J.P.; Corcoran, K.D.; Vogel, A.P. Coronavirus-like virions associated with a wasting syndrome in guinea pigs. Lab. Anim. Sci. 1990, 40, 375–378. [Google Scholar]
- Liang, L.; He, C.; Lei, M.; Li, S.; Hao, Y.; Zhu, H.; Duan, Q. Pathology of guinea pigs experimentally infected with a novel reovirus and coronavirus isolated from SARS patients. DNA Cell Biol. 2005, 24, 485–490. [Google Scholar] [CrossRef]
- Kerr, P.J.; Donnelly, T.M. Viral Infections of Rabbits. Vet. Clin. N. Am. Exot. Anim. Pract. 2013, 16, 437–468. [Google Scholar] [CrossRef] [PubMed]
- Descôteaux, J.P.; Lussier, G. Experimental infection of young rabbits with a rabbit enteric coronavirus. Can. J. Vet. Res. 1990, 54, 473–476. [Google Scholar]
- Cavanagh, D. Coronaviruses in poultry and other birds. Avian Pathol. 2005, 34, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Miłek, J.; Blicharz-Domańska, K. Coronaviruses in avian species-review with focus on epidemiology and diagnosis in wild birds. J. Vet. Res. 2018, 62, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavi. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Cho, K.O.; Ward, L.A.; Saif, L.J.; Saif, Y.M. Experimental bovine coronavirus in turkey poults and young chickens. Avian Dis. 2001, 45, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Tang, Y.; Saif, Y.M. Pathogenicity of Turkey coronavirus in Turkeys and chickens. Avian Dis. 2003, 47, 515–522. [Google Scholar] [CrossRef][Green Version]
- Chu, D.K.W.; Leung, C.Y.H.; Gilbert, M.; Joyner, P.H.; Ng, E.M.; Tse, T.M.; Guan, Y.; Peiris, J.S.M.; Poon, L.L.M. Avian Coronavirus in Wild Aquatic Birds. J. Virol. 2011, 85, 12815–12820. [Google Scholar] [CrossRef]
- Liais, E.; Croville, G.; Mariette, J.; Delverdier, M.; Lucas, M.N.; Klopp, C.; Lluch, J.; Donnadieu, C.; Guy, J.S.; Corrand, L.; et al. Novel avian coronavirus and fulminating disease in Guinea Fowl, France. Emerg. Infect. Dis. 2014, 20, 105–108. [Google Scholar] [CrossRef]
- Jonassen, C.M.; Kofstad, T.; Larsen, I.L.; Løvland, A.; Handeland, K.; Follestad, A.; Lillehaug, A. Molecular identification and characterization of novel coronaviruses infecting graylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anas platyrhynchos). J. Gen. Virol. 2005, 86, 1597–1607. [Google Scholar] [CrossRef]
- Jordan, B.J.; Hilt, D.A.; Poulson, R.; Stallknecht, D.E.; Jackwood, M.W. Identification of avian coronavirus in wild aquatic birds of the central and eastern USA. J. Wildl. Dis. 2015, 51, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, I.N.A.; van Beurden, S.J.; Weerts, E.A.W.S.; Verheije, M.H. The avian coronavirus spike protein. Virus Res. 2014, 194, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ducatez, M.F. Recommendations for a standardized avian coronavirus (AvCoV) nomenclature: Outcome from discussions within the framework of the European Union COST Action FA1207: “towards control of avian coronaviruses: Strategies for vaccination, diagnosis and surveilla. Avian Pathol. 2016, 45, 602–603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catroxo, M.H.B.; Martins, A.M.C.R.P.F.; Petrella, S.; Curi, N.A.; Melo, N. Research of Viral Agent in Free-living Pigeon Feces (Columba livia) in the City of São Paulo, SP, Brazil, for Transmission Electron Microscopy. Int. J. Morphol. 2011, 29, 628–635. [Google Scholar] [CrossRef]
- Zhuang, Q.; Liu, S.; Zhang, X.; Jiang, W.; Wang, K.; Wang, S.; Peng, C.; Hou, G.; Li, J.; Yu, X.; et al. Surveillance and taxonomic analysis of the coronavirus dominant in pigeons in China. Transbound. Emerg. Dis. 2020, 67, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.H.; Zhu, G.J.; Wu, L.Z.; Hua, G.X. Isolation and characterization of a coronavirus from pigeons with pancreatitis. Am. J. Vet. Res. 2006, 67, 1575–1579. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, G.; Liao, M.; Ren, T.; Xin, C. Study on the S1 gene of the two isolated stain of coronavirus from the wild birds. Chin. J. Prev. Vet. Med. 2005, 27, 380–384. [Google Scholar]
- Barr, D.; Reece, R.; O’Rourke, D.; Button, C.; Faragher, J. Isolation of infectious bronchitis virus from a flock of racing pigeons. Aust. Vet. J. 1988, 65, 228. [Google Scholar] [CrossRef]
- Cook, J.K.A.; Jackwood, M.; Jones, R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012, 41, 239–250. [Google Scholar] [CrossRef]
- Day, J.M.; Gonder, E.; Jennings, S.; Rives, D.; Robbins, K.; Tilley, B.; Wooming, B. Investigating turkey enteric coronavirus circulating in the Southeastern United States and Arkansas during 2012 and 2013. Avian Dis. 2014, 58, 313–317. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Chen, J.; Kong, X.; Shao, Y.; Han, Z.; Feng, L.; Cai, X.; Gu, S.; Liu, M. Isolation of avian infectious bronchitis coronavirus from domestic peafowl (Pavo cristatus) and teal (Anas). J. Gen. Virol. 2005, 86, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Ghany, H.M.A.; Elseddawy, N.M. Diagnostic Studies of Infectious Bronchitis Disease in Broilers using Pathological and Molecular Investigations in Kaliobeya Governorate, Egypt. Adv. Environ. Biol. 2019, 13, 1–6. [Google Scholar]
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, M. Review of infectious bronchitis virus around the world. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef] [PubMed]
- De Wit, J.J.S.; Cook, J.K.A.; van der Heijden, H.M.J.F. Infectious bronchitis virus variants: A review of the history, current situation and control measures. Avian Pathol. 2011, 40, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tian, Y.; Guan, R.; Gao, W.; Yang, X.; Zhou, L.; Wang, H. Infectious Bronchitis Virus Infection Induces Apoptosis during Replication in Chicken Macrophage HD11 Cells. Viruses 2017, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, A.; Abdul-Cader, M.S.; Almatrouk, Z.; van der Meer, F.; Cork, S.C.; Gomis, S.; Abdul-Careem, M.F. Induction of innate host responses characterized by production of interleukin (IL)-1β and recruitment of macrophages to the respiratory tract of chickens following infection with infectious bronchitis virus (IBV). Vet. Microbiol. 2018, 215, 1–10. [Google Scholar] [CrossRef]
- Reddy, V.R.A.P.; Trus, I.; Desmarets, L.M.B.; Li, Y.; Theuns, S.; Nauwynck, H.J. Productive replication of nephropathogenic infectious bronchitis virus in peripheral blood monocytic cells, a strategy for viral dissemination and kidney infection in chickens. Vet. Res. 2016, 47, 1–19. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 2019, 73, 529–557. [Google Scholar] [CrossRef]
- Xia, J.; He, X.; Du, L.J.; Liu, Y.Y.; You, G.J.; Li, S.Y.; Liu, P.; Cao, S.J.; Han, X.F.; Huang, Y. Preparation and protective efficacy of a chicken embryo kidney cell-attenuation GI-19/QX-like avian infectious bronchitis virus vaccine. Vaccine 2018, 36, 4087–4094. [Google Scholar] [CrossRef]
- Abou El-Fetouh, M.; Mohamed, M.; Refat, N.; Ahmed, M.; El-Zanaty, A.E. Pathological Studies on Infectious Bronchitis Disease in Chickens. Zagazig Vet. J. 2016, 44, 248–259. [Google Scholar] [CrossRef][Green Version]
- Shivaprasad, H.L. Pathology of birds—An overview. In Proceedings of the C.L. Davis Foundation Conference on Gross Morbid Anatomy of Animals, Washington, DC, USA, 8–12 April 2002; pp. 1–50. [Google Scholar]
- Jackwood, M.; De Wit, S. Infectious bronchitis. In Diseases of Poultry; Swayne, D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 167–188. [Google Scholar]
- Yu, L.; Wang, Z.; Jiang, Y.; Low, S.; Kwang, J. Molecular Epidemiology of Infectious Bronchitis Virus Isolates from China and Southeast Asia. Avian Dis. 2001, 45, 201. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.L.; Cowen, B.S.; Hattel, A.L.; Naqi, S.A.; Wilson, R.A. Detection of viral antigen following exposure of one-day-old chickens to the Holland 52 strain of infectious bronchitis virus. Avian Pathol. 1991, 20, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Toro, H.; Godoy, V.; Larenas, J.; Reyes, E.; Kaleta, E.F. Avian infectious bronchitis: Viral persistence in the harderian gland and histological changes after eyedrop vaccination. Avian Dis. 1996, 40, 114–120. [Google Scholar] [CrossRef]
- Van Ginkel, F.; Van Santen, V.; Gulley, S.; Toro, H. Infectious bronchitis virus in the chicken Harderian gland and lachrymal fluid: Viral load, infectivity, immune cell responses, and effects of viral immunodeficiency. Avian Dis. 2008, 52, 608–617. [Google Scholar] [CrossRef]
- Gough, R.E.; Cox, W.J.; Winkler, C.E.; Sharp, M.W.; Spackman, D. Isolation and identification of infectious bronchitis virus from pheasants. Vet. Rec. 1996, 138, 208–209. [Google Scholar] [CrossRef]
- Lister, S.; Beer, J.; Gough, R.; Holmes, R.; JMW, J.; Orton, R. Outbreaks of nephritis in pheasants (phasianus colchicus) with a possible coronavirus aetiology. Vet. Rec. 1985, 117, 612–613. [Google Scholar] [CrossRef]
- Cavanagh, D.; Mawditt, K.; Welchman, D.D.B.; Britton, P.; Gough, R.E. Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathol. 2002, 31, 81–93. [Google Scholar] [CrossRef]
- Spackman, D.; Cameron, I. Isolation of infectious bronchitis virus from pheasants. Vet. Rec. 1983, 113, 354–355. [Google Scholar] [CrossRef]
- Han, Z.; Liwen, X.; Ren, M.; Sheng, J.; Ma, T.; Sun, J.; Zhao, Y.; Liu, S. Genetic, antigenic and pathogenic characterization of avian coronaviruses isolated from pheasants (Phasianus colchicus) in China. Vet. Microbiol. 2020, 240, 108513. [Google Scholar] [CrossRef]
- Pennycott, T.W. Causes of mortality and culling in adult pheasants. Vet. Rec. 2000, 146, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Welchman, D.B.; Bradbury, J.M.; Cavanagh, D.; Aebischer, N.J. Infectious agents associated with respiratory disease in pheasants. Vet. Rec. 2002, 150, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Qian, H.; Yao, C. Isolation and identification of pigeon coronavirus. J. Shanghai Jiaotong Univ. Agric. Sci. 2005, 23, 275–279. [Google Scholar]
- Brown, P.A.; Courtillon, C.; Weerts, E.A.W.S.; Andraud, M.; Allée, C.; Vendembeuche, A.; Amelot, M.; Rose, N.; Verheije, M.H.; Eterradossi, N. Transmission Kinetics and histopathology induced by European Turkey Coronavirus during experimental infection of specific pathogen free turkeys. Transbound. Emerg. Dis. 2019, 66, 234–242. [Google Scholar] [CrossRef]
- Gomaa, M.H.; Yoo, D.; Ojkic, D.; Barta, J.R. Infection with a pathogenic turkey coronavirus isolate negatively affects growth performance and intestinal morphology of young turkey poults in Canada. Avian Pathol. 2009, 38, 279–286. [Google Scholar] [CrossRef]
- Gomaa, M.H.; Barta, J.R.; Ojkic, D.; Yoo, D. Complete genomic sequence of turkey coronavirus. Virus Res. 2008, 135, 237–246. [Google Scholar] [CrossRef]
- Chien, C.L.; Ching, C.W.; Tsang, L.L. Comparison of 3′-end encoding regions of Turkey coronavirus isolates from Indiana, North Carolina, and Minnesota with chicken infectious bronchitis coronavirus strains. Intervirology 2006, 49, 230–238. [Google Scholar]
- Wang, Y.; Cui, X.; Chen, X.; Yang, S.; Ling, Y.; Song, Q.; Zhu, S.; Sun, L.; Li, C.; Li, Y.; et al. A recombinant infectious bronchitis virus from a chicken with a spike gene closely related to that of a turkey coronavirus. Arch. Virol. 2020, 165, 703–707. [Google Scholar] [CrossRef]
- Bouwman, K.M.; Delpont, M.; Broszeit, F.; Berger, R.; Weerts, E.A.W.S.; Lucas, M.-N.; Delverdier, M.; Belkasmi, S.; Papanikolaou, A.; Boons, G.-J.; et al. Guinea Fowl Coronavirus Diversity Has Phenotypic Consequences for Glycan and Tissue Binding. J. Virol. 2019, 93, 1–11. [Google Scholar] [CrossRef]
- Circella, E.; Camarda, A.; Martella, V.; Bruni, G.; Lavazza, A.; Buonavoglia, C. Coronavirus associated with an enteric syndrome on a quail farm. Avian Pathol. 2007, 36, 251–258. [Google Scholar] [CrossRef]
- Torres, C.A.; Villarreal, L.Y.B.; Ayres, G.R.R.; Richtzenhain, L.J.; Brandão, P.E. An avian coronavirus in quail with respiratory and reproductive signs. Avian Dis. 2013, 57, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Gough, R.E.; Drury, S.E.; Culver, F.; Britton, P.; Cavanagh, D. Isolation of a coronavirus from a green-cheeked Amazon parrot (Amazon viridigenalis Cassin). Avian Pathol. 2006, 35, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Muradrasoli, S.; Bálint, Á.; Wahlgren, J.; Waldenström, J.; Belák, S.; Blomberg, J.; Olsen, B. Prevalence and Phylogeny of Coronaviruses in Wild Birds from the Bering Strait Area (Beringia). PLoS ONE 2010, 5, e13640. [Google Scholar] [CrossRef] [PubMed]
- Granzow, H.; Weiland, F.; Fichtner, D.; Schütze, H.; Karger, A.; Mundt, E.; Dresenkamp, B.; Martin, P.; Mettenleiter, T.C. Identification and ultrastructural characterization of a novel virus from fish. J. Gen. Virol. 2001, 82, 2849–2859. [Google Scholar] [CrossRef] [PubMed]
- Schütze, H. Coronaviruses in Aquatic Organisms. In Aquaculture Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 327–335. [Google Scholar]
- Huchzermeyer, F.W.; Gerdes, G.H.; Foggin, C.M.; Huchzermeyer, K.D.; Limper, L.C. Hepatitis in farmed hatchling Nile crocodiles (Crocodylus niloticus) due to chlamydial infection. J. S. Afr. Vet. Assoc. 1994, 65, 20–22. [Google Scholar] [PubMed]
- Chams, N.; Chams, S.; Badran, R.; Shams, A.; Araji, A.; Raad, M.; Mukhopadhyay, S.; Stroberg, E.; Duval, E.J.; Barton, L.M.; et al. COVID-19: A Multidisciplinary Review. Front. Public Heal. 2020, 8, 1–20. [Google Scholar] [CrossRef]
- Marraha, F.; Al Faker, I.; Gallouj, S. A Review of the Dermatological Manifestations of Coronavirus Disease 2019 (COVID-19). Dermatol. Res. Pract. 2020, 2020, 9360476. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain. Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef]
- Gu, J.; Korteweg, C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007, 170, 1136–1147. [Google Scholar] [CrossRef]
- Hwang, D.M.; Chamberlain, D.W.; Poutanen, S.M.; Low, D.E.; Asa, S.L.; Butany, J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod. Pathol. 2005, 18, 1–10. [Google Scholar] [CrossRef]
- Van Den Brand, J.M.A.; Smits, S.L.; Haagmans, B.L. Pathogenesis of Middle East respiratory syndrome coronavirus. J. Pathol. 2015, 235, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Alsaad, K.O.; Hajeer, A.H.; Al Balwi, M.; Al Moaiqel, M.; Al Oudah, N.; Al Ajlan, A.; AlJohani, S.; Alsolamy, S.; Gmati, G.E.; Balkhy, H.; et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection—Clinicopathological and ultrastructural study. Histopathology 2018, 72, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, X.; Tong, Q.; Li, W.; Wang, B.; Sutter, K.; Trilling, M.; Lu, M.; Dittmer, U.; Yang, D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020, 92, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; Najafian, B.; Deutsch, G.; Lacy, J.M.; Williams, T.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 2020, 396, 320–332. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.M.D.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Hanley, B.; Naresh, K.N.; Roufosse, C.; Nicholson, A.G.; Weir, J.; Cooke, G.S.; Thursz, M.; Manousou, P.; Corbett, R.; Goldin, R.; et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 2020, 1, e245–e253. [Google Scholar] [CrossRef]
- Ghosh, A.; Colling, R. An overview of COVID-19 for diagnostic pathologists: Clinicopathological correlation and diagnostic techniques. Diagnostic Histopathol. 2020, 1–8. [Google Scholar] [CrossRef]
- Martines, R.B.; Ritter, J.M.; Matkovic, E.; Gary, J.; Bollweg, B.C.; Bullock, H.; Goldsmith, C.S.; Silva-Flannery, L.; Seixas, J.N.; Reagan-Steiner, S.; et al. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, united states. Emerg. Infect. Dis. 2020, 26, 2005–2015. [Google Scholar] [CrossRef]
- Infection with SARS-CoV-2 in Animals. Available online: https://www.oie.int/fileadmin/Home/MM/A_Factsheet_SARS-CoV-2__1_.pdf (accessed on 1 October 2020).
- McMahon, B.J.; Morand, S.; Gray, J.S. Ecosystem change and zoonoses in the Anthropocene. Zoonoses Public Health 2018, 65, 755–765. [Google Scholar] [CrossRef]
- Gouilh, M.A.; Puechmaille, S.J.; Gonzalez, J.P.; Teeling, E.; Kittayapong, P.; Manuguerra, J.C. SARS-Coronavirus ancestor’s foot-prints in South-East Asian bat colonies and the refuge theory. Infect. Genet. Evol. 2011, 11, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Baric, R.S.; Fu, K.; Chen, W.; Yount, B. High Recombination and Mutation Rates in Mouse Hepatitis Virus Suggest that Coronaviruses may be Potentially Important Emerging Viruses. In Corona- and Related Viruses. Advances in Experimental Medicine and Biology; Talbot, P., Levy, G., Eds.; Springer: Boston, MA, USA, 1995; pp. 571–576. [Google Scholar]
- Baric, R.S.; Yount, B.; Hensley, L.; Peel, S.A.; Chen, W. Episodic evolution mediates interspecies transfer of a murine coronavirus. J. Virol. 1997, 71, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Thackray, L.B.; Holmes, K.V. Amino acid substitutions and an insertion in the spike glycoprotein extend the host range of the murine coronavirus MHV-A59. Virology 2004, 324, 510–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woo, P.C.Y.; Lau, S.K.P.; Huang, Y.; Yuen, K.Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. 2009, 234, 1117–1127. [Google Scholar] [CrossRef]
- Morse, S.S.; Mazet, J.A.; Woolhouse, M.; Parrish, C.R.; Carroll, D.; Karesh, W.B.; Zambrana-Torrelio, C.; Lipkin, W.I.; Daszak, P. Prediction and prevention of the next pandemic zoonosis. Lancet 2012, 380, 1956–1965. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).