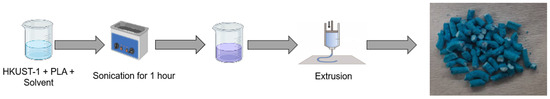
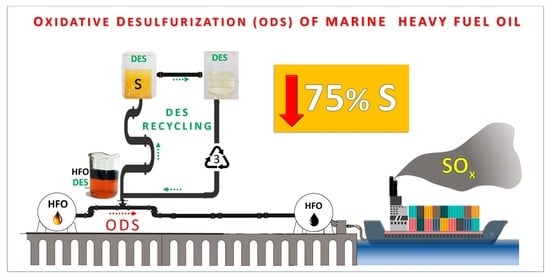
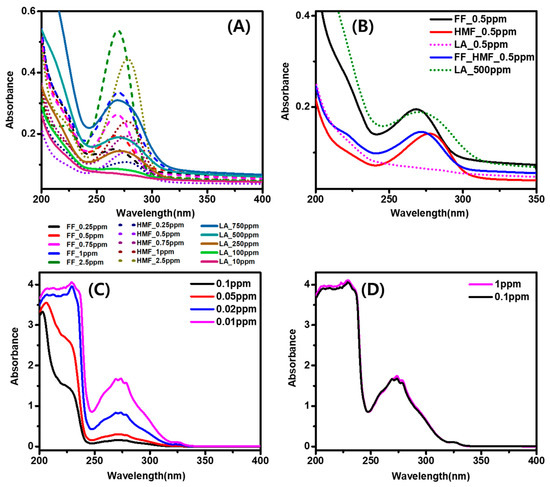
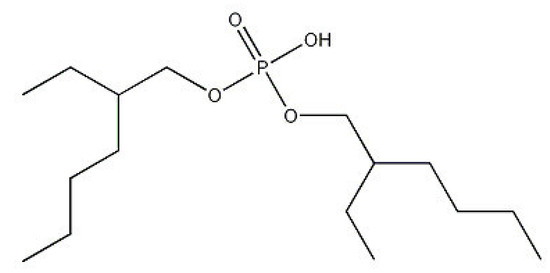
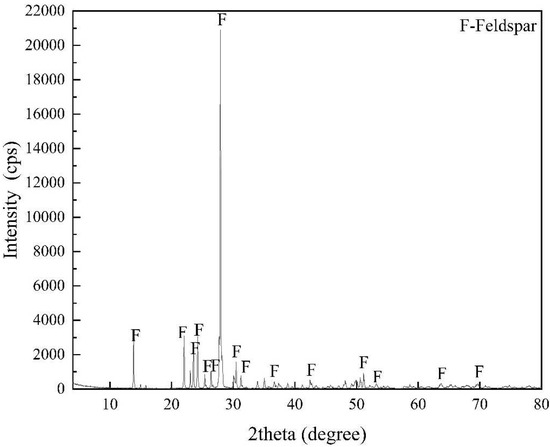
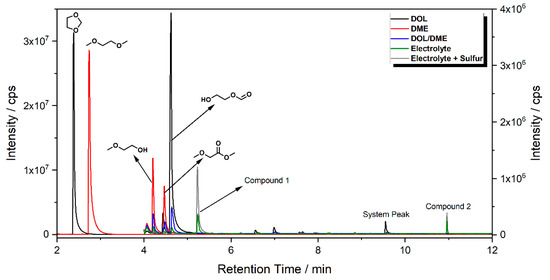
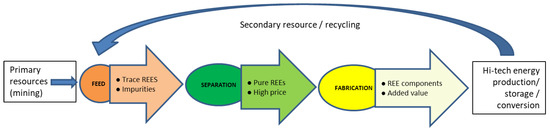
State of the Art in Separation and Analysis of Energies
A topical collection in Separations (ISSN 2297-8739). This collection belongs to the section "Analysis of Energies".
Viewed by 37361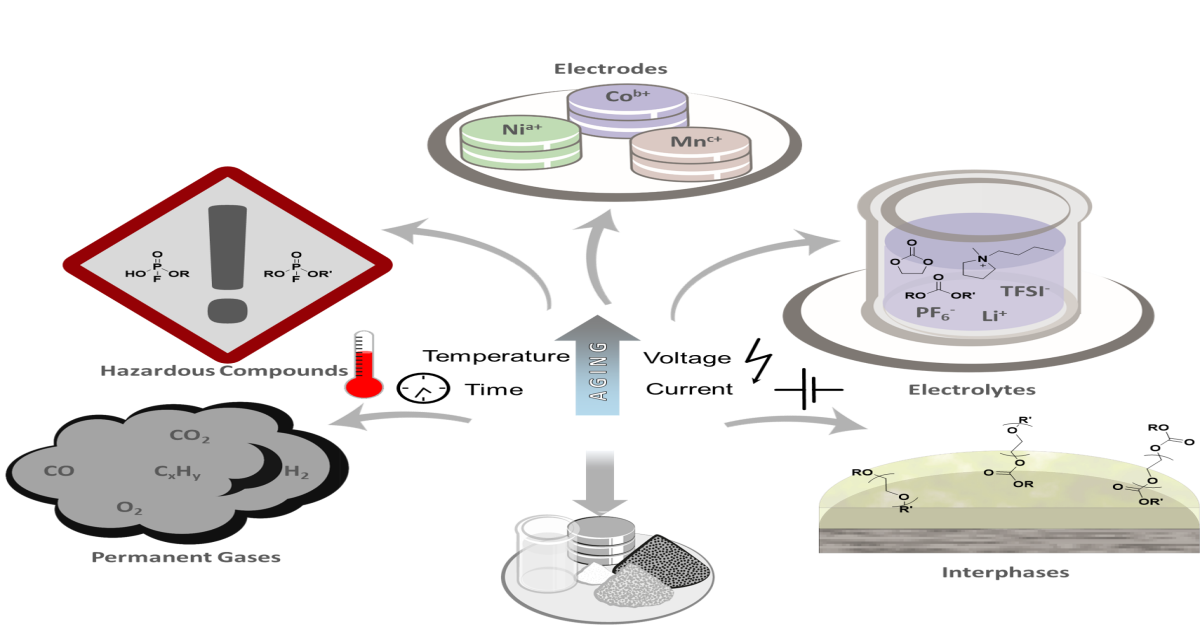
Editors
Interests: lithium-ion batteries; ageing; analytics; electrolyte; recycling
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
We are pleased to introduce a new topical collection of Separations on the topic “State of the Art in Separation and Analysis of Energies”.
In general terms, “energies” can be defined as all developments and applications with regard to energy supply, conversion, application and storage.
In the topical collection “State of the Art in Separation and Analysis of Energies”, we are welcoming original research and review articles on the development and application of separation methods in this field.
The Separation and analysis of energies is a broad field and can cover all separation techniques and detection methods. These methods can be based on analysis approaches or chemical/physical separations of elements or compounds. The identification/quantification or enhanced separation of elements and compounds are of particular interest. Reports on quality control and the standardization of energy products or materials will also be considered on the basis of their significance in the field.
In all cases, novelty will be the major suitability criterion of submitted articles. Authors should always address the novelty of their proposed methodology and draw a comparison with previously reported methods.
Dr. Sascha Nowak
Dr. Yannick Philipp Stenzel
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Separations is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- analysis of energies
- gas chromatography
- high-performance liquid chromatography
- ion chromatography
- capillary electrophoresis
- mass spectrometry
- sample preparation
- solid-phase extraction and microextraction
- ionic liquids
- battery electrolytes
- lithium-ion batteries