Abstract
Thallium (Tl) is an extremely toxic rare metal to the eco-environment. Trace thallium impurity in ammonium perrhenate is harmful to the high-temperature mechanical properties of rhenium metal used for aeroengine single crystal blade. The di(2-ethylhexyl) phosphoric acid (P204) extraction to remove thallium in ammonium perrhenate solution without additive was innovatively proposed. The migration behavior of trace thallium with the concentration of P204, saponification degree and organic/aqueous phase (O/A) ratio, distribution law of thallium in the extraction system of P204, and mechanism of thallium removal were revealed. It was found Tl removal was rapidly increased to 98.5%, at conditions of P204 0.75 mol/L saponified 70% by ammonia, Tl 3.27 mg/L, O/A 1:1, T 298.15 ± 2 K, 250 rpm, and 3 min. McCabe-Thiele Tl extraction equilibrium isotherms indicates Tl concentration of raffinate less than 18.7 μg/L, a theoretical extraction of two stages and a theoretical stripping of two stages are required when both O/A work lines were at 1.0. Therefore, the method of the P204 solvent extraction system can effectively extract Tl in the forms of TlA(org), TlA3(org), TlOHA2(org), and Tl(OH)2A(org). Meanwhile, the new approach can be a promising process for ammonium rhenate refining.
1. Introduction
Thallium (Tl) is a soft metal with a low melting point and tensile strength [1], and an extremely toxic rare metal [2]. Tl can enter mica, alunite, potassium feldspar, and jarosite by isomorphic substitution, replacing K+ owing to their similar ionic radii: 1.49 Å for Tl+ and 1.33 Å for K+. The sulfur-binding property promotes the coexistence of Tl with Pb, Zn, Cu, As, Sb, Fe, Hg, and Au in various sulfide minerals, where it is widely distributed, for it is a lithophilic property [3,4].
Copper concentrate contains thallium [5,6,7]. Therefore, part of trace thallium turned into soot in the copper smelting process [8] is migrated to dirty acid by acid washing, finally to the ammonium rhenate solid by crystallization preparation [9]. Those trace thallium impurity characterized by low melting point and tensile strength is a hazardous material [10] for high purity rhenium metals, characterized by high melting temperature (3180 °C), high ductility, and high temperature tensile strength [11]. Rhenium metal is a candidate material for typical commercial Ni-based superalloys, as known as high-temperature alloys with γ/γ′ two-phases structure, significantly critical for the manufacturing of turbine blades in aero engines and industrial gas turbines (IGT) [12,13]. Therefore, it is very important to study the removal of trace thallium from ammonium rhenate solution to guarantee the mechanical properties of the material of the superalloy.
The currently available techniques for the Tl removal from water mainly include adsorption [14,15], ion exchange [14,16,17], oxidative precipitation [18,19], and solvent extraction [20,21,22]. In conclusion, the removal of thallium by the four methods in aqueous solutions mainly follows three kinds of mechanisms: electrostatic attraction, redox reaction, and ion exchange. Among them, the adsorption technology and ion exchange are both popular due to their simplicity of operation and quick emergency treatment capability. However, there are several problems with the Tl(I) removal from wastewater by adsorbents and ion exchange [23]: (a) The Tl distribution coefficient is generally low, resulting in low Tl(I) adsorption efficiency in the presence of coexisting cations due to their severe competition during the electrostatic attraction or ion exchange process. (b) The desorption and regeneration after the Tl(I) adsorption process are difficult. The remaining thallium will further affect the microstructure and adsorption capacity of amorphous hydrous manganese dioxide, resulting in inefficient adsorbent regeneration. Meanwhile, solvent extraction technology is the popular one in the metallurgy field due to its high distribution coefficient, treatment capability, and organic phase easy regeneration. At present, many types of research focus on the treatment of thallium-containing wastewater. By contrast, less attention has been placed on thallium removal in ammonium rhenate aqueous.
This paper is mainly focused on Tl removal in ammonium rhenate aqueous. We set out to test the hypothesis that the di(2-ethylhexyl) phosphoric acid (P204, Figure 1) plays an important role in extracting the thallium in ammonium rhenate aqueous. A hydrogen bond dimer structure of P204 is used to mask the presence of its polar phosphate group, and is usually also involved in the reaction in the form of dimer when complexed with metal ions. Such extractant was extensively employed in extraction procedures of metals [24,25]. We specifically pay attention to thallium and rhenium behavior, in order to find a new method for purification and impurity removal from ammonium rhenate solution. It was shown that high-tech industries need rhenium metal for turbine blades in aero engines and industrial gas turbine manufacturing [12,13] and the thallium migration and enrichment through solvent extraction is also important for environmental protection [26,27]. Herein, we carried out experiments and an expression analysis of Tl removal by using P204 extraction for the ammonium rhenate refining. As well, these results of the experiments establish a central role of the Tl extraction in the organic phase.
Figure 1.
P204 chemical structural formula.
2. Experimental
2.1. Materials
2.1.1. Ammonium Perrhenate Solution
Original crude ammonium perrhenate solid sample supplied by an ammonium rhenate production plant. Its main impurity element composition was shown in Table 1. Take 30.00 g of the above ammonium rhenate sample and dissolve it in 1.0 L of high purity water to prepare ammonium rhenate solution for this experimental study, and its concentration of the main element and the main impurity element of the solution is shown in Table 2.

Table 1.
Original crude ammonium perrhenate solid main impurity element composition.

Table 2.
The concentration of the main value metal and main impurity element.
From Table 1, the Re content is 69.06%, and the theoretical rhenium content of ammonium rhenate is 69.42%. It can be calculated that the purity of ammonium rhenate in this solid sample is about 99.49%. The calculated Re/Tl mass ratio (Re 69.06% to Tl 0.0142%) value is 4861.47, which is much less than the Re/Tl mass ratio of 999,999.00 required in 99.99% purity rhenium metal [28]. Similarly, it can be calculated that the Re/K mass ratio (Re 69.06% to K 0.0048%) is 14,387.77, less than a Re/K mass ratio of 199,980.00 required in 99.99% purity rhenium metal [28].
The main impurities in crude ammonium rhenate are thallium and potassium. This paper mainly studies the migration mechanism of thallium.
2.1.2. Solvent Extraction System
For the solvent extraction system, P204 was used as received (i.e., without purification, AR, >95%, Luoyang Zhongda Chemical Co., Ltd., Luoyang, China), and the 260# (Shell Co., Ltd, 100% aliphatic diluent, Luoyang, China) was used as the diluent.
The ammonia saponified organic phase was obtained by stirring 0.5 h with the amount of ammonia (NH3.H2O, GR, 25–28%, Fuchen Chemical in China) required for the addition of the P204 organic phase. The hydrochloric acid (HCl, GR, 36–38%, sinopharm Chemical Reagent Co., Ltd, Beijing, China) was used as the stripping reagent.
2.2. Solvent Extraction Method
Both extraction and stripping experiments were carried out in a pear-shaped funnel with an SXL-70 oscillator at 298 ± 2 K.
The metal concentrations in the organic phase C2 (mg/L) are calculated according to Equation (1). The metal removal E (%) is calculated according to Equation (2).
where C0 and C1 (mg/L) represent the metal concentration in the feed solution and the raffinate solution, V0, V1, and V2 (mL) represent the feed volume, raffinate volume, and organic volume, respectively.
The theoretical saponification degree S (%) of the P204 organic phase is calculated according to Equation (3).
where CNH4OH is the concentration of concentrated ammonia water settled as 14.0 mol/L, Cp204 (mol/L) represents the P204 original concentration in the organic phase, and V3 (mL) represents the feed volume of the concentrated ammonia water.
The P204 and Tl molar ratio β are calculated according to Equation (4).
where CTl (mg/L) represents the Tl initial concentration in the feed aqueous phase.
The Tl concentration difference ΔCTl (mg/L) are calculated according to Equation (5).
where CTl(org) (mg/L) represents the Tl concentration in the organic phase, and CTl(o) (mg/L) represents the Tl initial concentration in the feed aqueous phase.
2.3. Analysis
The content of metal elements in crude ammonium rhenate solid sample is obtained by Inductively coupled plasma atomic emission spectrometer (ICP-AES, Atomscan Advantage, Thermo Jarrell Ash, Boston, MA, USA) and inductively coupled plasma-Mass Spectrometry (ICP-MS, Plasma Quant MS Q, Jena, Thuringia, Germany). The sum content of N, H, O, S, P, and C elements is calculated according to the mass conservation of matter. The concentrations of metal ions (Tl, K, mg/L) in the aqueous phase were determined by Inductively coupled plasma-Mass Spectrometry (ICP-MS).
2.4. Mechanism of P204 Extraction Metals
The mechanisms of P204 extraction metals are shown as Equations (6)–(8).
where HA(org) denotes the P204.
HA(org) + NH4OH(aq) = NH4A(org) + H+
nNH4A(org) + Men+ = MeAn(org) + nNH4+
MeAn (org) + nH+ = nHA(org) + Men+
3. Results and Discussion
3.1. Effects of Factor Variables on Tl Removal
3.1.1. Saponification Degree of P204 on Tl Removal
P204 is an acidic extractant, and its extraction amount is very low. The amount of metal extracted can only be increased by neutralizing the acid formed with the base. The effects of saponification degree of P204 in the organic phase on Tl removal were studied, and the results of the experiments are shown in Figure 2.
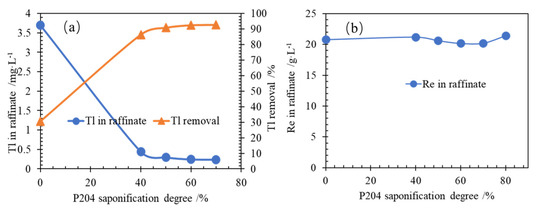
Figure 2.
(a) Effect of P204 saponification degree on Tl extraction; (b) Re concentration in raffinate at conditions of Tl 3.27 mg/L in aqueous, P204 0.6 mol/L, O/A 1:1, T 298.15 ± 2 K for 3 min, and aqueous pH 5.6.
It is shown in Figure 2a that the Tl removal changes slightly when the saponification degree of P204 increases. When the saponification degree of P204 is higher than 50%, the Tl removal is more than 90%. Moreover, when the saponification degree of P204 is higher than 60%, the Tl removal did not change significantly. It is shown in Figure 2b that at the same time as the extraction of thallium, the rhenium concentration in the raffinate is basically unchanged, indicating that P204 extraction will not cause a large amount of rhenium metal loss, which is beneficial for technical industrial applications. In order to keep the efficiency of organic phase stable extraction of thallium, 70% saponification degree of P204 organic was selected for the following experiments.
3.1.2. P204 Concentration on Tl Removal
The effects of P204 concentration on Tl extraction and Tl concentration in raffinate were studied and the results of the experiments are shown in Figure 3.
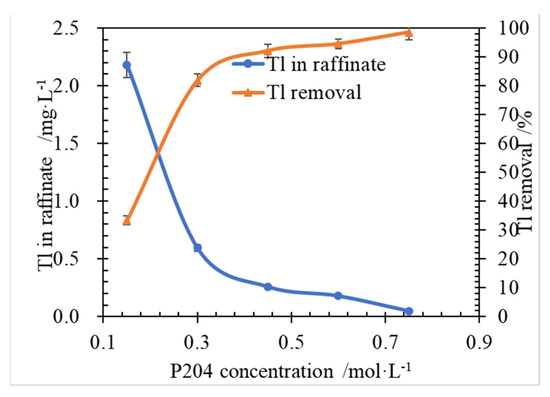
Figure 3.
Effect of P204 concentration on Tl extraction, at conditions of Tl 3.27 mg/L, O/A 1:1, ammonia saponified degree of the organic phase of 70%, T 298.15 ± 2 K, 250 rpm, and 3 min.
As shown in Figure 3, the Tl initial concentration was 3.27 mg/L (0.016 mmol/L), and P204 original concentration was 150 mmol/L, it can be calculated that the molar ratio value of β is 9375 far larger than the theoretical stoichiometric mole ratio of P204 to Tl of 1.0. At the condition, the Tl removal was low, and reached 33.3%. Moreover, Tl removal was rapidly increased to 98.5% (Tl 0.049 mg/L in raffinate), when P204 concentration was 0.75 mol/L and the molar ratio β is 46,875. It was indicated that it is necessary to provide a large amount of chemical energy, so as to increase the probability of collision contact between the extraction agent and Tl impurity ions. It was revealed that P204 concentration takes a positive role in Tl removal.
3.1.3. HCl Concentration on Tl Stripping
An organic loaded Tl 3.02 mg/L, was used in the feed of stripping experiment. Here, HCl solution was used as the stripping reagent. The effect of HCl concentration on Tl stripping was carried out, and the results were shown in Figure 4.
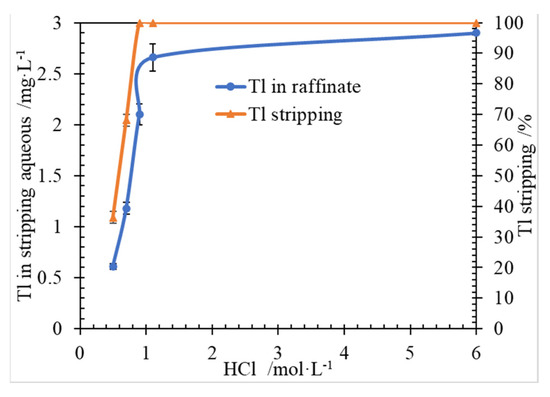
Figure 4.
Effect of HCl concentration on Tl stripping, at conditions of Tl 3.02 mg/L in ammonia saponified organic phase, O/A 1/1, T 298.15 ± 2 K, 250 rpm for 3 min.
As shown in Figure 4, Tl concentration in stripping aqueous was increased with the HCl concentration increasing, when the initial HCl concentration was lower than 0.9 mol/L. And Tl in organic was absolutely stripped, when the initial HCl concentration was higher than 0.9 mol/L. It was indicated that HCl concentration should be more than 0.9 mol/L to keep absolutely stripping Tl in organic.
3.2. McCabe-Thiele Extraction-Stripping Equilibrium Isotherms
3.2.1. Tl Extraction Equilibrium Isotherms
In order to achieve optimal extraction efficiency and resource efficiency, the effects of phase ratio (O/A) on metal extraction efficiency were researched. The results are shown in Figure 5.
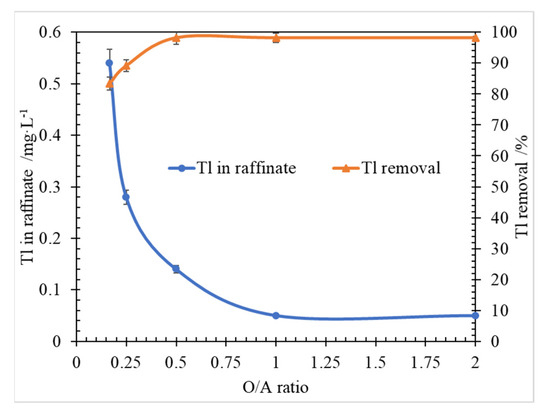
Figure 5.
Effect of O/A ratio on Tl extraction, at conditions of Tl 3.27 mg/L, P204 0.6 mol/L, saponified degree of 70%, T 298.15 ± 2 K, 250 rpm for 3 min.
According to the results in Figure 4, when the O/A ratio was larger than 0.2, with the O/A ratio increasing, the Tl removal was increasing, and the Tl concentration in raffinate was decreasing. At the condition, the molar ratio β of P204 concentration (600.0 mmol/L) to Tl concentration (0.204 mmol/L) was 2941.2. It can be inferred that by changing the molar ratio β values, the O/A ratio in the range from 0.2 to 0.5 takes an important role in Tl removal which can be at a high level of 98%, when O/A was larger than 0.5.
McCabe-Thiele Tl extraction equilibrium isotherms were drawn in Figure 6, according to the experimental results (Figure 5) of O/A comparison conditions. As shown in Figure 6, when O/A works at 1.0, a theoretical extraction of 2 stages is required to obtain the Tl concentration of raffinate less than 18.7 μg/L.
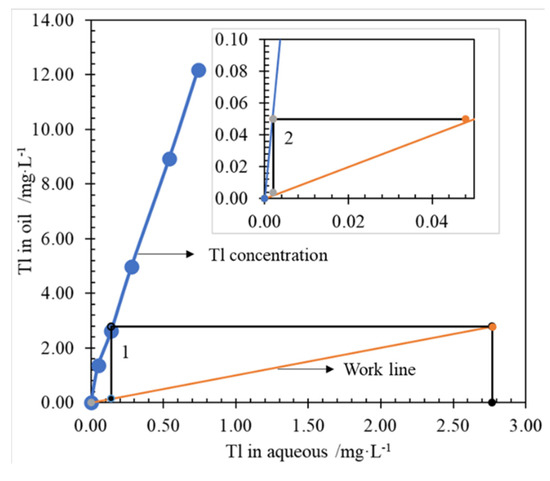
Figure 6.
McCabe-Thiele Tl extraction equilibrium isotherms, at conditions of Tl 3.27 mg/L, P204 0.6 mol/L, ammonia saponified degree of 70%, T 298.15 ± 2 K, 250 rpm for 3 min.
3.2.2. Tl Stripping Equilibrium Isotherms
A 5.0 L capacity beaker was used to stir and mix the extraction according to the above extraction conditions at a speed of 500 r/min to obtain a loaded phase containing Tl 2.23 mg/L for stripping equilibrium isotherms experiments. It should be noted here that due to the difference in stirring method and experimental scale, the Tl content of the load phase obtained this time is lower than the mixed load phase contained Tl 3.02 mg/L of the small oscillatory extraction. It is indicated that in industrial applications, it is necessary to select extraction equipment with good mixing efficiency.
The effects of O/A on metal stripping efficiency were researched. The results are shown in Figure 7. According to the results in Figure 7, when the O/A ratio was larger than 2.0, with the O/A ratio increasing, the Tl stripping was reduced, and the Tl concentration in raffinate was increasing. When the O/A ratio was 2, Tl removal reached 97.0% (Tl 4.30 mg/L in stripping aqueous). When the O/A ratio was 6.0, Tl removal decreased to 12.1% (Tl 1.62 mg/L in stripping aqueous).
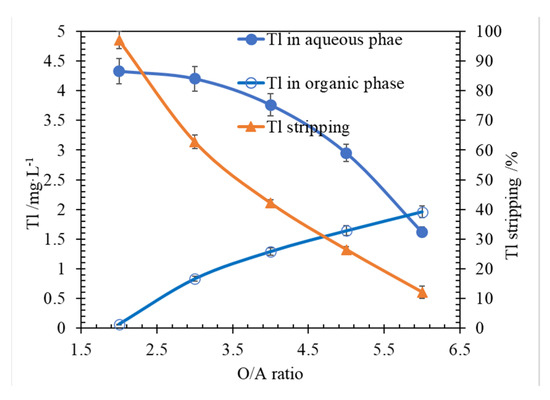
Figure 7.
Effect of O/A ratio on Tl stripping, at conditions of Tl 2.23 mg/L in ammonia saponified organic phase, O/A 1/1, T 298.15 ± 2 K, 250 rpm for 3 min.
McCabe-Thiele Tl stripping equilibrium isotherms of NH4-organic phase were drawn in Figure 8, according to the experimental results of O/A comparison conditions (Figure 7). As shown in Figure 8, when O/A works at 1.0, a theoretical stripping stage of 2 is required to achieve the Tl removal larger than 95.5%. Here, after stripping stage 2, the residual Tl uneasily stripped in the organic phase was 0.02 mg/L, and the P204 organic phase needed to be recycled by 6.0 mol/L hydrochloric acid solution to remove the residual trace Tl.
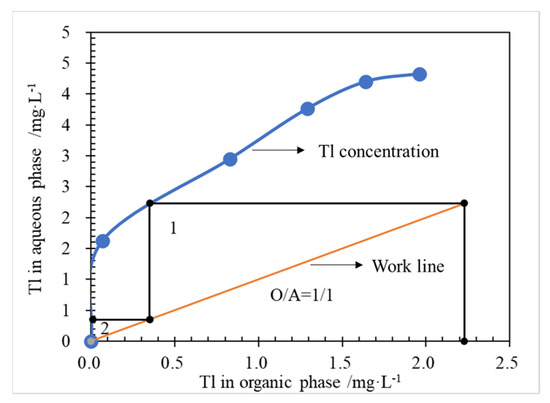
Figure 8.
McCabe-Thiele Tl extraction equilibrium isotherms, at conditions of Tl 2.23 mg/L in ammonia saponified organic phase, O/A 1/1, T 298.15 ± 2 K, 250 rpm for 3 min.
3.3. Saturation Capacity of Thallium in Organic Phase
In Section 3.1.1, the molar ratio β was 17,875, which is greatly larger than the theoretical measurement ratio of 1.0. Therefore, the organic phase may accommodate a large amount of Tl. Here, the same organic phase was used to extract the fresh aqueous phase of ammonium perrhenate continuously until the organic phase becomes saturated (Figure 9). The Tl removal performance of the same organic phase was evaluated by investigating the extraction times.
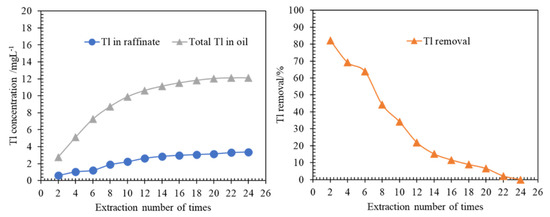
Figure 9.
(a) Saturation capacity of thallium in the organic phase; (b) Effect of extraction times on Tl removal at conditions of Tl 3.27 mg/L, P204 0.6 mol/L, ammonia saponified degree of 70%, T 298.15 ± 2 K, 250 rpm for 3 min.
As shown in Figure 9, at the second extraction of a fresh aqueous phase, where the initial molar ratio β is 17,646, Tl removal reached 82.2% (Tl 0.60 mg/L in raffinate), which was reduced with the extraction times increasing. It was investigated that Tl removal was low even at a large initial molar ratio β. This may be due to the Tl concentration difference (ΔCTl) between Tl initial concentration in the organic phase and Tl initial concentration in the aqueous phase. As calculated, the ΔCTl of the second time is −0.50, while the ΔCTl changed to 1.83 at the fourth time, and ΔCTl values of the rest times were 3.98, 5.47, 6.62, 7.36, 7.87, 8.26, 8.56, 8.78, 8.85 and 8.85, respectively.
At the seventh extraction of the new solution, the Tl removal was about 50%, and the ΔCTl value was 6.8. When the ΔCTl value was less than 6.8, the Tl removal was greater than 50%. It can be deduced that the ΔCTl is the main factor in Tl removal, and the larger the ΔCTl is, the lower the Tl removal when the same organic was used to extract the fresh aqueous. It can be seen in Figure 9 that after the 22nd extraction, the accumulated Tl concentration in the organic phase almost remained unchanged, and the loaded Tl of the organic phase tended to be saturated. Under experimental conditions, the saturated Tl capacity of the organic phase was 12.1 mg/L.
3.4. Mechanism of Thallium Removal by Solvent Extraction
The ion species distribution of Tl and Re in aqueous solution at different pH values drawn with software Medusa (Trial version, KTH Royal Institute of Technology, Stockholm, Sweden) were shown in Figure 10 and Figure 11. The extraction mechanism of Tl by P204 dissolved in kerosene is cation exchange extraction, and can be described in Figure 12. It can be seen in Figure 10 that Tl exists in the form of Tl+, Tl(OH)2+, Tl(OH)2+1, and Tl3+. The presence of Tl+ concentration in the pH range of 0 and 12.0 is basically unchanged, and NH4A(org) [29] can be extracted in the form of TlA(org) to remove thallium (Figure 11). In the pH range of 0–4.7, thallium is mainly present in the form of Tl+, Tl(OH)2+, Tl(OH)2+1 and Tl3+ (Figure 10), NH4A(org) can extracts Tl in form of TlA(org), TlA3(org), Tl(OH)2A (org) and TlOHA2 (org) (Figure 12), Tl+ concentrations is higher than Tl(OH)2+, Tl(OH)2+1 and Tl3+ ion concentrations (Figure 10). it is speculated that TlA (org) is the main extract. It is indicated in Figure 10 that rhenium mainly exists in the form of ReO4− at the pH range from 1.0 to 12.0. Based on the difference in charge properties between Tl+, Tl(OH)2+, Tl(OH)2+1 and ReO4−. P204 as a cationic extractant, may be used to extract Tl+, Tl(OH)2+ and Tl(OH)2+1 separating from ReO4− ion.
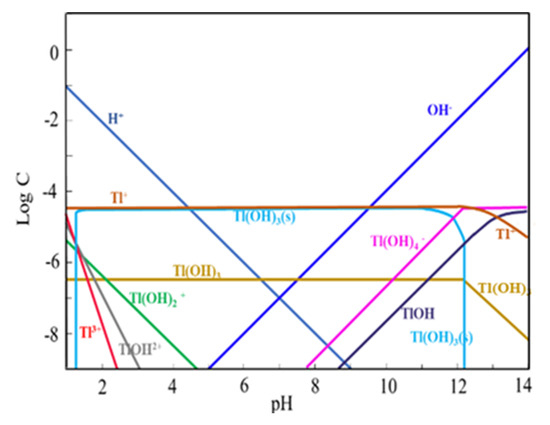
Figure 10.
Tl ion species in different pH rage.
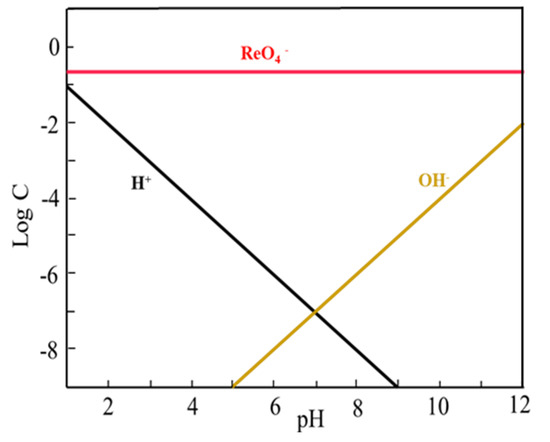
Figure 11.
Re ion species in different pH rage.
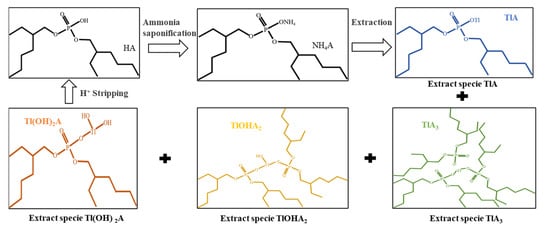
Figure 12.
Mechanism of thallium removal by P204 (HA) solvent extraction.
Infrared spectrum analysis was carried out on samples of unloaded organic of P204 + kerosene mixed and Tl loaded organic of P204 + kerosene + Tl mixed. The spectral diagram is shown in Figure 13. The differences between the spectra of these organic samples are absorption peaks at wave number 3371.62 cm−1 and 1640.47 cm−1 in the spectra of Tl loaded organic phase of P204 + kerosene + Tl mixed, which is mainly caused by the substitution of Tl for NH4+ ion in the carbon chain of P204 for -NH stretching vibration and -NH in-plane bending vibration.
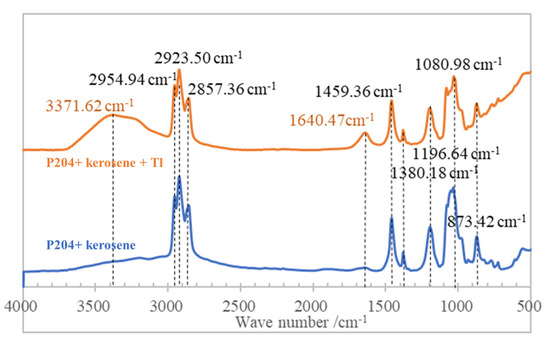
Figure 13.
FTIR Spectra of different organics.
4. Conclusions
In this study, a novel method for Tl removal by solvent extraction with P204 was proposed for ammonium perrhenate solution. By studying experimental mechanism elaboration and factor condition experiment, the conclusions were as followed:
For thallium in ammonium perrhenate solution, P204 selective extraction of thallium can be used to remove thallium effectively and separate rhenium, with the Re initial concentration of 18.7 g/L, in an unregulated solution environment (pH, ligand, and temperature).
Under the experimental conditions of P204 0.6 mol/L, Tl 3.27 mg/L, O/A 1:1, T 298.15 ± 2 K, 250 rpm for 3 min, the Tl removal reached 96%, and rhenium was hardly extracted. The main factors affecting the Tl removal were P204 concentration and the O/A ratio. when O/A works at 1.0, a theoretical extraction stage of 2 is required to obtain the Tl concentration of raffinate less than 18.7 μg/L. The organic loaded thallium 2.23 mg/L, can be extremely stripped by 1.1 mol/L HCl solution with two stages, at conditions of O/A 1/1, T 298.15 ± 2 K for 3 min.
With 0.6 mol/L P204 saponified degree of 70% in the organic phase, the saturated Tl capacity of the organic phase was 12.1 mg/L. The extraction mechanism of thallium by ammonia saponified P204 organic phase is ion exchange. The NH4+ ions in the organic phase exchange with Tl+, Tl3+, Tl(OH)2+1, and Tl(OH)2+ to achieve the migration of Tl from the aqueous phase to the organic phase. Ammonia saponified P204 organic phase NH4A(org), mainly extracts to remove thallium in the form of extract TlA(org).
Author Contributions
Conceptualization, Y.L.; methodology, Y.Y.; investigation, Y.L.; resources, C.S.; data curation, H.J. and C.S.; writing—original draft preparation, A.Y.; writing—review and editing, A.Y. and S.C.; supervision, Y.L.; project administration, Y.Y.; funding acquisition, X.Y.; funding application, X.Y.; project financial support, C.S. and X.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by GRINM Group Corporation Limited Youth Fund (NO. G12620213129037), Northwest Research Institute of Mining and Metallurgy Fund and National Natural Science Foundation of China Youth Science Foundation Project (NO. 51904271).
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nguyen, V.N.; Ho, K.H. The melting curves of tin, uranium, cadmium, thallium and indium metals under pressure. Chem. Phys. 2022, 553, 111389. [Google Scholar]
- Keith, L.; Telliard, W. ES&T special report: Priority pollutants: I a perspective view. Environ. Sci. Technol. 1979, 13, 416–423. [Google Scholar]
- Casiot, C.; Egal, M.; Bruneel, O.; Verma, N.; Parmentier, M.; Elbaz-Poulichet, F. Predominance of aqueous Tl(I) species in the river system downstream from the abandoned Carnoulès mine (Southern France). Environ. Sci. Technol. 2011, 45, 2056–2064. [Google Scholar] [CrossRef]
- Xiong, Y.L. The aqueous geochemistry of thallium: Speciation and solubility of thallium in low temperature systems. Environ. Chem. 2009, 6, 44–451. [Google Scholar] [CrossRef]
- Shirai, M. Electronic structure of thallium copper chalcogenides T1Cu2S2 and TICu2Se2. Synth. Met. 1995, 71, 1857–1858. [Google Scholar] [CrossRef]
- Rader, S.T.; Mazdab, F.K.; Barton, M.D. Minera logical thallium geochemistry and isotope variations from igneous, metamorphic, and metasomatic systems. Geochim. Cosmochim. Acta 2018, 243, 42–65. [Google Scholar] [CrossRef]
- George, L.L.; Biagioni, C.; Lepore, G.O.; Lacalamita, M.; Agrosì, G.; Capitani, G.C.; Bonaccorsi, E.; D’Acapito, F. The speciation of thallium in (Tl,Sb,As)-rich pyrite. Ore Geol. Rev. 2019, 107, 364–380. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Dong, X.; Yin, M.; Tsang, D.C.; Sun, J.; Liu, J.; Song, G.; Liu, Y. Temporal sedimentary record of thallium pollution in an urban lake: An emerging thallium pollution source from copper metallurgy. Chemosphere 2020, 242, 125–172. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.L.; Ning, R. Experimental study on thallium removal from ammonium perrhenate. Nonferr. Metals Sci. Eng. 2019, 10, 21–25. [Google Scholar]
- Shuang, G.A.O.; Hou, J.S.; Guo, Y.A.; Zhou, L.Z. Phase precipitation behavior and tensile properties of as-cast Ni-based superalloy during heat treatment. Trans. Nonferr. Metal. SOC 2018, 28, 1735–1744. [Google Scholar]
- Wei, Z.; Zhang, L.; Li, X.; Long, Y.; Qu, X. Effect of grain size on deformation behavior of pure rhenium. Mater. Sci. Eng. A 2022, 829, 142–170. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, T.; Lu, F.; Cao, K.; Wang, D.; Zhang, J.; Zhang, J.; Su, H.; Liu, L. The effect of rhenium on the microstructure stability and γ/γ′ interfacial characteristics of Ni-based single crystal superalloys during long-term aging. J. Alloys Compd. 2021, 876, 160114. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Ai, C. Nickel-Based Superalloys, Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Li, H.; Chen, Y.; Long, J.; Li, X.; Jiang, D.; Zhang, P.; Qi, J.; Huang, X.; Liu, J.; Xu, R.; et al. Removal of thallium from aqueous solutions using Fe-Mn binary oxides. J. Hazard. Mater. 2017, 338, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Su, M.; Chen, D.; Zhu, L.; Pang, Y.; Chen, Y. Highly-efficient and easy separation of hexahedral sodium dodecyl sulfonate/δ-FeOOH colloidal particles for enhanced removal of aqueous thallium and uranium ions: Synergistic effect and mechanism study. J. Hazard. Mater. 2021, 402, 123800. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, P.; Borthwick, A.G.; Chen, H.; Ni, J. Adsorption mechanisms of thallium(I) and thallium(III) by titanate nanotubes: Ion-exchange and coprecipitation. J. Colloid Interface Sci. 2014, 423, 67–75. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Long, J.; Jiang, D.; Liu, J.; Li, S.; Qi, J.; Zhang, P.; Wang, J.; Gong, J.; et al. Simultaneous removal of thallium and chloride from a highly saline industrial wastewater using modified anion exchange resins. J. Hazard. Mater. 2017, 333, 179–185. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, B.; Borthwick, A.G.; Li, Y.; Liu, W. Electrochemical oxidation of thallium(I) in groundwater by employing single-chamber microbial fuel cells as renewable power sources. Int. J. Hydrogen Energy 2017, 42, 29454–29462. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wang, X.; Huang, Z.; Xu, C.; Yang, T.; Zhao, X.; Qi, J.; Ma, J. Highly efficient removal of trace thallium from contaminated source waters with ferrate: Role of in situ formed ferric nanoparticle. Water Res. 2017, 124, 149–157. [Google Scholar] [CrossRef]
- Tereshatov, E.E.; Mazan, V.; Boltoeva, M.; Folden, C.M. Effect of hydrophobic ionic liquids aqueous solubility on metal extraction from hydrochloric acid media: Mathematical modelling and trivalent thallium behavior. Sep. Purif. Technol. 2021, 255, 117650. [Google Scholar] [CrossRef]
- Escudero, L.B.; Wuilloud, R.G.; Olsina, R.A. Sensitive determination of thallium species in drinking and natural water by ionic liquid-assisted ion-pairing liquid–liquid microextraction and inductively coupled plasma mass spectrometry. J. Hazard. Mater. 2013, 244, 380–386. [Google Scholar] [CrossRef]
- Sato, T.; Yasumura, H.; Mizuno, Y.; Nishimura, T. Solvent extraction of trivalent gallium, indium, and thallium from hydrochloric acid solutions by TOPO and TBP. Talanta 1996, 59, 905–912. [Google Scholar]
- Zhao, Z.; Xiong, Y.; Cheng, X.; Hou, X.; Yang, Y.; Tian, Y.; You, J.; Xu, L. Adsorptive removal of trace thallium(I) from wastewater: A review and new perspectives. J. Hazard. Mater. 2020, 393, 122378. [Google Scholar] [CrossRef] [PubMed]
- Belova, V.V.; Petyaeva, M.M.; Tsareva, J.V.; Kostanyan, A.E. Solvent extraction of lanthanides(III) from chloride and nitrate media with di(2-ethylhexyl) phosphoric acid and ionic liquids in three-component biphasic solvent systems. Hydrometallurgy 2021, 199, 105526. [Google Scholar] [CrossRef]
- Ghosh, A.; Datta, D.; Uslu, H.; Bamufleh, H.S.; Kumar, S. Separation of copper ion (Cu2+) from aqueous solution using tri-n-butyl phosphate and di-2-ethylhexyl phosphoric acid as extractants. J. Mol. Liq. 2018, 258, 147–154. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Xiao, T.; Chen, Y.; Long, J.; Zhang, G.; Zhang, P.; Li, C.; Zhuang, L.; Li, K. Efficient removal of thallium(I) from wastewater using flower-like manganese dioxide coated magnetic pyrite cinder. Chem. Eng. J. 2018, 353, 867–877. [Google Scholar] [CrossRef]
- Peter, A.L.J.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef]
- YS/T 1017–2015; Rhenium Powder. Standardization Administration of China: Beijing, China, 2015.
- Le, T.; Xiao, B.; Ju, S.; Peng, J.; Jiang, F. Separation of indium from impurities in T-type microreactor with D2EHPA. Hydrometallurgy 2018, 183, 79–86. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).