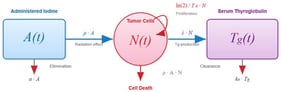
Background/Objectives: Radioactive iodine (RAI) therapy is widely used to treat metastatic differentiated thyroid cancer. To investigate physiological determinants of treatment response, a mechanistic model was developed, formulated as a system of coupled ordinary differential equations. Methods: The model captures the interactions between tumor burden, thyroglobulin (
Tg) production and clearance, and radioactive iodine activity within a pharmacokinetic–pharmacodynamic framework. Model parameters were estimated using the Monte Carlo Stochastic Approximation Expectation–Maximization (MCMCSAEM) algorithm, based on clinical data from a cohort of 50 patients. Results: Tumor radiosensitivity (
ρ) and initial tumor burden (
N0) consistently emerged as the most influential factors in both responder and non-responder groups classified by disease doubling time under RAI (
Td). A reduced model using only these two parameters preserved the principal response patterns of the full model. Other parameters influenced transient dynamics but had limited effect on overall
Tg variance. Conclusions: These results support the use of a reduced calibration approach focused on
ρ,
N0, and the effective doubling time
Td. The findings establish a theoretical foundation for developing tractable dynamic surrogates that reproduce the main treatment kinetics and support model-based clinical decision-making in RAI therapy.