Feature Papers in Pathophysiology
Share This Topical Collection
Editor
 Prof. Dr. Jonathan Steven Alexander
Prof. Dr. Jonathan Steven Alexander
 Prof. Dr. Jonathan Steven Alexander
Prof. Dr. Jonathan Steven Alexander
E-Mail
Website
Collection Editor
Department of Molecular and Cellular Physiology, LSU Health Shreveport, Shreveport, LA 71103, USA
Interests: neurophysiology; neurodegeneration; cellular neuroscience; neurobiology; stroke therapy; kidney and liver transplantation; 3D tissue engineering
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Topical Collection, “Feature Papers in Pathophysiology”, intends to collect high-quality research articles, short communications, and reviews in all scientific fields related to pathophysiology, with a focus on clinical and pre-clinical research. Since the aim of this Topical Collection is to curate, through the selection of outstanding works, premier research in diagnostics, mechanisms, theoretical models and biomarkers. We encourage Editorial Board Members of Pathophysiology and members of the International Society for Pathophysiology and the Japanese Society for Pathophysiology to contribute manuscripts which cover the leading progress in their field of research, or to reach out to authorities and contemporaries who may wish to contribute. Topics include, but are not limited to:
- Diagnostics;
- Novel biomarkers;
- Novel therapies;
- Alzheimer’s disease;
- Stroke and stroke therapy;
- Neurovascular diseases;
- Imaging modalities in disease diagnosis;
- Educational approaches to teaching pathophysiology;
- Transplantation;
- Inflammation and immunity;
- Behavior and neurological pathways;
- Gravity and space biology;
- Cancer biology;
- DNA repair mechanisms.
We are looking forward to your contributions to this Special Issue.
Dr. Jonathan Steven Alexander
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pathophysiology is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 1400 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- diagnostics
- novel biomarkers
- novel therapies
- Alzheimer’s disease
- stroke and stroke therapy
- neurovascular diseases
- imaging modalities in disease diagnosis
- educational approaches to teaching pathophysiology
- transplantation
- inflammation and immunity
- behavior and neurological pathways
- gravity and space biology
- cancer biology
- DNA repair mechanisms
Published Papers (12 papers)
Open AccessCase Report
Secondary Prostate Lymphoma Mimicking Prostate Cancer Successfully Managed by Transurethral Resection to Relieve Urinary Retention
by
Lorand-Tibor Reman, Ovidiu Malau, Daniel Porav-Hodade, Calin Chibelean, Arpad-Oliver Vida, Ciprian Todea, Veronica Ghirca, Alexandru Laslo, Raul-Dumitru Gherasim, Rares Vascul, Orsolya-Brigitta Katona, Raluca-Diana Hagău and Orsolya Martha
Viewed by 1078
Abstract
Secondary lymphoma of the prostate is described as the involvement of the prostate gland by lymphomatous spread from a primary site. This condition is exceedingly rare and often presents diagnostic and therapeutic challenges. The symptoms often mimic those of benign prostatic hyperplasia or
[...] Read more.
Secondary lymphoma of the prostate is described as the involvement of the prostate gland by lymphomatous spread from a primary site. This condition is exceedingly rare and often presents diagnostic and therapeutic challenges. The symptoms often mimic those of benign prostatic hyperplasia or prostate cancer, including LUTS (lower urinary tract symptoms) and even complete urinary retention. Here, we present a rare case of a 62-year-old male patient undergoing chemotherapy for stage IV mantle cell stomach lymphoma and subsequently secondary prostatic involvement. The patient presented with complete urinary retention, accompanied by biochemical (PSA = 11.7 ng/mL) and imaging (Magnetic Resonance Imaging-PIRADS V lesion) suspicion for prostate cancer. Histopathologic analysis of the MRI-targeted prostate fusion biopsy revealed secondary prostatic lymphoma. The chosen treatment was transurethral resection of the prostate (TUR-P) for relief of symptoms, which significantly improved urinary function (postoperative IPSS = 5 and Qmax = 17 mL/s). This case underscores the importance of considering prostatic lymphoma in the differential diagnosis of bladder outlet obstruction, especially in patients with a known lymphoma history. This report also provides a focused review of the literature on secondary prostatic lymphoma, highlighting the diagnostic challenges, treatment options, and clinical outcomes.
Full article
►▼
Show Figures
Open AccessReview
Mitochondrial Dysfunction in Diabetes: Shedding Light on a Widespread Oversight
by
Franklyn Nonso Iheagwam, Amarachi Joy Joseph, Eniola Deborah Adedoyin, Olawumi Toyin Iheagwam and Samuel Akpoyowvare Ejoh
Cited by 8 | Viewed by 8312
Abstract
Diabetes mellitus represents a complicated metabolic condition marked by ongoing hyperglycemia arising from impaired insulin secretion, inadequate insulin action, or a combination of both. Mitochondrial dysfunction has emerged as a significant contributor to the aetiology of diabetes, affecting various metabolic processes critical for
[...] Read more.
Diabetes mellitus represents a complicated metabolic condition marked by ongoing hyperglycemia arising from impaired insulin secretion, inadequate insulin action, or a combination of both. Mitochondrial dysfunction has emerged as a significant contributor to the aetiology of diabetes, affecting various metabolic processes critical for glucose homeostasis. This review aims to elucidate the complex link between mitochondrial dysfunction and diabetes, covering the spectrum of diabetes types, the role of mitochondria in insulin resistance, highlighting pathophysiological mechanisms, mitochondrial DNA damage, and altered mitochondrial biogenesis and dynamics. Additionally, it discusses the clinical implications and complications of mitochondrial dysfunction in diabetes and its complications, diagnostic approaches for assessing mitochondrial function in diabetics, therapeutic strategies, future directions, and research opportunities.
Full article
►▼
Show Figures
Open AccessArticle
Early Radiation-Induced Changes in Lung Tissue and Intercellular Junctions: Implications for Tissue Repair and Fibrosis
by
Ekaterina S. Karetnikova, Alexandra A. Livanova, Arina A. Fedorova and Alexander G. Markov
Viewed by 1752
Abstract
Early changes in lung tissue following ionizing radiation (IR) initiate processes that may lead to either regeneration or fibrosis. Intercellular junction proteins play a crucial role in the organization and function of epithelial tissues, both under normal conditions and after injuries. Alterations in
[...] Read more.
Early changes in lung tissue following ionizing radiation (IR) initiate processes that may lead to either regeneration or fibrosis. Intercellular junction proteins play a crucial role in the organization and function of epithelial tissues, both under normal conditions and after injuries. Alterations in the expression and localization of these proteins can influence the fate of epithelial cells. This study aims to investigate the effects of IR on lung tissue structure, as well as on the levels and distribution of intercellular junction proteins. Wistar rats were subjected to total X-ray irradiation at doses of 2 and 10 Gy. Lung tissue samples were collected for Western blot and histological analysis 72 h post-IR. IR at doses of 2 and 10 Gy led to structural changes in lung tissue and elevated levels of E-cadherin. The 10 Gy IR resulted in increased claudin-4 and occludin in lung parenchyma, decreased claudin-8 and claudin-12 in bronchial epithelium and endothelium, and suppression of apoptosis. Data evaluation indicated that alterations in the protein composition of intercellular junctions are essential processes in lung tissue at early stages after IR, and at least some of these alterations are associated with adaptation.
Full article
►▼
Show Figures
Open AccessReview
Bronchial Asthma and COVID-19: Etiology, Pathological Triggers, and Therapeutic Considerations
by
Anna Starshinova, Anastasia Borozinets, Anastasia Kulpina, Vitaliy Sereda, Artem Rubinstein, Igor Kudryavtsev and Dmitry Kudlay
Cited by 3 | Viewed by 4811
Abstract
Bronchial asthma (BA) continues to be a difficult disease to diagnose. Various factors have been described in the development of BA, but to date, there is no clear evidence for the etiology of this chronic disease. The emergence of COVID-19 has contributed to
[...] Read more.
Bronchial asthma (BA) continues to be a difficult disease to diagnose. Various factors have been described in the development of BA, but to date, there is no clear evidence for the etiology of this chronic disease. The emergence of COVID-19 has contributed to the pandemic course of asthma and immunologic features. However, there are no unambiguous data on asthma on the background and after COVID-19. There is correlation between various trigger factors that provoke the development of bronchial asthma. It is now obvious that the SARS-CoV-2 virus is one of the provoking factors. COVID-19 has affected the course of asthma. Currently, there is no clear understanding of whether asthma progresses during or after COVID-19 infection. According to the results of some studies, a significant difference was identified between the development of asthma in people after COVID-19. Mild asthma and moderate asthma do not increase the severity of COVID-19 infection. Nevertheless, oral steroid treatment and hospitalization for severe BA were associated with higher COVID-19 severity. The influence of SARS-CoV-2 infection is one of the protective factors. It causes the development of severe bronchial asthma. The accumulated experience with omalizumab in patients with severe asthma during COVID-19, who received omalizumab during the pandemic, has strongly suggested that continued treatment with omalizumab is safe and may help prevent the severe course of COVID-19. Targeted therapy for asthma with the use of omalizumab may also help to reduce severe asthma associated with COVID-19. However, further studies are needed to prove the effect of omalizumab. Data analysis should persist, based on the results of the course of asthma after COVID-19 with varying degrees of severity.
Full article
►▼
Show Figures
Open AccessReview
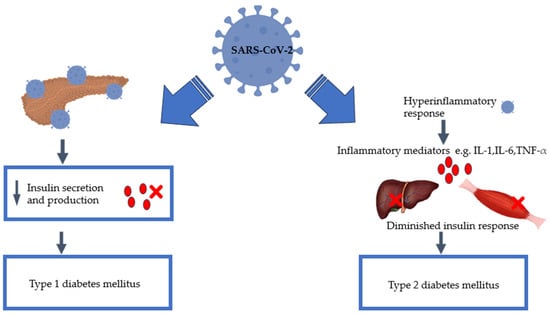
COVID-19-Induced Diabetes Mellitus: Comprehensive Cellular and Molecular Mechanistic Insights
by
Praise Tatenda Nhau, Mlindeli Gamede and Ntethelelo Sibiya
Cited by 8 | Viewed by 4424
Abstract
Despite evidence demonstrating the risks of developing diabetes mellitus because of SARS-CoV-2, there is, however, insufficient scientific data available to elucidate the relationship between diabetes mellitus and COVID-19. Research indicates that SARS-CoV-2 infection is associated with persistent damage to organ systems due to
[...] Read more.
Despite evidence demonstrating the risks of developing diabetes mellitus because of SARS-CoV-2, there is, however, insufficient scientific data available to elucidate the relationship between diabetes mellitus and COVID-19. Research indicates that SARS-CoV-2 infection is associated with persistent damage to organ systems due to the systemic inflammatory response. Since COVID-19 is known to induce these conditions, further investigation is necessary to fully understand its long-term effects on human health. Consequently, it is essential to consider the effect of the COVID-19 pandemic when predicting the prevalence of diabetes mellitus in the future, especially since the incidence of diabetes mellitus was already on the rise before the pandemic. Additional research is required to fully comprehend the impact of SARS-CoV-2 infection on glucose tolerance and insulin sensitivity. Therefore, this article delves deeper into the current literature and links the perceived relationship between SARS-CoV-2 and diabetes. In addition, the article highlights the necessity for further research to fully grasp the mechanisms that SARS-CoV-2 utilises to induce new-onset diabetes. Where understanding and consensus are reached, therapeutic interventions to prevent the onset of diabetes could be proposed. Lastly, we propose advocating for the regular screening of diabetes and pre-diabetes, particularly for the high-risk population with a history of COVID-19 infection.
Full article
►▼
Show Figures
Open AccessBrief Report
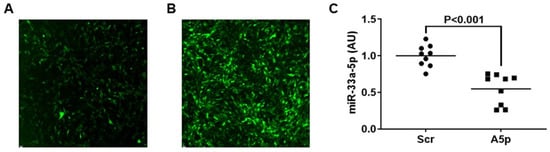
Inhibition of miR-33a-5p in Macrophage-like Cells In Vitro Promotes apoAI-Mediated Cholesterol Efflux
by
Olanrewaju Oladosu, Emma Chin, Christian Barksdale, Rhonda R. Powell, Terri Bruce and Alexis Stamatikos
Cited by 5 | Viewed by 2274
Abstract
Atherosclerosis is caused by cholesterol accumulation within arteries. The intima is where atherosclerotic plaque accumulates and where lipid-laden foam cells reside. Intimal foam cells comprise of both monocyte-derived macrophages and macrophage-like cells (MLC) of vascular smooth muscle cell (VSMC) origin. Foam cells can
[...] Read more.
Atherosclerosis is caused by cholesterol accumulation within arteries. The intima is where atherosclerotic plaque accumulates and where lipid-laden foam cells reside. Intimal foam cells comprise of both monocyte-derived macrophages and macrophage-like cells (MLC) of vascular smooth muscle cell (VSMC) origin. Foam cells can remove cholesterol via apoAI-mediated cholesterol efflux and this process is regulated by the transporter ABCA1. The microRNA miR-33a-5p is thought to be atherogenic via silencing ABCA1 which promotes cholesterol retention and data has shown inhibiting miR-33a-5p in macrophages may be atheroprotective via enhancing apoAI-mediated cholesterol efflux. However, it is not entirely elucidated whether precisely inhibiting miR-33a-5p in MLC also increases ABCA1-dependent cholesterol efflux. Therefore, the purpose of this work is to test the hypothesis that inhibition of miR-33a-5p in cultured MLC enhances apoAI-mediated cholesterol efflux. In our study, we utilized the VSMC line MOVAS cells in our experiments, and cholesterol-loaded MOVAS cells to convert this cell line into MLC. Inhibition of miR-33a-5p was accomplished by transducing cells with a lentivirus that expresses an antagomiR directed at miR-33a-5p. Expression of miR-33a-5p was analyzed by qRT-PCR, ABCA1 protein expression was assessed via immunoblotting, and apoAI-mediated cholesterol efflux was measured using cholesterol efflux assays. In our results, we demonstrated that lentiviral vector-mediated knockdown of miR-33a-5p resulted in decreasing expression of this microRNA in cultured MLC. Moreover, reduction of miR-33a-5p in cultured MLC resulted in de-repression of ABCA1 expression, which caused ABCA1 protein upregulation in cultured MLC. Additionally, this increase in ABCA1 protein expression resulted in enhancing ABCA1-dependent cholesterol efflux through increasing apoAI-mediated cholesterol efflux in cultured MLC. From these findings, we conclude that inhibiting miR-33a-5p in MLC may protect against atherosclerosis by promoting ABCA1-dependent cholesterol efflux.
Full article
►▼
Show Figures
Open AccessReview
CKD Urine Metabolomics: Modern Concepts and Approaches
by
Elena Y. Danilova, Anna O. Maslova, Andrey N. Stavrianidi, Alexander E. Nosyrev, Larisa D. Maltseva and Olga L. Morozova
Cited by 7 | Viewed by 4613
Abstract
One of the primary challenges regarding chronic kidney disease (CKD) diagnosis is the absence of reliable methods to detect early-stage kidney damage. A metabolomic approach is expected to broaden the current diagnostic modalities by enabling timely detection and making the prognosis more accurate.
[...] Read more.
One of the primary challenges regarding chronic kidney disease (CKD) diagnosis is the absence of reliable methods to detect early-stage kidney damage. A metabolomic approach is expected to broaden the current diagnostic modalities by enabling timely detection and making the prognosis more accurate. Analysis performed on urine has several advantages, such as the ease of collection using noninvasive methods and its lower protein and lipid content compared with other bodily fluids. This review highlights current trends in applied analytical methods, major discoveries concerning pathways, and investigated populations in the context of urine metabolomic research for CKD over the past five years. Also, we are presenting approaches, instrument upgrades, and sample preparation modifications that have improved the analytical parameters of methods. The onset of CKD leads to alterations in metabolism that are apparent in the molecular composition of urine. Recent works highlight the prevalence of alterations in the metabolic pathways related to the tricarboxylic acid cycle and amino acids. Including diverse patient cohorts, using numerous analytical techniques with modifications and the appropriate annotation and explanation of the discovered biomarkers will help develop effective diagnostic models for different subtypes of renal injury with clinical applications.
Full article
►▼
Show Figures
Open AccessReview
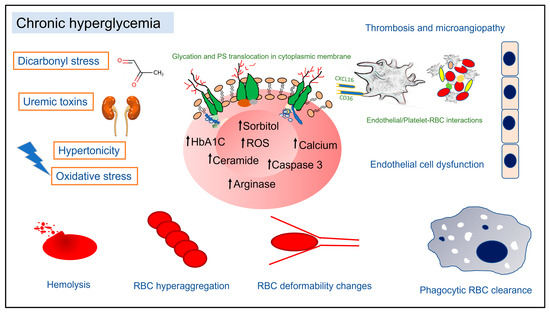
Pathophysiology of Red Blood Cell Dysfunction in Diabetes and Its Complications
by
Alyssa Williams, Rosi Bissinger, Hala Shamaa, Shivani Patel, Lavern Bourne, Ferruh Artunc and Syed M. Qadri
Cited by 36 | Viewed by 14412
Abstract
Diabetes Mellitus (DM) is a complex metabolic disorder associated with multiple microvascular complications leading to nephropathy, retinopathy, and neuropathy. Mounting evidence suggests that red blood cell (RBC) alterations are both a cause and consequence of disturbances related to DM-associated complications. Importantly, a significant
[...] Read more.
Diabetes Mellitus (DM) is a complex metabolic disorder associated with multiple microvascular complications leading to nephropathy, retinopathy, and neuropathy. Mounting evidence suggests that red blood cell (RBC) alterations are both a cause and consequence of disturbances related to DM-associated complications. Importantly, a significant proportion of DM patients develop varying degrees of anemia of confounding etiology, leading to increased morbidity. In chronic hyperglycemia, RBCs display morphological, enzymatic, and biophysical changes, which in turn prime them for swift phagocytic clearance from circulation. A multitude of endogenous factors, such as oxidative and dicarbonyl stress, uremic toxins, extracellular hypertonicity, sorbitol accumulation, and deranged nitric oxide metabolism, have been implicated in pathological RBC changes in DM. This review collates clinical laboratory findings of changes in hematology indices in DM patients and discusses recent reports on the putative mechanisms underpinning shortened RBC survival and disturbed cell membrane architecture within the diabetic milieu. Specifically, RBC cell death signaling, RBC metabolism, procoagulant RBC phenotype, RBC-triggered endothelial cell dysfunction, and changes in RBC deformability and aggregation in the context of DM are discussed. Understanding the mechanisms of RBC alterations in DM provides valuable insights into the clinical significance of the crosstalk between RBCs and microangiopathy in DM.
Full article
►▼
Show Figures
Open AccessArticle
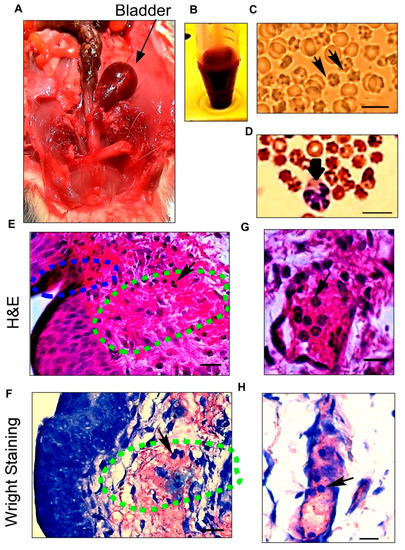
Sex Differences in Immune Cell Infiltration and Hematuria in SCI-Induced Hemorrhagic Cystitis
by
Hadi Askarifirouzjaei, Leila Khajoueinejad, Elena Wei, Sruti Cheruvu, Carlos Ayala, Ning Chiang, Thomas Theis, Dongming Sun, Mehdi Fazeli and Wise Young
Cited by 1 | Viewed by 3132
Abstract
Rats manifest a condition called hemorrhagic cystitis after spinal cord injury (SCI). The mechanism of this condition is unknown, but it is more severe in male rats than in female rats. We assessed the role of sex regarding hemorrhagic cystitis and pathological chronic
[...] Read more.
Rats manifest a condition called hemorrhagic cystitis after spinal cord injury (SCI). The mechanism of this condition is unknown, but it is more severe in male rats than in female rats. We assessed the role of sex regarding hemorrhagic cystitis and pathological chronic changes in the bladder. We analyzed the urine of male and female Sprague-Dawley and Fischer 344 rats after experimental spinal cord contusion, including unstained microscopic inspections of the urine, differential white blood cell counts colored by the Wright stain, and total leukocyte counts using fluorescent nuclear stains. We examined bladder histological changes in acute and chronic phases of SCI, using principal component analysis (PCA) and clustered heatmaps of Pearson correlation coefficients to interpret how measured variables correlated with each other. Male rats showed a distinct pattern of macroscopic hematuria after spinal cord injury. They had higher numbers of red blood cells with significantly more leukocytes and neutrophils than female rats, particularly hypersegmented neutrophils. The histological examination of the bladders revealed a distinct line of apoptotic umbrella cells and disrupted bladder vessels early after SCI and progressive pathological changes in multiple bladder layers in the chronic phase. Multivariate analyses indicated immune cell infiltration in the bladder, especially hypersegmented neutrophils, that correlated with red blood cell counts in male rats. Our study highlights a hitherto unreported sex difference of hematuria and pathological changes in males and females’ bladders after SCI, suggesting an important role of immune cell infiltration, especially neutrophils, in SCI-induced hemorrhagic cystitis.
Full article
►▼
Show Figures
Open AccessArticle
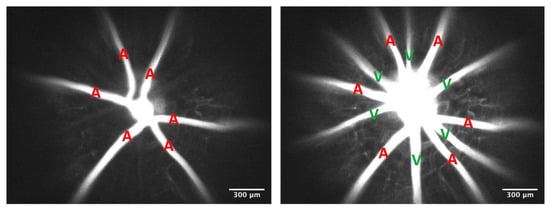
The Endothelial Glycocalyx and Retinal Hemodynamics
by
Gaganpreet Kaur, Wendy Leskova and Norman R. Harris
Cited by 5 | Viewed by 2690
Abstract
Purpose. Previous studies suggest that the endothelial glycocalyx adds to vascular resistance, inhibits thrombosis, and is critical for regulating homogeneous blood flow and ensuring uniform red blood cell (RBC) distribution. However, these functions and consequences of the glycocalyx have not been examined in
[...] Read more.
Purpose. Previous studies suggest that the endothelial glycocalyx adds to vascular resistance, inhibits thrombosis, and is critical for regulating homogeneous blood flow and ensuring uniform red blood cell (RBC) distribution. However, these functions and consequences of the glycocalyx have not been examined in the retina. We hypothesize that the endothelial glycocalyx is a critical regulator of retinal hemodynamics and perfusion and decreases the propensity for retinal thrombus formation. Methods. Hyaluronidase and heparinase, which are endothelial glycocalyx-degrading enzymes, were infused into mice. Fluorescein isothiocyanate–dextran (2000 kDa) was injected to measure lumen diameter, while RBC velocity and distribution were measured using fluorescently labeled RBCs. The diameters and velocities were used to calculate retinal blood flow and shear rates. Mean circulation time was calculated by measuring the difference between arteriolar and venular mean transit times. Rose Bengal dye was infused, followed by illumination with a green light to induce thrombosis. Results. The acute infusion of hyaluronidase and heparinase led to significant increases in both arteriolar (7%) and venular (16%) diameters in the retina, with a tendency towards increased arteriolar velocity. In addition, the degradation caused a significant decrease in the venular shear rate (14%). The enzyme infusion resulted in substantial increases in total retinal blood flow (26%) and retinal microhematocrit but no changes in the mean circulation time through the retina. We also observed an enhanced propensity for retinal thrombus formation with the removal of the glycocalyx. Conclusions. Our data suggest that acute degradation of the glycocalyx can cause significant changes in retinal hemodynamics, with increases in vessel diameter, blood flow, microhematocrit, pro-thrombotic conditions, and decreases in venular shear rate.
Full article
►▼
Show Figures
Open AccessReview
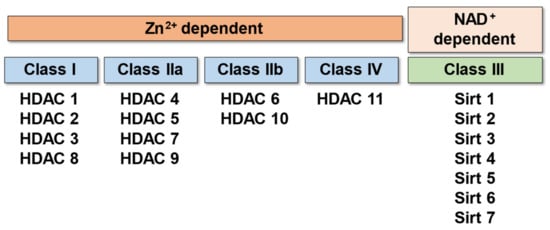
Critical Functions of Histone Deacetylases (HDACs) in Modulating Inflammation Associated with Cardiovascular Diseases
by
Supaporn Kulthinee, Naohiro Yano, Shougang Zhuang, Lijiang Wang and Ting C. Zhao
Cited by 29 | Viewed by 5183
Abstract
Histone deacetylases (HDACs) are a superfamily of enzymes that catalyze the removal of acetyl functional groups from lysine residues of histone and non-histone proteins. There are 18 mammalian HDACs, which are classified into four classes based on the primary homology with yeast HDACs.
[...] Read more.
Histone deacetylases (HDACs) are a superfamily of enzymes that catalyze the removal of acetyl functional groups from lysine residues of histone and non-histone proteins. There are 18 mammalian HDACs, which are classified into four classes based on the primary homology with yeast HDACs. Among these groups, Class I and II HDACs play a major role in lysine deacetylation of the N-terminal histone tails. In mammals, HDACs play a pivotal role in the regulation of gene transcription, cell growth, survival, and proliferation. HDACs regulate the expression of inflammatory genes, as evidenced by the potent anti-inflammatory activity of pan-HDAC inhibitors, which were implicated in several pathophysiologic states in the inflammation process. However, it is unclear how each of the 18 HDAC proteins specifically contributes to the inflammatory gene expression. It is firmly established that inflammation and its inability to converge are central mechanisms in the pathogenesis of several cardiovascular diseases (CVDs). Emerging evidence supports the hypothesis that several different pro-inflammatory cytokines regulated by HDACs are associated with various CVDs. Based on this hypothesis, the potential for the treatment of CVDs with HDAC inhibitors has recently begun to attract attention. In this review, we will briefly discuss (1) pathophysiology of inflammation in cardiovascular disease, (2) the function of HDACs in the regulation of atherosclerosis and cardiovascular diseases, and (3) the possible therapeutic implications of HDAC inhibitors in cardiovascular diseases. Recent studies reveal that histone deacetylase contributes critically to mediating the pathophysiology of inflammation in cardiovascular disease. HDACs are also recognized as one of the major mechanisms in the regulation of inflammation and cardiovascular function. HDACs show promise in developing potential therapeutic implications of HDAC inhibitors in cardiovascular and inflammatory diseases.
Full article
►▼
Show Figures
Open AccessArticle
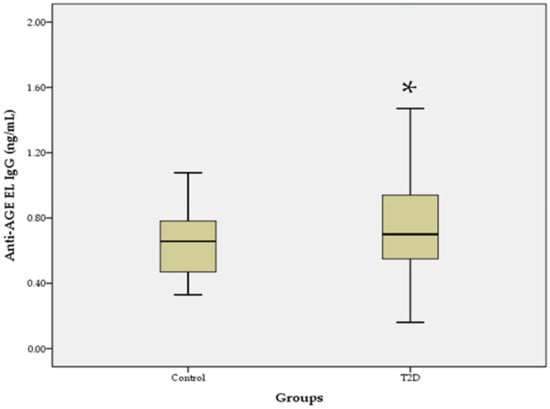
Elevated IgG and IgM Autoantibodies to Advanced Glycation End Products of Vascular Elastin in Hypertensive Patients with Type 2 Diabetes: Relevance to Disease Initiation and Progression
by
Krasimir Kostov and Alexander Blazhev
Cited by 6 | Viewed by 3807
Abstract
The increased glycation of elastin is an important factor in vascular changes in diabetes. Using the ELISA method, we determined serum levels of IgM and IgG autoantibodies to advanced glycation end products of vascular elastin (anti-AGE EL IgM and anti-AGE EL IgG) in
[...] Read more.
The increased glycation of elastin is an important factor in vascular changes in diabetes. Using the ELISA method, we determined serum levels of IgM and IgG autoantibodies to advanced glycation end products of vascular elastin (anti-AGE EL IgM and anti-AGE EL IgG) in 59 hypertensive patients with type 2 diabetes (T2D) and 20 healthy controls. Serum levels of matrix metalloproteinases-2 and -9 (MMP-2 and MMP-9) and the C-reactive protein (CRP) were also determined. The levels of anti-AGE EL IgM antibodies in the T2D group were similar to those in the control group, while those of anti-AGE EL IgG antibodies were significantly higher (
p = 0.017). Significant positive correlations were found between the levels of anti-AGE EL IgM antibodies and MMP-2 (
r = 0.322;
p = 0.013) and between the levels of anti-AGE EL IgG antibodies and CRP (
r = 0.265;
p = 0.042). Our study showed that elevated anti-AGE EL IgG antibody levels may be an indicator of the enhanced AGE-modification and inflammatory-mediated destruction of vascular elastin in hypertensive patients with T2D. Anti-AGE EL IgM antibodies may reflect changes in vascular MMP-2 activity, and their elevated levels may be a sign of early vascular damage.
Full article
►▼
Show Figures