Early Radiation-Induced Changes in Lung Tissue and Intercellular Junctions: Implications for Tissue Repair and Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Western Blot
2.3. Histological Analysis and Morphometry
2.4. Immunofluorescent Staining
2.5. Statistics
3. Results
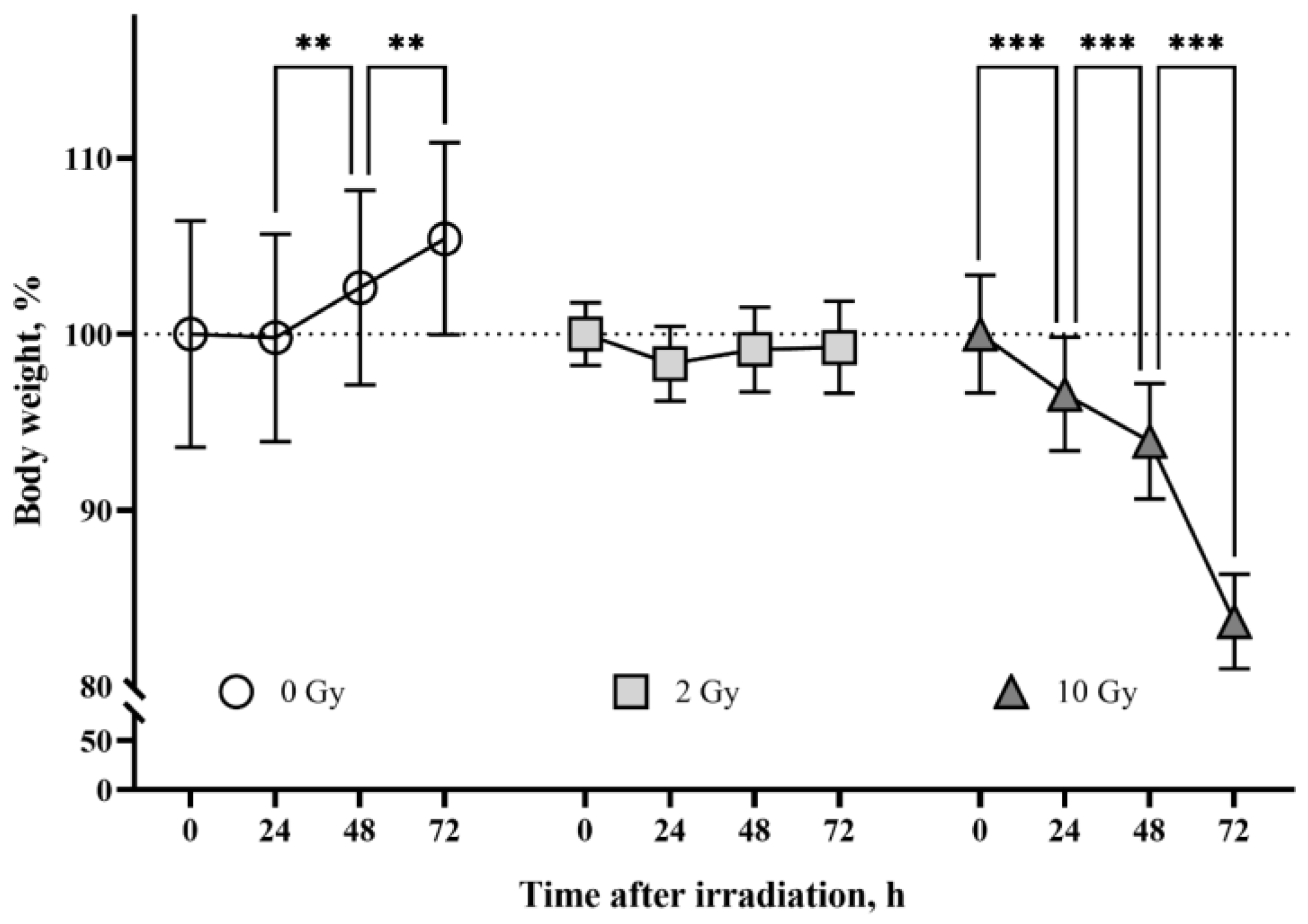
3.1. IR Affects Body Weight in Rats
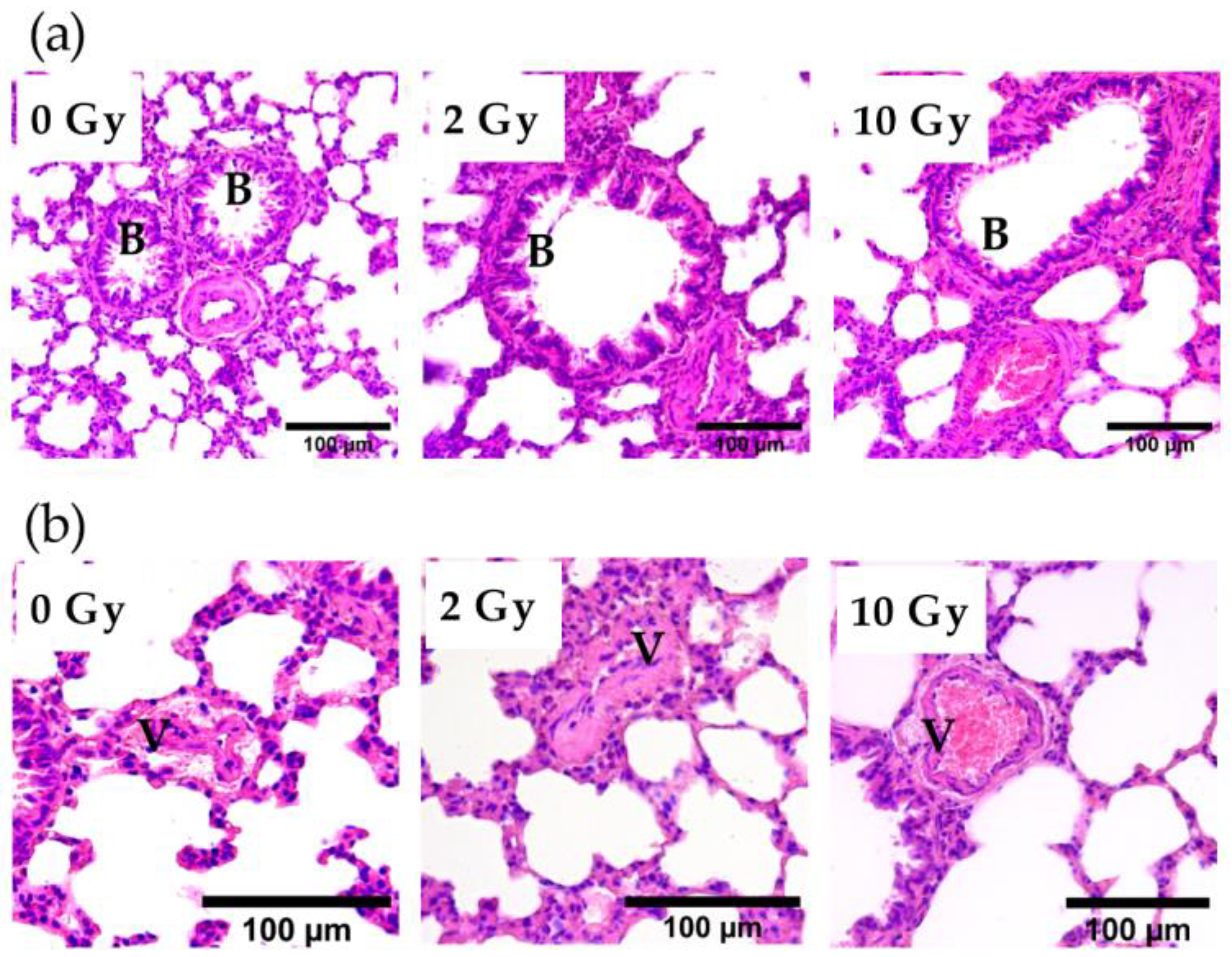
3.2. Early Changes in Lung Tissue after IR
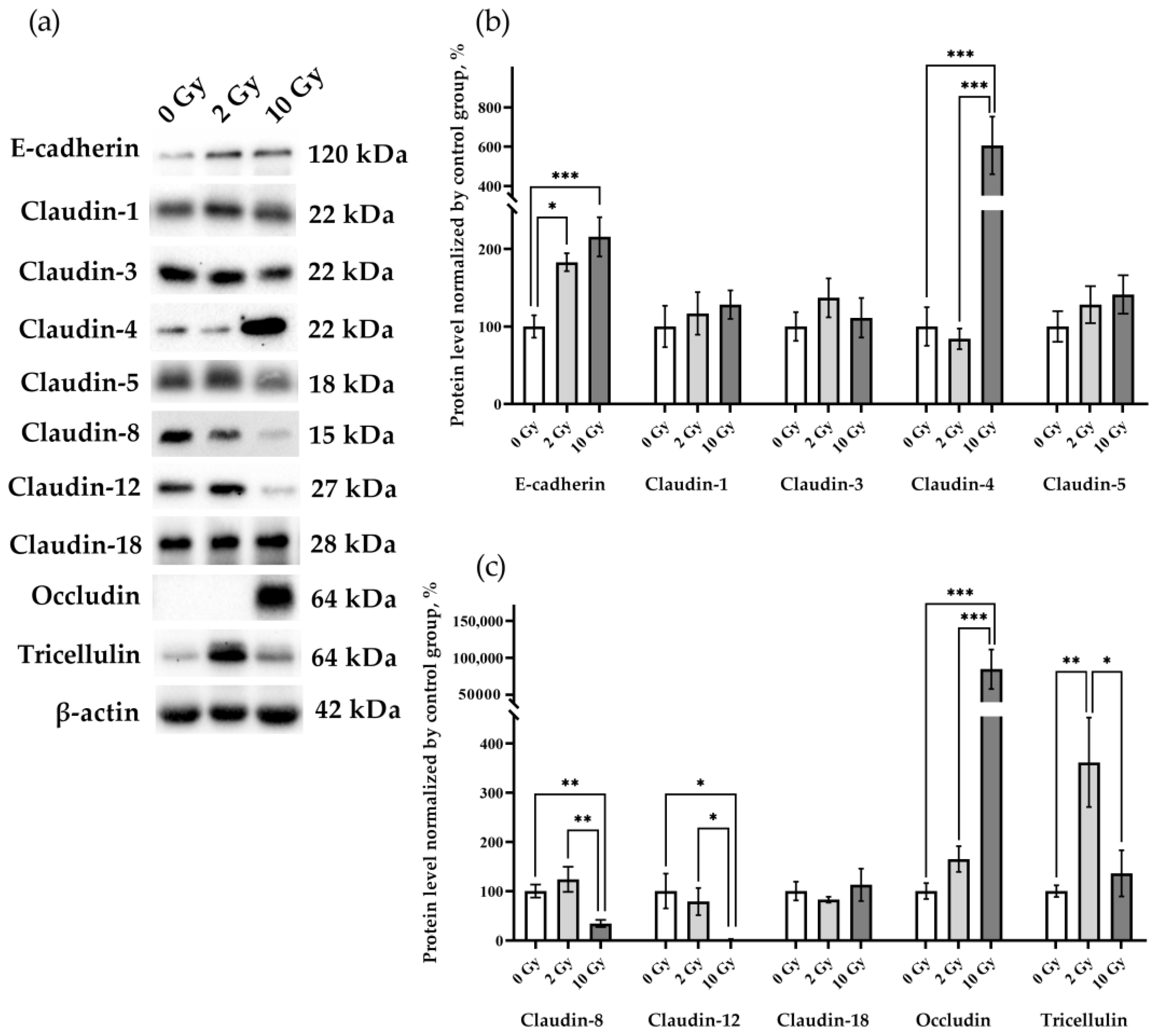
3.3. IR Causes Specific Changes in the Levels of Intercellular Junction Proteins in Lung Tissue
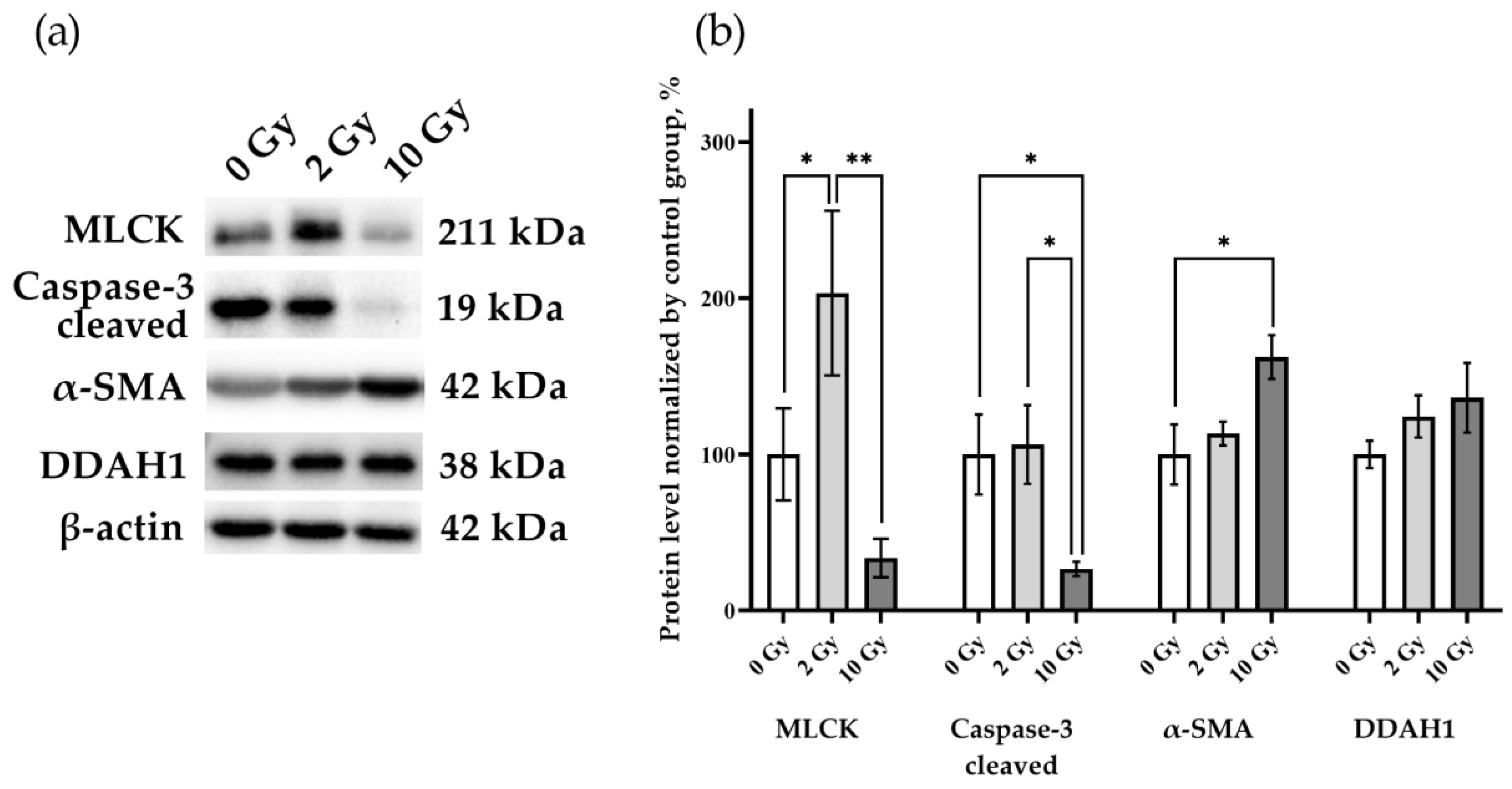
3.4. Evaluation of Processes Induced by IR
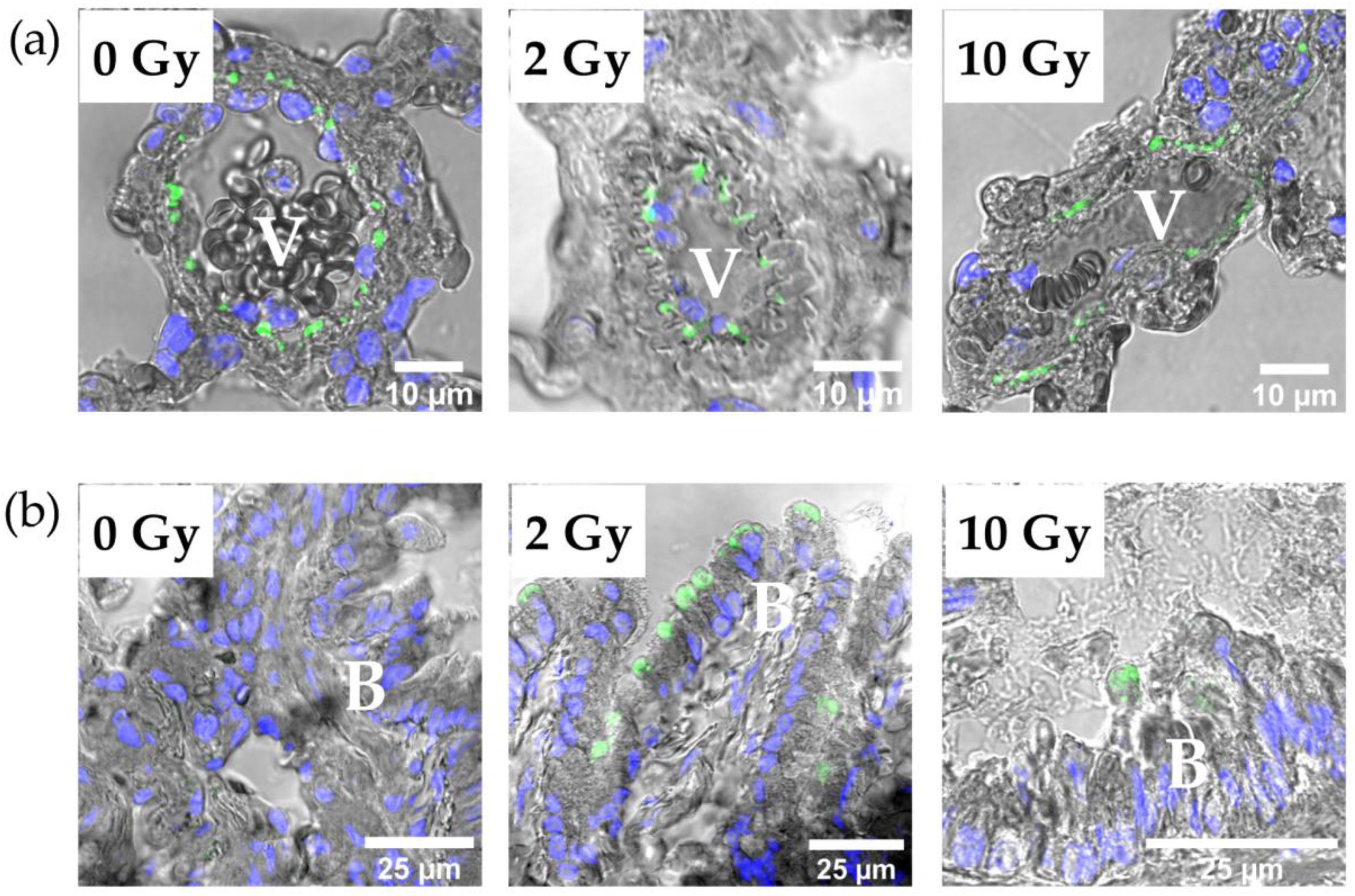
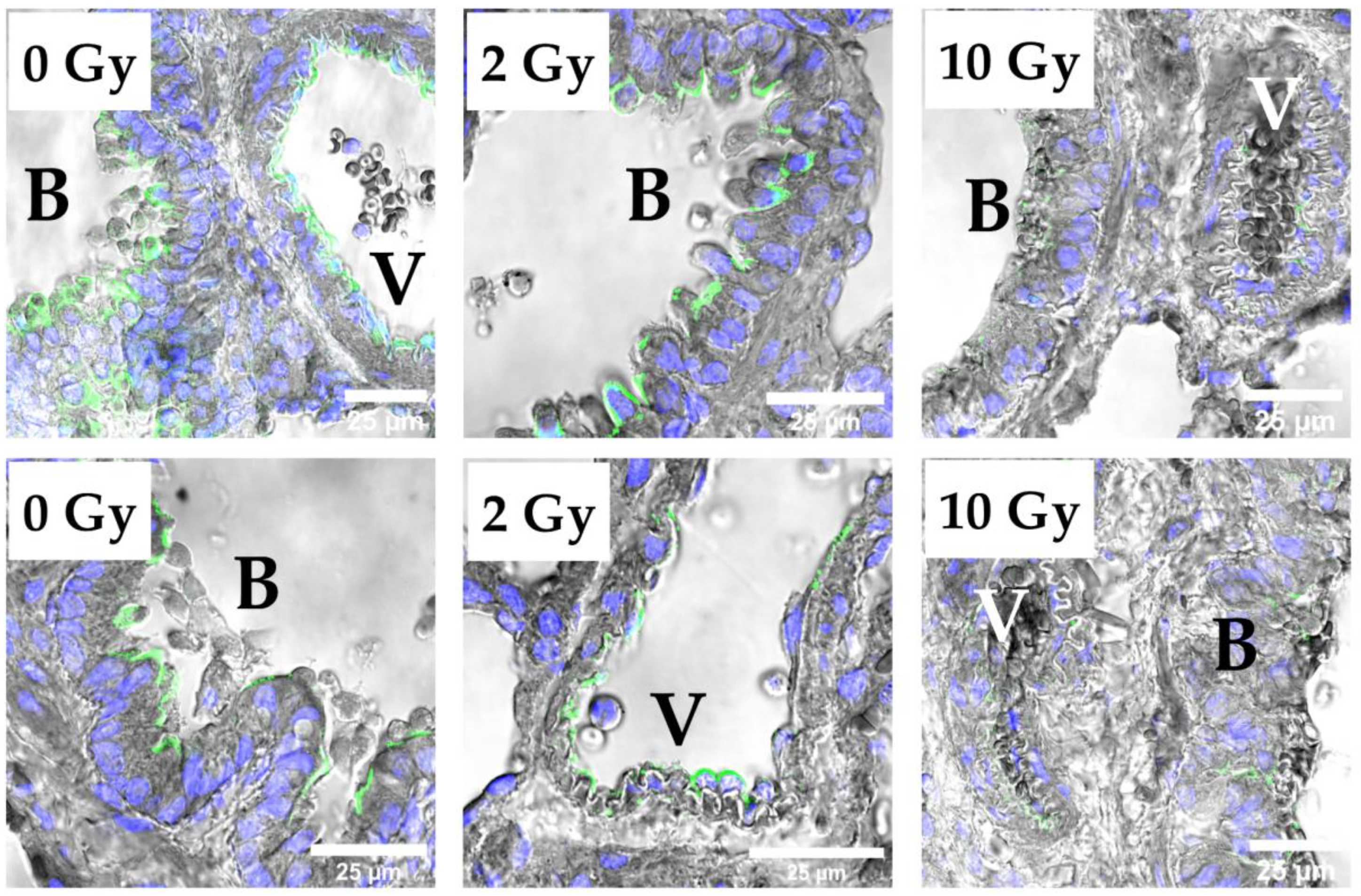
3.5. Distribution of Intercellular Junction Proteins in Rat Lung Tissue
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.J.; Novaes, P.E.; Gadia, R.; Motta, R. Guidelines for the treatment of lung cancer using radiotherapy. Rev. Da Assoc. Medica Bras. 2017, 63, 729–732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tyldesley, S.; Delaney, G.; Foroudi, F.; Barbera, L.; Kerba, M.; Mackillop, W. Estimating the need for radiotherapy for patients with prostate, breast, and lung cancers: Verification of model estimates of need with radiotherapy utilization data from British Columbia. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Latini, P.; Aristei, C.; Aversa, F.; Checcaglini, F.; Maranzano, E.; Raymondi, C.; Panizza, B.M.; Perrucci, E.; Martelli, M.F. Lung damage following bone marrow transplantation after hyperfractionated total body irradiation. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1991, 22, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.T.; Hanania, N.A.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef]
- Kasmann, L.; Dietrich, A.; Staab-Weijnitz, C.A.; Manapov, F.; Behr, J.; Rimner, A.; Jeremic, B.; Senan, S.; De Ruysscher, D.; Lauber, K.; et al. Radiation-induced lung toxicity—Cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat. Oncol. 2020, 15, 214. [Google Scholar] [CrossRef]
- Marks, L.B.; Bentzen, S.M.; Deasy, J.O.; Kong, F.M.; Bradley, J.D.; Vogelius, I.S.; El Naqa, I.; Hubbs, J.L.; Lebesque, J.V.; Timmerman, R.D.; et al. Radiation dose-volume effects in the lung. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S70–S76. [Google Scholar] [CrossRef]
- Beach, T.A.; Groves, A.M.; Williams, J.P.; Finkelstein, J.N. Modeling radiation-induced lung injury: Lessons learned from whole thorax irradiation. Int. J. Radiat. Biol. 2020, 96, 129–144. [Google Scholar] [CrossRef]
- Jarzebska, N.; Karetnikova, E.S.; Markov, A.G.; Kasper, M.; Rodionov, R.N.; Spieth, P.M. Scarred Lung. An Update on Radiation-Induced Pulmonary Fibrosis. Front. Med. 2020, 7, 585756. [Google Scholar] [CrossRef]
- Jiao, Y.; Cao, F.; Liu, H. Radiation-induced Cell Death and Its Mechanisms. Health Phys. 2022, 123, 376–386. [Google Scholar] [CrossRef]
- Lierova, A.; Jelicova, M.; Nemcova, M.; Proksova, M.; Pejchal, J.; Zarybnicka, L.; Sinkorova, Z. Cytokines and radiation-induced pulmonary injuries. J. Radiat. Res. 2018, 59, 709–753. [Google Scholar] [CrossRef]
- Yan, Y.; Fu, J.; Kowalchuk, R.O.; Wright, C.M.; Zhang, R.; Li, X.; Xu, Y. Exploration of radiation-induced lung injury, from mechanism to treatment: A narrative review. Transl. Lung Cancer Res. 2022, 11, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Catravas, J.D.; Burch, S.E.; Spurlock, B.O.; Mills, L.R. Early effects of ionizing radiation on pulmonary endothelial angiotensin-converting enzyme and 5′-nucleotidase, in vivo. Toxicol. Appl. Pharmacol. 1988, 94, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.L.; Zhang, X.; Hadley, C.; Rabbani, Z.N.; Zhang, Y.; Marks, S.; Vujaskovic, Z. Temporal expression of hypoxia-regulated genes is associated with early changes in redox status in irradiated lung. Free Radic. Biol. Med. 2012, 53, 337–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhatt, T.; Rizvi, A.; Batta, S.P.; Kataria, S.; Jamora, C. Signaling and mechanical roles of E-cadherin. Cell Commun. Adhes. 2013, 20, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.H. Molecular Mobility-Mediated Regulation of E-Cadherin Adhesion. Trends Biochem. Sci. 2020, 45, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.G.; Aschenbach, J.R.; Amasheh, S. The epithelial barrier and beyond: Claudins as amplifiers of physiological organ functions. IUBMB Life 2017, 69, 290–296. [Google Scholar] [CrossRef]
- Almeida, C.; Nagarajan, D.; Tian, J.; Leal, S.W.; Wheeler, K.; Munley, M.; Blackstock, W.; Zhao, W. The role of alveolar epithelium in radiation-induced lung injury. PLoS ONE 2013, 8, e53628. [Google Scholar] [CrossRef]
- Guarino, M.; Tosoni, A.; Nebuloni, M. Direct contribution of epithelium to organ fibrosis: Epithelial-mesenchymal transition. Hum. Pathol. 2009, 40, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.G.; Aschenbach, J.R.; Amasheh, S. Claudin clusters as determinants of epithelial barrier function. IUBMB Life 2015, 67, 29–35. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef]
- Zuo, L.; Kuo, W.T.; Turner, J.R. Tight Junctions as Targets and Effectors of Mucosal Immune Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef] [PubMed]
- He, W.Q.; Wang, J.; Sheng, J.Y.; Zha, J.M.; Graham, W.V.; Turner, J.R. Contributions of Myosin Light Chain Kinase to Regulation of Epithelial Paracellular Permeability and Mucosal Homeostasis. Int. J. Mol. Sci. 2020, 21, 993. [Google Scholar] [CrossRef] [PubMed]
- Varedi, M.; Pajouhi, N.; Owji, M.; Naghibalhossaini, F.; Omrani, G.H.R. Differential modulation of claudin 4 expression and myosin light chain phosphorylation by thyroid function in lung injury. Clin. Respir. J. 2017, 11, 797–804. [Google Scholar] [CrossRef]
- Cohen, T.S.; Gray Lawrence, G.; Margulies, S.S. Cultured alveolar epithelial cells from septic rats mimic in vivo septic lung. PLoS ONE 2010, 5, e11322. [Google Scholar] [CrossRef]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Park, C.S.; Jang, A.S. Impact of ozone on claudins and tight junctions in the lungs. Environ. Toxicol. 2018, 33, 798–806. [Google Scholar] [CrossRef]
- Titto, M.; Ankit, T.; Saumya, B.; Gausal, A.; Sarada, S. Curcumin prophylaxis refurbishes alveolar epithelial barrier integrity and alveolar fluid clearance under hypoxia. Respir. Physiol. Neurobiol. 2020, 274, 103336. [Google Scholar] [CrossRef]
- Pao, H.P.; Liao, W.I.; Tang, S.E.; Wu, S.Y.; Huang, K.L.; Chu, S.J. Suppression of Endoplasmic Reticulum Stress by 4-PBA Protects Against Hyperoxia-Induced Acute Lung Injury via Up-Regulating Claudin-4 Expression. Front. Immunol. 2021, 12, 674316. [Google Scholar] [CrossRef]
- Wray, C.; Mao, Y.; Pan, J.; Chandrasena, A.; Piasta, F.; Frank, J.A. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L219–L227. [Google Scholar] [CrossRef]
- Yokoyama, M.; Narita, T.; Sakurai, H.; Katsumata-Kato, O.; Sugiya, H.; Fujita-Yoshigaki, J. Maintenance of claudin-3 expression and the barrier functions of intercellular junctions in parotid acinar cells via the inhibition of Src signaling. Arch. Oral Biol. 2017, 81, 141–150. [Google Scholar] [CrossRef]
- Zhou, B.; Flodby, P.; Luo, J.; Castillo, D.R.; Liu, Y.; Yu, F.X.; McConnell, A.; Varghese, B.; Li, G.; Chimge, N.O.; et al. Claudin-18-mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis. J. Clin. Investig. 2018, 128, 970–984. [Google Scholar] [CrossRef] [PubMed]
- van Roy, F.; Berx, G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. CMLS 2008, 65, 3756–3788. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Y.; Kahn, M.; Ann, D.K.; Han, A.; Wang, H.; Nguyen, C.; Flodby, P.; Zhong, Q.; Krishnaveni, M.S.; et al. Interactions between beta-catenin and transforming growth factor-beta signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). J. Biol. Chem. 2012, 287, 7026–7038. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cai, W.; Zhou, S.; Xu, L.; Jiang, C. Protective effect of bone marrow derived mesenchymal stem cells in lipopolysaccharide-induced acute lung injury mediated by claudin-4 in a rat model. Am. J. Transl. Res. 2016, 8, 3769–3779. [Google Scholar] [PubMed]
- Srinivas, C.; Kumar, A.; Rai, R.; Kini, J.; Kumarchandra, R. Standardization of Mean Lethal Dose (LD 50/30) of X -rays using Linear Accelerator (LINIAC) in Albino Wistar Rat Model Based on Survival Analysis Studies and Hematological Parameters. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1215–1219. [Google Scholar]
- National Institutes of Health. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection; National Institutes of Health: Washington, DC, USA, 2011.
- Matsuu, M.; Shichijo, K.; Ikeda, Y.; Ito, M.; Naito, S.; Okaichi, K.; Nakashima, M.; Nakayama, T.; Sekine, I. Sympathetic hyperfunction causes increased sensitivity of the autonomic nervous system to whole-body X irradiation. Radiat. Res. 2005, 163, 137–143. [Google Scholar] [CrossRef]
- Trajkovic, S.; Dobric, S.; Jacevic, V.; Dragojevic-Simic, V.; Milovanovic, Z.; Dordevic, A. Tissue-protective effects of fullerenol C60(OH)24 and amifostine in irradiated rats. Colloids Surf. B Biointerfaces 2007, 58, 39–43. [Google Scholar] [CrossRef]
- Chapman, W.H.; Jerome, E.A. An analysis of the effects of tota-body x-irradiation on the body weight of white Swiss mice. II. Body-weight changes of male mice as a biological dosimeter. Radiat. Res. 1956, 4, 519–531. [Google Scholar] [CrossRef]
- Frank, J.A. Claudins and alveolar epithelial barrier function in the lung. Ann. N. Y. Acad. Sci. 2012, 1257, 175–183. [Google Scholar] [CrossRef]
- Soini, Y. Claudins in lung diseases. Respir. Res. 2011, 12, 70. [Google Scholar] [CrossRef]
- Han, L.; Luo, H.; Huang, W.; Zhang, J.; Wu, D.; Wang, J.; Pi, J.; Liu, C.; Qu, X.; Liu, H.; et al. Modulation of the EMT/MET Process by E-Cadherin in Airway Epithelia Stress Injury. Biomolecules 2021, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Zhang, H.; Guo, X.W.; Lu, Y.; Wang, S.N.; Cheng, B.; Dong, S.H.; Lyu, X.L.; Li, F.S.; Li, Y.W. Mechanically Activated Calcium Channel PIEZO1 Modulates Radiation-Induced Epithelial-Mesenchymal Transition by Forming a Positive Feedback With TGF-beta1. Front. Mol. Biosci. 2021, 8, 725275. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Son, Y.; Jung, M.G.; Jeong, Y.J.; Kim, S.H.; Lee, S.J.; Lee, Y.J.; Lee, H.J. Geranylgeranylacetone alleviates radiation-induced lung injury by inhibiting epithelial-to-mesenchymal transition signaling. Mol. Med. Rep. 2016, 13, 4666–4670. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Hu, K.; Liu, W.; Jiang, J.; Chen, Y.; Wang, R. Role of matrix metalloproteinases in radiation-induced lung injury in alveolar epithelial cells of Bama minipigs. Exp. Ther. Med. 2015, 10, 1437–1444. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Zhou, J.; Xiao, Z.; Li, Y.; Zhang, Y.; Yue, H.; Li, Z.; Tian, J. beta-Catenin/Lin28/let-7 regulatory network determines type II alveolar epithelial stem cell differentiation phenotypes following thoracic irradiation. J. Radiat. Res. 2021, 62, 119–132. [Google Scholar] [CrossRef]
- Kielgast, F.; Schmidt, H.; Braubach, P.; Winkelmann, V.E.; Thompson, K.E.; Frick, M.; Dietl, P.; Wittekindt, O.H. Glucocorticoids Regulate Tight Junction Permeability of Lung Epithelia by Modulating Claudin 8. Am. J. Respir. Cell Mol. Biol. 2016, 54, 707–717. [Google Scholar] [CrossRef]
- Sun, W.; Wu, W.; Fang, X.; Ge, X.; Zhang, Y.; Han, J.; Guo, X.; Zhou, L.; Yang, H. Disruption of pulmonary microvascular endothelial barrier by dysregulated claudin-8 and claudin-4: Uncovered mechanisms in porcine reproductive and respiratory syndrome virus infection. Cell. Mol. Life Sci. CMLS 2024, 81, 240. [Google Scholar] [CrossRef]
- Koval, M. Claudin heterogeneity and control of lung tight junctions. Annu. Rev. Physiol. 2013, 75, 551–567. [Google Scholar] [CrossRef]
- Rokkam, D.; Lafemina, M.J.; Lee, J.W.; Matthay, M.A.; Frank, J.A. Claudin-4 levels are associated with intact alveolar fluid clearance in human lungs. Am. J. Pathol. 2011, 179, 1081–1087. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Overgaard, C.E.; Ward, C.; Margulies, S.S.; Koval, M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L40–L49. [Google Scholar] [CrossRef]
- Karetnikova, E.S.; Jarzebska, N.; Rodionov, R.N.; Spieth, P.M.; Markov, A.G. Transcriptional Levels of Intercellular Junction Proteins in an Alveolar Epithelial Cell Line Exposed to Irradiation or Bleomycin. Bull. Exp. Biol. Med. 2024, 176, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Hao, S.; Xu, X.; Zhou, J.; Liu, Z.; Lu, H.; Wang, L.; Jin, W.; Li, S. Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability. Acta Pharmacol. Sin. 2019, 40, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jamal, M.; Guo, P.; Jin, Z.; Zheng, F.; Song, X.; Zhan, J.; Wu, H. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed. Pharmacother. 2019, 118, 109363. [Google Scholar] [CrossRef]
- Liu, M.; Gu, C.; Wang, Y. Upregulation of the tight junction protein occludin: Effects on ventilation-induced lung injury and mechanisms of action. BMC Pulm. Med. 2014, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Yan, J.; Wang, Y. Aerobic exercise alleviates ventilator-induced lung injury by inhibiting NLRP3 inflammasome activation. BMC Anesthesiol. 2022, 22, 369. [Google Scholar] [CrossRef]
- Zhang, X.D.; Yu, W.H.; Liu, M.M.; Liu, R.; Wu, H.; Wang, Z.; Hai, C.X. Pentoxifylline inhibits phosgene-induced lung injury via improving hypoxia. Drug Chem. Toxicol. 2022, 46, 1100–1107. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karetnikova, E.S.; Livanova, A.A.; Fedorova, A.A.; Markov, A.G. Early Radiation-Induced Changes in Lung Tissue and Intercellular Junctions: Implications for Tissue Repair and Fibrosis. Pathophysiology 2024, 31, 531-544. https://doi.org/10.3390/pathophysiology31040039
Karetnikova ES, Livanova AA, Fedorova AA, Markov AG. Early Radiation-Induced Changes in Lung Tissue and Intercellular Junctions: Implications for Tissue Repair and Fibrosis. Pathophysiology. 2024; 31(4):531-544. https://doi.org/10.3390/pathophysiology31040039
Chicago/Turabian StyleKaretnikova, Ekaterina S., Alexandra A. Livanova, Arina A. Fedorova, and Alexander G. Markov. 2024. "Early Radiation-Induced Changes in Lung Tissue and Intercellular Junctions: Implications for Tissue Repair and Fibrosis" Pathophysiology 31, no. 4: 531-544. https://doi.org/10.3390/pathophysiology31040039
APA StyleKaretnikova, E. S., Livanova, A. A., Fedorova, A. A., & Markov, A. G. (2024). Early Radiation-Induced Changes in Lung Tissue and Intercellular Junctions: Implications for Tissue Repair and Fibrosis. Pathophysiology, 31(4), 531-544. https://doi.org/10.3390/pathophysiology31040039






