Abstract
The increased glycation of elastin is an important factor in vascular changes in diabetes. Using the ELISA method, we determined serum levels of IgM and IgG autoantibodies to advanced glycation end products of vascular elastin (anti-AGE EL IgM and anti-AGE EL IgG) in 59 hypertensive patients with type 2 diabetes (T2D) and 20 healthy controls. Serum levels of matrix metalloproteinases-2 and -9 (MMP-2 and MMP-9) and the C-reactive protein (CRP) were also determined. The levels of anti-AGE EL IgM antibodies in the T2D group were similar to those in the control group, while those of anti-AGE EL IgG antibodies were significantly higher (p = 0.017). Significant positive correlations were found between the levels of anti-AGE EL IgM antibodies and MMP-2 (r = 0.322; p = 0.013) and between the levels of anti-AGE EL IgG antibodies and CRP (r = 0.265; p = 0.042). Our study showed that elevated anti-AGE EL IgG antibody levels may be an indicator of the enhanced AGE-modification and inflammatory-mediated destruction of vascular elastin in hypertensive patients with T2D. Anti-AGE EL IgM antibodies may reflect changes in vascular MMP-2 activity, and their elevated levels may be a sign of early vascular damage.
1. Introduction
Diabetes mellitus is a chronic disease with an increasing frequency over the last decade [], with type 2 diabetes (T2D) accounting for more than 90% of all diagnosed cases []. In the long term, patients with T2D are at increased risk of developing cardiovascular disease (CVD), and the identification of specific biomarkers may improve their treatment []. One group of biomarkers that can be used are the autoantibodies to advanced glycation end products (AGEs) [,].
AGEs are formed by non-enzymatic reactions between the carbonyl groups of reducing sugars, such as glucose, and the free amino groups of a number biomolecules in the body, via the Maillard reaction []. This reaction is followed by the generation of a reversible Schiff-base adduct, which rearranges into a more stable and covalently bonded Amadori product. The Amadori product then undergoes irreversible chemical modifications that generate AGEs []. The glycation process can affect all proteins in the body, including circulating, extracellular, and intracellular proteins, such as hemoglobin, albumin, insulin, immunoglobulins, low-density lipoproteins, lens crystalline proteins, collagen (COL), and elastin (EL) [,,]. Other biomolecules, such as lipids and DNA, can also be modified in a similar way []. Particularly vulnerable to glycation are long-lived molecules such as COL and EL in the vascular extracellular matrix (ECM), due to the slow rate of their turnover [,]. In diabetes, AGEs can also be formed through the polyol pathway, where intermediates are even more potent glycation agents than glucose [,]. The non-enzymatic glycation of biomolecules is accelerated in patients with diabetes, but also occurs in non-diabetic subjects [].
EL is the main structural element of the arteries and has the lowest turnover rate of all components of vascular ECM (half-life of about 40 years) []. Its mechanical properties are crucial for normal arterial function, and for this reason, it is widely involved in the pathogenesis of CVD []. Biochemical analyses showed that after only twelve days of incubation at a sugar concentration of 250 mmol/L, one of the five available lysines per elastin monomer was already glycated. At longer incubation times, the generation of AGEs increases, which can significantly alter the physical properties of EL []. Changes in vascular EL in diabetes and the formation of cross-links with AGEs may contribute to its fragility and fragmentation, which may be amplified by concomitant hypertension [].
Structural changes in biomolecules due to AGE modifications are associated with the formation of new epitopes that make them potential targets of the immune system. Anti-AGE antibodies that can be used as a biomarker for vascular damage have been found in the sera of patients with diabetes [,,,]. Due to their immunogenicity, AGEs can cause inflammation by stimulating the AGE receptor (RAGE), which triggers a series of signaling cascades and activates pro-inflammatory genes [,,]. Inflammation, in turn, may enhance the activity of matrix metalloproteinases (MMPs) in the vascular wall [].
In our study, we used as an antigen human aortic α-elastin, glycated in vitro, to determine the serum levels of IgM and IgG autoantibodies to AGEs of vascular elastin (anti-AGE EL IgM antibodies and anti-AGE EL IgG antibodies) in hypertensive patients with T2D. We also measured serum levels of MMP-2, MMP-9, and the C-reactive protein (CRP) as indirect biomarkers for elastase activity and low-grade systemic inflammation.
2. Materials and Methods
2.1. Screening of the Patients and Controls
The studied clinical contingent includes patients with T2D who were admitted for periodic control and monitoring at the Dr. Georgi Stranski University Hospital in Pleven. Control subjects were clinically healthy age-matched volunteers. The patients and controls were screened for hypertension according to the 2018 ESC/ESH Clinical Practice Guidelines. Blood pressure (BP) was measured on the left arm in a sitting position after 5–10 min of rest. Hypertension was defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg, or if the patients had been diagnosed or had taken antihypertensive drugs at any time during the preceding six months. Normal BP was defined as systolic BP 120–129 mmHg and diastolic BP 80–84 mmHg.
2.2. Immunological and Biochemical Assays
To measure the levels of anti-AGE EL IgM and anti-AGE EL IgG antibodies and the other laboratory parameters, blood was drawn into vacutainer tubes and was centrifuged at 2500 rpm for 10 min to separate the serum. Biochemical analyzes were performed immediately, and serum samples for the immunoassay were stored at −70 °C until testing.
2.2.1. Determination of Anti-AGE EL IgM and Anti-AGE EL IgG Antibodies
AGE-elastin was obtained via the incubation of human aortic α-elastin (1.33 mg/mL) with 100 mmol/L glucose for 30 days, as described by Baydanoff et al. []. A blocking ELISA was used for the detection of IgM and IgG autoantibodies to AGEs of vascular EL. The 96-well plates were coated with AGE-elastin (5 μg/mL) and incubated with 100 μL of human sera (diluted 1:20) for 1 h at 37 °C. Then, 100 µL of goat anti-human IgM Ab, Fc5µ, HRP conjugate (AP114P, Sigma-Aldrich, St. Louis, MO, USA) and goat anti-human IgG Ab, Fc, HRP conjugate (AP113P, SigmaAldrich, St. Louis, MO, USA), respectively, were added to each well. Immunoconjugates were diluted 1:10,000 and ortho-phenylenediamine was used as the chromogen. The reaction was stopped by adding 50 μL/well of sulfuric acid (4 M H2SO4), and the optical density was measured on a Coulter Microplate Reader UV Max (Molecular Devices Corp., Menlo Park, CA, USA) at a wavelength of 492 nm. All samples were tested in triplicate.
2.2.2. Determination of MMP-2 and MMP-9
Serum levels of MMP-2 and MMP-9 were determined by ELISA kits from R&D Systems (MMP-2, cat. no. DMP2F0 and MMP-9, cat. no. DMP900). The samples were analyzed on a Coulter Microplate Reader UV Max at a wavelength of 450 nm.
2.2.3. Biochemical Analysis
Serum CRP levels were measured by particle enhanced turbidimetry. Glycated haemoglobin (HbA1c) levels were determined by a turbidimetric inhibition immunoassay. Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) were measured by enzymatic methods. All samples were analyzed on a Cobas Integra 400 system (Roche Diagnostics, Basel, Switzerland).
2.3. Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics version 23.0 software (SPSS, Inc., Chicago, IL, USA). The differences between the means of two groups were assessed by an unpaired Student’s t-test. Correlation analysis was performed with Pearson’s correlation test. p values of less than 0.05 were considered statistically significant.
3. Results
3.1. Characteristics of the Study Population
The study population consisted of 59 hypertensive patients with T2D (age 60.8 ± 14.7 years; mean disease duration of 10.1 ± 7.8 years) and 20 healthy controls (mean age 61.5 ± 11.4 years). The clinical characteristics of the groups are shown in Table 1.

Table 1.
Clinical characteristics of the groups.
3.2. Comparison of Anti-AGE EL Antibody Levels between the T2D Group and Controls
The levels of anti-AGE EL IgM antibodies in the T2D group were similar to those in the control group, and the difference was not statistically significant (0.46 ± 0.18 vs. 0.45 ± 0.13; p = 0.923). In contrast, the levels of anti-AGE EL IgG antibodies were significantly higher in the T2D group than in the control group (0.84 ± 0.48 vs. 0.65 ± 0.20; p = 0.017; Figure 1).
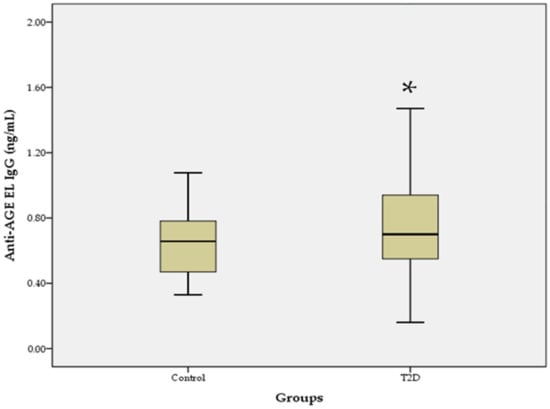
Figure 1.
Serum levels of anti-AGE EL IgG antibodies in the T2D group compared to the control group. Data are represented as mean ± SD. * p < 0.05.
3.3. Correlation between Anti-AGE EL Antibody Levels and Clinical Features
In the T2D group, we found significant positive correlations between the levels of anti-AGE EL IgM antibodies and MMP-2 (r = 0.322; p = 0.013; Figure 2), as well as between the levels of anti-AGE EL IgG antibodies and CRP (r = 0.265; p = 0.042; Figure 3).
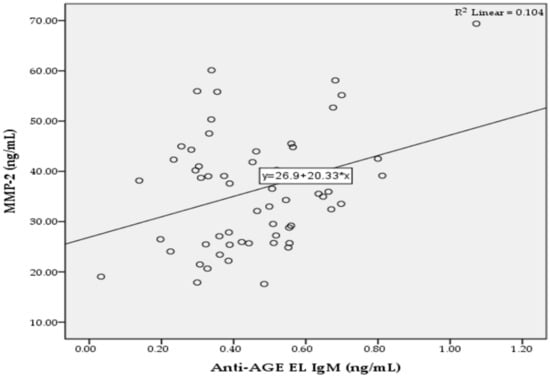
Figure 2.
Correlation between the serum levels of anti-AGE EL IgM antibodies and MMP-2 in the T2D group.
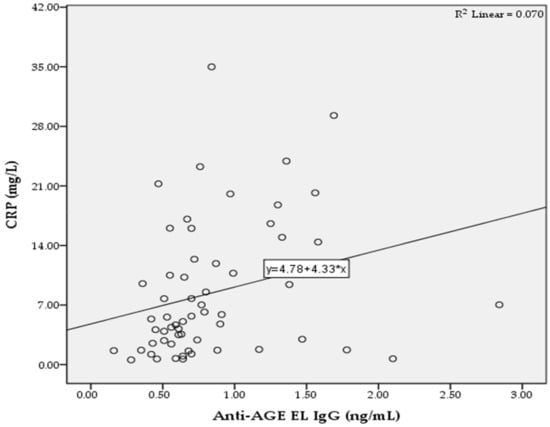
Figure 3.
Correlation between the serum levels of anti-AGE EL IgG antibodies and CRP in the T2D group.
4. Discussion
Autoimmunity is considered the major factor in the pathogenesis of type 1 diabetes (T1D), but also plays a role in T2D. A hallmark of autoimmune involvement in T2D is the presence of circulating autoantibodies []. The detection of elevated levels of autoantibodies to AGEs in patients with T2D raises the question of their role in the pathophysiology of the disease. Nikolov et al. have found that serum levels of total anti-AGE antibodies were significantly higher in hypertensive patients with T2D with microvascular complications than healthy controls and patients without such complications []. Similar results have also been reported in patients with T1D [,].
The accumulation of AGEs on long-lived proteins of the vascular tissue is closely related to the development of diabetic vascular complications, which makes the measurement of serum levels of total and class-specific autoantibodies to AGE EL important for the assessment of increased vascular risk in patients with T2D. Because elastin is a main structural element of arteries and is a potential target for the formation of AGEs [], we investigated serum levels of IgM and IgG autoantibodies to AGEs of vascular EL (AGE EL) in patients with advanced T2D and hypertension, who are at increased cardiovascular risk. The results showed that the levels of anti-AGE EL IgG antibodies were significantly higher in the T2D group compared to the control group, while the levels of anti-AGE EL IgM antibodies were similar to those in the controls (Figure 1). The non-enzymatic glycation of vascular EL is a spontaneous process [] that leads to the formation of autoantibodies against epitopes of AGE EL [], both in normal aging and in diabetes []. The IgM class of autoantibodies predominates in the early stage of the immune response and later undergoes switching to the IgG class, which has the same antigen specificity []. Because T2D is a chronic disease, the levels of anti-AGE EL IgG antibodies are significantly higher than those of anti-AGE EL IgM antibodies compared to the controls. We also found a positive correlation between the serum levels of anti-AGE EL IgM antibodies and MMP-2, suggesting that these antibodies may serve as a biomarker for vascular damage in T2D (Figure 2). MMP-2 is an important ECM enzyme that can break down various substrates, such as COL, EL, fibronectin, and laminin []. A number of studies have shown that the dysregulation of MMP-2 may contribute to the development of diabetic vascular complications [,,]. Elastases include five MMPs (MMP-2, -7, -9, -12, and-14), and serine and cysteine proteinases. They can cleave EL, leading to the formation of EL-derived peptides. Interestingly, these peptides are able to promote insulin resistance and the appearance of characteristic features of T2D, as well as to promote atherogenesis []. They can also promote angiogenesis, cell adhesion, proliferation, chemotaxis, protease activity, and apoptosis []. Our results showed that serum levels of MMP-2 and MMP-9 were significantly higher in the T2D group than in the control group, which may be an indirect sign of increased EL destruction in the arterial wall (Table 1).
The increased accumulation of AGEs in diabetic vascular tissue causes an inflammatory response characterized by leukocyte activation and the release of proinflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-α [,,]. In response to these cytokines, the liver produces CRP, which is considered an important biomarker for systemic inflammation. IL-6 is the major inducer of CRP gene expression, with IL-1 potentiating this effect []. Our data show that there is a significant positive correlation between the levels of anti-AGE EL IgG antibodies and CRP as a marker for systemic inflammation (Figure 3). Therefore, this correlation suggests the presence of a direct relationship between the degree of inflammatory response and the levels of anti-AGE EL IgG antibodies. Our data also show that CRP levels were significantly higher in the T2D group compared to the control group (Table 1). Pickup et al. reported that IL-6 and CRP were elevated in the serum of patients with non-insulin-dependent diabetes mellitus []. In addition, elevated serum concentrations of AGEs in patients with T2D are an independent determinant of CRP levels []. CRP causes numerous proinflammatory and proatherogenic effects in endothelial cells, such as the decreased production of nitric oxide and prostacyclin, increased production of endothelin-1, and increased expression of adhesion molecules, monocyte chemotactic protein-1, interleukin-8, and plasminogen activator inhibitor-1 [].
The exact role of anti-AGE antibodies in the pathophysiology of diabetes is not fully understood. It is thought that they may be part of a defense mechanism that serves to remove damaged or dysfunctional proteins as a result of enhanced AGE modifications []. In this regard, the likely role of anti-AGE EL IgM and anti-AGE EL IgG antibodies is that they may be involved in the removal of damaged glycated vascular EL and its metabolites through the formation of circulating immune complexes and their subsequent elimination by a mononuclear phagocytic system. An additional mechanism of complement activation and K-cell-mediated antibody-dependent cytotoxicity may contribute to the further destruction of EL in the arterial wall, and specific T- and B-lymphocytes may also be involved in this process [,,].
A limitation of the study is the relatively small number of individuals studied, which requires these results to be confirmed in a larger cohort.
5. Conclusions
Our results showed that the levels of anti-AGE EL IgG antibodies were significantly higher in the T2D group compared to the control group, which can be explained by the chronic course of the disease. A positive correlation was found between the levels of anti-AGE EL IgG antibodies and CRP, suggesting a direct relationship between the levels of these antibodies and the grade of systemic inflammation in T2D patients. The levels of anti-AGE EL IgM antibodies may predominate in the early stages of the immune response, and the existence of a positive correlation between them and MMP-2 suggests that they may serve as predictors of early vascular damage. Therefore, it can be concluded that the measurement of serum levels of class-specific autoantibodies against AGE EL may be important for the overall assessment of vascular risk in T2D patients.
Author Contributions
Conceptualization, K.K.; methodology, A.B.; software, K.K.; formal analysis, K.K.; investigation, K.K.; resources, A.B.; data curation, K.K.; writing—original draft preparation, K.K.; writing—review and editing, K.K.; visualization, K.K.; project administration, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Medical University-Pleven, Bulgaria.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of Medical University-Pleven (Protocol No. 247, 24 April 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors confirm that the data supporting the findings of this report are available within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Cho, N.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Dougherty, T.; Heile, M. Type 2 diabetes in the US managed care setting: The burden of disease and rationale for an oral glucagon-like peptide-1 receptor agonist. Am. J. Manag. Care 2020, 26, S325–S334. [Google Scholar] [PubMed]
- Bachmann, K.N.; Wang, T.J. Biomarkers of cardiovascular disease: Contributions to risk prediction in individuals with diabetes. Diabetologia 2018, 61, 987–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turk, Z.; Ljubic, S.; Turk, N.; Benko, B. Detection of autoantibodies against advanced glycation end products and AGE-immune complexes in serum of patients with diabetes mellitus. Clin. Chim. Acta 2001, 303, 105–115. [Google Scholar] [CrossRef]
- Prasad, A.; Bekker, P.; Tsimikas, S. Advanced glycation end products and diabetic cardiovascular disease. Cardiol. Rev. 2012, 20, 177–183. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation damage: A possible hub for major pathophysiological disorders and aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef] [Green Version]
- Peppa, M.; Raptis, S.A. Advanced glycation end products and cardiovascular disease. Curr. Diabetes Rev. 2008, 4, 92–100. [Google Scholar] [CrossRef]
- Sell, D.R.; Monnier, V.M. Molecular basis of arterial stiffening: Role of glycation—A mini-review. Gerontology 2012, 58, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, M.S.; Akhter, F.; Khan, M.S.; Khan, A.; Ashraf, J.M.; Pandey, R.P.; Shahab, U. Glycoxidation of biological macromolecules: A critical approach to halt the menace of glycation. Glycobiology 2014, 24, 979–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snedeker, J.G.; Gautieri, A. The role of collagen crosslinks in ageing and diabetes—The good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014, 4, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Brüel, A.; Oxlund, H. Changes in biomechanical properties, composition of collagen and elastin, and advanced glycation endproducts of the rat aorta in relation to age. Atherosclerosis 1996, 127, 155–165. [Google Scholar] [CrossRef]
- Hamada, Y.; Araki, N.; Horiuchi, S.; Hotta, N. Role of polyol pathway in nonenzymatic glycation. Nephrol. Dial. Transplant. 1996, 11, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Misciagna, G.; Michele, G.D.; Trevisan, M. Non enzymatic glycated proteins in the blood and cardiovascular disease. Curr. Pharm. Des. 2007, 13, 3688–3695. [Google Scholar] [CrossRef]
- Briones, A.M.; Arribas, S.M.; Salaices, M. Role of extracellular matrix in vascular remodeling of hypertension. Curr. Opin. Nephrol. Hypertens. 2010, 19, 187–194. [Google Scholar] [CrossRef]
- Cocciolone, A.J.; Hawes, J.Z.; Staiculescu, M.C.; Johnson, E.O.; Murshed, M.; Wagenseil, J.E. Elastin, arterial mechanics, and cardiovascular disease. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H189–H205. [Google Scholar] [CrossRef]
- Winlove, C.; Parker, K.; Avery, N.; Bailey, A. Interactions of elastin and aorta with sugars in vitro and their effects on biochemical and physical properties. Diabetologia 1996, 39, 1131–1139. [Google Scholar] [CrossRef]
- Duca, L.; Blaise, S.; Romier, B.; Laffargue, M.; Gayral, S.; El Btaouri, H.; Kawecki, C.; Guillot, A.; Martiny, L.; Debelle, L.; et al. Matrix ageing and vascular impacts: Focus on elastin fragmentation. Cardiovasc. Res. 2016, 110, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Nicoloff, G.; Weiss, A.S.; Iotova, V.; Tzaneva, V.; Petrova, C.; Domuschieva, N.; Nikolov, A.; Tzvetanov, P.; Christova, P. Abnormal levels of serum antielastin antibodies in children with diabetes mellitus type 1. J. Investig. Med. 2006, 54, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, A.; Blazhev, A.; Tzekova, M.; Kostov, K.; Popovski, N. Serum levels of antibodies to advanced glycation end products in patients with type 2 diabetes mellitus and hypertension. Folia Med. 2020, 62, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Nicoloff, G.; Baydanoff, S.; Stanimirova, N.; Petrova, C.; Christova, P. An association of anti-elastin IgA antibodies with development of retinopathy in diabetic children. Gen. Pharmacol. 2000, 35, 83–87. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamamoto, H. Receptor for advanced glycation end-products-mediated inflammation and diabetic vascular complications. J. Diabetes Investig. 2011, 2, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Park, S.; Lakatta, E.G. RAGE signaling in inflammation and arterial aging. Front. Biosci. 2009, 14, 1403–1413. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Baydanoff, S.; Konova, E.; Dosheva, I.; Dorovski, P. Non-enzymatic glycation of elastin. Glycosylation Dis. 1994, 1, 53–58. [Google Scholar] [CrossRef]
- Itariu, B.K.; Stulnig, T.M. Autoimmune aspects of type 2 diabetes mellitus—A mini-review. Gerontology 2014, 60, 189–196. [Google Scholar] [CrossRef]
- Baydanoff, S.; Konova, E.; Ivanova, N. Determination of anti-AGE antibodies in human serum. Glycoconj. J. 1996, 13, 335–339. [Google Scholar] [CrossRef]
- Nicoloff, G.; Baydanoff, S.; Petrova, C.; Christova, P. Antibodies to advanced glycation end products in children with diabetes mellitus. Vascul. Pharmacol. 2002, 39, 39–45. [Google Scholar] [CrossRef]
- Prasad, K.; Mishra, M. Do advanced glycation end products and its receptor play a role in pathophysiology of hypertension? Int. J. Angiol. 2017, 26, 1–11. [Google Scholar] [PubMed] [Green Version]
- Ahmad, M.N.; Farah, A.I.; Al-Qirim, T.M. The cardiovascular complications of diabetes: A striking link through protein glycation. Rom. J. Intern. Med. 2020, 58, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geha, R.S.; Rosen, F.S. The genetic basis of immunoglobulin-class switching. N. Engl. J. Med. 1994, 330, 1008–1009. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Patron, C.; Radomski, M.W.; Davidge, S.T. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ. Res. 1999, 85, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Thrailkill, K.M.; Bunn, R.C.; Moreau, C.S.; Cockrell, G.E.; Simpson, P.M.; Coleman, H.N.; Frindik, J.P.; Kemp, S.F.; Fowlkes, J.L. Matrix metalloproteinase-2 dysregulation in type 1 diabetes. Diabetes Care 2007, 30, 2321–2326. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Fernandez, N.; Jacobs-Cachá, C.; Mora-Gutiérrez, J.M.; Vergara, A.; Orbe, J.; Soler, M.J. Matrix metalloproteinases in diabetic kidney disease. J. Clin. Med. 2020, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, G.; Kowluru, R.A. Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab. Investig. 2010, 90, 1365–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelzer, C.E.; Duca, L. Elastic fibers: Formation, function, and fate during aging and disease. FEBS J. 2021, 298, 3704–3730. [Google Scholar] [CrossRef]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial dysfunction in diabetic retinopathy. Front. Endocrinol. 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Pertyńska-Marczewska, M.; Kiriakidis, S.; Wait, R.; Beech, J.; Feldmann, M.; Paleolog, E.M. Advanced glycation end products upregulate angiogenic and pro-inflammatory cytokine production in human monocyte/macrophages. Cytokine 2004, 28, 35–47. [Google Scholar] [CrossRef]
- Ganter, U.; Arcone, R.; Toniatti, C.; Morrone, G.; Ciliberto, G. Dual control of C-reactive protein gene expression by interleukin-1 and interleukin-6. EMBO J. 1989, 8, 3773–3779. [Google Scholar] [CrossRef]
- Pickup, J.; Mattock, M.; Chusney, G.; Burt, D. NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997, 40, 1286–1292. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.C.B.; Chow, W.S.; Tam, S.; Bucala, R.; Betteridge, J. Association between acute-phase reactants and advanced glycation end products in type 2 diabetes. Diabetes Care 2004, 27, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jialal, I.; Devaraj, S.; Venugopal, S.K. C-reactive protein: Risk marker or mediator in atherothrombosis? Hypertension 2004, 44, 6–11. [Google Scholar] [CrossRef]
- Korça, E.; Piskovatska, V.; Börgermann, J.; Navarrete Santos, A.; Simm, A. Circulating antibodies against AGE-modified proteins in patients with coronary atherosclerosis. Sci. Rep. 2020, 10, 17105. [Google Scholar] [CrossRef]
- Kostov, K.; Blazhev, A. Use of glycated hemoglobin (A1c) as a biomarker for vascular risk in type 2 diabetes: Its relationship with matrix metalloproteinases-2, -9 and the metabolism of collagen IV and elastin. Medicina 2020, 56, 231. [Google Scholar] [CrossRef]
- Peterszegi, G.; Mandet, C.; Texier, S.; Robert, L.; Bruneval, P. Lymphocytes in human atherosclerotic plaque exhibit the elastin-laminin receptor: Potential role in atherogenesis. Atherosclerosis 1997, 135, 103–107. [Google Scholar] [CrossRef]
- Peterszegi, G.; Texier, S.; Robert, L. Human helper and memory lymphocytes exhibit an inducible elastin-laminin receptor. Int. Arch. Allergy Immunol. 1997, 114, 218–223. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).