- 4.6Impact Factor
- 8.6CiteScore
- 16 daysTime to First Decision
Mesoionic Carbenes: Exceptionally Electron-Rich Carbon-Donor Ligands in Synthesis, Catalysis, and Materials Science
This special issue belongs to the section “Organometallic Chemistry“.
Special Issue Information
Dear Colleagues,
Ligands have the ability to control the electronic and structural properties of metal complexes and, therefore, to regulate the way they react. Consequently, major advances in molecular science in general are directly related to the emergence of new types of innovative ligands. 1,3-Imidazole derived N-heterocyclic carbenes (NHCs) (A) are a prominent example and ranked as very versatile carbon-donor ligands in organometallic chemistry and catalysis. NHCs typically bind to metals at the C2-carbon atom and are generally regarded as normal or classical NHCs. The strong σ-donor ability of NHCs leads to the formation of a rather robust M‒C(NHC) bond, giving rise to very stable metal complexes. In addition, it also enhances the reactivity of metal complexes by making the metal atom more electron rich. NHCs have also been utilized in main-group chemistry and numerous molecules with fascinating structures and intriguing bonding situations have been stabilized by the use of NHCs. This is clearly because of the strong σ-donor strength, as well as auspicious steric features of NHCs. Over the past few years, a relatively new type of carbenes (B), in which the carbene carbon atom is located at the unusual C4-position, has received significant attention, as they are even stronger σ-donors than their classical (C2) analogs A. Similarly, 1,2,3-triazole derived C4-carbenes (D) have also been shown as very powerful ligands in catalysis and organometallic chemistry. These so-called abnormal-NHCs (aNHCs) are also referred to as mesoionic carbenes (MICs), as no neutral Lewis form without the introduction of formal charges can be written for B and D.
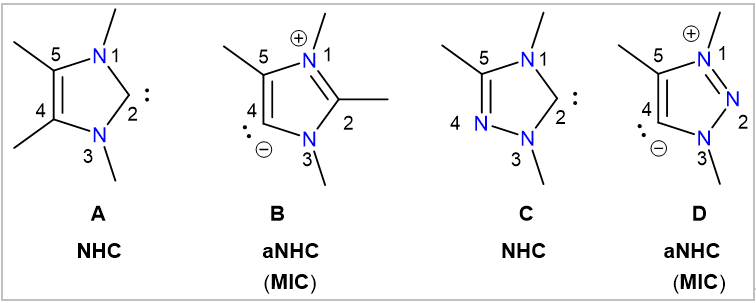
While D are stronger σ-donors than classical NHCs A and C, aNHCs (or MICs) B are exceptional. Whether described as aNHCs or MICs, these C4 carbenes B and D have already been recognized as very powerful ligands in synthesis and catalysis. Moreover, the scope of MICs is continuously expanding with the availability of their efficient synthetic methods. In addition, the possibility of incorporating new functionalities in MICs offers unique opportunities for controlling/modifying the stability, reactivity, and other properties of derived complexes.
The central aim of this Special Issue is to compile the original cutting-edge research in MIC chemistry that encompasses the structural and electronic investigations of unique molecular entities, catalysis, small molecular activation, organic synthesis, theoretical analysis, and molecular materials, among others.
Prof. Dr. Rajendra S. Ghadwal
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Molecules is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Carbene
- Mesoionic carbenes (MICs)
- Abnormal carbenes (aNHCs)
- C4-/C5-carbenes
- Anionic dicarbenes
- Di- and multi-topic carbenes
- Heteroatom functionalized carbenes
- Electronic and steric properties
- Stabilization of reactive main-group species
- Transition metal complexes
- Main-group compounds
- Computational study
- Bond activation and functionalization
- Small molecule activation
- Organic synthesis
- Structure-reactivity correlations
- Catalysis
- Mechanistic insight
Benefits of Publishing in a Special Issue
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- Reprint: MDPI Books provides the opportunity to republish successful Special Issues in book format, both online and in print.


