Journal Description
Targets
Targets
is an international, peer-reviewed, open access journal on chemical measurement science, biology, material science, pharmacy, clinical diagnostics, molecular medicine and biomedicine published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 20.9 days after submission; acceptance to publication is undertaken in 3.8 days (median values for papers published in this journal in the second half of 2025).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Companion journal: Sensors.
- Journal Clusters of Oncology: Cancers, Current Oncology, Onco and Targets.
Latest Articles
Single-Cell Transcriptomics and Computational Frameworks for Target Discovery in Cancer
Targets 2026, 4(1), 6; https://doi.org/10.3390/targets4010006 - 3 Feb 2026
Abstract
►
Show Figures
Single-cell transcriptomics has redefined our understanding of cancer by exposing the complexity of tumor ecosystems and their therapeutic vulnerabilities. scRNA-seq studies have identified lineage hierarchies, immune evasion programs, and resistance-associated states across solid and liquid tumors, informing biomarker development and drug discovery. Advanced
[...] Read more.
Single-cell transcriptomics has redefined our understanding of cancer by exposing the complexity of tumor ecosystems and their therapeutic vulnerabilities. scRNA-seq studies have identified lineage hierarchies, immune evasion programs, and resistance-associated states across solid and liquid tumors, informing biomarker development and drug discovery. Advanced computational frameworks integrate these data with longitudinal profiling, RNA velocity, and network diffusion to prioritize targets and predict therapeutic response. Emerging multi-omics approaches further expand the scope of precision oncology by linking genetic alterations, protein-level markers, and spatial context to functional states. This narrative review aims to synthesize current applications of single-cell transcriptomics for target discovery, highlight computational frameworks that translate high-dimensional data into actionable insights, and explore how multi-omics integration is shaping future directions. By bridging molecular complexity with target prioritization, these approaches hold promise for translating single-cell insights into clinically actionable biomarkers and therapeutic strategies for personalized cancer treatment and rational drug development.
Full article
Open AccessReview
Comprehensive Roles of ZIP and ZnT Zinc Transporters in Metabolic Inflammation
by
Susmita Barman, Seetur R. Pradeep and Krishnapura Srinivasan
Targets 2026, 4(1), 5; https://doi.org/10.3390/targets4010005 - 27 Jan 2026
Abstract
Zinc homeostasis is fundamental to metabolic health, orchestrated by the coordinated actions of two major zinc transporter families: ZIP (Zrt- and Irt-like proteins) and ZnT (zinc transporters). ZIP transporters facilitate zinc influx into the cytosol from the extracellular space or from the lumen
[...] Read more.
Zinc homeostasis is fundamental to metabolic health, orchestrated by the coordinated actions of two major zinc transporter families: ZIP (Zrt- and Irt-like proteins) and ZnT (zinc transporters). ZIP transporters facilitate zinc influx into the cytosol from the extracellular space or from the lumen of intracellular organelles, whereas ZnT transporters control zinc efflux from the cytosol to the extracellular space or facilitate its sequestration into intracellular vesicles and organelles, concurrently harboring the meticulous intracellular zinc homeostasis. This equilibrium is essential for all critical functions like cellular response, metabolic control, and immune pathway alteration. Disruption of this homeostasis is a driver of different pathological alterations like metabolic inflammation, a chronic low-grade inflammatory state underlying obesity; type 2 diabetes; and nonalcoholic fatty liver disease. Recent studies revealed that ZIP and ZnT transporters dynamically regulate metabolic and inflammatory cues, with their tissue-specific expression varying by tissue and acclimating to different physiological and pathological conditions. Recent advanced research in molecular and genetic understanding has helped to deepen our knowledge of the interplay of activity between ZIP and ZnT transporters and their crosstalk in metabolic tissues, underscoring the potential therapeutic prospect for restoring zinc balance and ameliorating metabolic inflammation. This review provides a comprehensive overview that covers the function, regulation, and interactive crosstalk of ZIP and ZnT zinc transporters in metabolic tissues and their pathological conditions.
Full article
(This article belongs to the Special Issue Comprehending Molecular Targets: Mechanisms and Actions in Drug Development)
►▼
Show Figures

Graphical abstract
Open AccessReview
The Indole Scaffold in Biochemistry and Therapeutics: A Privileged Structure with Diverse Chemical, Biological, and Clinical Significance
by
Cristina Manuela Drăgoi, Alina-Crenguţa Nicolae and Ion-Bogdan Dumitrescu
Targets 2026, 4(1), 4; https://doi.org/10.3390/targets4010004 - 21 Jan 2026
Abstract
The indole scaffold represents a privileged structural motif in medicinal chemistry, celebrated for its remarkable chemical versatility, biological ubiquity, and clinical relevance. This review provides a comprehensive analysis of the recent research on the indole nucleus, emphasizing its physicochemical properties, reactivity patterns, and
[...] Read more.
The indole scaffold represents a privileged structural motif in medicinal chemistry, celebrated for its remarkable chemical versatility, biological ubiquity, and clinical relevance. This review provides a comprehensive analysis of the recent research on the indole nucleus, emphasizing its physicochemical properties, reactivity patterns, and capacity to interact with a wide array of biological targets. Found in key endogenous compounds such as serotonin and melatonin, indole serves as a cornerstone in neurochemical signaling, circadian regulation, and chrono-metabolic homeostasis. Beyond its physiological roles, synthetic indole derivatives have shown extensive therapeutic potential across diverse domains, including oncology, infectious diseases, neurodegenerative disorders, immunomodulation, and metabolic syndromes. The review explores structure–activity relationships (SAR), pharmacokinetics, and the molecular mechanisms by which indole-based compounds exert their tremendous effects, that are ranging from enzyme inhibition to receptor modulation. Special focus is given to current clinical applications and emerging strategies for enhancing drug specificity, bioavailability, and safety through indolic frameworks. Additionally, we highlight the translational potential of indole-containing molecules in personalized medicine, underscoring opportunities for future drug discovery. By integrating insights from medicinal chemistry, biochemistry, pharmacology, and clinical science, this review affirms the indole ring’s enduring value as a central scaffold in therapeutic innovation.
Full article
(This article belongs to the Special Issue Comprehending Molecular Targets: Mechanisms and Actions in Drug Development)
►▼
Show Figures

Figure 1
Open AccessArticle
Oral Candida Colonisation in Radiotherapy-Treated Head and Neck Cancer Patients: Prevalence, Species Diversity and Antifungal Resistance Compared with Healthy Controls
by
Tanya Pereira-Riveros, Alicia Lozano Borbalas, Eric Fernández-De la Cruz, Josep M. Sierra and Teresa Vinuesa
Targets 2026, 4(1), 3; https://doi.org/10.3390/targets4010003 - 21 Jan 2026
Abstract
Head and neck cancer (HNC) patients frequently experience alterations in the oral environment following radiotherapy, including xerostomia and impaired mucosal integrity, which may favour fungal overgrowth. This study aimed to characterise oral Candida colonisation in radiotherapy-treated HNC patients and compare it with that
[...] Read more.
Head and neck cancer (HNC) patients frequently experience alterations in the oral environment following radiotherapy, including xerostomia and impaired mucosal integrity, which may favour fungal overgrowth. This study aimed to characterise oral Candida colonisation in radiotherapy-treated HNC patients and compare it with that of healthy individuals. Unstimulated saliva samples from 61 HNC patients and 100 controls were cultured on chromogenic agar, and isolates were identified using API 20C AUX or MALDI-TOF. Salivary flow was measured to quantify xerostomia. A representative subset of isolates (10 per group) underwent antifungal susceptibility testing by disk diffusion according to CLSI/EUCAST criteria. Candida colonisation was significantly higher in HNC patients than in controls (64.6% vs. 20%, p < 0.001), with greater species diversity and increased detection of non-albicans yeasts, including C. tropicalis, C. parapsilosis, C. glabrata, and C. krusei. All HNC patients exhibited reduced salivary flow. Azole resistance was more frequent among HNC isolates (26%) than among controls (10%), whereas all isolates remained susceptible to amphotericin B and nystatin. These findings indicate that radiotherapy-associated xerostomia substantially alters the oral mycobiota and underscore the importance of routine species-level identification and antifungal susceptibility testing in HNC patients to guide clinical decision-making.
Full article
(This article belongs to the Special Issue Multidisciplinary Approach to Oral Cavity Cancer: A Hard Enemy)
►▼
Show Figures

Figure 1
Open AccessArticle
Patient-Derived Microtumors: How Can We Continue to Personalize Treatment for Ovarian Cancer Patients?
by
Emily O'Brien, Dhruva Dave, Abbie Kleckley, Fibiana Oladipo, Christopher M. Mayer, Rebecca Henderson, Blanca Vasquez, Elizabeth Lucas, Jeffrey A. Thomas, Rony Thomas, Raj Singh, Jingsong Chen, Michael D. Toboni, Charles A. Leath III and Rebecca C. Arend
Targets 2026, 4(1), 2; https://doi.org/10.3390/targets4010002 - 12 Jan 2026
Abstract
►▼
Show Figures
Background/Objectives: This pilot study investigates the feasibility of using patient-derived microtumors (PDMs) to assess chemotherapy response in epithelial ovarian cancer. Methods: Fresh tissue from 10 patients was used to develop PDMs, which were then tested against carboplatin/paclitaxel, carboplatin/docetaxel, and carboplatin/pegylated liposomal doxorubicin (PLD).
[...] Read more.
Background/Objectives: This pilot study investigates the feasibility of using patient-derived microtumors (PDMs) to assess chemotherapy response in epithelial ovarian cancer. Methods: Fresh tissue from 10 patients was used to develop PDMs, which were then tested against carboplatin/paclitaxel, carboplatin/docetaxel, and carboplatin/pegylated liposomal doxorubicin (PLD). Of the 10 PDMs, 3 were obtained from primary debulking surgery (PDS), and 7 were obtained at the time of interval debulking surgery following neoadjuvant chemotherapy. Results: When looking at PDMs derived from tissue collected at the time of PDS, we found that 100% of PDMs demonstrated a full response to carboplatin/PLD, while 30% showed a full response to all regimens, all of which were derived from high-grade serous carcinoma during PDS. The remaining PDMs showed moderate responses to carbo/taxol and carbo/doce. Conclusions: This study suggests that PDMs can be used to assess the efficacy of chemotherapy regimens, as a hypothesis-generating step toward future predictive validation.
Full article

Graphical abstract
Open AccessArticle
Molecular Modeling Reveals Selective AChE Inhibitor Against Bemisia tabaci Pest
by
Fernanda F. de Souza, Juliana F. Vilachã, Othon S. Campos and Heberth de Paula
Targets 2026, 4(1), 1; https://doi.org/10.3390/targets4010001 - 31 Dec 2025
Abstract
►▼
Show Figures
Acetylcholinesterase (AChE) is an important molecular target in the development of insecticides, but due to also being found in the human body, it is necessary to characterize the inhibitory profile of compounds to achieve selectivity. In this study, we employed molecular modeling and
[...] Read more.
Acetylcholinesterase (AChE) is an important molecular target in the development of insecticides, but due to also being found in the human body, it is necessary to characterize the inhibitory profile of compounds to achieve selectivity. In this study, we employed molecular modeling and 3D-QSAR approaches to identify novel compounds that inhibit AChE1 in Bemisia tabaci, a common agricultural pest in tropical and subtropical crops. We conducted molecular docking simulations and quantitative structure–activity relationship analysis (QSAR) to identify compounds with potential inhibitory activity and to develop a predictive model for the activity of these new compounds. The validated model demonstrated remarkable predictive performance. Using the model, we screened a library of novel moieties in favorable regions of the most active molecules in the dataset and identified promising candidates, including FS168. We performed molecular dynamics simulations with FS168 bound to the AChE1 of B. tabaci and observed stabilization and interaction with important catalytic amino acids, indicating a potential inhibition mechanism. Our results showcase the potential of combining molecular modeling and 3D-QSAR approaches for discovering new potential AChE1 inhibitors in Bemisia tabaci as selective agrochemicals.
Full article

Graphical abstract
Open AccessReview
Navigating the Molecular and Cellular Landscape of Breast Cancer in India: From Unique Pathogenesis to the Promise of Personalized Medicine and Future Technologies
by
Anichavezhi Devendran and Sivasankar Perumal
Targets 2025, 3(4), 38; https://doi.org/10.3390/targets3040038 - 15 Dec 2025
Abstract
Breast cancer is a substantial and growing public health issue in India, with epidemiological data demonstrating distinct and often severe disease characteristics in contrast to Western countries. Contrary to the global trend, Indian women frequently develop the disease at an earlier age and
[...] Read more.
Breast cancer is a substantial and growing public health issue in India, with epidemiological data demonstrating distinct and often severe disease characteristics in contrast to Western countries. Contrary to the global trend, Indian women frequently develop the disease at an earlier age and tend to present with more advanced stages, emphasizing important variations in disease pathophysiology. This review compiles and critically evaluates the current literature to describe the specific pathophysiology of breast cancer in the Indian population. We investigate the unique cellular and molecular landscapes, evaluate the impact of specific Indian demographic and genetic features, and highlight crucial gaps in knowledge, diagnostic tools, and therapeutic approaches. The assessment reveals a molecular landscape determined by the incidence of specific tumor subtypes; triple-negative breast cancer, for instance, is frequently diagnosed in younger women, and genetic profiling research suggests variations in its susceptibility genes and mutation patterns when compared to global populations. While this paper brings together recent advancements, it highlights the challenges of adopting global diagnostic and treatment guidelines in the Indian healthcare system. These challenges are largely due to variances and specific demographic and socioeconomic discrepancies that create substantial hurdles for timely diagnosis and patient care. We highlight significant gaps, such as the need for more complete multi-omics profiling of Indian patient cohorts, an absence of uniform and readily available screening programs, and shortcomings in healthcare infrastructure and qualified oncology experts. Furthermore, the review highlights the crucial need for therapeutic strategies tailored to the distinct genetic and demographic profiles of Indian breast cancer patients. We present significant strategies for addressing these challenges, with a focus on integrating multi-omics data and clinical characteristics to gain deeper insight into the underlying causes of the disease. Promising avenues include using artificial intelligence and advancements in technology to improve diagnostics, developing indigenous and affordable treatment options, and establishing context-specific research frameworks for the Indian population. This review also underlines the necessity for personalized strategies to improve breast cancer outcomes in India.
Full article
(This article belongs to the Special Issue Comprehending Molecular Targets: Mechanisms and Actions in Drug Development)
►▼
Show Figures

Figure 1
Open AccessCase Report
Fatal Early Toxicity After Allogeneic Stem Cell Transplantation in Heavily Pretreated Follicular Lymphoma: Clinical Decision-Making Between Bispecific Antibodies and CAR T-Cell Therapy
by
Martina Canichella, Raffaella Cerretti, Monika Malgorzata Trawinska, Mariagiovanna Cefalo, Luca Cupelli, Carla Mazzone, Alessandra Checcoli, Alice Di Rocco, Paolo de Fabritiis and Elisabetta Abruzzese
Targets 2025, 3(4), 37; https://doi.org/10.3390/targets3040037 - 10 Dec 2025
Abstract
►▼
Show Figures
For patients with relapsed/refractory (R/R) follicular lymphoma (FL) after ≥2 prior lines of therapy, T-cell-redirecting therapies—including the bispecific CD3xCD20 antibody (BsAbs) mosunetuzumab (mosu) and chimeric antigen receptor T-cell (CAR-T) therapies such as axicabtagene ciloleucel (axi-cel), lisocabtagen maraleucel (liso-cel), and tisagenlecleucel (tisa-cel)—are approved by
[...] Read more.
For patients with relapsed/refractory (R/R) follicular lymphoma (FL) after ≥2 prior lines of therapy, T-cell-redirecting therapies—including the bispecific CD3xCD20 antibody (BsAbs) mosunetuzumab (mosu) and chimeric antigen receptor T-cell (CAR-T) therapies such as axicabtagene ciloleucel (axi-cel), lisocabtagen maraleucel (liso-cel), and tisagenlecleucel (tisa-cel)—are approved by the FDA and EMA. Treatment selection should consider patient-related factors, prior therapeutic exposure, and toxicity profiles. We describe the 20-year history of a patient with R/R FL. At the fourth relapse, both BsAbs and CAR-T cells were available; however, due to the cumulative toxic burden and the high risk of cytopenias, mosu was selected as the preferred option. During mosu, the patient developed pure red cell aplasia unrelated to infections. Despite achieving a partial response after eight cycles of mosu, this complication led to the decision to proceed with allogeneic stem cell transplantation (allo-HSCT). The course was ultimately complicated by severe early toxicity with massive hemoptysis, acute respiratory failure, and hemorrhagic alveolitis, resulting in a fatal outcome. This case illustrates the delicate balance required in selecting between BsAbs and CAR-T therapy in R/R FL. Contributing factors to the patient’s fragility included profound immune status, transfusion-dependent red cell aplasia, prior cumulative chemotherapy, and pulmonary toxicity associated with conditioning regimens. The case underscores the importance of individualized treatment strategies and suggests that earlier integration of novel T-cell-redirecting therapies may mitigate cumulative toxicity and infection risk. Individualized therapeutic planning is critical in heavily pretreated R/R FL. In select cases, bridging strategies using BsAbs can provide disease control and facilitate transplantation. Still, careful assessment of patient fitness, marrow reserve, and cumulative toxicity is essential to minimize the risk of fatal complications.
Full article

Figure 1
Open AccessArticle
Long-Term Outcomes of Co-Testing with 3-Type HPV mRNA (16/18/45) and Cytology in Women Under 40: A Real-World Cohort from Northern Norway (8–10 Years of Follow-Up)
by
Marie Bostrøm, Gunnar Skov Simonsen and Sveinung Wergeland Sørbye
Targets 2025, 3(4), 36; https://doi.org/10.3390/targets3040036 - 6 Dec 2025
Abstract
►▼
Show Figures
In Norway, organized cervical cancer screening was cytology-based until 2023, and women screened in 2013–2015 were largely unvaccinated. We conducted a retrospective, population-based cohort study to assess whether co-testing with a 3-type HPV mRNA assay improves detection of high-grade cervical lesions in women
[...] Read more.
In Norway, organized cervical cancer screening was cytology-based until 2023, and women screened in 2013–2015 were largely unvaccinated. We conducted a retrospective, population-based cohort study to assess whether co-testing with a 3-type HPV mRNA assay improves detection of high-grade cervical lesions in women < 40 years. Among 11,395 women screened in Northern Norway and followed for 8–10 years, 2807 formed a co-testing cohort (ThinPrep cytology plus PreTect SEE; HPV16/18/45) and 8588 formed a cytology-only cohort. The endpoint was histologically confirmed CIN2+. Sensitivity for CIN2+ was 63.7% with cytology alone and 71.0% with co-testing (absolute +7.3 percentage points; p = 0.034). In the co-testing cohort, HPV mRNA was detected in 10.2% of women, of whom 46.0% developed CIN2+, while CIN2+ risk in HPV mRNA-negative women was 5.2%. Co-testing produced wide risk gradients: CIN2+ risk was 58.3% in double-positive women (HPV mRNA-positive and ASC-US+) and 3.3% in double-negative women (HPV mRNA-negative and normal cytology), with no cervical cancers observed in the latter group. In this cytology-based, largely unvaccinated setting, co-testing with a 3-type HPV mRNA assay improved detection performance and long-term risk stratification in women < 40 years, supporting its use as a quality-assurance and triage tool within organized screening programs.
Full article
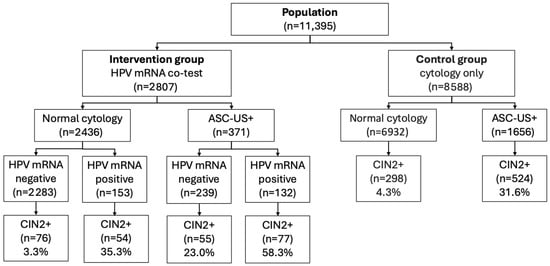
Figure 1
Open AccessArticle
Discovery of Novel FGFR1 Inhibitors via Pharmacophore Modeling and Scaffold Hopping: A Screening and Optimization Approach
by
Xingchen Ji, Jiahua Tao, Na Zhang, Linxin Wang, Xiyi Zheng and Lianxiang Luo
Targets 2025, 3(4), 35; https://doi.org/10.3390/targets3040035 - 27 Nov 2025
Abstract
►▼
Show Figures
Aberrant activation of fibroblast growth factor receptor 1 (FGFR1) drives tumor progression in multiple cancer types, yet existing FGFR1 inhibitors suffer from suboptimal target selectivity and dose-limiting toxicities. This study describes an integrated computational approach for the identification of novel FGFR1 inhibitors. We
[...] Read more.
Aberrant activation of fibroblast growth factor receptor 1 (FGFR1) drives tumor progression in multiple cancer types, yet existing FGFR1 inhibitors suffer from suboptimal target selectivity and dose-limiting toxicities. This study describes an integrated computational approach for the identification of novel FGFR1 inhibitors. We established a computational pipeline incorporating ligand-based pharmacophore modeling, multi-tiered virtual screening with hierarchical docking (HTVS/SP/XP), and MM-GBSA binding energy calculations to evaluate interactions within the FGFR1 kinase domain. From an initial library of 9019 anticancer compounds, three hit compounds exhibited superior FGFR1 binding affinity compared to the reference ligand 4UT801. Scaffold hopping was performed to generate 5355 structural derivatives, among which candidate compounds 20357a–20357c showed improved bioavailability and reduced toxicity as predicted by absorption, distribution, metabolism, excretion, and toxicity (ADMET) profiling. Molecular dynamics (MD) simulations validated stable binding modes and favorable interaction energies for these candidates. Collectively, our study identifies structurally novel FGFR1 inhibitors with optimized pharmacodynamic and safety profiles, thereby advancing targeted anticancer drug discovery.
Full article

Figure 1
Open AccessReview
Galectin-3: A Multitasking Protein Linking Cardiovascular Diseases, Immune Disorders and Beyond
by
Mariarosaria Morello, Gisella Titolo, Saverio D’Elia, Silvia Caiazza, Ettore Luisi, Achille Solimene, Chiara Serpico, Andrea Morello, Francesco Natale, Paolo Golino, Plinio Cirillo and Giovanni Cimmino
Targets 2025, 3(4), 34; https://doi.org/10.3390/targets3040034 - 15 Nov 2025
Abstract
►▼
Show Figures
In recent decades, the novel role of Galectin-3 (Gal-3) in both physiological and pathological conditions has emerged. Gal-3 is a key protein involved in immunity, inflammation, cell adhesion, proliferation, differentiation, and apoptosis. Its physiological role is crucial for the regulation of these cellular
[...] Read more.
In recent decades, the novel role of Galectin-3 (Gal-3) in both physiological and pathological conditions has emerged. Gal-3 is a key protein involved in immunity, inflammation, cell adhesion, proliferation, differentiation, and apoptosis. Its physiological role is crucial for the regulation of these cellular functions. In pathological settings, elevated levels of Gal-3 are associated with diseases such as cancer, heart failure, and fibrotic diseases, making it an important diagnostic and prognostic biomarker in these conditions. It seems that Gal-3 acts as a bridge between different diseases. Because of its pro-inflammatory and pro-tumorigenic properties, it connects atherosclerosis and cancer, regulating inflammation, cell proliferation, immune evasion, angiogenesis and survival in both diseases. Specifically, in atherosclerosis, Gal-3 promotes plaque formation by driving inflammation, oxidative stress, lipid deposition, and vascular cell migration. In cancer, Gal-3 influences tumor growth and metastasis by modulating an immunosuppressive tumor microenvironment, increasing cell survival, and enhancing cell–matrix and cell–cell interactions. Moreover, by stimulating fibroblasts, Gal-3 favors matrix deposition and tissue fibrosis that together with the inflammatory properties contributes to adverse ventricular remodeling leading to heart failure. Finally, taking into account its role in pathogen recognition and immune cells (B and T cells) modulation, Gal-3 might be a critical factor in host defense, disease progression, and the development of autoimmune conditions. Thus, targeting Gal-3 might be a promising therapeutic strategy to pursue for management of different pathological scenarios.
Full article

Figure 1
Open AccessArticle
Simulated Pharmacokinetic Compatibility of Tamoxifen and Estradiol: Insights from a PBPK Model in Hormone-Responsive Breast Cancer
by
Beatriz Gomes and Nuno Vale
Targets 2025, 3(4), 33; https://doi.org/10.3390/targets3040033 - 30 Oct 2025
Abstract
►▼
Show Figures
Although traditionally contraindicated, the coadministration of tamoxifen and estradiol may hold clinical relevance in specific contexts, particularly in breast cancer survivors with premature menopause and a high risk of osteoporosis, thereby justifying the need to re-evaluate this therapeutic combination. This study presents an
[...] Read more.
Although traditionally contraindicated, the coadministration of tamoxifen and estradiol may hold clinical relevance in specific contexts, particularly in breast cancer survivors with premature menopause and a high risk of osteoporosis, thereby justifying the need to re-evaluate this therapeutic combination. This study presents an innovative physiologically based pharmacokinetic (PBPK) modeling approach to evaluate the coadministration of tamoxifen and estradiol in women with breast cancer and a high risk of osteoporosis. Using GastroPlus® software, PBPK models were developed and validated for both drugs, based on physicochemical and kinetic data obtained from the literature and, where necessary, supplemented by estimates generated in ADMET Predictor®. The simulations considered different hormonal profiles (pre and postmenopausal) and therapeutic regimens, evaluating potential interactions mediated by the CYP3A4 enzyme. Analysis of the pharmacokinetic parameters (F, Cmax, Tmax and AUC) revealed strong agreement between the simulated and experimental values, with prediction errors of less than twofold. The drug interaction studies, carried out in dynamic and stationary modes, indicated that estradiol does not significantly alter the pharmacokinetics of tamoxifen, even at increasing doses or in enlarged virtual populations. These results represent the first in silico evidence that, under certain conditions, the concomitant use of estradiol does not compromise the pharmacokinetic efficacy of tamoxifen. Although the study is computational, it provides a solid scientific basis for re-evaluating this therapeutic combination and proposes a pioneering model for personalized strategies in complex oncological contexts. All simulations assumed average enzyme abundance/activity without CYP polymorphism parameterization; findings are restricted to parent-tamoxifen pharmacokinetics and do not infer metabolite (e.g., endoxifen) exposure or phenotype effects.
Full article

Figure 1
Open AccessReview
Extracellular Vesicle-Associated miRNAs in Cornea Health and Disease: Diagnostic Potential and Therapeutic Implications
by
Nagendra Verma, Swati Arora, Anurag Kumar Singh and Amrendra Kumar
Targets 2025, 3(4), 32; https://doi.org/10.3390/targets3040032 - 17 Oct 2025
Cited by 5
Abstract
Extracellular Vesicle-associated microRNAs (EV-miRNAs) are emerging as pivotal regulators of corneal health and disease, holding exceptional promise for transforming both diagnostics and therapeutics. These vesicles carry distinct miRNA signatures in biofluids such as tears, offering a powerful, non-invasive approach for early detection, risk
[...] Read more.
Extracellular Vesicle-associated microRNAs (EV-miRNAs) are emerging as pivotal regulators of corneal health and disease, holding exceptional promise for transforming both diagnostics and therapeutics. These vesicles carry distinct miRNA signatures in biofluids such as tears, offering a powerful, non-invasive approach for early detection, risk stratification, and dynamic monitoring of corneal disorders. In addition, EV-miRNAs act as key mediators of critical biological processes, including inflammation, fibrosis, and tissue repair. Consequently, they represent attractive therapeutic targets; for example, engineered EVs loaded with miRNA mimics or inhibitors can precisely modulate these pathways to promote regeneration and suppress disease progression. Yet, despite this considerable promise, the translation of EV-miRNA research into clinical practice remains constrained by several challenges. Topmost among these are the lack of standardized EV isolation methods, variability in miRNA quantification, and the pressing need for regulatory frameworks tailored to the complexity of these biological therapeutics. Addressing these barriers is essential to ensure reproducibility, scalability, and safety in clinical applications. Accordingly, this review synthesizes current knowledge on EV-miRNA profiles in corneal diseases, critically evaluates their diagnostic and therapeutic potential, and highlights strategies to overcome existing technical and regulatory limitations. Ultimately, the successful integration of EV-miRNA-based approaches into personalized medicine frameworks could revolutionize the management of corneal diseases and substantially improve patient outcomes.
Full article
(This article belongs to the Topic Biomarkers of Disease: Discovery and Clinical Applications)
►▼
Show Figures

Graphical abstract
Open AccessReview
Integrating Nanotechnology and Artificial Intelligence for Early Detection and Prognostication of Glioblastoma: A Translational Perspective
by
Meghraj Vivekanand Suryawanshi, Imtiyaz Bagban and Akshata Yashwant Patne
Targets 2025, 3(4), 31; https://doi.org/10.3390/targets3040031 - 14 Oct 2025
Cited by 1
Abstract
►▼
Show Figures
Glioblastoma (GBM) is the most common and aggressive malignant brain tumor in adults. This review explains the connections between the genesis and progression of GBM and particular cellular tumorigenic mechanisms, such as angiogenesis, invasion, migration, growth factor overexpression, genetic instability, and apoptotic disorders,
[...] Read more.
Glioblastoma (GBM) is the most common and aggressive malignant brain tumor in adults. This review explains the connections between the genesis and progression of GBM and particular cellular tumorigenic mechanisms, such as angiogenesis, invasion, migration, growth factor overexpression, genetic instability, and apoptotic disorders, as well as possible therapeutic targets that help predict the course of the disease. Glioblastoma multiforme (GBM) diagnosis relies heavily on histopathological features, molecular markers, extracellular vesicles, neuroimaging, and biofluid-based glial tumor identification. In order to improve miRNA stability and stop the proliferation of cancer cells, nanoparticles, magnetic nanoparticles, contrast agents, gold nanoparticles, and nanoprobes are being created for use in cancer treatments, neuroimaging, and biopsy. Targeted nanoparticles can boost the strength of an MRI signal by about 28–50% when compared to healthy tissue or controls in a preclinical model like mouse lymph node metastasis. Combining the investigation of CNAs and noncoding RNAs with deep learning-driven global profiling of genes, proteins, RNAs, miRNAs, and metabolites presents exciting opportunities for creating new diagnostic markers for malignancies of the central nervous system. Artificial intelligence (AI) advances precision medicine and cancer treatment by enabling the real-time analysis of complex biological and clinical data through wearable sensors and nanosensors; optimizing drug dosages, nanomaterial design, and treatment plans; and accelerating the development of nanomedicine through high-throughput testing and predictive modeling.
Full article

Graphical abstract
Open AccessReview
The Expanding E3 Ligase-Ligand Landscape for PROTAC Technology
by
Zhenzhen Li, Xiaoli Huang, Xuchi Zhao, Yunxiu Zhang and Ping Li
Targets 2025, 3(4), 30; https://doi.org/10.3390/targets3040030 - 27 Sep 2025
Abstract
Proteolysis-targeting chimeras (PROTACs) are a transformative therapeutic modality that co-opts the ubiquitin-proteasome system for selective protein degradation. To date, the development of PROTACs has been overwhelmingly dominated by the recruitment of four canonical E3 ligases: CRBN, VHL, MDM2, and IAP. This limited repertoire
[...] Read more.
Proteolysis-targeting chimeras (PROTACs) are a transformative therapeutic modality that co-opts the ubiquitin-proteasome system for selective protein degradation. To date, the development of PROTACs has been overwhelmingly dominated by the recruitment of four canonical E3 ligases: CRBN, VHL, MDM2, and IAP. This limited repertoire represents a critical bottleneck, restricting the scope of degradable proteins and potential therapeutic applications. Addressing this challenge, recent years have witnessed a surge in the successful recruitment of novel E3 ligases. This review provides a dedicated and comprehensive summary of this progress, focusing exclusively on the emerging E3 ligases and their cognate ligands reported for PROTAC technology outside of the well-established quartet. We detail their discovery and strategic application, highlighting how this rapidly expanding toolbox promises to overcome existing limitations and unlock the full potential of targeted protein degradation.
Full article
(This article belongs to the Special Issue Comprehending Molecular Targets: Mechanisms and Actions in Drug Development)
►▼
Show Figures

Figure 1
Open AccessArticle
Contrast-Enhanced Mammography as a Functional Biomarker in Breast Cancer: Correlation of Enhancement Patterns with Ki-67 and Histological Grade
by
Marina Balbino, Manuela Montatore, Federica Masino, Antonietta Ancona, Francesca Anna Carpagnano, Giulia Capuano, Riccardo Guglielmi and Giuseppe Guglielmi
Targets 2025, 3(3), 29; https://doi.org/10.3390/targets3030029 - 17 Sep 2025
Abstract
►▼
Show Figures
Background: Contrast-Enhanced Spectral Mammography (CESM) combines anatomical and functional imaging, showing promise in breast cancer diagnosis. Despite well-established lesion detection accuracy, few studies have investigated the link between CESM enhancement patterns and tumor aggressiveness biomarkers. Methods: We retrospectively evaluated 100 patients (mean age
[...] Read more.
Background: Contrast-Enhanced Spectral Mammography (CESM) combines anatomical and functional imaging, showing promise in breast cancer diagnosis. Despite well-established lesion detection accuracy, few studies have investigated the link between CESM enhancement patterns and tumor aggressiveness biomarkers. Methods: We retrospectively evaluated 100 patients (mean age 59.5 years) undergoing CESM with complete histopathological data. Lesions were categorized by enhancement intensity (high, medium, low) and contrast homogeneity (homogeneous vs. heterogeneous), correlated with Ki-67 index and histological grade. Results: Lesion size measured by CESM closely matched histology (mean 2.16 cm vs. 2.25 cm). Mass-like lesions corresponded mainly to invasive ductal carcinoma, while non-mass patterns aligned with lobular or in situ carcinomas. Enhancement intensity correlated moderately with Ki-67 (Spearman ρ = 0.56, p < 0.001), and contrast heterogeneity showed a weaker but significant correlation with tumor grade (ρ = 0.22, p < 0.05). Conclusions: CESM accurately assesses tumor size and provides functional insight into tumor biology. Enhancement intensity may serve as a non-invasive proliferation marker, while contrast heterogeneity offers additional prognostic data, supporting CESM’s role in personalized breast cancer management.
Full article

Figure 1
Open AccessReview
The Use of Particle Radiotherapy and Radiation Sensitizers for Treatment of Chordomas: A Narrative Review
by
Aarti Kishore Jain, Sahdev S. Baweja, Beatrice Campilan, Madison J. Michles, Aviva Berkowitz and Patricia L. Zadnik Sullivan
Targets 2025, 3(3), 28; https://doi.org/10.3390/targets3030028 - 15 Aug 2025
Abstract
►▼
Show Figures
Chordomas are primary tumors of the skull base and vertebral column typically derived from the notochord. Treatment options consist of surgical resection, radiotherapy, and chemotherapy. This study reviews clinical trials focused on radiotherapy techniques, such as photon therapy and carbon ion radiotherapy, as
[...] Read more.
Chordomas are primary tumors of the skull base and vertebral column typically derived from the notochord. Treatment options consist of surgical resection, radiotherapy, and chemotherapy. This study reviews clinical trials focused on radiotherapy techniques, such as photon therapy and carbon ion radiotherapy, as well as the concomitant use of radia-tion sensitizers. We completed a literature review on all published clinical trials on the usage of photon, proton, and carbon ion radiotherapy (CIRT) for chordoma in adults and all published literature on radiation sensitizers used for treatment in chordoma from 2000 to 2025. We reviewed all nine current clinical trials on radiotherapy for chordoma in adults. All clinical trials were able to achieve an overall survival rate above 50% at 3-year follow-up. Seven publications were found on the use of radiation sensitizers for chordomas, both in vitro and in vivo. The completed clinical trials evaluate the effectiveness of proton, photon, and CIRT for treatment of the skull base, spine, and sacral chordoma. Current trials continue these efforts and compare the different radiotherapies and determine appropriate doses. Research on radiation sensitizers for chordomas shows various therapies, ranging from hyperthermia to pharmaceutical options, that require further study.
Full article

Figure 1
Open AccessReview
Approaches for Identifying LncRNA-Associated Proteins for Therapeutic Targets and Cancer Biomarker Discovery
by
Mohammad Shabir Hussain, Puneet Vij, Sudhir Kotnala, Shadab Ahmad, Subhash C. Chauhan and Manish K. Tripathi
Targets 2025, 3(3), 27; https://doi.org/10.3390/targets3030027 - 11 Aug 2025
Abstract
►▼
Show Figures
Long non-coding RNAs (lncRNAs) are increasingly recognized as key regulators of gene expression and cellular signaling in cancer. Their functions are primarily mediated through interactions with specific protein partners that modulate chromatin structure, epigenetic remodeling, transcription, and signal transduction. In this review, we
[...] Read more.
Long non-coding RNAs (lncRNAs) are increasingly recognized as key regulators of gene expression and cellular signaling in cancer. Their functions are primarily mediated through interactions with specific protein partners that modulate chromatin structure, epigenetic remodeling, transcription, and signal transduction. In this review, we explore reports and strategies for the proteomic characterization of lncRNA-associated proteins, particularly emphasizing high-throughput liquid chromatography–mass spectrometry (LC-MS)-based techniques. Affinity-based methods such as RNA pull-down, ChIRP MS, RAP-MS, BioID-MS, and SILAC-MS enable sensitive and specific mapping of lncRNA and protein complexes. These approaches reveal cancer-specific proteomic signatures, post-translational modifications, and mechanistic insights into tumor biology. The use of label-free quantification, bituminization, and crosslinking strategies further enhances the resolution of dynamic RNA–protein networks. Validation tools following bioinformatic analyses, such as Western blotting, immunohistochemistry, immunofluorescence, and ELISA, are used to prioritize and confirm findings. Candidate biomarkers from hepatocellular carcinoma to colorectal and prostate cancers, profiling lncRNA-associated proteins, hold promise for identifying clinically actionable biomarkers and therapeutic targets. This review highlights the translational relevance of lncRNA protein studies and advocates for their broader adoption in oncological research. In LC-MS workflows, proteins bound to lncRNAs are enzymatically digested into peptides, separated via nano-LC, and analyzed using high-resolution tandem MS. Label-free or isotope-labeled methods quantify differential enrichment, followed by bioinformatics-driven pathway annotation.
Full article

Graphical abstract
Open AccessReview
Skeletal Health in Pituitary and Neuroendocrine Diseases: Prevention and Treatments of Bone Fragility
by
Flavia Costanza, Antonella Giampietro, Laura De Marinis, Antonio Bianchi, Sabrina Chiloiro and Alfredo Pontecorvi
Targets 2025, 3(3), 26; https://doi.org/10.3390/targets3030026 - 8 Aug 2025
Abstract
►▼
Show Figures
Bone loss is common in patients affected by pituitary and neuroendocrine disorders as both hormone excess and hormone deficiency can affect bone structure. There is increasing evidence that pituitary hormones directly influence bone cells turnover by bypassing endocrine organs. Osteopenia, osteoporosis, and vertebral
[...] Read more.
Bone loss is common in patients affected by pituitary and neuroendocrine disorders as both hormone excess and hormone deficiency can affect bone structure. There is increasing evidence that pituitary hormones directly influence bone cells turnover by bypassing endocrine organs. Osteopenia, osteoporosis, and vertebral fractures often result from these skeletal changes; however, diagnosing and managing bone frailty in pituitary and neuroendocrine disorders is still challenging because of the unpredictable outcomes in terms of fracture risk, even after the improvement of pituitary dysfunction, and the limited evidence for the use of bone-active drugs in these pathologies. The use of vitamin D supplements for fracture prevention is still debated in these secondary forms of bone frailty, although some studies have shown similar benefits to those derived in the general population. This review offers an overview on the characteristics of bone fragility in different pituitary and neuroendocrine diseases, and focuses on the prevention and treatment of skeletal disorders with bone-active drugs and vitamin D formulations currently available in this setting.
Full article

Figure 1
Open AccessReview
Bacteriophages: Potential Candidates for the Dissemination of Antibiotic Resistance Genes in the Environment
by
Shahid Sher, Husnain Ahmad Khan, Zaman Khan, Muhammad Sohail Siddique, Dilara Abbas Bukhari and Abdul Rehman
Targets 2025, 3(3), 25; https://doi.org/10.3390/targets3030025 - 22 Jul 2025
Abstract
The invention of antibacterial agents (antibiotics) was a significant event in the history of the human race, and this invention changed the way in which infectious diseases were cured; as a result, many lives have been saved. Recently, antibiotic resistance has developed as
[...] Read more.
The invention of antibacterial agents (antibiotics) was a significant event in the history of the human race, and this invention changed the way in which infectious diseases were cured; as a result, many lives have been saved. Recently, antibiotic resistance has developed as a result of excessive use of antibiotics, and it has become a major threat to world health. ARGs are spread across biomes and taxa of bacteria via lateral or horizontal gene transfer (HGT), especially via conjugation, transformation, and transduction. This review concerns transduction, whereby bacteriophages or phages facilitate gene transfer in bacteria. Bacteriophages are just as common and many times more numerous than their bacterial prey, and these phages are much more influential in controlling the population of bacteria. It is estimated that 25% of overall genes of Escherichia coli have been copied by other species of bacteria due to the HGT process. Transduction may take place via a generalized or specialized mechanism, with phages being ubiquitous in nature. Phage and virus-like particle (VLP) metagenomics have uncovered the emergence of ARGs and mobile genetic elements (MGEs) of bacterial origins. These genes, when transferred to bacteria through transduction, confer resistance to antibiotics. ARGs are spread through phage-based transduction between the environment and bacteria related to people or animals, and it is vital that we further understand and tackle this mechanism in order to combat antimicrobial resistance.
Full article
(This article belongs to the Special Issue Small-Molecule Antibiotic Drug Development)
►▼
Show Figures

Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Biomedicines, Cancers, Diagnostics, JCM, Metabolites, Targets
Biomarkers of Disease: Discovery and Clinical Applications
Topic Editors: Ioannis Kanakis, Andreas TsakalofDeadline: 30 June 2026

Special Issues
Special Issue in
Targets
Multidisciplinary Approach to Oral Cavity Cancer: A Hard Enemy
Guest Editors: Francesco Perri, Agostino GuidaDeadline: 20 February 2026
Special Issue in
Targets
Molecular Spectroscopy-Based Targeted Detection
Guest Editors: Zhuo Chen, Dingbin Liu, Ping LiDeadline: 30 June 2026









