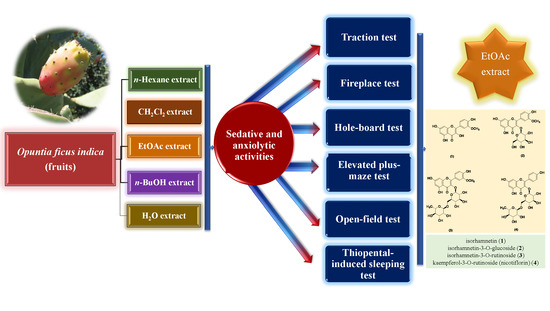
Sedative and Anxiolytic Activities of Opuntia ficus indica (L.) Mill.: An Experimental Assessment in Mice
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Fractionation Processes for the Bioassays
3.3. Chromatographic Separation and Isolation of the Active Compounds
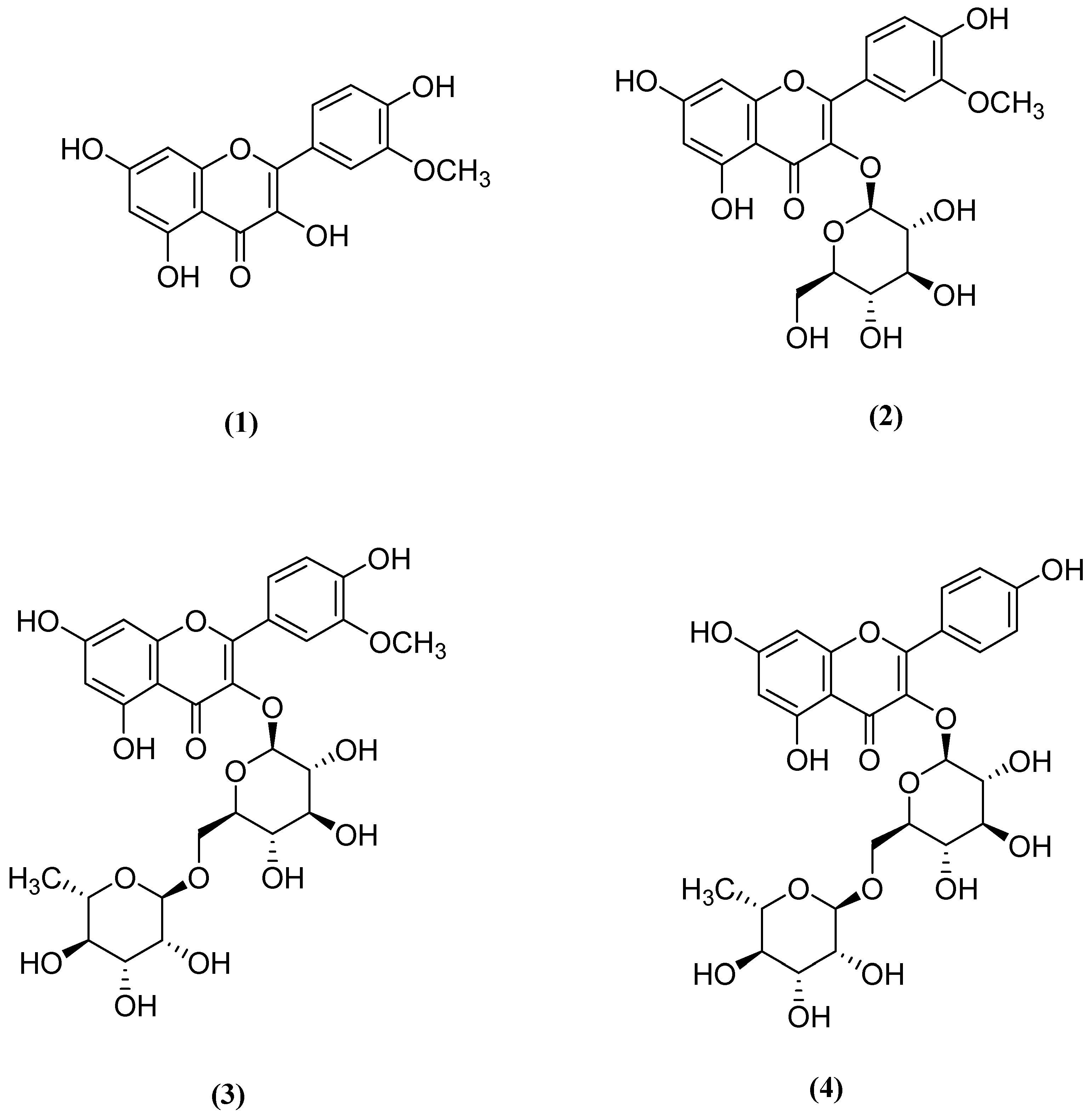
3.4. Structure Elucidation of the Compounds 1–4
3.5. Biological Activity Studies
3.5.1. Animals
3.5.2. Preparation of Test Samples
3.5.3. Traction Test
3.5.4. Fireplace Test
3.5.5. Hole-Board Test
3.5.6. Elevated Plus-Maze Test
3.5.7. Open-Field Test
3.5.8. Thiopental-Induced Sleeping Test
3.6. Statistical Analysis of Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lieb, R.; Becker, E.; Altamura, C. The epidemiology of generalized anxiety disorder in Europe. Eur. Neuropsychopharmacol. 2005, 15, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Katzung, B.G.; Masters, S.B.; Trevor, A.J. Basic and Clinical Pharmacology, 11th ed.; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Woods, J.H. Benzodiazepines: Use, abuse, and consequences. Pharmacol. Rev. 1992, 44, 151–347. [Google Scholar] [PubMed]
- Dhawan, K.; Dhawan, S.; Chhabra, S. Attenuation of benzodiazepine dependence in mice by a tri-substituted benzoflavone moiety of Passiflora incarnata Linneaus: A non-habit forming anxiolytic. J. Pharm. Pharm. Sci. 2003, 6, 215–222. [Google Scholar] [PubMed]
- Duru, B.; Turker, N. Changes in physical properties and chemical composition of cactus pear (Opuntia ficus-indica) during maturation. J. Prof. Assoc. Cactus 2005, 7, 22–33. [Google Scholar]
- Halmi, S.; Benlakssira, B.; Bechtarzi, K.; Djerrou, Z.; Djeaalab, H.; Riachi, F.; Pacha, Y.H. Antihyperglycemic activity of prickly pear (Opuntia ficus-indica) aqueous extract. IJMAP 2012, 2, 540–543. [Google Scholar]
- Galati, E.M.; Monforte, M.T.; Tripodo, M.M.; d’Aquino, A.; Mondello, M.R. Antiulcer activity of Opuntia ficus indica (L.) Mill. (Cactaceae): Ultrastructural study. J. Ethnopharmacol. 2001, 76, 1–9. [Google Scholar] [CrossRef]
- Rodriguez-Felix, A. Postharvest physiology and technology of cactus pear fruits and cactus leaves. Acta Hortic. 2000, 191–199. [Google Scholar] [CrossRef]
- Russell, C.E.; Felker, P. The prickly-pears (Opuntia spp., Cactaceae)—A source of human and animal food in semiarid regions. Econ. Bot. 1987, 41, 433–445. [Google Scholar] [CrossRef]
- Le Houérou, H.N. Cacti (Opuntia spp.) as a fodder crop for marginal lands in the Mediterranean basin. Acta Hortic. 2002, 581, 21–46. [Google Scholar] [CrossRef]
- Le Houérou, H.N. Utilization of fodder trees and shrubs in the arid and semiarid zones of West Asia and North Africa. Arid Soil Res. Rehab. 2000, 14, 101–135. [Google Scholar] [CrossRef]
- Gregory, R.A.; Felker, P. Crude protein and phosphorus contents of eight contrasting Opuntia forage clones. J. Arid Environ. 1992, 22, 323–331. [Google Scholar] [CrossRef]
- Guevara, J.C.; Yahia, E.M.; De La Fuente, E.B. Modified atmosphere packaging of prickly pear cactus stems (Opuntia spp.). LWT-Food Sci. Technol. 2001, 34, 445–451. [Google Scholar] [CrossRef][Green Version]
- Parish, J.; Felker, P. Fruit quality and production of cactus pear (Opuntia spp.) fruit clones selected for increased frost hardiness. J. Arid Environ. 1997, 37, 123–143. [Google Scholar] [CrossRef]
- Yahia, E.M.; Mondragon-Jacobo, C. Nutr.itional components and anti-oxidant capacity of ten cultivars and lines of cactus pear fruit (Opuntia spp.). Food Res. Int. 2011, 44, 2311–2318. [Google Scholar] [CrossRef]
- El-Samahy, S.K.; El-Hady, E.A.; Habiba, R.A.; Moussa-Ayoub, T.E. Some functional, chemical, and sensory characteristics of cactus pear rice-based extrudates. J. Prof. Assoc. Cactus 2007, 9, 136–147. [Google Scholar]
- El-Samahy, S.K.; Youssef, K.M.; Moussa-Ayoub, T.E. Producing ice cream with concentrated cactus pear pulp: A preliminary study. J. Prof. Assoc. Cactus 2009, 11, 1–12. [Google Scholar]
- Moßhammer, M.R.; Stintzing, F.C.; Carle, R. Cactus pear fruits (Opuntia spp.): A review of processing technologies and current uses. J. Prof. Assoc. Cactus 2006, 8, 1–25. [Google Scholar]
- Kim, J.H.; Park, S.M.; Ha, H.J.; Moon, C.J.; Shin, T.K.; Kim, J.M.; Lee, N.H.; Kim, H.C.; Jang, K.J.; Wie, M.B. Opuntia ficus-indica attenuates neuronal injury in in vitro and in vivo models of cerebral ischemia. J. Ethnopharmacol. 2006, 104, 257–262. [Google Scholar] [CrossRef]
- Gurdal, B.; Kultur, S. An ethnobotanical study of medicinal plants in Marmaris (Mugla, Turkey). J. Ethnopharmacol. 2013, 146, 113–126. [Google Scholar] [CrossRef]
- Osorio-Esquivel, O.; Alicia-Ortiz-Moreno; Alvarez, V.B.; Dorantes-Alvarez, L.; Giusti, M.M. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 2011, 44, 2160–2168. [Google Scholar] [CrossRef]
- El Kossori, R.L.; Villaume, C.; El Boustani, E.; Sauvaire, Y.; Mejean, L. Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus indica sp.). Plant Foods Hum. Nutr. 1998, 52, 263–270. [Google Scholar] [CrossRef] [PubMed]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for Nutr.ition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Nassar, A. Chemical composition and functional properties of prickly pear (Opuntia ficus indica) seeds flour and protein concentrate. World J. Dairy Food Sci. 2008, 3, 11–16. [Google Scholar]
- Piga, A. Cactus pear: A fruit of Nutraceutical and functional importance. J. Prof. Assoc. Cactus 2004, 6, 9–22. [Google Scholar]
- Ramadan, M.F.; Mörsel, J.T. Oil cactus pear (Opuntia ficus-indica L.). Food Chem. 2003, 82, 339–345. [Google Scholar] [CrossRef]
- StIntzing, F.C.; Schieber, A.; Carle, R. Phytochemical and Nutr.itional significance of cactus pear. Eur. Food Res. Technol. 2001, 212, 396–407. [Google Scholar] [CrossRef]
- Uchoa, A.F.; Souza, P.A.; Zarate, R.M.; Gomes-Filho, E.; Campos, F.A. Isolation and characterization of a reserve protein from the seeds of Opuntia ficus-indica (Cactaceae). Braz. J. Med. Biol. Res. 1998, 31, 757–761. [Google Scholar] [CrossRef]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Impellizzeri, G.; Piattelli, M. Biosynthesis of indicaxanthin in Opuntia ficus-indica fruits. Phytochemistry 1972, 11, 2499–2502. [Google Scholar] [CrossRef]
- StIntzing, F.C.; Schieber, A.; Carle, R. Identification of betalains from yellow beet (Beta vulgaris L.) and cactus pear [Opuntia ficus-indica (L.) Mill.] by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2002, 50, 2302–2307. [Google Scholar] [CrossRef]
- Garzón, G.A.; Wrolstad, R.E. The stability of pelargonidin-based anthocyanins at varying water activity. Food Chem. 2001, 75, 185–196. [Google Scholar] [CrossRef]
- Tokgöz, H.; Gölükcü, M.; Toker, R. Kan portakalı suyunun bazı kalite parametreleri üzerine ışık, pH, depolama, sıcaklık ve süresinin etkisi. Gıda Teknol. Elektron. Derg. 2013, 8, 1–9. [Google Scholar]
- Galati, E.M.; Tripodo, M.M.; Trovato, A.; Miceli, N.; Monforte, M.T. Biological effect of Opuntia ficus indica (L.) Mill. (Cactaceae) waste matter. Note I: Diuretic activity. J. Ethnopharmacol. 2002, 79, 17–21. [Google Scholar] [CrossRef]
- Loro, J.F.; del Rio, I.; Perez-Santana, L. Preliminary studies of analgesic and anti-inflammatory properties of Opuntia dillenii aqueous extract. J. Ethnopharmacol. 1999, 67, 213–218. [Google Scholar] [CrossRef]
- Park, E.H.; Kahng, J.H.; Lee, S.H.; Shin, K.H. An anti-inflammatory principle from cactus. Fitoterapia 2001, 72, 288–290. [Google Scholar] [CrossRef]
- Galati, E.M.; Pergolizzi, S.; Miceli, N.; Monforte, M.T.; Tripodo, M.M. Study on the increment of the production of gastric mucus in rats treated with Opuntia ficus indica (L.) Mill. cladodes. J. Ethnopharmacol. 2002, 83, 229–233. [Google Scholar] [CrossRef]
- Lee, E.B.; Hyun, J.E.; Li, D.W.; Moon, Y.I. Effects of Opuntia ficus-indica var. saboten stem on gastric damages in rats. Arch. Pharm. Res. 2002, 25, 67–70. [Google Scholar] [CrossRef]
- Cardenas Medellin, M.L.; Serna Saldivar, S.O.; Velazco de la Garza, J. Effect of raw and cooked nopal (Opuntia ficus indica) ingestion on growth and profile of total cholesterol, lipoproteins, and blood glucose in rats. Arch. Latinoam. Nutr. 1998, 48, 316–323. [Google Scholar]
- Frati, A.C.; Jimenez, E.; Ariza, C.R. Hypoglycemic effect of Opuntia-ficus-indica in non-insulin-dependent diabetes-mellitus patients. Phytother. Res. 1990, 4, 195–197. [Google Scholar] [CrossRef]
- Cao, X.; Wei, Y.; Ito, Y. Preparative isolation of isorhamnetin from Stigma Maydis using high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 273–280. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Tastan, H.; Kupeli Akkol, E. Bioactivity-guided isolation of flavonoids from Urtica dioica L. and their effect on endometriosis rat model. J. Ethnopharmacol. 2019, 243, 112100. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Atikur Rahman, M.; Ferdous, A. Evaluation of sedative and hypnotic activity of ethanolic extract of Scoparia dulcis Linn. Evid. Based Complement. Altern. Med. 2015, 2015, 873954. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Tsuji, M.; Matsumiya, T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur. J. Pharmacol. 1998, 350, 21–29. [Google Scholar] [CrossRef]
- Viola, H.; Wasowski, C.; Destein, M.L.; Wolfman, C.; Silveira, R.; Dajas, F.; Medina, J.H.; Paladini, A.C. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995, 61, 213–216. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M.; Khan, H.; Haq, I. Evaluation of n-hexane extract of Viola betonicifolia for its neuropharmacological properties. J. Nat. Med. 2013, 67, 1–8. [Google Scholar] [CrossRef]
- Carvajal, C.C.; Vercauteren, F.; Dumont, Y.; Michalkiewicz, M.; Quirion, R. Aged neuropeptide Y transgenic rats are resistant to acute stress but maInt.ain spatial and non-spatial learning. Behav. Brain Res. 2004, 153, 471–480. [Google Scholar] [CrossRef]
- Li, X.L.; Aou, S.; Hori, T.; Oomura, Y. Spatial memory deficit and emotional abnormality in OLETF rats. Physiol. Behav. 2002, 75, 15–23. [Google Scholar] [CrossRef]
- Moragrega, I.; Carrasco, M.C.; Vicens, P.; Redolat, R. Spatial learning in male mice with different levels of aggressiveness: Effects of housing conditions and nicotine administration. Behav. Brain Res. 2003, 147, 1–8. [Google Scholar] [CrossRef]
- Rimondini, R.; Ågren, G.; Börjesson, S.; Sommer, W.; Heilig, M. Persistent behavioral and autonomic supersensitivity to stress following prenatal stress exposure in rats. Behav. Brain Res. 2003, 140, 75–80. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Giladi, E.; Pick, C.G.; Gozes, I. Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci. Lett. 2004, 361, 128–131. [Google Scholar] [CrossRef]
- Ferguson, G.D.; Herschman, H.R.; Storm, D.R. Reduced anxiety and depression-like behavior in synaptotagmin IV (−/−) mice. Neuropharmacology 2004, 47, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Cao, B.J.; Dalvi, A.; Holmes, A. Animal models of anxiety: An ethological perspective. Braz. J. Med. Biol. Res. 1997, 30, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Candland, D.K.; Nagy, Z.M. The open field: Some comparative data. Ann. N. Y. Acad. Sci. 1969, 159, 831–851. [Google Scholar] [CrossRef]
- Lieben, C.K.J.; van Oorsouw, K.; Deutz, N.E.P.; Blokland, A. Acute tryptophan depletion induced by a gelatin-based mixture impairs object memory but not affective behavior and spatial learning in the rat. Behav. Brain Res. 2004, 151, 53–64. [Google Scholar] [CrossRef]
- Islam, N.U.; Khan, I.; Rauf, A.; Muhammad, N.; Shahid, M.; Shah, M.R. Antinociceptive, muscle relaxant and sedative activities of gold nanoparticles generated by methanolic extract of Euphorbia milii. BMC Complement. Altern. Med. 2015, 15, 160. [Google Scholar] [CrossRef]
- Sivam, S.P.; Nabeshima, T.; Ho, I.K. Acute and chronic effects of pentobarbital in relation to postsynaptic GABA receptors: A study with muSci.mol. J. Neurosci. Res. 1982, 7, 37–47. [Google Scholar] [CrossRef]
- Steinbach, J.H.; Akk, G. Modulation of GABAA receptor channel gating by pentobarbital. J. Physiol. 2001, 537, 715–733. [Google Scholar] [CrossRef]
- Awad, R.; Ahmed, F.; Bourbonnais-Spear, N.; Mullally, M.; Ta, C.A.; Tang, A.; Merali, Z.; Maquin, P.; Caal, F.; Cal, V.; et al. Ethnopharmacology of Q’eqchi’ Maya antiepileptic and anxiolytic plants: Effects on the GABAergic system. J. Ethnopharmacol. 2009, 125, 257–264. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Lopez-Rubalcava, C.; Rocha, L.; Heinze, G.; Gonzalez Esquinca, A.R.; Martinez-Vazquez, M. Anxiolytic-like and sedative actions of Rollinia mucosa: Possible involvement of the GABA/benzodiazepine receptor complex. Pharm. Biol. 2010, 48, 70–75. [Google Scholar] [CrossRef]
- Fernandez, S.; Wasowski, C.; Paladini, A.C.; Marder, M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol. Biochem. Behav. 2004, 77, 399–404. [Google Scholar] [CrossRef]
- Kahnberg, P.; Lager, E.; Rosenberg, C.; Schougaard, J.; Camet, L.; Sterner, O.; Ostergaard Nielsen, E.; Nielsen, M.; Liljefors, T. Refinement and evaluation of a pharmacophore model for flavone derivatives binding to the benzodiazepine site of the GABA(A) receptor. J. Med. Chem. 2002, 45, 4188–4201. [Google Scholar] [CrossRef] [PubMed]
- Trofimiuk, E.; Walesiuk, A.; Braszko, J.J. St John’s wort (Hypericum perforatum) diminishes cognitive impairment caused by the chronic restraint stress in rats. Pharmacol. Res. 2005, 51, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandah, H.; Hosseini, M.; Doulati, K. Hypnotic effect of Rosa damascena in mice. Iran. J. Pharm. Res. 2004, 3, 181–185. [Google Scholar]
- Girish, C.; Raj, V.; Arya, J.; Balakrishnan, S. Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. Eur. J. Pharmacol. 2013, 710, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.H.; Viola, H.; Wolfman, C.; Marder, M.; Wasowski, C.; Calvo, D.; Paladini, A.C. Overview—Flavonoids: A new family of benzodiazepine receptor ligands. Neurochem. Res. 1997, 22, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Noguerón-Merino, M.; Jiménez-Ferrer, E.; Román-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Herrera-Ruiz, M. Interactions of a standardized flavonoid fraction from Tilia americana with serotoninergic drugs in elevated plus maze. J. Ethnopharmacol. 2015, 164, 319–327. [Google Scholar] [CrossRef]
- Shephard, R.A. Behavioral effects of GABA agonists in relation to anxiety and benzodiazepine action. Life Sci. 1987, 40, 2429–2436. [Google Scholar] [CrossRef]
- Aguirre-Hernández, E.; González-Trujano, M.E.; Terrazas, T.; Santoyo, J.H.; Guevara-Fefer, P. Anxiolytic and sedative-like effects of flavonoids from Tilia americana var. mexicana: GABAergic and serotonergic participation. Salud Ment. 2016, 39, 37–46. [Google Scholar]
- Priprem, A.; Watanatorn, J.; Sutthiparinyanont, S.; Phachonpai, W.; Muchimapura, S. Anxiety and cognitive effects of quercetin liposomes in rats. Nanomedicine 2008, 4, 70–78. [Google Scholar] [CrossRef]
- Salgueiro, J.B.; Ardenghi, P.; Dias, M.; Ferreira, M.B.C.; Izquierdo, I.; Medina, J.H. Anxiolytic natural and synthetic flavonoid ligands of the central benzodiazepine receptor have no effect on memory tasks in rats. Pharmacol. Biochem. Behav. 1997, 58, 887–891. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Dok-Go, H.; Lee, K.H.; Kim, H.J.; Lee, E.H.; Lee, J.; Song, Y.S.; Lee, Y.H.; Jin, C.; Lee, Y.S.; Cho, J. Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res. 2003, 965, 130–136. [Google Scholar] [CrossRef]
- Ha, H.J.; Kwon, Y.S.; Park, S.M.; Shin, T.; Park, J.H.; Kim, H.C.; Kwon, M.S.; Wie, M.B. Quercetin attenuates oxygen–glucose deprivation-and excitotoxin-induced neurotoxicity in primary cortical cell cultures. Biol. Pharm. Bull. 2003, 26, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.E.D.; Ramsay, R.R. Dietary inhibitors of monoamine oxidase A. J. Neural. Transm. 2011, 118, 1031–1041. [Google Scholar] [CrossRef]
- Asha, D.; Sumathi, T. Isorhamnetin (IRN) attenuates cognitive dysfunction induced by the Int.racerebroventricular injection of amyloid beta 25–35 (Aβ 25–35) in Sprague Dawley rats. J. Pharm. Sci. Res. 2015, 7, 130–136. [Google Scholar]
- Li, R.; Guo, M.; Zhang, G.; Xu, X.; Li, Q. Nicotiflorin reduces cerebral ischemic damage and upregulates endothelial nitric oxide synthase in primarily cultured rat cerebral blood vessel endothelial cells. J. Ethnopharmacol. 2006, 107, 143–150. [Google Scholar] [CrossRef]
- Nakayama, M.; Aihara, M.; Chen, Y.N.; Araie, M.; Tomita-Yokotani, K.; Iwashina, T. Neuroprotective effects of flavonoids on hypoxia-, glutamate-, and oxidative stress-induced retinal ganglion cell death. Mol. Vis. 2011, 17, 1784–1793. [Google Scholar]
- Huang, J.L.; Fu, S.T.; Jiang, Y.Y.; Cao, Y.B.; Guo, M.L.; Wang, Y.; Xu, Z. Protective effects of nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats. Pharmacol. Biochem. Behav. 2007, 86, 741–748. [Google Scholar] [CrossRef]
- Courvoisier, S.; Ducrot, R.; Julou, L. Psychotropic Drugs; Elsevier: Amsterdam, The Netherlands, 1957; pp. 373–391. [Google Scholar]
- Laroche, M.J.; Rousselet, F. Les Animaux de Laboratoire: Éthique et Bonnes Pratiques; Masson: Paris, France, 1990. [Google Scholar]
- Hoffmann, G. Les Animaux de Laboratoire: Précis; Vigot Freres: Paris, France, 1963. [Google Scholar]
- Clark, G.; Koester, A.G.; Pearson, D.W. Exploratory behavior in chronic disulfoton poisoning in mice. Psychopharmacologia 1971, 20, 169–171. [Google Scholar] [CrossRef]
- File, S.E.; Wardill, A.G. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacologia 1975, 44, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Emamghoreishi, M.; Khasaki, M.; Aazam, M.F. Coriandrum sativum: Evaluation of its anxiolytic effect in the elevated plus-maze. J. Ethnopharmacol. 2005, 96, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Consolini, A.E.; Ragone, M.I.; Migliori, G.N.; Conforti, P.; Volonte, M.G. Cardiotonic and sedative effects of Cecropia pachystachya Mart. (ambay) on isolated rat hearts and conscious mice. J. Ethnopharmacol. 2006, 106, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Khan, I.A. Pharmacological evaluation of sedative and hypnotic activities of methanolic extract of Lycopus europaeus in mice. J. Phytopharm. 2013, 2, 8–12. [Google Scholar]
- Herrera-Ruiz, M.; Gutierrez, C.; Enrique Jimenez-Ferrer, J.; Tortoriello, J.; Miron, G.; Leon, I. Central nervous system depressant activity of an ethyl acetate extract from Ipomoea stans roots. J. Ethnopharmacol. 2007, 112, 243–247. [Google Scholar] [CrossRef]
- Williamson, E.M.; Okpako, D.T.; Evans, F.J. Selection, Preparation and Pharmacological Evaluation of Plant Material; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
Sample Availability: Not available. |
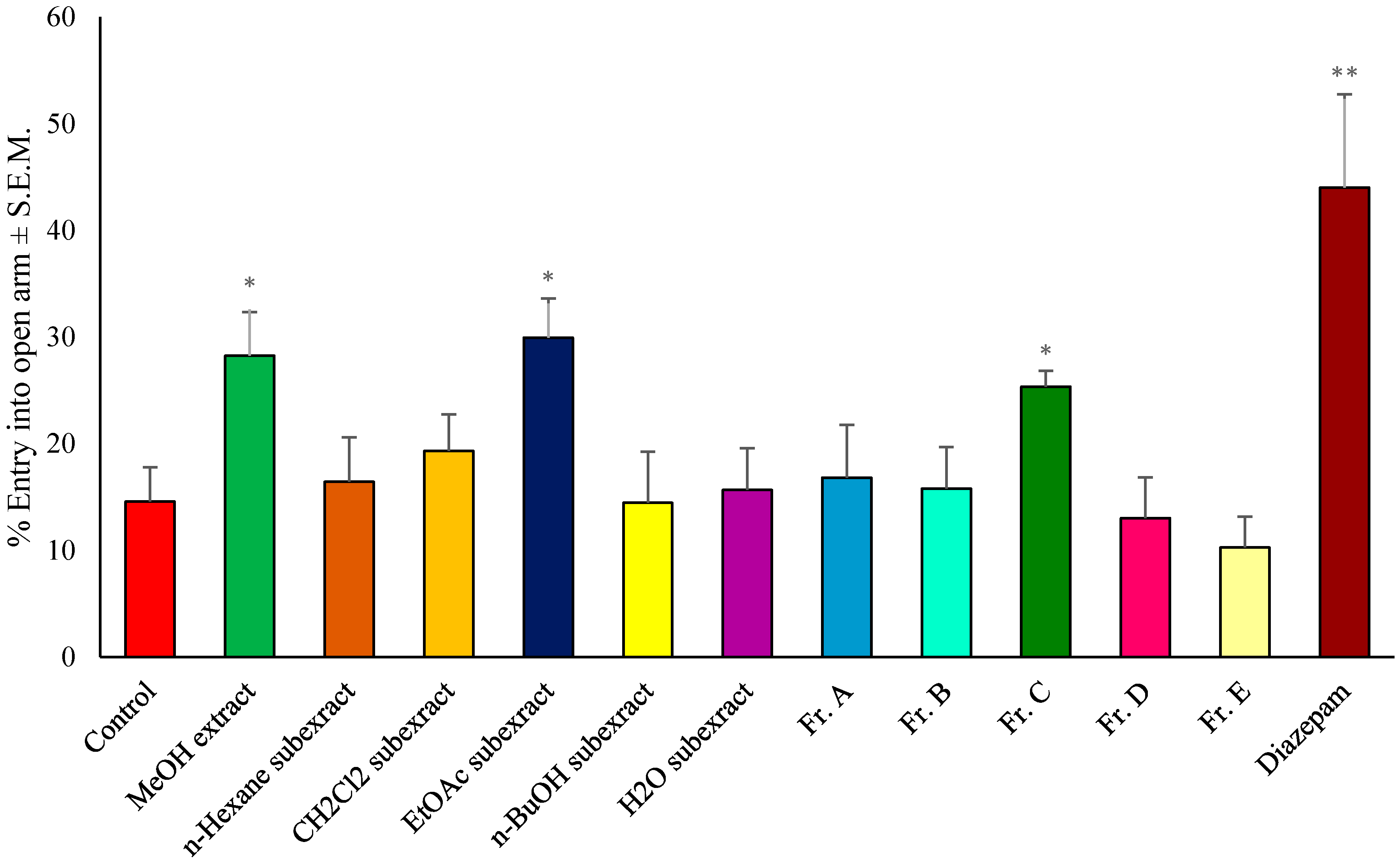
| Test Material | Dose (mg/kg) | Traction Test | Fireplace Test | Hole Board Test |
|---|---|---|---|---|
| (Re-Establishment Time) (Sec) ± S.E.M | (Time to Go Back the Tube in Seconds) ± S.E.M | (Explored Holes During 5 min) ± S.E.M | ||
| Control | - | 0.25 ± 0.05 | 10.63 ± 2.68 | 56.22 ± 9.74 |
| MeOH extract | 100 | 4.52 ± 1.26 | 159.27 ± 9.94 *** | 8.78 ± 1.52 ** |
| n-Hexane subexract | 100 | 0.37 ± 0.11 | 14.79 ± 3.35 | 47.30 ± 10.97 |
| CH2Cl2 subexract | 100 | 1.74 ± 0.99 | 19.04 ± 2.81 | 39.04 ± 7.58 |
| EtOAc subexract | 100 | 3.04 ± 1.13 | 146.90 ± 11.04 *** | 7.51 ± 1.83 ** |
| n-BuOH subexract | 100 | 2.56 ± 0.82 | 28.15 ± 5.66 | 42.69 ± 6.16 |
| H2O subexract | 100 | 0.62 ± 0.14 | 8.71 ± 1.04 | 54.93 ± 10.39 |
| Fr. A | 100 | 2.55 ± 1.83 | 12.40 ± 1.33 | 38.85 ± 8.07 |
| Fr. B | 100 | 3.10 ± 1.57 | 14.85 ± 3.62 | 35.22 ± 7.04 |
| Fr. C | 100 | 4.30 ± 0.75 | 156.36 ± 8.41 | 7.90 ± 1.36 ** |
| Fr. D | 100 | 2.31 ± 1.19 | 13.99 ± 3.79 | 28.74 ± 13.66 |
| Fr. E | 100 | 1.98 ± 0.63 | 10.32 ± 2.86 | 48.64 ± 12.59 |
| Diazepam | 1 | 11.82 ± 1.54 *** | 174.61 ± 6.46 *** | 0.00 ± 0.00 *** |
| Test Material | Dose (mg/kg) | Number of Squares Crossed ± S.E.M. | |||
|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | ||
| Control | - | 78.84 ± 10.06 | 61.29 ± 9.93 | 43.31 ± 8.78 | 50.67 ± 7.02 |
| MeOH extract | 100 | 33.16 ± 7.42 * | 30.41 ± 7.18 * | 24.11 ± 6.07 * | 19.93 ± 4.35 ** |
| n-Hexane subexract | 100 | 85.29 ± 13.61 | 75.53 ± 11.12 | 51.80 ± 14.21 | 50.19 ± 12.69 |
| CH2Cl2subexract | 100 | 74.13 ± 9.89 | 66.90 ± 10.71 | 58.59 ± 8.66 | 48.26 ± 9.07 |
| EtOAc subexract | 100 | 31.73 ± 7.26 * | 28.82 ± 6.19 * | 21.63 ± 9.91 * | 16.07 ± 7.74 * |
| n-BuOH subexract | 100 | 74.90 ± 11.42 | 57.14 ± 9.43 | 40.30 ± 8.53 | 41.27 ± 9.05 |
| H2O subexract | 100 | 81.99 ± 13.36 | 66.72 ± 14.59 | 51.34 ± 10.26 | 48.15 ± 11.39 |
| Fr. A | 100 | 74.62 ± 8.94 | 55.19 ± 8.90 | 39.91 ± 10.69 | 38.19 ± 12.23 |
| Fr. B | 100 | 75.86 ± 11.70 | 63.44 ± 9.76 | 48.24 ± 7.61 | 49.78 ± 9.19 |
| Fr. C | 100 | 30.16 ± 6.91 * | 26.39 ± 5.82 ** | 21.74 ± 5.12 ** | 19.63 ± 4.85 ** |
| Fr. D | 100 | 79.83 ± 15.43 | 75.16 ± 12.41 | 68.60 ± 11.29 | 64.12 ± 9.01 |
| Fr. E | 100 | 71.10 ± 10.28 | 63.49 ± 8.29 | 60.31 ± 9.06 | 55.26 ± 10.92 |
| Diazepam | 1 | 25.14 ± 7.99 *** | 20.43 ± 7.34 *** | 14.35 ± 6.42 *** | 11.20 ± 3.87 *** |
| Test Material | Dose (mg/kg) | Onset of Sleeping (min) ± S.E.M | Sleeping Duration (min) ± S.E.M |
|---|---|---|---|
| Control | - | 60.22 ± 5.99 | 74.14 ± 9.01 |
| MeOH extract | 100 | 26.81 ± 2.65 ** | 255.62 ± 4.80 *** |
| n-Hexane subexract | 100 | 56.47 ± 7.16 | 81.49 ± 9.14 |
| CH2Cl2subexract | 100 | 47.35 ± 4.68 | 96.63 ± 13.43 |
| EtOAc subexract | 100 | 29.33 ± 1.94 ** | 234.91 ± 5.62 *** |
| n-BuOH subexract | 100 | 58.40 ± 9.39 | 76.28 ± 11.89 |
| H2O subexract | 100 | 62.11 ± 8.53 | 61.13 ± 9.91 |
| Fr. A | 100 | 55.83 ± 10.90 | 88.43 ± 8.24 |
| Fr. B | 100 | 48.29 ± 9.77 | 79.01 ± 10.40 |
| Fr. C | 100 | 23.24 ± 2.10 ** | 249.15 ± 6.47 *** |
| Fr. D | 100 | 41.35 ± 4.86 | 92.18 ± 12.63 |
| Fr. E | 100 | 68.91 ± 11.53 | 76.51 ± 9.94 |
| Diazepam | 1 | 12.04 ± 1.88 *** | 310.53 ± 8.62 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkol, E.K.; Ilhan, M.; Karpuz, B.; Genç, Y.; Sobarzo-Sánchez, E. Sedative and Anxiolytic Activities of Opuntia ficus indica (L.) Mill.: An Experimental Assessment in Mice. Molecules 2020, 25, 1844. https://doi.org/10.3390/molecules25081844
Akkol EK, Ilhan M, Karpuz B, Genç Y, Sobarzo-Sánchez E. Sedative and Anxiolytic Activities of Opuntia ficus indica (L.) Mill.: An Experimental Assessment in Mice. Molecules. 2020; 25(8):1844. https://doi.org/10.3390/molecules25081844
Chicago/Turabian StyleAkkol, Esra Küpeli, Mert Ilhan, Büşra Karpuz, Yasin Genç, and Eduardo Sobarzo-Sánchez. 2020. "Sedative and Anxiolytic Activities of Opuntia ficus indica (L.) Mill.: An Experimental Assessment in Mice" Molecules 25, no. 8: 1844. https://doi.org/10.3390/molecules25081844
APA StyleAkkol, E. K., Ilhan, M., Karpuz, B., Genç, Y., & Sobarzo-Sánchez, E. (2020). Sedative and Anxiolytic Activities of Opuntia ficus indica (L.) Mill.: An Experimental Assessment in Mice. Molecules, 25(8), 1844. https://doi.org/10.3390/molecules25081844