Abstract
Callicarpa giraldii Hesse ex Rehd. is an endemic plant in China and has long been used as a traditional medicine in several provinces. Although the plant has been reported to contain flavonoids, triterpenes, and alkaloids, this study represents the first report of the isolation of phyllocladane-type diterpenoids, a relatively rare class of compounds. In this study, 18 new phyllocladane-type diterpenoids (7–24) were isolated and structurally elucidated, including eight uncommon 3,4-seco phyllocladane-type diterpenoids (15–22) and two unusual phyllocladane-type diterpene dimers (23–24), along with six known analogues (1–6). Their structures were elucidated by a comprehensive analysis of 1D and 2D NMR, IR, and HRESIMS data. The absolute configurations were determined by single crystal X-ray diffraction experiments, DFT NMR calculations, and TDDFT ECD calculations. Based on the obtained and reported spectroscopic data, we refined a rule to distinguish phyllocladane-type diterpenoids from their diastereomeric ent-kaurane-type compounds. Additionally, the isolated compounds were evaluated for their in vitro anti-neuroinflammatory activity against lipopolysaccharide (LPS)-induced inflammation in BV-2 microglial cells. Compounds 5, 10, 13, 18, 19, and 20 showed moderate inhibitory activity at the concentration of 20 μM, with compounds 5 and 13 markedly reducing the mRNA levels of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α at this concentration.
1. Introduction
Phyllocladane-type diterpenoids, a rare class of tetracyclic diterpenes, have been scarcely found in nature. To date, fewer than 50 diterpenes with this unique skeletal structure have been reported, primarily distributed across the genera Callicarpa [1], Plectranthus [2], Anisomeles [3], and Cryptomeria [4]. The pharmacological activities of these diterpenoids mainly include promoting plant growth [5], antibacterial properties [3,4], and cytotoxicity [1,6]. It should be noted that phyllocladane-type diterpenoids are easily misidentified as the more common ent-kaurane due to their diastereomeric relationship [7].
The genus Callicarpa (Lamiaceae) consists of approximately 190 species worldwide, with 46 species found in China, predominantly distributed in regions south of the Yangtze River [8]. Callicarpa plants are rich in diterpenes, which have been widely reported from this genus in recent years, including labdane- [9,10], abietane- [11,12,13], clerodane- [14], and phyllocladane-type diterpenes [1]. In the previous study in our lab, 3,4-seco-isopimarane and 3,4-seco-pimarane diterpenoids were isolated from the whole plant of Callicarpa nudiflora [15].
Callicarpa giraldii Hesse ex Rehd. (Lamiaceae) is an endemic plant in China, widely distributed in Gansu, Hubei, Fujian, Guangdong, Guangxi, Sichuan, Guizhou, and Yunnan provinces. Traditionally, it has been recorded for its effects in dispelling wind, removing dampness, dispersing blood stasis, and detoxifying. C. giraldii has been reported to contain a diverse array of secondary metabolites, including flavonoids, triterpenes, and alkaloids [16]. However, no reports have previously documented the isolation of diterpenes from this plant.
In this study, a systematic investigation of the branches and leaves of C. giraldii was conducted, aiming to achieve an in-depth understanding of the chemical diversity of this plant, especially its diterpenoids (Figure 1), and the possible bioactivities relevant to its traditional usages. Herein, we describe the isolation and structural elucidation of these diterpenoids, as well as their anti-neuroinflammatory properties against lipopolysaccharide (LPS)-induced inflammation in BV-2 microglial cells.
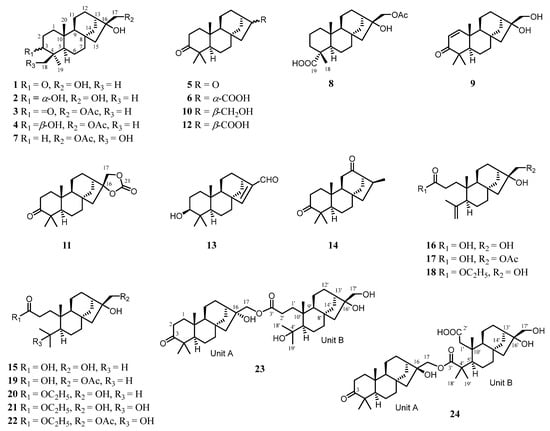
Figure 1.
Structures of compounds 1–24 from C. giraldii.
2. Results
2.1. Spectroscopic Differentiation Between Phyllocladane- and Ent-Kaurane-Type Diterpenoids
Phyllocladane-type diterpenoids are a relatively rare class of compounds and are often misidentified as ent-kaurane type diterpenoids due to their diastereoisomeric relationship. It is necessary to refine simple spectroscopic rules to quickly distinguish these two types of diterpenes rather than relying mostly on single crystal X-ray diffraction experiments.
In 2000, Liu found that some of the reported NMR rules were not reliable for the determination of phyllocladane-type diterpenoids [17], based on the isolation and synthesis of several phyllocladane-type diterpenes. Dong further summarized that “the carbon spectra of the two diterpenes show obvious differences, particularly in C-14, C-15, and the methyl group at C-20” [7]. However, due to the limited variety of phyllocladane-type diterpenoids, the rule based on specific NMR data ranges for distinguishing these two diterpenes was not substantially refined.
In this study, six known phyllocladane-type diterpenes (1–6) were separated from C. giraldii and identified as calliterpenone (1) [18], (3β,16α)-phyllocladane-3,16,17-triol (2) [19], calliterpenone monoacetate (3) [1], (3β,16α)-phyllocladane-3,16,17-triol17-acetate (4) [19], 17-norphyllocladane-3,16-dion (5) [19], and (16R)-3-oxophyllocladan-17-oic acid (6) [17], respectively, by comparison of their spectroscopic data with those in literature. Fortunately, suitable single crystals of compounds 1, 2, and 5 were successfully obtained, which fully confirmed their absolute configurations. These data, in combination with the concrete data reported in the previous literature (Figure 2), provided more comprehensive 13C NMR rules to rapidly determine phyllocladane- or ent-kaurane-type diterpenoids (Table 1).
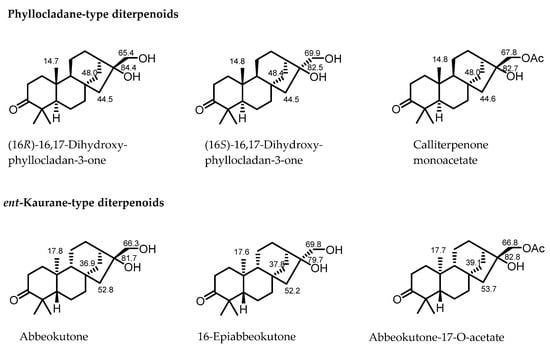
Figure 2.
13C NMR data in CDCl3 of some known Phyllocladane- and ent-Kaurane-type diterpenoids [17,18,20].

Table 1.
Summary of the 13C NMR data differences of phyllocladane- and ent-kaurane-type diterpenoids.
The chemical shift of C-14 exceeds 40 ppm in phyllocladane-type diterpenoids, while it is below 40 ppm in ent-kaurane-type diterpenoids.
The chemical shift of C-15 ranges from 40 to 50 ppm in phyllocladane-type diterpenoids, while it exceeds 50 ppm in ent-kaurane-type diterpenoids.
The chemical shift of Me-20 ranges from 14 to 15 ppm in phyllocladane-type diterpenoids, while it is around 17 ppm in ent-kaurane-type diterpenoids.
For phyllocladane-type diterpenoids substituted by a hydroxyl or an acetoxy group at C-17, the chemical shift of C-17 ranged from 65 to 68 ppm in compounds with rel-16R, while it exceeded 69 ppm in compounds with rel-16S.
2.2. Structural Elucidation of New Compounds 7–24
Compound 7 was isolated as colorless needle crystals. Its molecular formula was determined as C22H36O4 by HRESIMS data (m/z 387.2498 [M + Na]+, calcd for C22H36O4Na, 387.2511), indicative of five degrees of unsaturation. The IR spectrum (Figure S2) showed absorption bands for hydroxy (3428 cm−1) and carbonyl groups (1713 cm−1). The 1H NMR data (Table 2) showed three methyl groups (δH 2.10, 0.96, 0.85) and two oxygenated methylenes (δH 4.24, 4.16; δH 3.74, 3.41). The 13C NMR and DEPT data (Table 3) revealed 22 carbon atom signals, including three methyls, 11 methylenes, three methines, and five quaternary carbons (one ester carbonyl). These observations accounted for one degree of unsaturation, and the remaining four required a tetracyclic scaffold for compound 7.

Table 2.
1H NMR data of compounds 7–10 in CDCl3 (δ in ppm, J in Hz).

Table 3.
13C NMR data of compounds 7–14 in CDCl3 (125 MHz, δ in ppm).
The 1H–1H COSY spectrum revealed three spin-coupling systems of H2-1/H2-2/H2-3, H-5/H2-6/H2-7, and H-9/H2-11/H2-12 (Figure 3 and Figure S5). The HMBC spectrum (Figure 3 and Figure S7) exhibited key correlations from H3-20 to C-1/C-5/C-9/C-10, from H3-18 to C-3/C-4/C-5/C-19, and from H2-7 to C-8/C-9, which indicated the existence of two fused six-membered carbon rings, where Me-20 was located at C-10 while Me-18 and a hydroxymethyl (-CH2OH) were located at C-4. HMBC correlations from H2-14 to C-8/C-9/C-12/C-13 constructed another six-membered ring, which fused to ring C through C-8 and C-9. In addition, HMBC correlations from H2-15 to C-8/C-9/C-13/C-14/C-16 constructed a five-membered ring. Moreover, an acetoxy group connected to C-17 and a hydroxyl group located at C-16 were deduced from the HMBC correlations from H2-17 and the methyl of the acetoxy group to the carbonyl carbon (δC 171.4). Thus, the planer structure of compound 7 was established.
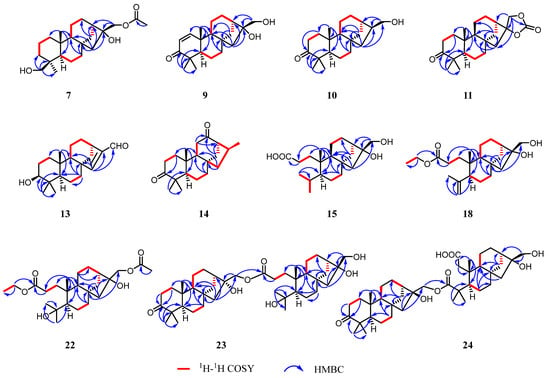
Figure 3.
Key 1H-1H COSY and HMBC correlations of compounds 7, 9–11, 13–15, 18, and 22–24.
The NOESY correlations (Figure 4 and Figure S8) of H-5/H3-19 suggested that these protons were co-facial and α-oriented, while the cross-peak of H2-18/H3-20 indicated that they were on the opposite face and β-oriented. To figure out the orientation of C-16, DFT NMR calculation was carried out on two possible conformations of 7a (16R) and 7b (16S). The Boltzmann-weighted average NMR data of 7a and 7b were compared with the experimental data using the improved statistical method DP4+ probability [19]. Compound 7 gave 100% possibilities (H data, C data, and all data) for 7a. The absolute configuration was further proved by a single-crystal X-ray diffraction experiment with Cu Kα radiation (CCDC 2389914, Figure 5). Therefore, the structure of compound 7 was fully defined and named calligirlin A.
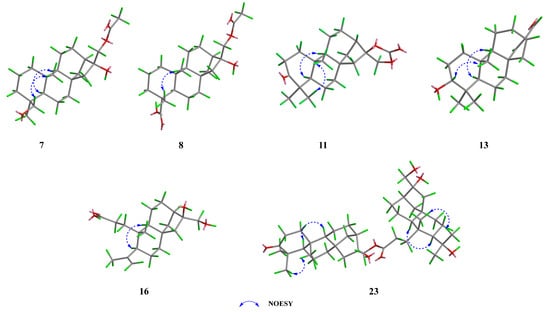
Figure 4.
Key NOESY correlations of compounds 7, 8, 11, 13, 16, and 23. The 3D structure modeling was produced by PerkinElmer Chem3D by using MM2 minimization calculation.
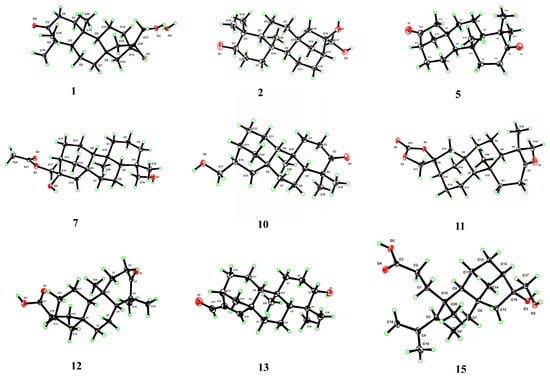
Figure 5.
Perspective ORTEP drawings for compounds 1, 2, 5, 7, 10, 11, 12, 13, and 15.
Compound 8 was isolated as a white solid. The HRESIMS and 13C NMR data gave a molecular formula of C22H34O5 with six degrees of unsaturation. The 13C and 1H NMR data of 8 (Table 2 and Table 3) also suggested a phyllocladane-type diterpene. Compared with compound 7, a carboxyl group instead of a hydroxymethyl group was located at C-19, which was supported by the HMBC correlation (Figure 3 and Figure S17) from H3-18 to C-19 (δC 181.9). The NOESY correlation (Figure 4 and Figure S18) of H3-18/H3-20 suggested that these two methyls were on the same face and β-oriented, while the carboxyl group was on the other face and α-oriented. According to the spectroscopic rule (Table 2), the relative configuration of C-16 was designated as R by the chemical shift of C-17 (δC 67.9). Therefore, given the biosynthetic consideration, the full structure of compound 8 was defined and named calligirlin B.
The molecular formula of 9 was established as C20H30O3 by HRESIMS. The NMR data comparison of 9 and 1 (Table 2 and Table 3) suggested that they might share the same skeleton [21]. The carbonyl carbon (δC 205.3) and two typical olefinic carbons (δC 158.2, 125.8; δH 6.99, 5.83) revealed the presence of an α, β-unsaturated δ-ketone moiety located at C-1/C-2/C-3, which was evidenced by the HMBC correlations from H-1 to C-3/C-5/C-10, from H-2 to C-4/C-10 (Figure 3 and Figure S25). The NOESY correlation of H-5/H-9 suggested that these protons were co-facial and α-oriented (Figure S26). The chemical shift of C-17 (δC 65.6) suggested a rel-16R for C-16. To further determine the absolute configurations, TDDFT ECD calculation was performed on the structure of 9 with assigned relative configurations. The non-redundant advantageous conformers were re-optimized at the M062X/6-31G(d) level using the SMD solvent model for methanol. Then, ECD calculations were performed at the CAM-B3LYP/TZVP level on the same solvent model for methanol. The results showed that the experimental ECD curve of compound 9 matched well with its calculated one (Figure 6). Therefore, the structure of 9 was fully established and named calligirlin C.
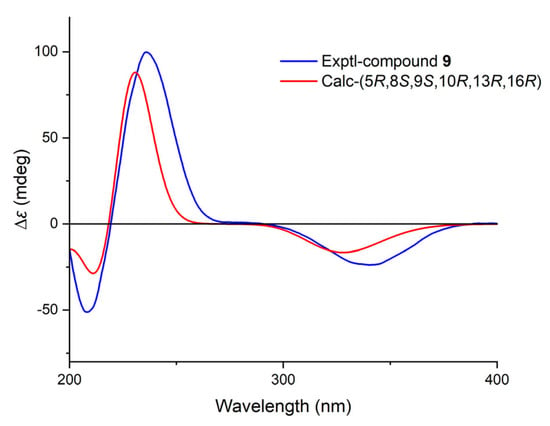
Figure 6.
Comparison of experimental and calculated ECD spectra for compound 9 in methanol.
Compound 10 was isolated as colorless orthorhombic crystals and had a molecular formula of C20H32O2 based on its HRESIMS data, one less oxygen atom than that of 1. Its NMR data (Table 2 and Table 3) were highly similar to those of 1, except for some signals of ring D. The absence of OH-16 was evidenced by the upfield shift of C-16 (δC 36.9) (Table 3). The NOESY correlation of H-5/H-9 suggested that these protons were co-facial and α-oriented (Figure S34). The chemical shift of C-17 (δC 67.6) suggested that the relative configuration of C-16 was R. The full structure, including the absolute configuration of compound 10, was confirmed by a single-crystal X-ray diffraction experiment with Cu Kα radiation (CCDC 2389915, Figure 5). Therefore, the structure of compound 10 was proposed and named calligirlin D.
Compound 11 was isolated as light-yellow needle crystals. Its molecular formula was determined as C21H30O4 by HRESIMS, indicative of seven degrees of unsaturation. Its NMR data (Table 3 and Table 4) were similar to those of a known compound 16α, 17-isopropylideno-3-oxo-phyllocladane [22], suggesting it might also possess an additional 1,3-dioxolane located at C-16. When comparing their NMR data, a quaternary carbon (δC 154.7) in 11, instead of a quaternary carbon (δC 108.7) and two methyl groups (δC 26.9, 26.9) in the known compound, was observed, implying the additional five-membered ring in 11 was a 1, 3-dioxolane-2-one. HMBC correlations from H2-17 to C-13/C-15/C-16/C-21 (Figure 3 and Figure S41) further supported such elucidation. The NOESY correlation of H3-18/H3-20, H-5/H3-19, and H-5/H-9 inferred the relative configurations (Figure 4 and Figure S42). The absolute configuration was finally established by a single-crystal X-ray diffraction experiment with Cu Kα radiation (CCDC 2390159, Figure 5). Therefore, the full structure of compound 11 was defined and named calligirlin E.

Table 4.
1H NMR data of compounds 11–14 in CDCl3 (δ in ppm, J in Hz).
The molecular formula of 12 was established as C20H30O3 by its HRESIMS data, the same as that of the known compound 6. The 1H and 13C NMR data (Table 3 and Table 4) of 12 were almost identical to 6, except for the differences observed for the chemical shifts of the carboxylic acid group. A suitable single crystal of compound 12 was obtained, and the single-crystal X-ray diffraction experiment with Cu Kα radiation finally confirmed the full structure of 12 (CCDC 2389922, Figure 5). Accordingly, compound 12 was ultimately identified as an isomer of 6, with the carboxyl group at the C-16 having the opposite configuration compared to that in 6, and named calligirlin F.
Compound 13 was isolated as a white solid. The HRESIMS and 13C NMR data gave a molecular formula of C20H30O2 with six degrees of unsaturation. Its NMR data (Table 3 and Table 4) were indicative of a phyllocladane-type diterpene skeleton. Detailed analysis revealed the presence of an α, β-unsaturated δ-aldehyde (δC 156.9, 147.6, 190.0) and an oxygenated methine (δC 79.0). HMBC correlations (Figure 3 and Figure S57) from H-17 to C-13/C-16 and from H-15 to C-8/C-13/C-14/C-16/C-17 indicated the existence of the 15,16-ene-17-aldehyde moiety. HMBC correlations from H-3 to C-2 and C-4 suggested a hydroxy group attached to C-3. The NOESY correlation of H-3/H-5 suggested that the hydroxy group was β-oriented (Figure 4 and Figure S58). Fortunately, a suitable single crystal of compound 13 was obtained, and the single-crystal X-ray diffraction experiment with Cu Kα radiation finally confirmed the full structure of 13 (CCDC 2389916, Figure 5). The structure of compound 13 was then established and named calligirlin G.
Compound 14 was isolated as a white solid. Its molecular formula was determined as C20H30O2 by HRESIMS. A detailed NMR data analysis (Table 3 and Table 4) suggested that 14 was an analogue of 5 but with an additional methyl group. Further analysis of its NMR data revealed the existence of Me-17 and 12-ketone, which could be supported by the HMBC correlations (Figure 3 and Figure S65) from H3-17 to C-13/C-15/C-16, from H-13 to C-12/C-15/C-14, from H2-11/to C-9/C-12/C-13, and from H2-14 to C-8/C-9/C-12/C-13. To figure out the orientation of C-16, DFT calculation of 1H and 13C NMR chemical shifts was carried out on two possible conformations of 14a (16S) and 14b (16R). The Boltzmann-weighted average NMR data of 14a and 14b were compared with the experimental data using the improved statistical method DP4+ probability. The result gave 100% possibilities (H data, C data, and all data) for 14a. Therefore, given the biosynthetic consideration, the structure and absolute configuration of compound 14 were proposed and named calligirlin H.
Compound 15 was isolated as colorless needle crystals. Its molecular formula was determined as C20H34O4 by HRESIMS, corresponding to four degrees of unsaturation. A detailed analysis of its NMR data (Table 5 and Table 6) was conducted, and the 13C NMR and DEPT data (Table 6) only revealed 19 carbon atom signals, including three methyls, nine methylenes, four methines, and three quaternary carbons. The missing carbon was identified as C-3 by the HMBC correlations from H2-1 and H2-2 to the carbonyl carbon at δC 178.6. As one degree of unsaturation was occupied by the carboxyl group, the remaining three suggested a three-ring skeleton. The planer structure of 15 was established by 2D NMR data. The 1H–1H COSY spectrum (Figure 3 and Figure S73) revealed three spin-coupling systems of H2-1/H2-2, H-5/H2-6/H2-7 and H-9/H2-11/H2-12, H-13/H2-14. HMBC correlations (Figure 3 and Figure S75) from H3-20 to C-1/C-2/C-5/C-9/C-10, and from H2-7 to C-5/C-6/C-8/C-9/C-10 indicated a six-membered ring. HMBC correlations from H-9 to C-8/C-11/C-12/C-13 indicated another six-membered ring, which was fused to the former one through C-8 and C-9. HMBC correlations from H2-15 to C-8/C-9/C-12/C-14/C-16, and from H2-17 to C-13/C-16, constructed a five-membered ring with a hydroxymethyl (C-17) and a hydroxyl group both located at C-16. In addition, an isopropyl was located at C-5, which was deduced from the 1H-1H COSY correlations of H3-18/H-4/H3-19 and the HMBC correlations from the H3-18 to C-5/C-19. Besides Me-20, a propanoic acid group was attached to C-10, which was established by the HMBC correlations from H2-2 to C-1/C-3 and from Me-20 to C-1. Thus, the structure of 15 was constructed as a 3,4-seco phyllocladane diterpene, a rare type of phyllocladane derivative. The NOESY correlation (Figure S76) of H-5/H-9 suggested that these protons were co-facial and α-oriented. The absolute configuration was established by a single-crystal X-ray diffraction experiment with Cu Kα radiation (CCDC 2389933, Figure 5). Therefore, the structure of compound 15 was fully defined and named calligirlin I.

Table 5.
1H NMR data of compounds 15–18 (δ in ppm, J in Hz).

Table 6.
13C NMR data of compounds 15–22 (δ in ppm).
Compound 16 was obtained as a white solid. Its molecular formula was determined to be C20H32O4 with five degrees of unsaturation by HRESIMS. The 1H and 13C NMR data of 16 (Table 5 and Table 6) showed high similarities to those of 15, suggesting 16 was also a 3,4-seco phyllocladane-type diterpene. Comparison of their NMR data revealed the presence of a terminal double bond (δC 114.2, 148.8, δH 4.67, 4.88) and a singlet methyl (δC 24.3, δH 1.75) in 15, replacing a methine (δC 26.7, δH 1.91) and two doublet methyl (δC 25.2, 19.5, δH 0.93, 0.87) as found in 16. HMBC correlations (Figure 3 and Figure S83) from the olefinic proton (δH 4.88, 4.67) to C-19/C-4/C-5 indicated an isopropenyl attached to C-5. The NOESY correlation (Figure 4 and Figure S84) of H-5/H-9 suggested that these protons were α-oriented. According to the above-mentioned spectroscopic rules, the chemical shift of C-17 (δC 66.2) implied a rel-R-configuration for C-16. Therefore, the whole structure of 16 was proposed and named calligirlin J.
Comprehensive analysis of NMR data of compound 17 (Table 5 and Table 6) revealed that 17 was an acetylated derivative of 16. The acetoxy group was connected to C-17 owing to the HMBC correlations (Figure 3 and Figure S91) from the methyl (δH 2.10) and H2-17 to the carbonyl carbon (δC 171.4). The chemical shift of C-17 (δC 67.9) suggested that the relative configuration of C-16 was R (Figure 4). Therefore, the whole structure of compound 17 was proposed and named calligirlin K.
Compound 18 was obtained as a white solid. The 1H and 13C NMR data (Table 5 and Table 6) of 18 showed high similarities to those of 16, suggesting that they might possess the same skeleton. The HMBC correlations (Figure 3 and Figure S99) from the quartet oxygenated methylene (δH 4.10) to the carbonyl carbon (δC 113.9, C-3), and from the triplet methyl (δH 1.24) to the oxymethylene carbon (δC 60.5), indicated that 18 was an ethyl ester derivative of 16, and the esterification happened at C-3. The NOESY correlation (Figure S100) of H-5/H-9 was observed, suggesting α-orientations for both protons. The chemical shift of C-17 (δC 65.8) suggested the typical rel-16R in a phyllocladane-type diterpene. Therefore, the structure of compound 18 was proposed and named calligirlin L.
Compound 19 was obtained as a white solid. Its molecular formula was determined to be C22H36O5 with five degrees of unsaturation by HRESIMS. The 1H and 13C NMR data (Table 6 and Table 7) of 19 showed high similarities to those of compound 15, except for the presence of an additional acetyl group (δH 2.11, s; δC 171.4, 21.1) in 19. HMBC correlations (Figure 3 and Figure S107) from H2-17 to the carbonyl carbon (δC 171.4, C) suggested that an acetoxyl rather than a hydroxy was connected to C-17. The NOESY correlation (Figure S108) of H-5/H-9 and the chemical shift of C-17 (δC 67.9) suggested the same relative configurations with compound 5. Therefore, given the biosynthetic consideration, the whole structure of compound 19 was established and named calligirlin M.

Table 7.
1H NMR data of compounds 19–22 in CDCl3 (δ in ppm, J in Hz).
Compound 20 was obtained as a white solid. Its molecular formula was determined to be C22H38O4 with four degrees of unsaturation by HRESIMS. The 1H and 13C NMR data analysis (Table 6 and Table 7) revealed that 20 was also an ethyl ester derivative of compound 15, similar to the above-mentioned compounds 16 and 18. Such elucidation was supported by the HMBC correlations (Figure 3 and Figure S115) from the oxygenated methylene (δH 4.14) to the carbonyl carbon (δC 174.5, C-3) and from the triplet methyl (δH 1.28) to the oxymethylene carbon (δC 60.5). Therefore, the whole structure of compound 20 was proposed and named calligirlin N.
The molecular formula of compound 21, determined by HRESIMS, was established as C22H38O5, one more oxygen atom than that of 20. Its 1H and 13C NMR data (Table 6 and Table 7) showed high similarities to those of 20, expect that two doublet methyls (δC 25.0, 19.1, δH 0.92, 0.80) and one methine (δC 25.2, δH 1.88) were observed in 21, replacing two singlet methyl (δC 34.2, 27.8, δH 1.28, 1.22) and one oxygenated quaternary carbon (δC 75.9) compared to 20. HMBC correlations (Figure 3 and Figure S123) from H-5 to C-4/C-10/C-6/C-18/C-19/C-20 and from H3-18 to C-4/C-5 indicated a hydroxyl group located at C-4. Accordingly, the structure of compound 21 was fully established and named calligirlin O.
The molecular formula of compound 22 was determined to be C24H40O6 with five degrees of unsaturation by HRESIMS. The detailed analysis of the 1H and 13C NMR data of 22 (Table 6 and Table 7) revealed that 22 possessed a similar skeleton to that of 21, except for an additional acetoxy group observed in 22. HMBC correlations (Figure 3 and Figure S131) from the methyl (δH 2.10) and H2-17 to the carbonyl carbon (δC 171,4) supported that the acetoxyl group was attached to C-17. Therefore, the structure of compound 22 was proposed and named calligirlin P.
Compound 23 was isolated as a white solid. Its molecular formula was determined as C40H64O7 by the pseudo molecular ion peak (m/z 679.4544 [M + Na]+, calcd for C40H64O7Na 679.4550) in the HRESIMS, corresponding to nine degrees of unsaturation. The IR spectrum showed absorption bands for hydroxy (3440 cm−1) and carbonyl groups (1704 cm−1). The 1H NMR data (Table 8) showed the signals of six methyl groups. The 13C NMR and DEPT data (Table 8) revealed the resonances of 40 carbon signals ascribed to six methyls, 18 methylenes, six methines, and 10 quaternary carbons (a carbonyl and an ester carbonyl carbon). A detailed analysis of these NMR data revealed that compound 23 might be a compound dimerized from two different monomeric units of phyllocladane-type diterpene.

Table 8.
1H and 13C NMR data of compounds 23 and 24 (δ in ppm, J in Hz).
The 1H–1H COSY cross-peaks revealed four spin systems of H2-1/H2-2, H2-6/H2-7, H-9/H2-11/H2-12, H-13/H2-14 (Figure 3 and Figure S137). HMBC correlations from H3-20 to C-1/C-9/C-10, from H3-18 to C-3/C-4/C-5/C-19, from H2-7 to C-6/C-8/C-9, from H2-14 to C-8/C-11/C-12/C-13/C-16, and from H2-15 to C-8/C-9/C-14/C-16/C-17 were observed (Figure 3 and S139). These data constructed a half fragment of Unit A, as shown in Figure 1. Similarly, the spin systems of H2-1’/H2-2’, H-9’/H2-11’/H2-12’ and H-13’/H2-14’ deduced by 1H–1H COSY correlations, along with the HMBC correlations from H3-20’ to C-1’/C-5’/C-9’/C-10’, from H2-7’ to C-5’/C-6’/C-8’/C-9’, from H-9’ to C-8’/C-11’/C-13’, from H2-15’ to C-8’/C-9’/C-13’/C-14’/C-16’, from H2-17’ to C-16’/C-13’, from H3-18’ to C19’/C-4’/C-5’, and from H2-2’ to C-1’/C-3’ manifested the other fragment of Unit B (Figure 1). Furthermore, the HMBC correlations from H2-17 (δH 4.19) to C-3’ provided strong evidence for the connection of two fragments via an ester carbonyl group. Thereby determining the planar structure of 23.
The NOESY correlations (Figure 4 and Figure S140) of H-5/H-9 and H-5’/H-9’ suggested that these protons were co-facial and α-oriented. The chemical shift of C-17 (δC 67.6) and C-17’ (δC 65.7) suggested the β-orientation for both C-17 and C-17’. Therefore, given the biosynthetic consideration, the full structure of compound 23 was proposed and named calligirlin Q.
Compound 24 was isolated as a colorless oil. Its molecular formula was determined as C40H62O8 by HRESIMS, indicating 10 degrees of unsaturation. A detailed analysis of its 1H and 13C NMR spectra (Table 8), with the aid of HSQC data, revealed 40 carbon resonances, including six methyls, 17 methylenes, six methines, and 11 quaternary carbons. The above characteristic resonances suggested that 24 could also be a phyllocladane-type diterpenoid dimer. The same Unit A (Figure 1) was constructed by the 1H–1H COSY correlations (Figure 3 and Figure S145), indicating four spin systems of H2-1/H2-2, H2-6/H2-7, H-9/H2-11, and H2-12/H2-13, and the HMBC correlations from H3-20 to C-1/C-9/C-10, from H3-18 to C-3/C-5/C-19, from H2-7 to C-5/C-8/C-9, from H2-14 to C-8/C-9/C-12/, and from H2-15 to C-8/C-9/C-14/C-16/C-17 (Figure 3 and Figure S147). The Unit B of 24 (Figure 1) was established by three spin-coupling systems of H-5’/H2-6’, H-9’/H2-11’, and H-13’/H-14’ (Figure 3 and Figure S147), and the HMBC correlations from H2-1’ to C-2’/C-5/C-9’/C-10’/C-20’ and from H3-20 to C-2’/C-5/C-9’/C-10’ (Figure 3 and Figure S147). HMBC correlations from H3-18’ to C-3’/C-5/C-19’ suggested that one carbonyl group was located at C-3’ (δC 180.7). The other one (δC 175.8) was placed at C-2’ by H3-20 to C-2’/C-5/C-9’/C-10’. Thus, the moiety of Unit B was a 2,3-seco phyllocladane diterpene. HMBC correlation from H2-17 to C-3’ further confirmed that these two units were also connected through the ester carbonyl group. The NOESY correlations (Figure 4 and Figure S148) of H-5/H-9 and H-5’/H-9’ suggested that these protons were co-facial and α-oriented. The chemical shift of C-17 (δC 69.0) suggested that C-17 was β-oriented. Therefore, given the biosynthetic consideration, the full structure of compound 24 was proposed and named calligirlin R.
2.3. Anti-neuroinflammatory Activity Evaluation
The anti-neuroinflammatory activity of compounds 1–7, 10–13, 15, 18–20, and 22–24 was evaluated in vitro against lipopolysaccharide (LPS)-induced inflammation-related BV-2 microglial cells. Compounds 5, 10, 13, 18, 19, and 20 at 20 μM exhibited obvious inhibitory activity on NO production against LPS-induced inflammation-related BV-2 microglial cells (Table 9). In addition, compounds 5 and 13 at 20 μM markedly reduced the mRNA levels of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in LPS-stimulated BV-2 microglial cells (Figure 7), which further demonstrated their inhibitory activities against microglial inflammation.

Table 9.
Anti-inflammatory effect of compounds 5, 10, 13, 18, 19, and 20 in LPS-induced murine microglial BV-2 Cells a.
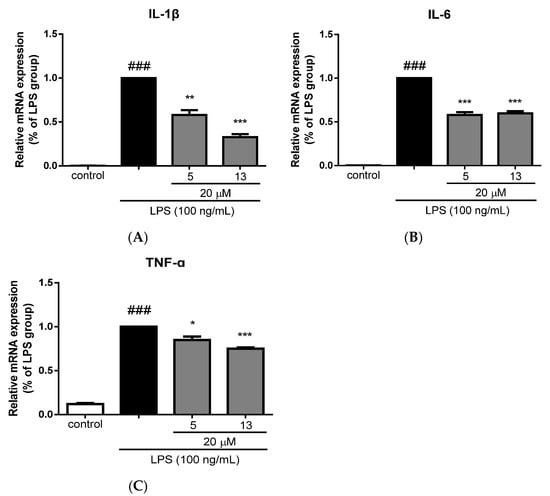
Figure 7.
Inhibitory effects of compounds 5 and 13 on mRNA levels of IL-1β (A), IL-6 (B), and TNF-α (C) in BV-2 cells stimulated by LPS. Data were normalized by the LPS group and presented as means ± SEM, n = 3. ### p < 0.001 vs. the control group; * p < 0.05, ** p < 0.01, *** p < 0.001 compared to the LPS group.
3. Discussion
In summary, the systematic investigation of C. giraldii led to the first report about the isolation of 24 phyllocladane-type diterpenoids, including 18 new derivatives, from the title plant. Among the new compounds, 15–22 were unusual 3,4-seco phyllocladane-type diterpenoids, while 23 and 24 were identified as two new phyllocladane-type diterpenoids dimers. Notably, unit B of 24 possessed the first identified 2,3-seco phyllocladane fragment.
By analyzing NMR data of the isolated compounds in this study and those reported in previous literature, spectroscopic rules were refined for distinguishing the phyllocladane- from the ent-kaurane type diterpenoids based on the chemical shifts of C-14, C-15, and C-20. The chemical shift of C-17 could be used to identify the stereochemistry of C-16. These rules were also applicable to 3,4-seco phyllocladane-type diterpenoids. The reliability of these rules was further confirmed by the single-crystal X-ray diffraction experiment results of new compounds 7, 10, 11, 12, 13, and 15 in this study.
In comparing carbon NMR data of 3,4-seco and typical phyllocladane-type diterpenoids, it was found that the C-20 chemical shift in 3,4-seco phyllocladane-type diterpenoids (with unchanged H-5 relative configuration after ring opening) was between 18 and 20 ppm. However, for (5β,16α)-4,16,17-trihydroxy-3,4-seco-phyllocladan-3-oic acid, where the H-5 relative configuration is reversed, the C-20 chemical shift was 17.4 ppm.
Compounds 5, 10, 13, 18, 19, and 20 exhibited anti-neuroinflammatory activity, as evidenced by their downregulation of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in LPS-stimulated murine microglial BV-2 cells at the tested concentrations.
4. Materials and Methods
4.1. General Experimental Procedures
Optical rotations were measured with a Rudolph Research Analytical Autopol VI automatic polarimeter (Hackettstown, NJ, USA). IR spectra were recorded with a Thermo Nicolet FTIR IS5 spectrometer (Thermo Fisher, Waltham, MA, USA). NMR spectra were recorded on Bruker Avance III-500, Bruker Avance III-600, or III-800 spectrometers. (BRUKER BIOSPIN AG, Fällanden, Switzerland). Chemical shifts were reported in ppm (δ) with coupling constants in hertz. HRESIMS data were acquired on a Waters Synapt G2 Si Q-TOf mass detector. Single-crystal X-ray diffraction measurements were conducted on a Bruker Smart Apex II diffractometer with a graphite monochromator. LCESIMS analysis was performed on a Waters 2695 system equipped with a 2998 PDA detector, a Waters 2424 ELSD, and Waters 3100 MS detectors. Preparative HPLC was run on a Waters system equipped with a Waters 2767 autosampler, a Waters 2545 pump, and a Waters 2489 PDA, using a Waters Sunfire RP C18 column (5 μm, 30 × 150 mm, flow rate 30 mL/min). AB-8 macroporous resin (Shandong Lu Kang Chemical Co., Ltd., Jining, China). MCI gel CHP20P (75–150 μm, Mitsubishi Chemical Industries, Tokyo, Japan). ODS gel AAG12S50 (12 nm, s-50 μm, YMC Co., Ltd., Kyoto, Japan). Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden), Toyopearl HW-40F (30–60 2 μm, TOSOH corporation, Tokyo, Japan). Silica gel (200–300 meshe, and 300–400 mesh, Qingdao Marine Chemical Inc. Qingdao, China). All solvents used for CC were of analytical grade (Shanghai Chemical Reagents Co., Ltd., Shanghai, China), and solvents used for HPLC were of HPLC grade (Merck KGaA, Darmstadt, Germany).
4.2. Plant Material
The branches and leaves of C. giraldii were collected from Jinghong City in Xishuangbanna Prefecture, Yunnan Province, China, in January 2020 and identified by Jun Zhang, an associate researcher from Kunming Zhifen Biotechnology Co., Ltd. (Kunming, China) A voucher (No. 20200115001) was deposited at the Herbarium of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
4.3. Extraction and Isolation
The dried branches and leaves of C. giraldii (30 kg) were extracted with 95% ethanol (3 × 60 L, 7 days each) at room temperature. The ethanol extracts were concentrated under reduced pressure to obtain 831.5 g of extract. The extract was suspended in water and partitioned successively with petroleum ether (PE), dichloromethane (DCM), and ethyl acetate, yielding a PE extract (228.9 g), a DCM extract (260.6 g), an EA extract (68.5 g), and a water-soluble fraction. Then, the PE fraction was partitioned with 70% aqueous methanol to afford a 70% methanol fraction (53.2 g). LC-MS and TLC analyses indicated that the DCM extract and the 70% methanol extract showed high similarity in the chemical compositions, so these two extracts were combined to give a new fraction (313.8 g). The new fraction was subject to column chromatography (CC) over AB-8 macroporous resin, eluted with aqueous EtOH (20%, 50%, 75%, 95%) in a stepwise manner, then with acetone, finally yielding five subfractions Frs. A-E.
Fr. C (110.7 g) was applied to an MCI gel column, eluted with a gradient system of aqueous EtOH from 25% to 75%, resulting in subfractions Fr. C1–Fr. C14. Combined Fr. C6 and Fr. C7 (total 2.7 g) were subjected to CC over Sephadex LH-20 gel eluting with MeOH, and subsequently to CC over HW-40F gel eluting with MeOH, then purified by preparative HPLC to give compounds 15 (6.0 mg) and 16 (3.3 mg) (MeCN/H2O with 0.1% formic acid from 30 to 50%, 25 min).
Fr. C8 (9.4 g) was fractionated using an ODS gel column eluted with a gradient elution of aqueous MeCN (32–95%) to afford subfractions Fr. C8A–Fr. C8H. Fr. C8H (1.2 g) was subsequently separated by chromatography on Sephadex LH-20 gel eluted with MeOH to afford Fr. C8H1–Fr. C8H7. Fr. C8H3 (286.7 mg) was purified by preparative HPLC to afford compound 21 (10.0 mg, MeCN/H2O with 0.1% formic acid, from 23 to 43%, 25 min). Fr. C8H4 (759.7 mg) was separated by using a HW-40F column (eluted with MeOH) and further purified by preparative HPLC to give compound 2 (2.3 mg, MeCN/H2O with 0.1% formic acid, from 21 to 41%, 25 min). Fr. C8H4E (190.3 mg) was subjected to CC over silica gel (200–300 mesh, CH2Cl2/MeOH, gradient from 200:1 to 30:1) and then purified by preparative HPLC (MeCN/H2O with 0.1% formic acid, from 25% to 45%, 25 min) to give compound 9 (2.3 mg).
Fr. C10 (6.0 g) was applied for CC over Sephadex LH-20 gel eluted with CHCl3: MeOH = 1:1, yielding seven subfractions Fr. C10A- Fr. C10G. Fr. C10B (596.9 mg) was separated by using a HW-40F gel column eluted with MeOH and finally preparative HPLC (MeCN/H2O with 0.1% formic acid, from 34 to 59%, 25 min) to afford compound 22 (9.9 mg). Fr. C10C (773.8 mg) was also separated by using a HW-40F gel column (MeOH), and then purified by silica gel CC (200–300 mesh), with isocratic elution of CH2Cl2/MeOH (180:1–50:1) and then purified by preparative HPLC to give compounds 4 (22.1 mg) and 7 (2.9 mg) (MeCN/H2O with 0.1% formic acid, from 39 to 64%, 25 min).
Fr. C10D (3.3 g) was subjected to CC over HW-40F gel eluted with MeOH to afford Fr. C10D6 (678.0 mg), and then was treated with CC over HW-40F eluted with MeOH again to yield Fr. C10D6A–Fr. C10D6H. Fr. C10D6C (44.0 mg) was purified by preparative HPLC to give compound 19 (5.4 mg, MeCN/H2O with 0.1% formic acid, from 38 to 63%, 25 min). Fr. C10D6D (381.0 mg) was subjected to silica gel CC (200–300 mesh) with isocratic elution of CH2Cl2/acetone (150:1) and then purified by preparative HPLC to afford compounds 8 (1.4 mg) and 17 (2.2 mg) (MeCN/H2O with 0.1% formic acid, from 30 to 50%, 46–66%, 25 min, respectively).
Fr. C11 (13.3 g) was treated with CC over Sephadex LH-20 gel eluted with CHCl3: MeOH = 1:1 to yield Fr. C11A- Fr. C11H, which was further separated by CC over ODS gel (MeCN/H2O with 0.1% formic acid, from 30% to 95%) to give a subfraction of Fr. C11C2 (245.9 mg). Compounds 1 (44.3 mg) and 3 (9.8 mg) were obtained from Fr. C11C2 by preparative HPLC (MeCN/H2O with 0.1% formic acid, from 32 to 52%, 44 to 64%, 25 min, respectively). Fr. C11C7 (2.8 g) was subjected to a CC over silica gel (200–300 mesh, CH2Cl2/acetone, from 30:1 to 1:1) and separated by CC over silica gel (200–300 mesh, CH2Cl2/MeOH, from 150:1 to 30:1) and then purified by preparative HPLC (MeCN/H2O with 0.1% formic acid, from 42 to 62%, 25 min) to afford compound 20 (5.3 mg).
Fr. C12 (25.1 g) was separated by CC over ODS gel (MeCN/H2O with 0.1% formic acid, from 20% to 95%) to give 18 subfractions of Fr. C12A-Fr. C12R. Compounds 5 (37.3 mg), 6 (8.3 mg), 18 (5.4 mg), 23 (22.8 mg), and 24 (6.6 mg) were obtained by CC over a HW-40F gel (MeOH) and then preparative HPLC (MeCN/H2O with 0.1% formic acid, from 47 to 67%, 47–67%, 45–65%, 46–66%, 46–66%, 25 min, respectively) from Fr. C12J (2.2 g). Fr. C12L (1.1 g) was further purified by CC over Sephadex LH-20 eluted with MeOH, resulting in subfractions Frs. C12L1-7. Fr. C12L5 (281.7 mg) was subjected to separation by chromatography on HW-40F gel eluted with MeOH to afford Fr. C12L5F and Fr. C12L5J. Fr. C12L5F (66.1 mg) was purified by a silica gel column (200–300 mesh) using a gradient solvent system of CH2Cl2/MeOH (200:1–30:1) and then purified by preparative HPLC to afford compound 12 (3.1 mg) (MeCN/H2O with 0.1% formic acid, from 51 to 71%, 25 min). Compound 11 (1.5 mg) was isolated from Fr. C12L5J (25.5 mg) by preparative HPLC (MeCN/H2O with 0.1% formic acid, from 49 to 69%, 25 min). Fr. C12M (2.2 g) was separated by chromatography on Sephadex LH-20 gel eluted with MeOH to afford nine subfractions of Fr. C12M1-Fr. C12M9. Compounds 10 (65.1 mg) and 13 (7.3 mg) were isolated from Fr. C12M6 (665.7 mg) by repeated CC over silica gel (200–300 mesh, PE/acetone, 30:1–1:1) and then preparative HPLC (MeCN/H2O with 0.1% formic acid, from 50 to 70%, 51 to 71%, 25 min, respectively). In a similar way, compound 14 (1.0 mg) was isolated from Fr. C12M6E (104.0 mg) by repeated CC over silica gel (200–300 mesh, PE/acetone, from 30:1 to 1:1) and finally preparative HPLC (MeCN/H2O with 0.1% formic acid, from 52 to 72%, 25 min).
4.3.1. Calligirlin A (7)
4.3.2. Calligirlin B (8)
4.3.3. Calligirlin C (9)
4.3.4. Calligirlin D (10)
4.3.5. Calligirlin E (11)
4.3.6. Calligirlin F (12)
4.3.7. Calligirlin G (13)
4.3.8. Calligirlin H (14)
4.3.9. Calligirlin I (15)
4.3.10. Calligirlin J (16)
4.3.11. Calligirlin K (17)
4.3.12. Calligirlin L (18)
4.3.13. Calligirlin M (19)
4.3.14. Calligirlin N (20)
4.3.15. Calligirlin O (21)
4.3.16. Calligirlin P (22)
4.3.17. Calligirlin Q (23)
White solid; [α + 36 (c 0.1, MeOH); IR (KBr) vmax 3440, 2930, 2860, 1704, 1457, 1384, 1301, 1263, 1181, 1150, 1102, 1073, 1036, 737 cm−1; 1H and 13C NMR, see Table 8; HRESIMS m/z 679.4544 ([M + Na]+, calcd for C40H64O7Na, 679.4450).
4.3.18. Calligirlin R (24)
White solid; [α + 22 (c 0.1, MeOH); IR (KBr) vmax 3459, 2928, 2861, 1713, 1457, 1387, 1301, 1262, 1192, 1146, 1102, 1072, 1037, 737 cm−1; 1H and 13C NMR, see Table 8; HRESIMS m/z 693.4337 ([M + Na]+, calcd for C40H62O8Na, 693.4342).
4.4. Computational Section
The DFT NMR calculations and TDDFT ECD calculations were performed with the Gaussian 16 program [23]. The conformational searching was conducted by the Conflex 8.0 software using the MMFF (Merck Molecular Force Field) force field within an energy window of 5.0 kcal/mol [24]. The conformers with the Boltzmann population above 1.0% were selected for re-optimization at the B3LYP/6-31G(d) level in vacuo. DFT NMR calculations were run at the level of mPW1PW91/6-311G (d,p) with the PCM solvent mode for chloroform. A possible configuration was specified using DP4+ probability analysis [21]. TDDFT ECD calculations were run at the CAM-B3LYP/TZVP level after re-optimization at the M06-2X/6-31G(d) level, both using the SMD solvent model for methanol. Calculated ECD spectra were generated using the SpecDis Version 17.1 [22,25].
4.5. X-Ray Crystallographic Analyses
Crystals of Compounds 1, 2, 5, 7, 10, 11, 12, 13, and 15 were obtained from their methanol or acetone solutions, respectively. Suitable crystals were selected for the X-ray crystallographic analysis. With the use of the Bruker SHELXTL (2014) software package, the structure was settled and refined. Copies of crystallographic data of every crystal can be obtained free of charge via the Internet at www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 9–11 August 2025) or on application to the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [tel: (+44) 1223-336-408; fax: (+44) 1223-336-033; e-mail: deposit@ccdc.cam.ac.uk].
4.5.1. Crystal Data for Compound 1
C40H66O7 (M = 658.92 g/mol): monoclinic, space group C2 (no. 5), a = 13.3789(4) Å, b = 6.2918(2) Å, c = 20.9753(7) Å, β = 101.6040(10)°, V = 1729.56(10) Å3, Z = 2, T = 100 K, μ(Cu Kα) = 0.667 mm−1, Dcalc = 1.265 g/cm3, 13502 reflections measured (4.3° ≤ 2Θ ≤ 148.814°), 3462 unique (Rint = 0.0793, Rsigma = 0.0635) which were used in all calculations. The final R1 was 0.0452 (I > 2σ(I)) and wR2 was 0.1185. Flack parameter: 0.08(11). Crystallographic data for 1 have been deposited at the Cambridge Crystallographic Data Centre with deposit no. CCDC 2389726.
4.5.2. Crystal Data for Compound 2
C40H70O7 (M = 662.96 g/mol): monoclinic, space group C2 (no. 5), a = 12.9964(8) Å, b = 6.2092(4) Å, c = 25.0541(15) Å, β = 96.763(3)°, V = 2007.7(2) Å3, Z = 2, T = 170 K, μ(Cu Kα) = 0.575 mm−1, Dcalc = 1.097 g/cm3, 17974 reflections measured (3.552° ≤ 2Θ ≤ 136.474°), 3673 unique (Rint = 0.0905, Rsigma = 0.0680) which were used in all calculations. The final R1 was 0.0635 (I > 2σ(I)) and wR2 was 0.1774. Flack parameter:0.16. Crystallographic data for 2 have been deposited at the Cambridge Crystallographic Data Centre with deposit no. CCDC 2389913.
4.5.3. Crystal Data for Compound 5
C19H28O2 (M = 288.41 g/mol): orthorhombic, space group P212121 (no. 19), a = 7.4821(2) Å, b = 10.3278(3) Å, c = 20.0847 [18] Å, V = 1552.02(8) Å3, Z = 4, T = 170 K, μ(Cu Kα) = 0.602 mm−1, Dcalc = 1.234 g/cm3, 19921 reflections measured (8.806° ≤ 2Θ ≤ 140.104°), 2942 unique (Rint = 0.0673, Rsigma = 0.0390) which were used in all calculations. The final R1 was 0.0404 (I > 2σ(I)) and wR2 was 0.1038. Flack parameter: 0.04(13). Crystallographic data for 5 have been deposited at the Cambridge Crystallographic Data Centre with deposit no. CCDC 2389917.
4.5.4. Crystal Data for Compound 7
C22H36O4 (M = 364.51 g/mol): monoclinic, space group P21 (no. 4), a = 7.3566(3) Å, b = 19.9404(8) Å, c = 7.4739(3) Å, β = 116.2340(10)°, V = 983.44(7) Å3, Z = 2, T = 150 K, μ(Cu Kα) = 0.654 mm−1, Dcalc = 1.231 g/cm3, 18434 reflections measured (13.206° ≤ 2Θ ≤ 149.058°), 3975 unique (Rint = 0.0681, Rsigma = 0.0487) which were used in all calculations. The final R1 was 0.0444 (I > 2σ(I)) and wR2 was 0.1148. Flack parameter:0.03(10). Crystallographic data for 7 have been deposited at the Cambridge Crystallographic Data Centre with deposit no. CCDC 2389914.
4.5.5. Crystal Data for Compound 10
C20H32O2 (M = 304.45 g/mol): triclinic, space group P1 (no. 1), a = 6.4804(2) Å, b = 7.3872(3) Å, c = 10.3910(4) Å, α = 103.846(2)°, β = 96.885(2)°, γ = 115.760(2)°, V = 420.49(3) Å3, Z = 1, T = 150 K, μ(Cu Kα) = 0.577 mm−1, Dcalc = 1.202 g/cm3, 21250 reflections measured (9.068° ≤ 2Θ ≤ 149.562°), 3284 unique (Rint = 0.0689, Rsigma = 0.0478) which were used in all calculations. The final R1 was 0.0407 (I > 2σ(I)) and wR2 was 0.1080. Flack parameter:0.05(13). Crystallographic data for 10 have been deposited at the Cambridge Crystallographic Data Centre with deposit no. CCDC 2389915.
4.5.6. Crystal Data for Compound 11
C21H30O4 (M = 346.45 g/mol): orthorhombic, space group P212121 (no. 19), a = 6.16210(10) Å, b = 9.2762(2) Å, c = 30.6471(7) Å, V = 1751.81 [18] Å3, Z = 4, T = 150 K, μ(Cu Kα) = 0.713 mm−1, Dcalc = 1.314 g/cm3, 35528 reflections measured (5.768° ≤ 2Θ ≤ 149.2°), 3583 unique (Rint = 0.0922, Rsigma = 0.0445) which were used in all calculations. The final R1 was 0.0404 (I > 2σ(I)) and wR2 was 0.0942. Flack parameter: 0.12(12). Crystallographic data for 11 have been deposited at the Cambridge Crystallographic Data Centre with deposit no. CCDC 2390159.
4.5.7. Crystal Data for Compound 12
C20H30O3 (M = 318.44 g/mol): monoclinic, space group P21 (no. 4), a = 6.41320(10) Å, b = 19.0135(4) Å, c = 7.2649(2) Å, β = 105.8560(10)°, V = 852.16(3) Å3, Z = 2, T = 150.00 K, μ(Cu Kα) = 0.641 mm−1, Dcalc = 1.241 g/cm3, 20082 reflections measured (9.302° ≤ 2Θ ≤ 149.252°), 3438 unique (Rint = 0.0682, Rsigma = 0.0459) which were used in all calculations. The final R1 was 0.0360 (I > 2σ(I)), and wR2 was 0.0943 (all data). Flack parameter: 0.08(10). Crystallographic data for 12 have been deposited at the Cambridge Crystallographic Data Centre with deposit no. CCDC 2389922.
4.5.8. Crystal Data for Compound 13
C20H30O2 (M = 302.44 g/mol): monoclinic, space group P21 (no. 4), a = 6.99180(10) Å, b = 23.8335(4) Å, c = 15.1339(3) Å, β = 91.7220(10)°, V = 2520.76(7) Å3, Z = 6, T = 170 K, μ(Cu Kα) = 0.577 mm−1, Dcalc = 1.195 g/cm3, 49969 reflections measured (5.842° ≤ 2Θ ≤ 149.176°), 10263 unique (Rint = 0.0869, Rsigma = 0.0585) which were used in all calculations. The final R1 was 0.0424 (I > 2σ(I)), and wR2 was 0.1090 (all data). Flack parameter: −0.13(10). Crystallographic data for 13 have been deposited at the Cambridge Crystallographic Data Centre with deposit no. CCDC 2389916.
4.5.9. Crystal Data for Compound 15
C21H38O5 (M = 370.51 g/mol): triclinic, space group P1 (no. 1), a = 6.5343(2) Å, b = 7.1372(2) Å, c = 11.2987(3) Å, α = 84.2530(10)°, β = 82.0570(10)°, γ = 75.3600(10)°, V = 503.76(2) Å3, Z = 1, T = 100 K, μ(Cu Kα) = 0.681 mm−1, Dcalc = 1.221 g/cm3, 17254 reflections measured (7.918° ≤ 2Θ ≤ 148.822°), 3710 unique (Rint = 0.0493, Rsigma = 0.0445) which were used in all calculations. The final R1 was 0.0401 (I > 2σ(I)) and wR2 was 0.1058. Flack parameter: 0.05(8). Crystallographic data for 15 have been deposited at the Cambridge Crystallographic Data Centre with deposit no. CCDC 2389933.
4.6. Pharmacological Activity Assessment
The BV-2 cells (identifier: CSTR:19375.09.3101MOUGNM45) were a gift from Prof. L. Feng (Shanghai Institute of Materia Medica, Chinese Academy of Sciences), which was purchased from the National Collection of Authenticated Cell Cultures (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA), 60 mg/L ampicillin sodium, and 50 mg/L streptomycin sulfate at 37 °C in a humidified incubator with 5% CO2.
The NO production was tested by the Griess reaction. Briefly, a density of 2 × 10 5 cells/mL BV-2 cells was seeded per well in 96-well plates with a volume of 100 μL/well and cultured in a 37 °C constant-temperature incubator containing 5% CO2. After culturing for 24 h, the culture medium of BV-2 cells was replaced with fresh DMEM high-glucose medium containing 10% fetal bovine serum. The BV-2 cells were pretreated with test compounds (10 or 20 μM final concentration) or vehicle for 2 h, followed by LPS (100 ng/mL final concentration) exposure for 24 h. For the measurement of nitrite, 50 μL of culture medium taken from each well and 50 μL of Greiss buffer were mixed and incubated at room temperature for 15 min. The absorbance of each well was measured at 540 nm using a FlexStation 3 Microplate Reader, and the level of nitrite was calculated from a standard curve of sodium nitrite. The NO production in LPS was taken as 100%.
Quantitative real-time PCR (qRT–PCR) was employed to evaluate the mRNA levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α. BV-2 microglial cells were seeded in a 12-well plate at a density of 2 × 105 cells/mL and cultured overnight. The cells were then pretreated with 20 μM of compound 5 or compound 13 for 2 h and exposed to 100 ng/mL of LPS for 6 h. Total RNAs were extracted with Trizol reagent and reverse-transcribed with a PrimeScript RT Reagent Kit (Vazyme, Nanjing, China) to convert to cDNA. qRT-PCR assays were carried out with a real-time PCR detection system (Thermo Fisher Waltham, MA, USA) using a SYBR green kit (Vazyme, Nanjing, China). The gene expression values were normalized to those of GAPDH.
5. Conclusions
A systematic phytochemical investigation of C. giraldii led to the isolation and characterization of 18 new diterpenoids, including eight phyllocladane-type (7–14), eight 3,4-seco phyllocladane-type (15–22), and two unusual phyllocladane-type diterpene dimers (23, 24), along with six known analogues. Through detailed analysis, empirical rules were further improved for distinguishing the phyllocladane-type from ent-kaurane type diterpenoids. Furthermore, anti-inflammatory evaluations revealed that compounds 5 and 13 exhibited significant anti-neuroinflammatory activity by reducing the expression levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in LPS-stimulated BV-2 microglial cells. These findings not only expand the chemical diversity of the genus Callicarpa but also provide potential lead compounds for further development of anti-neuroinflammatory agents.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30071553/s1, Figure S1. The HRESIMS spectrum of compound 7; Figure S2. IR spectrum of compound 7; Figure S3. 1H NMR Spectrum of compound 7 in CDCl3; Figure S4. 13C NMR and DEPT 135 spectrum of compound 7 in CDCl3; Figure S5. 1H-1H COSY Spectrum of compound 7 in CDCl3; Figure S6. HSQC Spectrum of compound 7 in CDCl3; Figure S7. HMBC spectrum of compound 7 in CDCl3; Figure S8. NOESY Spectrum of compound 7 in CDCl3; Figure S9. Two possible conformations of compound 7; Figure S10. DP4+ probability analysis results for compound 7 (7a: rel- 4S, 5R, 8S, 9S, 10R, 13R, 16R). Figure S11. HRESIMS Spectrum of compound 8; Figure S12. IR spectrum of compound 8; Figure S13. 1H NMR Spectrum of compound 8 in CDCl3; Figure S14. 13C NMR and DEPT 135 spectrum of compound 8 in CDCl3; Figure S15. 1H-1H COSY Spectrum of compound 8 in CDCl3; Figure S16. HSQC Spectrum of compound 8 in CDCl3; Figure S17. HMBC Spectrum of compound 8 in CDCl3; Figure S18. NOESY Spectrum of compound 8 in CDCl3; Figure S19. HRESIMS Spectrum of compound 9; Figure S20. IR spectrum of compound 9; Figure S21. 1H NMR Spectrum of compound 9 in CDCl3; Figure S22. 13C NMR and DEPT 135 spectrum of compound 9 in CDCl3; Figure S23. 1H-1H COSY Spectrum of compound 9 in CDCl3; Figure S24. HSQC Spectrum of compound 9 in CDCl3; Figure S25. HMBC Spectrum of compound 9 in CDCl3; Figure S26. NOESY Spectrum of compound 9 in CDCl3; Figure S27. HRESIMS Spectrum of compound 10; Figure S28. IR spectrum of compound 10; Figure S29. 1H NMR Spectrum of compound 10 in CDCl3; Figure S30. 13C NMR and DEPT 135 spectrum of compound 10 in CDCl3; Figure S31. 1H-1H COSY Spectrum of compound 10 in CDCl3; Figure S32. HSQC Spectrum of compound 10 in CDCl3; Figure S33. HMBC Spectrum of compound 10 in CDCl3; Figure S34. NOESY Spectrum of compound 10 in CDCl3; Figure S35. HRESIMS Spectrum of compound 11; Figure S36. IR spectrum of compound 11; Figure S37. 1H NMR Spectrum of compound 11 in CDCl3; Figure S38. 13C NMR and DEPT 135 spectrum of compound 11 in CDCl3; Figure S39. 1H-1H COSY Spectrum of compound 11 in CDCl3; Figure S40. HSQC Spectrum of compound 11 in CDCl3; Figure S41. HMBC Spectrum of compound 11 in CDCl3; Figure S42. NOESY Spectrum of compound 11 in CDCl3; Figure S43. HRESIMS Spectrum of compound 12; Figure S44. IR spectrum of compound 12; Figure S45. 1H NMR Spectrum of compound 12 in CDCl3; Figure S46. 13C NMR and DEPT 135 spectrum of compound 12 in CDCl3; Figure S47. 1H-1H COSY Spectrum of compound 12 in CDCl3; Figure S48. HSQC Spectrum of compound 12 in CDCl3; Figure S49. HMBC Spectrum of compound 12 in CDCl3; Figure S50. NOESY Spectrum of compound 12 in CDCl3; Figure S51. HRESIMS Spectrum of compound 13; Figure S52. IR spectrum of compound 13; Figure S53. 1H NMR Spectrum of compound 13 in CDCl3; Figure S54. 13C NMR and DEPT 135 spectrum of compound 13 in CDCl3; Figure S55. 1H-1H COSY Spectrum of compound 13 in CDCl3; Figure S56. HSQC Spectrum of compound 13 in CDCl3; Figure S57. HMBC Spectrum of compound 13 in CDCl3; Figure S58. NOESY Spectrum of compound 13 in CDCl3; Figure S59. HRESIMS Spectrum of compound 14; Figure S60. IR spectrum of compound 14; Figure S61. 1H NMR Spectrum of compound 14 in CDCl3; Figure S62. 13C NMR and DEPT 135 spectrum of compound 14 in CDCl3; Figure S63. 1H-1H COSY Spectrum of compound 14 in CDCl3; Figure S64. HSQC Spectrum of compound 14 in CDCl3; Figure S65. HMBC Spectrum of compound 14 in CDCl3; Figure S66. NOESY Spectrum of compound 14 in CDCl3; Figure S67. Two possible conformations of compound 14; Figure S68. DP4+ probability analysis result for compound 14 (14a: rel- 5R, 8R, 9S, 10R, 13R, 16S); Figure S69. HRESIMS Spectrum of compound 15; Figure S70. IR spectrum of compound 15; Figure S71. 1H NMR Spectrum of compound 15 in CD3OD; Figure S72. 13C NMR and DEPT 135 spectrum of compound 15 in CD3OD; Figure S73. 1H-1H COSY Spectrum of compound 15in CD3OD; Figure S74. HSQC Spectrum of compound 15 in CD3OD; Figure S75. HMBC Spectrum of compound 15 in CD3OD; Figure S76. NOESY Spectrum of compound 15 in CD3OD; Figure S77. HRESIMS Spectrum of compound 16; Figure S78. IR spectrum of compound 16; Figure S79. 1H NMR Spectrum of compound 16 in CD3OD; Figure S80. 13C NMR and DEPT 135 spectrum of compound 16 in CD3OD; Figure S81. 1H-1H COSY Spectrum of compound 16 in CD3OD; Figure S82. HSQC Spectrum of compound 16 in CD3OD; Figure S83. HMBC Spectrum of compound 16 in CD3OD; Figure S84. NOESY Spectrum of compound 16 in CD3OD; Figure S85. HRESIMS Spectrum of compound 17; Figure S86. IR spectrum of compound 17; Figure S87. 1H NMR Spectrum of compound 17 in CDCl3; Figure S88. 13C NMR and DEPT 135 spectrum of compound 17 in CDCl3; Figure S89. 1H-1H COSY Spectrum of compound 17 in CDCl3; Figure S90. HSQC Spectrum of compound 17 in CDCl3; Figure S91. HMBC Spectrum of compound 17 in CDCl3; Figure S92. NOESY Spectrum of compound 17 in CDCl3; Figure S93. HRESIMS Spectrum of compound 18; Figure S94. IR spectrum of compound 18; Figure S95. 1H NMR Spectrum of compound 18 in CDCl3; Figure S96. 13C NMR and DEPT 135 spectrum of compound 18 in CDCl3; Figure S97. 1H-1H COSY Spectrum of compound 18 in CDCl3; Figure S98. HSQC Spectrum of compound 18 in CDCl3; Figure S99. HMBC Spectrum of compound 18 in CDCl3; Figure S100. NOESY Spectrum of compound 18 in CDCl3; Figure S101. HRESIMS Spectrum of compound 19; Figure S102. IR spectrum of compound 19; Figure S103. 1H NMR Spectrum of compound 19 in CDCl3; Figure S104. 13C NMR and DEPT 135 spectrum of compound 19 in CDCl3; Figure S105. 1H-1H COSY Spectrum of compound 19 in CDCl3; Figure S106. HSQC Spectrum of compound 19 in CDCl3; Figure S107. HMBC Spectrum of compound 19 in CDCl3; Figure S108. NOESY Spectrum of compound 19 in CDCl3; Figure S109. HRESIMS Spectrum of compound 20; Figure S110. IR spectrum of compound 20; Figure S111. 1H NMR Spectrum of compound 20 in CDCl3; Figure S112. 13C NMR and DEPT 135 spectrum of compound 20 in CDCl3; Figure S113. 1H-1H COSY Spectrum of compound 20 in CDCl3; Figure S114. HSQC Spectrum of compound 20 in CDCl3; Figure S115. HMBC Spectrum of compound 20 in CDCl3; Figure S116. NOESY Spectrum of compound 20 in CDCl3; Figure S117. HRESIMS Spectrum of compound 21; Figure S118. IR spectrum of compound 21; Figure S119. 1H NMR Spectrum of compound 21 in CDCl3; Figure S120. 13C NMR and DEPT 135 spectrum of compound 21 in CDCl3; Figure S121. 1H-1H COSY Spectrum of compound 21 in CDCl3; Figure S122. HSQC Spectrum of compound 21 in CDCl3; Figure S123. HMBC Spectrum of compound 21 in CDCl3; Figure S124. NOESY Spectrum of compound 21 in CDCl3; Figure S125. HRESIMS Spectrum of compound 22; Figure S126. IR spectrum of compound 22; Figure S127. 1H NMR Spectrum of compound 22 in CDCl3; Figure S128. 13C NMR and DEPT 135 spectrum of compound 22 in CDCl3; Figure S129. 1H-1H COSY Spectrum of compound 22 in CDCl3; Figure S130. HSQC Spectrum of compound 22 in CDCl3; Figure S131. HMBC Spectrum of compound 22 in CDCl3; Figure S132. NOESY Spectrum of compound 22 in CDCl3; Figure S133. HRESIMS Spectrum of compound 23; Figure S134. IR spectrum of compound 23; Figure S135. 1H NMR Spectrum of compound 23 in CDCl3; Figure S136. 13C NMR and DEPT 135 spectrum of compound 23 in CDCl3; Figure S137. 1H-1H COSY Spectrum of compound 23 in CDCl3; Figure S138. HSQC Spectrum of compound 23 in CDCl3; Figure S139. HMBC Spectrum of compound 23 in CDCl3; Figure S140. NOESY Spectrum of compound 23 in CDCl3; Figure S141. HRESIMS Spectrum of compound 24; Figure S142. IR spectrum of compound 24; Figure S143. 1H NMR Spectrum of compound 24 in CD3OD; Figure S144. 13C NMR and DEPT 135 spectrum of compound 24 in CD3OD; Figure S145. 1H-1H COSY Spectrum of compound 24 in CD3OD; Figure S146. HSQC Spectrum of compound 24 in CD3OD. Figure S147. HMBC Spectrum of compound 24 in CD3OD. Figure S148. NOESY Spectrum of compound 24 in CD3OD.
Author Contributions
X.L.: Investigation, Writing—original draft. Q.G.: Investigation, Writing—original draft. Y.X.: Investigation. J.M.: Investigation. C.T.: Investigation, Writing—review and editing, Validation. C.K.: Resources, Validation. B.H.: Investigation, Writing—review and editing, Validation. S.Y.: Project administration, Supervision. H.Z.: Conceptualization, Supervision. Y.Y.: Conceptualization, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key-Area Research and Development Program of Guangdong Province (2020B0303070002) and the National Key R&D Program’s “Strategic Scientific and Technological Innovation Cooperation” Key Project (2022YFE0203600) released by the Ministry of Science and Technology of China. Financial support from the National Natural Science Foundation of China (82173696, 82373766), Guangdong Basic and Applied Basic Research Foundation (2024A1515011348), and Zhongshan Municipal Bureau of Science and Technology (CXTD2023010) is also acknowledged.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Correction Statement
This article has been republished with a minor correction to the existing affiliation information. This change does not affect the scientific content of the article.
References
- Li, Q.; Shang, K.; Wang, J.; Xu, C.S.; Cai, Z.E.; Yuan, P.; Wang, C.G.; Gu, M.M.; Zhang, Y.; Liao, Z.X. Identification, structural revision and biological evaluation of the phyllocladane-type diterpenoids from Callicarpa longifolia var. floccosa. Tetrahedron 2024, 167, 134304. [Google Scholar] [CrossRef]
- Toyomasu, T.; Niida, R.; Kenmoku, H.; Kanno, Y.; Miura, S.; Nakano, C.; Shiono, Y.; Mitsuhashi, W.; Toshima, H.; Oikawa, H.; et al. Identification of diterpene biosynthetic gene clusters and functional analysis of labdane-related diterpene cyclases in Phomopsis amygdali. Biosci. Biotechnol. Biochem. 2008, 72, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.R.; Shurpali, K.; Gawde, R.L.; Sarkar, D.; Puranik, V.G.; Joshi, S.P. Phyllocladane diterpenes from Anisomeles heyneana. J. Asian Nat. Prod. Res. 2012, 14, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Chang, S.T.; Chang, S.C.; Chang, H.T. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Nat. Prod. Res. 2008, 22, 1085–1093. [Google Scholar] [CrossRef]
- Goel, M.K.; Kukreja, A.K.; Singh, A.K.; Khanuja, S.P.S. In vitro plant growth promoting activity of phyllocladane diterpenoids isolated from Callicarpa macrophylla Vahl. in shoot cultures of Rauwolfia serpentina. Nat. Prod. Commun. 2007, 2, 799–802. [Google Scholar] [CrossRef]
- Manríquez-Torres, J.J.; Hernández-Lepe, M.A.; Chávez-Méndez, J.R.; González-Reyes, S.; Serafín-Higuera, I.R.; Rodríguez-Uribe, G.; Torres-Valencia, J.M. Isolation and cytotoxic activity of phyllocladanes from the roots of Acacia schaffneri (Leguminosae). Molecules 2020, 25, 3944. [Google Scholar] [CrossRef]
- Sun, H.D. Diterpenoid Chemistry; Chemical Industry Press: Beijing, China, 2012; ISBN 9787122121394. [Google Scholar]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China; Science Press (Beijing) & Missouri Botanical Garden Press: Beijing, China, 2011. [Google Scholar]
- Dong, L.; Zhang, L.; Zhang, X.; Liu, M.; Wang, J.; Wang, Y. Two new 3,4-seco-labdane diterpenoids from Callicarpa nudiflora and their inhibitory activities against nitric oxide production. Phytochem. Lett. 2014, 10, 127–131. [Google Scholar] [CrossRef]
- Sun, X.; Liu, F.; Yang, X.; Wang, J.; Dong, B.; Xie, C.; Jin, D.Q.; Zhang, J.; Lee, D.; Ohizumi, Y.; et al. Seco-labdane diterpenoids from the leaves of Callicarpa nudiflora showing nitric oxide inhibitory activity. Phytochemistry 2018, 149, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Z.; Zhu, C.C.; Zhao, Z.X.; Li, X.H.; Xiong, T.Q.; Xia, Y.Y.; Ning, Y. Two new abietane diterpenoids from the caulis and leaves of Callicarpa kochiana. Fitoterapia 2012, 83, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, Y.; Wang, M.; Ren, Q.; Li, S.; Wang, H.; Sun, X.; Jin, D.Q.; Sun, H.; Ohizumi, Y.; et al. Bioactive diterpenoids from the leaves of Callicarpa macrophylla. J. Nat. Prod. 2015, 78, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, S.; Sun, X.; Ma, J.; Liu, F.; Tong, L.; Lee, D.; Ohizumi, Y.; Tuerhong, M.; Guo, Y. Diterpenoids from Callicarpa kwangtungensis and their NO inhibitory effects. Fitoterapia 2016, 113, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, J.; Wang, Q.; Shang, K.; Pu, D.B.; Zhang, R.H.; Li, X.L.; Dai, X.C.; Zhang, X.J.; Xiao, W.L. Clerodane diterpenoids with potential anti-inflammatory activity from the leaves and twigs of Callicarpa cathayana. Chin. J. Nat. Med. 2019, 17, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tang, C.P.; Ke, C.Q.; Shu, R.G.; Ye, Y. 3,4-seco-isopimarane and 3,4-seco-pimarane diterpenoids from Callicarpa nudiflora. Chin. J. Nat. Med. 2021, 19, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.G. Study of chemical constituents of two medical plants of Callicarpa Genus. Ph.D Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2019; pp. 72–77. [Google Scholar]
- Gui, L.; Ralph, M.; Peter, R. Chemical transformations of phyllocladane (=13β-Kaurane) diterpenoids. Helv. Chim. Acta 2003, 86, 420–438. [Google Scholar] [CrossRef]
- Singh, A.K.; Agrawal, P.K. 16α, l7-isopropylidenoI-3-oxo-phyllocladane, a diterpenoid from Callicarpa macrophylla. Phytochemistry 1994, 37, 587–588. [Google Scholar] [CrossRef]
- Shao, Y.; Hu, L.H.; Sim, L.Y.; Goh, S.H. Lignanoids and diterpenoids from Callicarpa furfuraceae. Helv. Chim. Acta 2006, 86, 64–71. [Google Scholar] [CrossRef]
- Bohlmannc, F.; Zderob, C.; Turner, B.L. Guaianolides and heliangolides from Hymenopappus newberryi. Phytochemistry 1984, 23, 1055–1058. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Organomet. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Goto, H.; Obata, S.; Kakayama, N.; Ohta, K. CONFLEX 8, CONFLEX Corporation: Tokyo, Japan, 2017.
- Pescitelli, T.G.; Bruhn, T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).