Abstract
Age-related impaired wounds often become infected with bacteria, leading to substantial mortality and morbidity in the elderly. The decline in androgen levels with increasing age is believed to exacerbate inflammation during wound infections. Despite its well-documented anti-inflammatory activities in wound repair, little is known about the effect of age-related androgen deprivation on bacterial phagocytosis in impaired chronic wounds. The aim of this study was to investigate the effect of age-related testosterone deprivation on the phagocytic functions of THP-1 monocyte-derived macrophages to eliminate Gram-positive and Gram-negative bacteria in vitro. Host–pathogen interaction experiments were conducted to quantify the macrophage-mediated clearance of two common wound-associated bacteria, methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa, under in vitro environments that model testosterone levels representative of those found in elderly males, healthy young adults and testosterone replacement therapy (TRT). Testosterone and its metabolite 5α-dihydrotestosterone (DHT) significantly dampened the macrophage-mediated phagocytosis of both MRSA and P. aeruginosa in a dose-dependent manner (p < 0.05). Blockade of the androgen receptor (AR) using enzalutamide confirmed that testosterone mediates bacterial clearance through binding to the AR. Blocking the conversion of testosterone to DHT through stimulation of macrophages with the 5-α-reductase inhibitor finasteride reversed the testosterone-mediated effects on bacterial clearance, which confirmed that testosterone could potentially dampen the innate phagocytic responses in macrophages through conversion to DHT. Novel findings in this study suggest that the selective manipulation of the AR and/or blockade of testosterone–DHT conversion may provide effective therapeutic treatments to combat wound infections in the elderly.
1. Introduction
Cutaneous wound healing is a dynamic biological process consisting of four sequential phases: hemostasis, inflammation, proliferation and remodeling [1]. The speed and quality of wound healing are influenced by numerous factors, including age [2]. Age-related delayed wounds are marked with an excessive prolonged inflammatory phase, highlighted by an overproduction of pro-inflammatory cytokines and chemokines, nitric oxide (NO) and Reactive Oxygen Species (ROS) by innate immune cells, such as macrophages and neutrophils [1,3]. While inflammatory responses become prolonged and ineffective during ageing, the predisposition for wound infections rises in the elderly, in part due to the delay in wound repair. Moreover, despite causing exaggerated pro-inflammatory environments, ageing processes have been shown to dampen key immunological processes such as phagocytosis, which leads to the development of wound infections and further contributes to the delay in wound repair. Despite the pronounced inflammatory response, evidence suggests that increasing age may result in an impaired ability of innate immune cells to eliminate bacteria from infected wounds [1]. Furthermore, the age-related decline in sex steroid hormones in both genders has been shown to be a key regulator of healing processes. In particular, androgen deficiency with increasing age is a key contributor to the development of impaired chronic wounds in both males and females [4,5,6]. It is suggested that the excessive presence of macrophages in age-related impaired wounds leads to an excessive matrix demolition due to the enhanced production of great amounts of NO and ROS, which can further delay wound healing and cause damage to the wound site [7,8].
Elderly males have been reported to heal slower than elderly females. This observation was associated with prolonged inflammatory responses and delayed matrix deposition and tissue remodeling [4,9]. While estrogen has been shown to accelerate wound healing in both humans and animals [10,11], androgens have been reported to dampen healing processes in both genders [4,5,6]. Additionally, elevated systemic concentrations of testosterone and DHT have been reported to reduce wound repair in human males [12,13]. Castrated mice were also found to heal faster compared to negative control mice [6]. These observations were associated with consistent enhanced inflammatory responses caused by reduced migration of immune cells to the wound site [14].
Impaired wounds often become colonized with a variety of microbial species, such as S. aureus and P. aeruginosa [15]. When the innate immune system functions appropriately, the eradication of wound bacteria is generally successful without medical intervention [16]. However, age-related comorbidities can compromise the host immune system, reducing the clearance of wound bacteria in the absence of effective treatments [17,18,19]. In particular, if wounds become heavily infected with Methicillin-resistant S. aureus, P. aeruginosa or other hospital-acquired pathogens, treatment might require aggressive medication with the last line of defense, antibacterial therapies [20,21]. Indeed, the treatment of MRSA/P. aeruginosa-infected wounds currently represents a significant challenge for modern healthcare organizations [21,22].
Testosterone and DHT signal predominantly through binding to the nuclear androgen receptor (AR) [6]. Despite being the most dominant androgen found in humans, testosterone has a lower binding affinity to the AR compared to DHT as testosterone–AR complexes are less stable compared to the DHT–AR complexes, which makes DHT the most potent androgen compared to testosterone [23]. The role of AR manipulation in wound repair has been well-documented. Stimulation of wound environments with the AR inhibitors was shown to accelerate repair via depression of inflammatory responses [6,14]. However, the effect of androgens and role of AR in inflammatory responses such as bacterial clearance during wound infection have gained little attention to date. Due to the presence of ARs on monocytes and macrophages, testosterone and DHT are suggested to have a direct effect on the innate immune functions of macrophages. Selective androgen receptor modulators (SARMs) such as ostarine are classes of AR ligands that possess strong AR-binding abilities to trigger androgenic effects in a tissue-specific manner. Selective androgen receptor degraders (SARDs) such as enzalutamide are AR-interacting molecules that have a high binding affinity to the AR and act to block tissue-specific androgenic signaling [24,25].
Testosterone is normally converted to its potent metabolite DHT by 5α-reductase, an enzyme produced in abundance in the skin and peripheral tissues [23,26]. Blocking the conversion of testosterone to DHT using 5α-reductase inhibitor has been shown to accelerate wound closure in both genders. However, little is known about the effect of 5α-reductase inhibition on the clearance of bacteria during wound infection. To the authors’ knowledge, novel findings in this study confirm for the first time that the blockade of the AR using enzalutamide and/or inhibition of 5α-reductase using finasteride reduce the phagocytosis of the wound-associated bacteria MRSA and P. aeruginosa by monocyte-derived macrophages. Our findings suggest that the selective inhibition of the AR and/or blockade of DHT production could potentially lead to the development of novel therapeutic strategies that combat wound infections in the elderly.
2. Materials and Methods
2.1. Cell Culture
THP-1 monocytes (ATCC, UK) were grown in RPMI-1640 medium containing 2% penicillin streptomycin and 10% Fetal Bovine Serum (Lonza, Slough, UK). Cells were resuspended in fresh complete RPMI-1640 medium (CM) at a density of 0.5 × 106 cells/mL twice a week. Cell viability was evaluated using 0.4% sterile-filtered trypan blue at a 1:1 ratio to monocyte suspension. An ATC10 automated cell counter (Bio-Rad, Hercules, CA, USA) was used to count the cells, with cell viability exceeding 90% for all experimental procedures.
2.2. Differentiation of Monocytes into Macrophages In Vitro
The THP-1 monocytes were incubated with 100 ng/mL phorbol 12-myristate 13-acetate (PMA) for 24 h at 37 °C and 5% CO2 in order to produce macrophage-like cells in vitro (Sigma Aldrich, Gillingham, Dorset, UK). The PMA-treated cells were washed twice with CM and allowed to settle in fresh PMA-free CM for an additional 48 h.
2.3. Flow Cytometry Analysis
The conversion of THP-1 monocytes into macrophage-like cells was demonstrated by flow cytometry via measurement of the macrophage-specific cell surface marker CD11c. The monocytes were seeded at 1 × 105 cells/mL and stimulated with PMA (100 ng/mL) as described above. Negative control (NC) cells consisting of monocytes incubated with fresh PMA-free CM were also seeded at a concentration of 1 × 105 cells/mL and incubated at 37 °C and 5% CO2 for 24 h. Trypsin EDTA (Lonza, UK) was added to the adherent PMA-treated cells prior to incubation for 5 min at 37 °C and 5% CO2. Detached cells were transferred into sterile Eppendorf tubes and centrifuged at 500× g for 7 min before resuspension in fresh PMA-free CM. The NC monocytes were also centrifuged at 500× g for 7 min and resuspended in fresh CM. Cells were fixed by incubation with 4% paraformaldehyde (Sigma-Aldrich, UK) for 20 min at room temperature (RT). Fixed cells were then washed with PBS and centrifuged at 500× g for 7 min. All cells were subsequently fixed with FITC-conjugated anti-human CD11c antibody diluted at 1:40 in 10% FBS for 30 min at RT following the manufacturer’s instructions (BioLegend, London, Greenwood Place UK). Stained NC and PMA-treated cells were washed twice with PBS prior to resuspension in PBS. The expression of the macrophage-specific surface marker CD11c was assessed on 10,000 live individual cells using a BD Accuri C6F1 cytometer (BD Biosciences, Bedford, MA, USA). The proportion of CD11c+ cells (%) and median fluorescence intensity (MFI) relative to NC monocytes were calculated. Results were analyzed using the FlowJo software (version 10.9.0).
2.4. Preparation of MRSA and P. aeruginosa Cultures
MRSA (strain F-182) and P. aeruginosa (strain PAO1) (ATCC Teddington, UK) were inoculated on nutrient agar (NA) plates at 37 °C for 18–24 h. Single bacterial colonies were inoculated into nutrient broth (NB) on an orbital shaker at 37 °C on for 18–24 h. A spectrophotometer was used to determine the concentration of MRSA and P. aeruginosa suspensions by measuring the optical density (OD) at 600 nm. A density of 1 × 105 CFU/mL of bacterial suspension was used for all experimental purposes.
2.5. Host–Pathogen Interaction Assays
The THP-1 monocyte-derived macrophages were seeded in CM at a concentration of 1 × 105 cells/mL in 12-well plates. The CM was discarded, and the cells were either treated with different testosterone or DHT concentrations (1 × 10−8 M, 1 × 10−7 M, 1 × 10−6 M and 1 × 10−5 M) in antibiotic-free RPMI-1640 medium for 24 h. NC macrophages consisting of cells incubated in antibiotic-free RPMI-1640 medium were also prepared and incubated for 24 h at 37 °C and 5% CO2. Supernatants were collected from all wells prior to incubating the macrophages with 1 × 104 CFU of MRSA or P. aeruginosa for 2 h at 37 °C and 5% CO2 to allow macrophage–bacteria interactions to occur. Subsequent to the 2 h host–pathogen interaction incubation, all macrophages were detached by incubation with trypsin EDTA for 5 min at 37 °C and 5% CO2. The trypsin was then neutralized with RPMI-1640 medium prior to inoculating the content of all wells into NA plates ensuring that macrophages are mechanically lysed [27,28]. The NA plates were then incubated at 37 °C overnight prior to counting the number of bacterial CFUs grown on the agar plates. The bacterial recovery in all wells was calculated following the period of macrophage–bacteria interactions, taking into account the following: (a) the number of bacterial cells that survived internalization by macrophages, and (b) the number of internalized bacterial cells that survived killing by macrophages.
2.6. Gentamicin Protection Assay
THP-1 monocytes were cultured inside a 48-well plate prior to differentiation into macrophages as described above. The PMA-differentiated cells were treated with testosterone (1 × 10−8 M, 1 × 10−7 M, 1 × 10−6 M and 1 h × 10−5 M) prior to incubation with MRSA or P. aeruginosa at a ratio of 10:1 (macrophage:bacteria) for 2 h as described above. A gentamicin protection assay was conducted according to methodologies described by Elsinghorst [29]. After the 2 h host–pathogen interactions, supernatants were collected from all wells prior to treatment with 0.1% Triton X-100 (Lonza, UK) for 10 min at 37 °C to detach the membrane-bound bacterial cells. The supernatants were collected, and the cells were treated with 50 µg/mL gentamicin in antibiotic-free media (Sigma-Aldrich, UK) for 30 min to eliminate extracellular and membrane-bound MRSA and P. aeruginosa colonies. The supernatants were removed prior to washing the macrophages with PBS. The wells were then incubated with Trypsin EDTA for 5 min at 37 °C and 5% CO2 to allow macrophage detachment. The trypsin was neutralized using antibiotic-free medium prior to cell collection. The macrophages and previously collected bacterial supernatants were plated into NA plates and incubated at 37 °C for 18–24 h to measure the MRSA and P. aeruginosa recovery (CFU/mL).
2.7. Androgen Receptor Stimulation/Blockade and 5α-Reductase Inhibition
THP-1 macrophages were grown at a density of 1 × 105 cells/mL prior to treatment with testosterone (1 × 10−6 M), the AR receptor agonist ostarine (1 × 10−6 M) or the AR antagonist enzalutamide (1 × 10−6 M) for 24 h as described above. Additional replicate macrophages (n = 6) were pre-treated with testosterone (1 × 10−6 M) for 24 h prior to incubation with finasteride (1 × 10−6 M) for a further 24 h at 37 °C and 5% CO2. The macrophages were subsequently infected by MRSA/P. aeruginosa for 2 h to allow host–pathogen interactions to occur. The bacterial recovery (CFU/mL) was determined as described above.
2.8. Statistical Analysis
Statistical analysis was conducted using IBM SPSS (Version 25). Statistical differences between treatments were determined using one-way ANOVA for parametric data or Mann–Whitney U tests for non-parametric data. A p value < 0.05 indicated significant differences between treatment groups.
3. Results
3.1. PMA Induces the Differentiation of Monocytes into Macrophages In Vitro
Prior to investigating the effect of testosterone and DHT on the innate immune functions of macrophages, it was important to produce a successful in vitro model of monocyte-derived macrophages. The conversion of THP-1 monocytes into macrophages was evaluated by flow cytometry through the detection of the macrophage-specific cell surface marker CD11c. The PMA-stimulated cells expressed elevated levels of CD11c (98.3%), while the NC THP-1 monocytes expressed negligible levels of CD11c (0.56%). PMA stimulation caused a significant increase in the MFI for CD11c in comparison with untreated NC monocytes (p < 0.05). These results confirm that PMA effectively converted the monocytic population into resting macrophages in vitro (Figure 1).
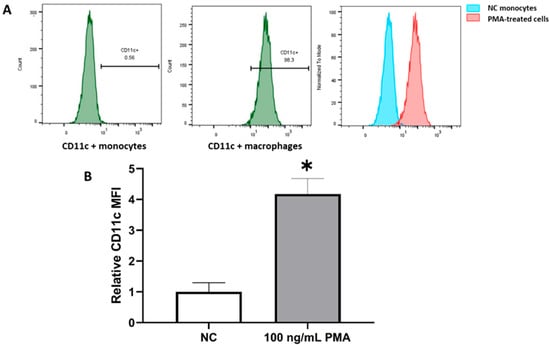
Figure 1.
PMA induces the differentiation of THP-1 monocytes into macrophage-like cells in vitro. The PMA-treated cells expressed high levels of CD11c (98.3%) compared to untreated NC monocytes (A). The MFI for CD11c was significantly elevated in PMA-treated monocytes in comparison with undifferentiated NC cells (B). Results are presented as the average of n = 4 experiments. * Designates significant differences in MFI of CD11b+ macrophages compared to CD11b+ monocytes. Error bars represent the standard error of the mean (StEM).
3.2. Testosterone Reduces the Uptake of MRSA and P. aeruginosa by THP-1 Macrophages In Vitro
The monocyte-derived macrophages were treated with testosterone at concentrations representative of testosterone deprivation (1 × 10−8 M), physiological levels found in healthy men (1 × 10−7 M), testosterone levels found in youth (1 × 10−6 M) and testosterone replacement therapy (TRT) levels (1 × 10−5 M) [30,31,32]. NC samples consisted of macrophages treated with 0 M testosterone. Cells were incubated with MRSA or P. aeruginosa for 2 h to allow host–pathogen interactions to happen. The bacterial recovery was measured by calculating the quantity of bacteria that survived the encounter with macrophages. This includes (a) bacterial cells that survived engulfment by macrophages and (b) bacterial cells that were engulfed by macrophages but survived intracellular killing.
Testosterone deprivation did not have any significant effect on the internalization of both MRSA and P. aeruginosa by macrophages compared to untreated NC cells. However, increasing testosterone concentrations resulted in a significant dose-dependent increase in the recovery of both MRSA and P. aeruginosa in comparison with NC samples (Figure 2).
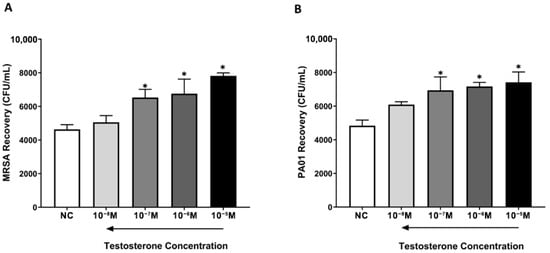
Figure 2.
Testosterone reduces the internalization of MRSA and P. aeruginosa by THP-1 monocyte-derived macrophages. NC macrophages and testosterone-treated macrophages were incubated with MRSA or P. aeruginosa for 2 h prior to growing the content of wells on NA plates. The number of recovered bacterial colonies was measured in all treatment groups. Testosterone significantly increased the recovery of MRSA (A) and P. aeruginosa (B) colonies in a concentration-dependent manner compared to untreated NC macrophages. Data are presented as the average of n = 6 experiments. * Indicates significant differences (p < 0.05) in bacterial recovery between treatments. Error bars represent the StEM.
3.3. Testosterone Dampens the Killing of MRSA and P. aeruginosa by Monocyte-Derived Macrophages In Vitro
A gentamicin protection assay was conducted to assess the effect of testosterone on the killing of MRSA and P. aeruginosa by monocyte-derived macrophages. Gentamicin was used to eliminate bacterial cells that were not internalized by macrophages (i.e., extracellular and membrane-bound bacteria) after the 2 h period of host–pathogen interactions. Macrophages were subsequently lysed to recover internalized bacteria. Gentamicin is unable to pass across the host cell membrane, so only bacteria that were successfully internalized but remained viable within phagocytes at the end of the host–pathogen interaction were recovered [29,33,34,35].
Although testosterone deprivation (1 × 10−8 M) had no significant impact on the killing of internalized MRSA and P. aeruginosa by macrophages (p > 0.05), physiological and supraphysiological testosterone levels significantly increased the number of viable internalized MRSA and P. aeruginosa by macrophages in a concentration-dependent manner compared to untreated NC macrophages (p < 0.05). These results confirm that testosterone not only reduced the internalization of MRSA and P. aeruginosa but diminished the phagocytic ability of macrophages to kill both bacterial species (Figure 3).
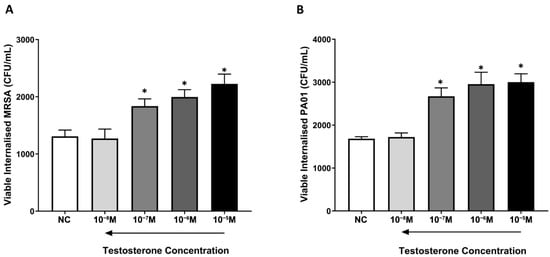
Figure 3.
Testosterone dampens the killing of MRSA and P. aeruginosa by THP-1 monocyte-derived macrophages. NC macrophages and testosterone-treated macrophages were incubated with MRSA or P. aeruginosa for 2 h prior to treating cells with gentamicin to eradicate extracellular and membrane-bound bacterial cells. The content of wells in all treatment groups was plated into NA plates prior to measuring the MRSA and P. aeruginosa recovery. Physiological and supraphysiological testosterone levels significantly reduced the phagocytosis of MRSA (A) and P. aeruginosa (B) colonies in a concentration-dependent manner compared to untreated NC macrophages. Data are presented as the average of n = 6 experiments. * Indicates significant differences (p < 0.05) in bacterial recovery between treatments. Error bars represent the StEM.
3.4. Inhibition of the Androgen Receptor Reverses the Testosterone-Mediated Decline in the Phagocytic Functions of Macrophages
The precise role of the AR on the testosterone-mediated reduction in bacterial phagocytosis by macrophages was elucidated in this study. To investigate the potential effect of AR stimulation/blockade on the phagocytosis of MRSA and P. aeruginosa, different sets of monocyte-derived macrophages were pre-treated with either supraphysiological testosterone (1 × 10−5 M), the AR modulator ostarine (1 × 10−6 M) or the AR inhibitor enzalutamide (1 × 10−6 M) for 24 h prior to incubation with MRSA or P. aeruginosa for 2 h at 37 °C and 5% CO2. Bacterial survival was measured after the host–pathogen interaction assays as described above.
Similar to the previously reported results, testosterone significantly increased MRSA and P. aeruginosa recovery compared to NC cells. The selective activation of the AR with ostarine mirrored the effects of testosterone on bacterial clearance by significantly increasing the recovery of MRSA and P. aeruginosa in comparison with the untreated NC macrophages (p < 0.05). Intriguingly, the selective AR antagonist enzalutamide reversed the testosterone-mediated decline in the phagocytosis of both MRSA and P. aeruginosa by macrophages, causing a significant reduction (p > 0.05) in the number of recovered MRSA and P. aeruginosa colonies compared to untreated NC macrophages or macrophages treated with testosterone/ostarine (p < 0.05). These findings confirm for the first time that AR inhibition enhances the phagocytosis of major wound-associated bacteria by in vitro macrophages (Figure 4).
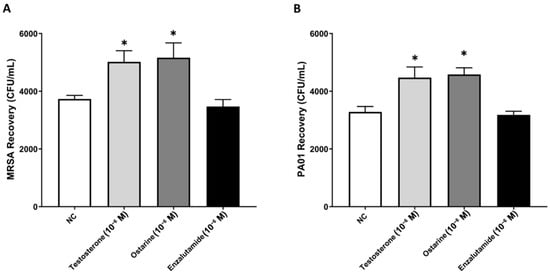
Figure 4.
Blockade of the AR enhances the phagocytosis of MRSA and P. aeruginosa by in vitro macrophages. Stimulation of cells with ostarine mirrored the testosterone-mediated effects on the phagocytic functions of macrophages by significantly increasing the recovery of MRSA (A) and P. aeruginosa (B) colonies compared to untreated NC cells. In contrast, blockade of the AR with enzalutamide significantly reduced the recovery of MRSA (A) and P. aeruginosa (B) compared to estrogen/astarine-treated cells. Data are presented as an average of six independent experiments (n = 6). * Indicates significant differences (p < 0.05) in MRSA (A) and P. aeruginosa (B) recovery between treatments. Error bars represent the StEM.
3.5. The 5-α-Reductase Inhibitor Finasteride Enhances the Phagocytic Functions of Macrophages In Vitro
5α-dihydrotestosterone (DHT), the most potent androgen, signals though binding to the nuclear AR. DHT strongly binds to the AR as DHT-AR complexes are known to be more stable compared to testosterone–AR complexes. Therefore, it is suggested that the testosterone-mediated reduction in wound-healing processes is associated with its conversion to DHT. Finasteride is a well-documented 5-α-reductase inhibitor that blocks the conversion of testosterone to its most potent metabolite DHT [36]. In order to better understand the mechanism by which testosterone inhibits the innate immune responses in macrophages, host–pathogen interaction experiments were repeated using testosterone/DHT-treated and finasteride-treated cells prior to measuring bacterial survival as described above.
As expected, treating cells with testosterone or DHT significantly reduced the phagocytosis of both MRSA and P. aeruginosa in comparison with untreated NC cells. This was illustrated in the significant increase in bacterial recovery observed with both treatments compared to untreated NC samples. Intriguingly, finasteride reversed the testosterone/DHT-mediated effect on the clearance of bacteria by significantly reducing the recovery of MRSA and P. aeruginosa compared to testosterone/DHT-treated cells (p < 0.05) (Figure 5). All together, these novel findings suggest that testosterone dampens the innate phagocytic functions of macrophages through binding to the AR and metabolic conversion to DHT.
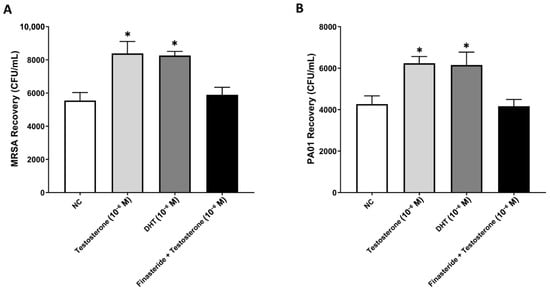
Figure 5.
Inhibition of 5-α-reductase improves the clearance of MRSA and P. aeruginosa by monocyte-derived macrophages. Blocking the conversion of testosterone to DHT through stimulation of macrophages with finasteride significantly reduced the recovery of MRSA (A) and P. aeruginosa (B) compared to testosterone/DHT-treated cells. Data are presented as an average of four independent experiments (n = 4). * Indicates significant differences (p < 0.05) between treatment groups. Error bars represent the StEM.
4. Discussion
Sex steroid hormones play a key modulatory role in wound healing. The age-related decline in circulating and peripheral androgen and estrogen levels is linked with dysregulated wound repair in the elderly [1]. While estrogens have been reported to accelerate wound healing in humans and animals, androgens such as testosterone and DHT have been shown to dampen wound repair in mice [6,37,38]. A study conducted by Gilliver, Ashworth [14] reported that castration of healthy male mice resulted in better healing outcomes compared with non-castrated controls. This observation was associated with reduced inflammatory responses in impaired wounds [14].
Testosterone and DHT have been shown to modulate inflammation, the major physiological process that contributes to the development of chronic wounds [6]. Both androgens have also been shown to boost the later stages of wound repair, including proliferation and collagen production [6]. Despite their well-documented influence on the inflammatory phase of wound repair, the effect of testosterone and DHT on the phagocytosis of bacteria by innate immune cells during wound infection has not been fully elucidated to date. Moreover, it is unclear if the age-related decline in androgen levels contributes to the development and/or exacerbation of wound infections.
Monocytes are the main reservoir of myeloid precursors for the generation of tissue macrophages and dendritic cells [39]. An in vitro model of THP-1 monocyte-derived macrophages was generated in this study. PMA is a well-documented chemical agent that is commonly used to stimulate the differentiation of monocytes into macrophages in vitro [40]. Flow cytometry data in this study demonstrate that PMA at a concentration of 100 ng/mL triggers the conversion of THP-1 monocytes into macrophages. The significantly higher expression of the macrophage-specific cell surface marker, CD11c, in PMA-treated cells compared to untreated monocytes confirmed the phenotypic differentiation of monocytes into macrophages. These results are supported by several studies reporting that PMA-stimulated monocytic cell lines express elevated levels of CD11c, CD11b and protein kinase-C (PKC) enzymes [41,42]. In addition, PMA-treated monocytes were reported to upregulate the levels of cell adhesion molecules such as the beta-2integrins (CD18, CD11a, CD11b and CD11c), which increase the adherence capabilities of cells in vitro [41]. PMA has also been reported to trigger phospholipid- and calcium-dependent isoforms of PKC and encourage cyclic AMP metabolism, which initiates the maturation of monocytes into macrophages [43].
Despite its well-documented influence on the inflammatory phase in wound repair, the effect of testosterone on the phagocytosis of wound-associated pathogens during wound infection remains unclear. Sex steroid hormones, particularly androgens, at the tested concentrations in this study, have been shown to have no significant influence on bacterial growth [27,44]. Results in this study demonstrated that testosterone and DHT reduce the ability of macrophages to engulf MRSA and P. aeruginosa in a dose-dependent manner compared with untreated controls. To further assess the effect of testosterone on bacterial killing, a gentamycin protection assay was conducted. Gentamicin is commonly used in host–pathogen interaction studies due to its ability to eradicate extracellular bacteria without affecting intracellular bacteria. This is mainly due to gentamicin’s poor capability to penetrate the cell membrane of macrophages and neutrophils [29]. Intriguingly, stimulating macrophages with increasing physiological and supraphysiological testosterone concentrations significantly reduced the number of engulfed viable intracellular MRSA and P. aeruginosa colonies compared to untreated NC cells. This demonstrates that testosterone reduces the clearance of internalized bacteria, not just their engulfment by macrophages.
Our findings are supported by several research studies highlighting the influence of androgens on innate immunity. Gomez et al. [39] studied the effect of four distinct androgens on the expression of FcγR in splenic mice macrophages. Both testosterone and DHT were shown to impair the clearance of IgG-sensitized macrophages by reducing the expression of FcγR1 and FcγR2 in splenic macrophages. Interestingly, DHT was more effective than testosterone [45]. Results in the present study also report that DHT mimics the actions of testosterone on the phagocytosis of bacteria by in vitro macrophages. It is well-established that androgens and estrogens are key regulators of wound-repair processes, including phagocytosis during infection [46]. While males commonly display weaker innate immune responses than females following infection, it is now well-accepted that males exhibit a higher susceptibility to microbial sepsis than their female counterparts [47,48]. Testosterone has also been shown to possess immunosuppressive effects on innate and adaptive immune responses, thus increasing susceptibility to S. aureus infection, which further supports our findings [49].
Males are reported to heal slower than females. They are also more predisposed to developing sepsis and multiple-organ failure subsequent to skin and soft tissue hemorrhagic damage, in part due to defective immune responses via abnormal activation of neutrophils [4,50,51]. Testosterone’s immunosuppressive effects on innate immune functions have also been observed in parasitic infections where elevated testosterone levels were reported to dampen immune responses and increased parasitic loads in men [52,53,54]. Furthermore, it is commonly accepted that females have a lower risk of developing bacterial, parasitic and viral infections compared to males [55,56,57,58]. However, the underlying mechanisms responsible for these gender-differentiated immune responses remain unclear. Testosterone and DHT were shown to reduce the production of pro-inflammatory cytokines, including TNF-α in murine monocytes and macrophages. Lai et al. [59] demonstrated that LPS induced the release of TNF-α by phagocytic macrophages and that this effect was reversed in AR-KO macrophages [59]. Similar observations were reported by D’agostino et al., [60] where testosterone was shown to reduce the expression of TNF-α in the murine macrophage cell line J774 [60]. Testosterone was also reported to decrease the LPS-induced NO levels in RAW 264.7 macrophages [61]. Several research studies also reported that testosterone had no beneficial impact on the migration of human monocytes and their phagocytic functions [62,63,64,65].
The effect of AR manipulations on inflammatory responses, such as bacterial clearance during wound infection, have gained little attention to date. AR agonists are AR-interacting molecules that have the ability to bind to the AR and achieve optimum androgen-signaling mechanisms to function as agonists of androgen in a tissue-specific manner [66,67]. AR antagonists also known as anti-androgens are molecules that have anti-androgenic affects in castrated animals [68]. Ostarine and enzalutamide are compounds commonly used to treat several AR-mediated diseases (e.g., prostate cancer) due to their tissue-specific responses [69]. Ostarine was used in this study to investigate the role of AR in the testosterone-mediated stimulation of bacterial clearance by monocyte-derived macrophages. The selective agonism of the AR with ostarine significantly reduced the bacterial clearance of both MRSA and P. aeruginosa in comparison with the untreated macrophages. Enzalutamide was used to block the binding of testosterone and/or DHT to the AR with an aim to investigate the effects of AR suppression in the testosterone/DHT-mediated phagocytosis of bacteria [69]. The selective antagonism of the AR using enzalutamide reversed the testosterone/ostarine-mediated deterioration in bacterial clearance by macrophages. Together, these results confirm that testosterone diminishes the phagocytic functions of macrophages through binding to the AR. To further elucidate the mechanistic pathways involved in the enzalutamide-mediated increase in phagocytosis, further experiments are warranted to examine the synergistic effect of testosterone–enzalutamide on the phagocytosis of MRSA and P. aeruginosa by in vitro and ex vivo macrophages. AR blockade in human macrophages has been shown to enhance the inflammasome-mediated phagocytosis of cancer cells in advanced prostate cancer [70]. siRNA knock down of AR was also shown to diminish chemotaxis of THP-1 monocytes in mice [71]. Although AR-KO mice were shown to express lower neutrophil counts compared to their wildtype counterparts, the cellular phagocytic abilities and respiratory burst reactions in mice remained intact post-AR knockdown [72].
In peripheral tissues, testosterone is converted to DHT through the action of 5α-reductase. Hindering the testosterone–DHT conversion using 5α-reductase was shown to enhance wound repair in mice [23,26]. However, little is known about the role of this metabolic conversion on innate immune responses during wound infection. Finasteride was utilized in this study to block the conversion of testosterone to its potent metabolite DHT in order to assess the potential impact of this enzymatic conversion on the phagocytosis of bacteria by macrophages.
Results in this study demonstrated that finasteride significantly enhanced the macrophage-mediated phagocytosis of both MRSA and P. aeruginosa in comparison with NC macrophages or testosterone/DHT-treated macrophages. This suggests that testosterone dampens the innate phagocytic functions of macrophages through metabolic conversion to DHT. Several mice gonadectomy studies have investigated the impact of androgens in microbial infections. Castration of male mice resulted in the production of higher levels of pro-inflammatory cytokines, including IL-12, IL-17, TNF-α, IFN γ and iNOS compared to non-castrated controls during tuberculosis infection [73]. In contrast, ovariectomized females did not display any differences in innate immune defenses during lung infection [74]. This suggests that androgens, particularly testosterone, could contribute to increased male vulnerability to lung infections such as tuberculosis.
DHT has been shown to enhance the alternative activations of macrophages, resulting in the production of anti-inflammatory M2 macrophages through binding to the AR [75]. Our results support these reports as we speculate that the testosterone/DHT-mediated decline in the phagocytosis of bacteria could be attributed to a possible androgen-mediated switch of macrophages to an M2 phenotype that is known to be less phagocytic and more regenerative. Further research studies investigating the precise effect of androgens on the polarization of macrophages are warranted.
The selective inhibition of AR with molecules such as enzalutamide and/or specific blockade of the testosterone–DHT conversion in phagocytes boosts their phagocytic innate immune functions, which may potentially lead to the development of effective therapeutic options to treat wound infections in the elderly with less reliance on antibiotics. Further studies are warranted to elucidate the precise molecular pathways involved in promoting the AR-mediated phagocytosis of bacteria during wound infections. Future in vivo experiments are also required to demonstrate the effects of AR manipulation and testosterone–DHT conversion on infected, impaired wounds.
Author Contributions
A.B. and M.E.M. conducted the experimental work, analyzed and interpreted the data and prepared the paper draft. J.A. contributed to the conception and design of the study. A.F., J.M., C.O. and A.M. contributed to data analysis and the preparation and revision of the paper. M.E.M. and J.A. were involved with all aspects of the work, including conception/design of the experiments, supervising experimental work, analyzing and interpreting the data and writing and revising the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Edge Hill University’s Research Investment Fund.
Data Availability Statement
The data presented in this paper mainly consist of flow cytometry histograms and CFU counts, which are displayed on the figures. Additional supplementary data can be accessed upon request from the corresponding author(s).
Acknowledgments
The authors would like to thank Manchester Metropolitan University and Edge Hill University for supporting this research study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- El Mohtadi, M.; Whitehead, K.; Dempsey-Hibbert, N.; Belboul, A.; Ashworth, J. Estrogen deficiency–a central paradigm in age-related impaired healing? EXCLI J. 2021, 20, 99. [Google Scholar] [PubMed]
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Fimmel, S.; Zouboulis, C. Influence of physiological androgen levels on wound healing and immune status in men. Aging Male 2005, 8, 166–174. [Google Scholar] [CrossRef]
- Toraldo, G.; Bhasin, S.; Bakhit, M.; Guo, W.; Serra, C.; Safer, J.D.; Bhawan, J.; Jasuja, R. Topical androgen antagonism promotes cutaneous wound healing without systemic androgen deprivation by blocking β-catenin nuclear translocation and cross-talk with TGF-β signaling in keratinocytes. Wound Repair Regen. 2012, 20, 61–73. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Mills, S.J. Androgen receptor–mediated inhibition of cutaneous wound healing. J. Clin. Investig. 2002, 110, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Soneja, A.; Drews, M.; Malinski, T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol. Rep. 2005, 57, 108. [Google Scholar]
- Witte, M.B.; Barbul, A. Role of nitric oxide in wound repair. Am. J. Surg. 2002, 183, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Gilliver, S.C.; Ruckshanthi, J.P.; Hardman, M.J.; Nakayama, T.; Ashcroft, G.S. Sex dimorphism in wound healing: The roles of sex steroids and macrophage migration inhibitory factor. Endocrinology 2008, 149, 5747–5757. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Greenwell-Wild, T.; Horan, M.A.; Wahl, S.M.; Ferguson, M.W. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am. J. Pathol. 1999, 155, 1137–1146. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Yang, X.; Glick, A.B.; Weinstein, M.; Letterio, J.J.; Mizel, D.E.; Anzano, M.; Greenwell-Wild, T.; Wahl, S.M.; Deng, C. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1999, 1, 260–266. [Google Scholar] [CrossRef]
- Gilliver, S.C. Differential Effects of Testosterone and 5α-DHT upon Cutaneous Wound Healing. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2005. [Google Scholar]
- Gilliver, S.C.; Ruckshanthi, J.P.; Hardman, M.J.; Zeef, L.; Ashcroft, G.S. 5α-Dihydrotestosterone (DHT) retards wound closure by inhibiting re-epithelialization. J. Pathol. 2009, 217, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Gilliver, S.C.; Ashworth, J.J.; Mills, S.J.; Hardman, M.J.; Ashcroft, G.S. Androgens modulate the inflammatory response during acute wound healing. J. Cell Sci. 2006, 119, 722–732. [Google Scholar] [CrossRef]
- Gomersall, J.; Mortimer, K.; Hassan, D.; Whitehead, K.A.; Slate, A.J.; Ryder, S.F.; Chambers, L.E.; El Mohtadi, M.; Shokrollahi, K. Ten-year analysis of bacterial colonisation and outcomes of major burn patients with a focus on Pseudomonas aeruginosa. Microorganisms 2023, 12, 42. [Google Scholar] [CrossRef]
- El Mohtadi, M.; Pilkington, L.; Liauw, C.M.; Ashworth, J.J.; Dempsey-Hibbert, N.; Belboul, A.; Whitehead, K.A. Differential engulfment of Staphylococcus aureus and Pseudomonas aeruginosa by monocyte-derived macrophages is associated with altered phagocyte biochemistry and morphology. EXCLI J. 2020, 19, 1372. [Google Scholar]
- Burnet, M.; Metcalf, D.G.; Milo, S.; Gamerith, C.; Heinzle, A.; Sigl, E.; Eitel, K.; Haalboom, M.; Bowler, P.G. A host-directed approach to the detection of infection in hard-to-heal wounds. Diagnostics 2022, 12, 2408. [Google Scholar] [CrossRef] [PubMed]
- Hornef, M.W.; Wick, M.J.; Rhen, M.; Normark, S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat. Immunol. 2002, 3, 1033–1040. [Google Scholar] [CrossRef]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.; Falanga, V.; Fife, C.; Gardner, S. Chronic wound repair and healing in older adults: Current status and future research. Wound Repair Regen. 2015, 23, 1–13. [Google Scholar] [CrossRef]
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent developments in methicillin-resistant Staphylococcus aureus (MRSA) treatment: A review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef]
- Agyeman, W.Y.; Bisht, A.; Gopinath, A.; Cheema, A.H.; Chaludiya, K.; Khalid, M.; Nwosu, M.; Konka, S.; Khan, S. A systematic review of antibiotic resistance trends and treatment options for hospital-acquired multidrug-resistant infections. Cureus 2022, 14, e29956. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, F.; Alzahrani, R.R.; Alsaadi, A.; Alrfaei, B.M.; Yassin, A.E.B.; Alkhulaifi, M.M.; Halwani, M. An explorative review on advanced approaches to overcome bacterial resistance by curbing bacterial biofilm formation. Infect. Drug Resist. 2023, 16, 19–49. [Google Scholar] [CrossRef] [PubMed]
- Kinter, K.J.; Anekar, A.A. Biochemistry, Dihydrotestosterone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Negro-Vilar, A. Selective androgen receptor modulators (SARMs): A novel approach to androgen therapy for the new millennium. J. Clin. Endocrinol. Metab. 1999, 84, 3459–3462. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S. Selective androgen receptor modulators as function promoting therapies. J. Frailty Aging 2015, 4, 121. [Google Scholar] [CrossRef]
- Handelsman, D.J. Androgen Physiology, Pharmacology, Use and Misuse. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- El Mohtadi, M. Effect of Estrogen on Host-Pathogen Interactions in ex vivo and in vitro Models of the Inflammatory Phase of Age-Related Impaired Healing. Ph.D. Thesis, Manchester Metropolitan University, Manchester, UK, 2019. [Google Scholar]
- Belboul, A. Effect of Hormone-Driven Ageing on Inflammatory Cell Clearance of Bacteria Under Hyperglycemic Conditions. Ph.D. Thesis, Manchester Metropolitan University, Manchester, UK, 2024. [Google Scholar]
- Elsinghorst, E.A. Measurement of invasion by gentamicin resistance. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1994; Volume 236, pp. 405–420. [Google Scholar]
- Bain, J. The many faces of testosterone. Clin. Interv. Aging 2007, 2, 567–576. [Google Scholar] [CrossRef]
- Gardner, I.H.; Safer, J.D. Progress on the road to better medical care for transgender patients. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Nassau, D.E.; Patel, P.; Ramasamy, R. Low testosterone in adolescents & young adults. Front. Endocrinol. 2020, 10, 494570. [Google Scholar] [CrossRef] [PubMed]
- Waldbeser, L.S.; Ajioka, R.S.; Merz, A.J.; Puaoi, D.; Lin, L.; Thomas, M.; So, M. The opaH locus of Neisseria gonorrhoeae MS11A is involved in epithelial cell invasion. Mol. Microbiol. 1994, 13, 919–928. [Google Scholar] [CrossRef]
- Hess, D.J.; Henry-Stanley, M.J.; Wells, C.L. Gentamicin promotes Staphylococcus aureus biofilms on silk suture. J. Surg. Res. 2011, 170, 302–308. [Google Scholar] [CrossRef]
- Oelschlaeger, T.A. Mechanisms of probiotic actions–a review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, X.; Jiang, C.; Shi, F.; Zhu, Y.; Yang, B.; Zhuo, J.; Jing, Y.; Luo, G.; Xia, S. Finasteride accelerates prostate wound healing after thulium laser resection through DHT and AR signalling. Cell Prolif. 2018, 51, e12415. [Google Scholar] [CrossRef]
- Gilliver, S.C.; Ashworth, J.J.; Ashcroft, G.S. The hormonal regulation of cutaneous wound healing. Clin. Dermatol. 2007, 25, 56–62. [Google Scholar] [CrossRef]
- Gilliver, S.; Ashcroft, G. Sex steroids and cutaneous wound healing: The contrasting influences of estrogens and androgens. Climacteric 2007, 10, 276–288. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Vasina, E.; Cauwenberghs, S.; Feijge, M.; Heemskerk, J.; Weber, C.; Koenen, R. Microparticles from apoptotic platelets promote resident macrophage differentiation. Cell Death Dis. 2011, 2, e211. [Google Scholar] [CrossRef] [PubMed]
- Sintiprungrat, K.; Singhto, N.; Sinchaikul, S.; Chen, S.-T.; Thongboonkerd, V. Alterations in cellular proteome and secretome upon differentiation from monocyte to macrophage by treatment with phorbol myristate acetate: Insights into biological processes. J. Proteom. 2010, 73, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Belboul, A.; Ashworth, J.; Fadel, A.; Mcloughlin, J.; Mahmoud, A.; El Mohtadi, M. Estrogen induces the alternative activation of macrophages through binding to estrogen receptor-alpha. Exp. Mol. Pathol. 2025, 143, 104971. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Chen, Z.; Sulaiman, K.; Feinberg, M.W.; Ballantyne, C.M.; Jain, M.K.; Simon, D.I. Integrin engagement regulates monocyte differentiation through the forkhead transcription factor Foxp1. J. Clin. Investig. 2004, 114, 408–418. [Google Scholar] [CrossRef]
- Parry, C. Androgens Inhibit Phagocytosis by Macrophages Via the Androgen Receptor in Vitro. Master’s Thesis, Manchester Metropolitan University, Manchester, UK, 2018. [Google Scholar]
- Gomez, F.; Ruiz, P.; Lopez, R.; Rivera, C.; Romero, S.; Bernal, J. Effects of androgen treatment on expression of macrophage Fcγ receptors. Clin. Diagn. Lab. Immunol. 2000, 7, 682–686. [Google Scholar] [CrossRef]
- Beery, T.A. Sex differences in infection and sepsis. Crit. Care Nurs. Clin. 2003, 15, 55–62. [Google Scholar] [CrossRef]
- Schröder, J.; Kahlke, V.; Staubach, K.-H.; Zabel, P.; Stüber, F. Gender differences in human sepsis. Arch. Surg. 1998, 133, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Eachempati, S.R.; Hydo, L.; Barie, P.S. Gender-based differences in outcome in patients with sepsis. Arch. Surg. 1999, 134, 1342–1347. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; de Souza Bittencout, R.; Arêas, I.D.; Pena, L.S.C.; Almeida, C.F.; de Brito Guimarães, B.C.; Dórea, R.S.D.M.; Correia, T.M.L.; Júnior, M.N.S.; Morbeck, L.L.B. Effects of 5α-dihydrotestosterone on the modulation of monocyte/macrophage response to Staphylococcus aureus: An in vitro study. Biol. Sex Differ. 2023, 14, 15. [Google Scholar] [CrossRef]
- Ashcroft, G.S. Sex differences in wound healing. Adv. Mol. Cell Biol. 2004, 34, 321–328. [Google Scholar]
- Engeland, C.G.; Sabzehei, B.; Marucha, P.T. Sex hormones and mucosal wound healing. Brain Behav. Immun. 2009, 23, 629–635. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Stefan Ekernas, L.; Creel, S. Unravelling complex associations between testosterone and parasite infection in the wild. Funct. Ecol. 2012, 26, 123–133. [Google Scholar] [CrossRef]
- Hillgarth, N.; Wingfield, J.C. Testosterone and immunosuppression in vertebrates: Implications for parasite-mediated sexual selection. In Parasites and Pathogens: Effects on Host Hormones and Behavior; Springer: Berlin/Heidelberg, Germany, 1997; pp. 143–155. [Google Scholar]
- Saino, N.; Møller, A.; Bolzerna, A. Testosterone effects on the immune system and parasite infestations in the barn swallow (Hirundo rustica): An experimental test of the immunocompetence hypothesis. Behav. Ecol. 1995, 6, 397–404. [Google Scholar] [CrossRef]
- Jacobsen, H.; Klein, S.L. Sex differences in immunity to viral infections. Front. Immunol. 2021, 12, 720952. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- vom Steeg, L.G.; Klein, S.L. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016, 12, e1005374. [Google Scholar] [CrossRef]
- Bernin, H.; Lotter, H. Sex bias in the outcome of human tropical infectious diseases: Influence of steroid hormones. J. Infect. Dis. 2014, 209, S107–S113. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-J.; Lai, K.-P.; Chuang, K.-H.; Chang, P.; Yu, I.-C.; Lin, W.-J.; Chang, C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-α expression. J. Clin. Investig. 2009, 119, 3739–3751. [Google Scholar] [CrossRef]
- D’agostino, P.; Milano, S.; Barbera, C.; Di Bella, G.; La Rosa, M.; Ferlazzo, V.; Farruggio, R.; Miceli, D.; Miele, M.; Castagnetta, L. Sex hormones modulate inflammatory mediators produced by macrophages a. Ann. N. Y. Acad. Sci. 1999, 876, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Friedl, R.; Brunner, M.; Moeslinger, T.; Spieckermann, P.G. Testosterone inhibits expression of inducible nitric oxide synthase in murine macrophages. Life Sci. 2000, 68, 417–429. [Google Scholar] [CrossRef]
- Viken, K. The effect of steroids on adhesiveness, rosette-forming ability and survival of cultured, human mononuclear cells. Acta Pathol. Microbiol. Scand. Sect. C Immunol. 1976, 84, 5–12. [Google Scholar] [CrossRef]
- Magri, B.; Viganò, P.; Rossi, G.; Somigliana, E.; Gaffuri, B.; Vignali, M. Comparative effect of the calcium antagonist verapamil and the synthetic steroids gestrinone and danazol on human monocyte phagocytosis in vitro. Gynecol. Obstet. Investig. 1997, 43, 6–10. [Google Scholar] [CrossRef]
- Yamada, K.; Hayashi, T.; Kuzuya, M.; Naito, M.; Asai, K.; Iguchi, A. Physiological concentration of 17 beta-estradiol inhibits chemotaxis of human monocytes in response to monocyte chemotactic protein 1. Artery 1996, 22, 24–35. [Google Scholar] [PubMed]
- Miyagi, M.; Aoyama, H.; Morishita, M.; Iwamoto, Y. Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. J. Periodontol. 1992, 63, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Kokal, M.; Mirzakhani, K.; Pungsrinont, T.; Baniahmad, A. Mechanisms of androgen receptor agonist-and antagonist-mediated cellular senescence in prostate cancer. Cancers 2020, 12, 1833. [Google Scholar] [CrossRef]
- Azhagiya Singam, E.R.; Tachachartvanich, P.; La Merrill, M.A.; Smith, M.T.; Durkin, K.A. Structural dynamics of agonist and antagonist binding to the androgen receptor. J. Phys. Chem. B 2019, 123, 7657–7666. [Google Scholar] [CrossRef]
- McLeod, D.G. Antiandrogenic drugs. Cancer 1993, 71, 1046–1049. [Google Scholar] [CrossRef]
- Christiansen, A.R.; Lipshultz, L.I.; Hotaling, J.M.; Pastuszak, A.W. Selective androgen receptor modulators: The future of androgen therapy? Transl. Androl. Urol. 2020, 9, S135. [Google Scholar] [CrossRef] [PubMed]
- Chaudagar, K.; Rameshbabu, S.; Mei, S.; Hirz, T.; Hu, Y.-M.; Argulian, A.; Labadie, B.; Desai, K.; Grimaldo, S.; Kahramangil, D. Androgen blockade primes NLRP3 in macrophages to induce tumor phagocytosis. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-K.; Pang, H.; Wang, L.; Niu, Y.; Luo, J.; Chang, E.; Sparks, J.D.; Lee, S.O.; Chang, C. New therapy via targeting androgen receptor in monocytes/macrophages to battle atherosclerosis. Hypertension 2014, 63, 1345–1353. [Google Scholar] [CrossRef]
- Chuang, K.-H.; Altuwaijri, S.; Li, G.; Lai, J.-J.; Chu, C.-Y.; Lai, K.-P.; Lin, H.-Y.; Hsu, J.-W.; Keng, P.; Wu, M.-C. Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor. J. Exp. Med. 2009, 206, 1181–1199. [Google Scholar] [CrossRef]
- Silver, R.F.; Li, Q.; Boom, W.H.; Ellner, J.J. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: Requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J. Immunol. 1998, 160, 2408–2417. [Google Scholar] [CrossRef]
- Bini, E.I.; Mata Espinosa, D.; Marquina Castillo, B.; Barrios Payán, J.; Colucci, D.; Cruz, A.F.; Zatarain, Z.L.; Alfonseca, E.; Pardo, M.R.; Bottasso, O. The influence of sex steroid hormones in the immunopathology of experimental pulmonary tuberculosis. PLoS ONE 2014, 9, e93831. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Díaz, M.; Strickland, A.B.; Keselman, A.; Heller, N.M. Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation. J. Immunol. 2018, 201, 2923–2933. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).