Abstract
Hepatitis B virus (HBV) infection is a silent epidemic; many infected people are asymptomatic and not aware of the infection. In 2022, it was reported that approximately 254 million people were living with chronic HBV infection globally, majority being in sub-Saharan Africa and Asia. In Kenya, the national HBV prevalence is estimated to be 3.5%. Our study was aimed at identifying key predictors and transmission trends that could inform the development of sustainable prevention models needed to address existing gaps in the national framework towards HBV elimination. We targeted participants seeking health services in Baringo and Kisumu county health facilities and conducted community mass testing in the two counties. Participants were interviewed using a study questionnaire and were tested for hepatitis B surface antigen (HBsAg) using an HBsAg rapid test. Venous blood was collected from participants who tested HBsAg+ for further infection confirmation and linkage to care. Logistic regression was performed to assess factors correlated with HBV infection. Out of 3034 participants, 192 tested positive for HBsAg and the prevalence of HBV infection was 6.3% (95% CI = 0.055–0.072). Intrafamilial infections in Baringo were 15.0%. HBV infection prevalence exceeded 10% among those aged 25–49 years, peaking at 13.1% in the 45–49-year age group and lowest at 1.8% in the 16–19-year age group. Overall, males had a higher prevalence in younger ages, while females above 60 years old were more affected. In multivariable logistic regression, individuals residing in Baringo (aPR = 8.1; 95% CI = 2.2–29.4), users of other injectable drugs (aPR = 6.7; 95% CI = 1.3–204.0), those traditionally circumcised (aPR 1.02; 95% CI = 0.56, 1.88), and staying >5 km from a health care facility (aPR = 10.4; 95% CI = 2.2–49.4) had significantly higher prevalence ratios of being infected with HBV. These different infection predictors underscore the need for different care and prevention approach models.
1. Introduction
Hepatitis B virus (HBV) infection remains a global public health concern, with approximately 1.2 million new cases reported annually and an estimated 1.1 million deaths each year resulting from HBV-related complications [1,2], despite the available stable and effective vaccine [3]. The burden and prevalence of HBV infection vary geographically, with countries in Asia and sub-Saharan Africa regions having the highest burden [4]. Kenya has a relatively lower national prevalence (3.5%) [5] compared to neighboring countries [6,7]; however, this prevalence varies across population types and geographical regions [5]. Higher rates are recorded among people with jaundice seeking health care services [8] and those living in the northern part of Kenya [5]. The prevalence is lower among expectant mothers [9] and those living in central and southern Kenya [5]. Exposure to different predisposing factors accounts for these variations.
HBV infection is a silent epidemic that may remain quiescent for many years [10,11]. Most of those infected are asymptomatic and not aware until the chronic stages of the infection, unknowingly transmitting the virus to unborn, spouses, partners, and other people via body fluids [10,11,12,13]. In Africa, most transmissions are believed to occur perinatally [4,14]. In the absence of intervention, 90% of perinatal infections develop into chronic HBV infection [15]. Additionally, in highly endemic areas, HBV transmission often occurs horizontally within households and through close contact with infected individuals [4,14,16]. This close contact can be through sharing sharp items like razors, unsafe sex with infected partners, or skin puncture using a contaminated sharp object and sharing unsafe injections [11,16]. These transmission modes are highly efficient, influenced by a combination of biological (viral load levels and age), behavioral (sexual practices and household exposures), health system (screening and diagnosis), and socio-economic factors [16,17,18]. However, the interaction of these factors during transmission remains unclear.
HBV infection progresses to chronic infection in approximately 5–10% of adults and more than 90% of children [1,2], while those who successfully clear the virus develop long-lasting protective antibodies [1,2,19]. The critical factors that predispose certain individuals to persistent infection, while others develop long-term immunity, remain unclear. This often yields conflicting results and appears to vary across populations and study settings [20,21]. Understanding these factors is very useful in developing structural countermeasures, tailored prevention strategies, long-term community engagement, and efficient programs that minimize resource wastage and sustained infection control. In 2016, the World Health Organization (WHO) adopted a global hepatitis strategy that aims to eliminate viral hepatitis as a public health threat by the year 2030, urging a 90% reduction in new infections and 65% decrease in deaths [1,4,22]. This initiative is yet to be realized in low- and middle-income countries (LMIC) [23]. In line with this global strategy, we sought to establish the rate of hepatitis B infection in Baringo and Kisumu counties in Kenya and to establish the transmission predictors that can be used to develop sustainable prevention models and disease management strategies.
2. Materials and Methods
2.1. Study Sites
The study was conducted in Baringo and Kisumu Counties of Kenya. Baringo County is located in the central Rift Valley region, with HIV prevalence ranging between 2.1 and 4.9% [24], and Kisumu County, which is located at the shores of Lake Victoria, where there is a comprehensive HIV program based on an estimated prevalence of 11.7% [24].
2.2. Study Population and Recruitment
Data from the community and selected health facility was collected for a period of one and a half years from September 2023 to February 2025. In the community, mass testing for HBV infection was performed in the villages and local shopping centers by trained health care workers. The communities (villages) were sensitized and educated about free HBV testing by the area’s public administration and health officers. During community testing, the health care worker team—nurses, laboratory technicians, medical officers, and pharmacists—visited the community to conduct mass testing.
All participants were aged 16 years and above and provided written informed consent or assent. For participants aged between 16 and 17 years, parental or guardian permission was obtained in addition to their written assent. A structured questionnaire was administered by trained study staff using Research Electronic Data Capture (REDCap) (Version 13.8.1) hosted at KEMRI on a tablet (https://projectredcap.org/software/, accessed on 24 August 2023). The questionnaire contained social demographic, behavioral, economic, and cultural characteristics. Finger-prick blood samples were collected from participants by trained laboratory officers and tested for the presence of HBsAg using the KEMRI HEPCELL Rapid test (KEMRI Production Unit, Nairobi, Kenya), according to the manufacturers’ instructions (sensitivity and specificity 98% and 99%, respectively) [25]. The test results were then recorded in the REDCap and electronically submitted to a central study database. All positive samples were confirmed using real-time PCR, and all who tested positive for the virus were linked to care. A similar process was followed to recruit and test patients in the outpatient departments (OPDs) of selected health facilities in the study counties. Anyone who tested HBsAg+ was linked to care.
2.3. Data Management and Analysis
Data from REDCap was downloaded from the central database repository to STATA software (Release 18) and coded. The estimated HBV prevalence in the population under study was calculated as a proportion of HBsAg+ participants and the total number of people tested in that region and stratified by gender and age group. The age-specific trends were calculated as age group differences stratified by gender and location, while the sex-specific trend was the difference in HBV prevalence between males and females. Intrafamilial clusters were members living in the same household or having common biological parents. Descriptive and inferential analysis was computed using STATA software. Multiple logistic regression modeling was performed to identify factors associated with HBV infection and presented as adjusted prevalence ratios (aPRs) with 95% confidence intervals (CIs). The variables were stratified by social demographic, behavioral, financial, and cultural characteristics. The significance level was set at a p-value of less than 0.05 and a 95% confidence interval for all statistical analyses.
2.4. Ethical Considerations
This study was approved by Kenya Medical Research Institute’s (KEMRI’s) Scientific and Ethics Review Unit (SERU) Protocol No. SERU4680 and NACOSTI License No. NACOSTI/P/23/27902. In addition, informed written consent was sought from participants aged above 18 years old and assent from those aged 17 years and below. Consent was obtained by trained personnel. All methods were performed in accordance with the Helsinki Declaration.
3. Results
A total of 3034 study participants were interviewed, of which 65.4% were female and 34.6% were male. Participant turnout was lower in Kisumu County (8.1%) compared to Baringo County (91.9%). Most of the participants had the highest education level as either primary, 1179 (46.1%), or secondary, 1031 (40.3%), with a small proportion having college, 334 (13.1%), and the rest had none (Table 1). More than 80% of the participants were married (n = 2431), of which there were more monogamous marriages (63.7%) than polygamous marriages (17.3%). There were 425 (14.2%) single and never married and the rest were once married but widowed(er) (Table 1). Of those who reported having children, 63.9% had children aged 5 years and below. When the participants were asked the number of siblings in their family, 1597 (55.6%) reported less than 5, 1153 (40.2%) had between 5 and 10, and the rest had more than 10 siblings (Table 1). The study was carried out in rural communities where the majority, 1838 (61.9%), were unemployed, of which only 383 (34.2%) had a net monthly income of more than USD 100 (Table 1). The mean age was 37 ± 5.93 years, ranging from 17 to 94 years. The proportion of participants within each age group is shown in Table 1.

Table 1.
Sociodemographic characteristics and prevalence of Hepatitis B.
3.1. Prevalence and Sociodemographic Factors Associated with Hepatitis B Infection
Out of the 3034 participants, 191 tested positive for HBsAg, resulting in 6.3% (95% CI = 0.055–0.072) prevalence of hepatitis B virus infection in the study regions. We assessed the association between demographic characteristics and HBV infection using a logistic regression model (Table 1). In the bivariable analysis demographic, factors that were associated with Hepatitis B infection (p-value ≤ 0.05) included respondents who were married (cPR = 2.18; 95% CI = 1.31, 3.60) in the marital status category, those whose education level was secondary (cPR = 0.70; 95% CI = 0.50, 0.98), respondents with 5 to 10 children (cPR = 1.90; 95% CI = 1.41, 2.55), respondents with more than 10 children (cPR = 2.07; 95% CI = 1.33, 3.22), respondents who live more than 5 km away from a health care facility (cOR = 7.3; 95% CI = 1.68–31.70), net monthly income of the unemployed between 51 and 100 USD (cPR = 1.81; 95% CI = 0.97, 3.39), and respondents who live in Baringo (cPR = 0.18; 95% CI = 0.06, 0.56), with Baringo having a higher prevalence (6.8% (95% CI = 0.058–0.077)) as compared to 1.2% (95% CI = −0.002–0.026) for Kisumu County. These factors were later subjected to a multivariable analysis.
3.2. Trends of Hepatitis B Infection by Age Group and Gender
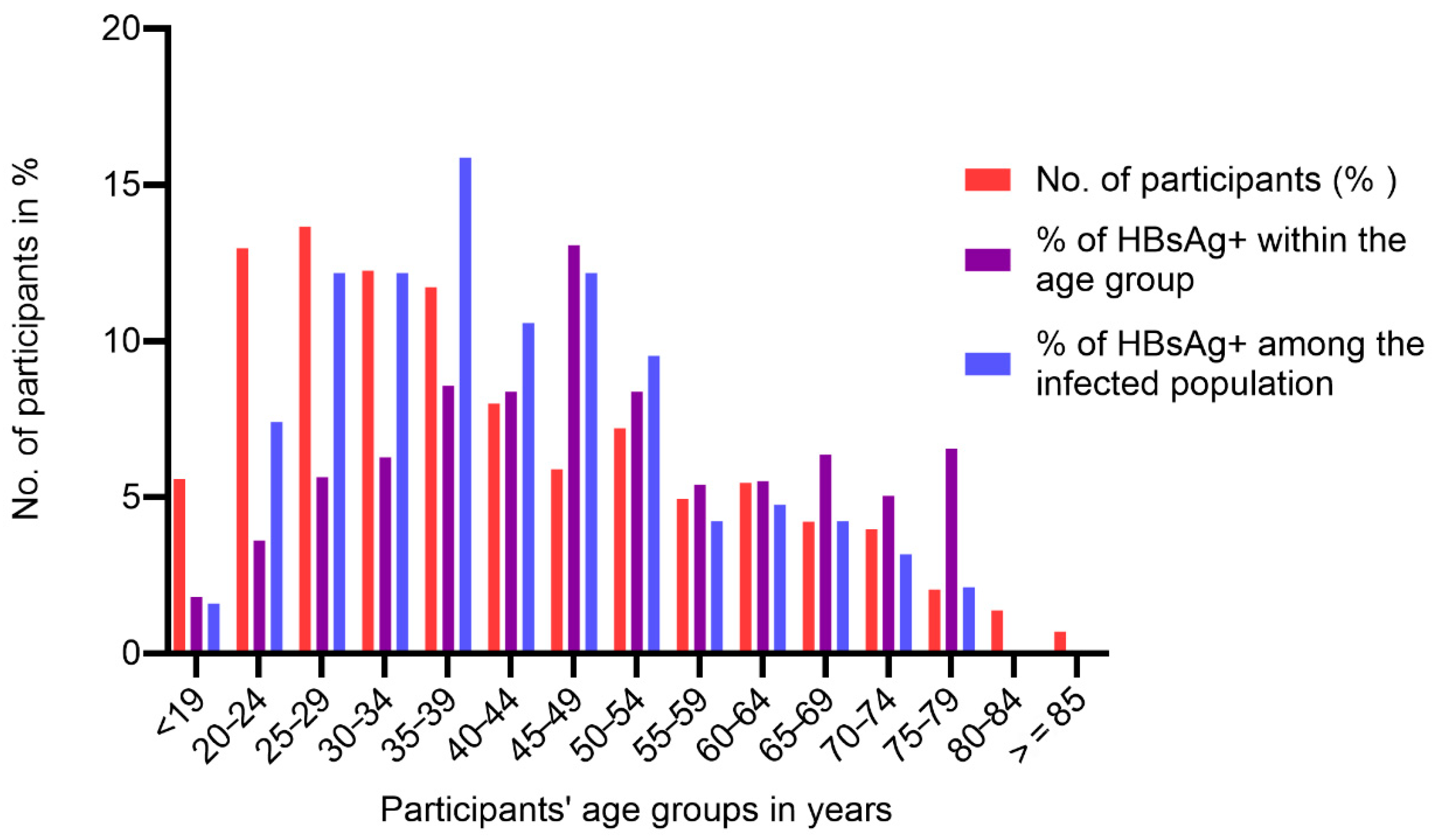
On stratifications of participants by age, the highest HBV prevalence of 13.1% was observed in individuals aged 45 to 49 years and the lowest of 1.8% was observed in participants ≤ 19 years old (Figure 1). Among the infected (HBsAg+) group, participants aged between 25 and 49 years had a prevalence of more than 10%, with a gradual decrease to no infection in participants ≥ 80 years old (Figure 1). Additionally, among HBsAg+, the age group of 35–39 years had the highest prevalence (15.9%), while those ≤19 years had the lowest (Figure 1).
Figure 1.
Trends of hepatitis B infection by age group.
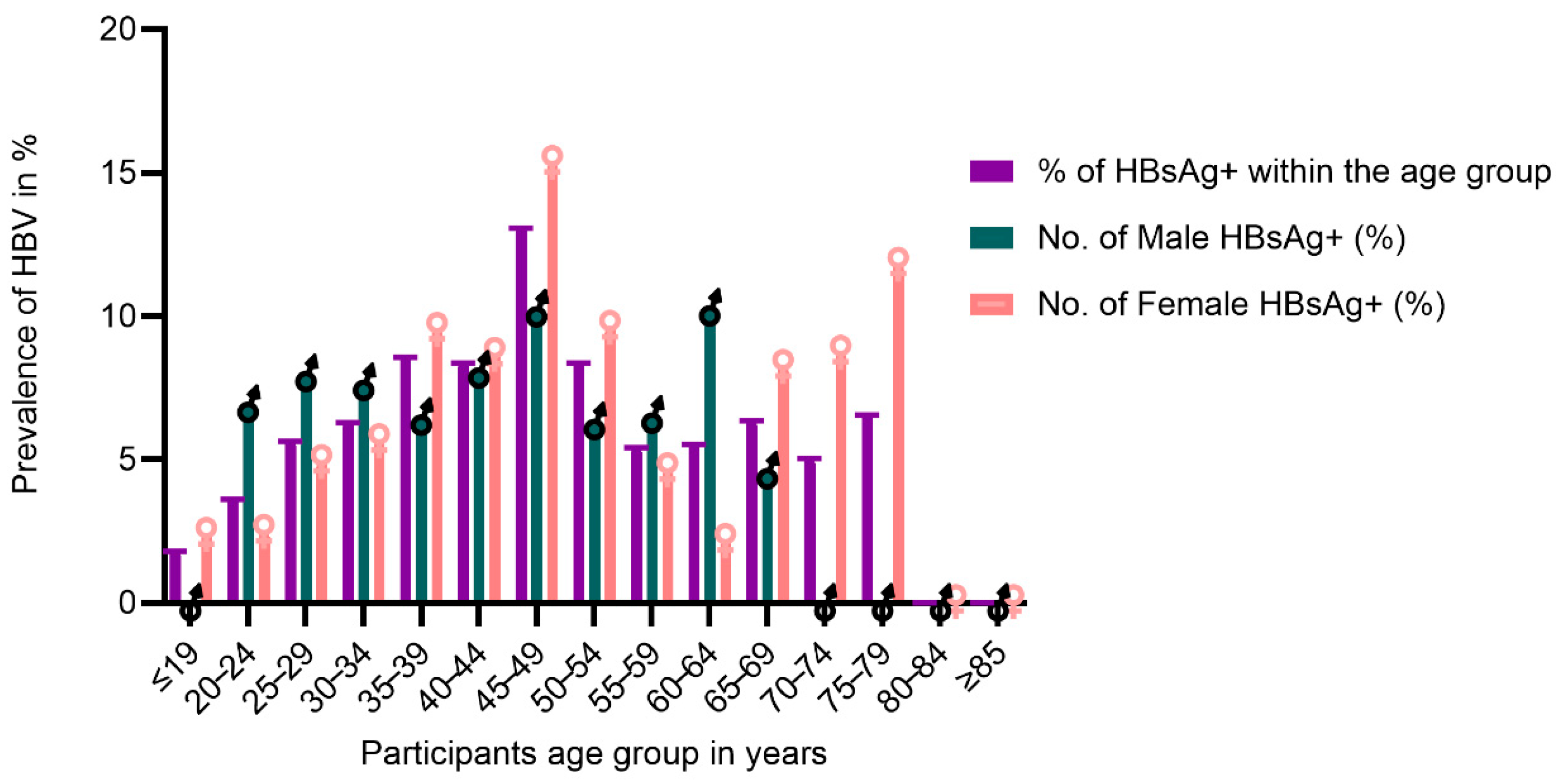
Regarding gender, the younger age groups of males had a higher prevalence up to 34 years, and females of older ages demonstrated a higher prevalence than males (Figure 2 and Table A1). The highest infected age group for males and females was 45–49 years, and while infected males were all under 70 years, females showed infections up to the age of 79 years (Figure 2 and Table A1). In bivariable analysis, being 30 years and above was associated with hepatitis B infection with respondents between 40 and 49 (cPR = 6.32; 95% CI = 1.93–20.66) being significantly affected with HBV infection (Table 1).
Figure 2.
Distribution of Hepatitis B infection by gender and age group.
3.3. Trends of HBV Infection Among Related Families
Among the families that participated in the study, 40 families were from the community mass testing, of which 6 families (15.0%) had two or more members infected. Among the six families, almost half (48.6%) of the family members and 56.7% of the siblings were infected. For parents, only half had at least one parent infected (Table 2). In one of the families where the mother had died of hepatocellular carcinoma, all the children were HBV-positive. Current infections among siblings were proportionately higher compared to the total family infections.

Table 2.
Distribution of HBV infection among related families.
3.4. Behavioral, Traditional, and Cultural Practices and Hepatitis B Infection
We assessed behavioral, traditional, and cultural practices that predispose participants to the hepatitis B virus. The majority (84.4%) of the participants shared items and sharps in the household, and the majority (87.9%) used public salons or barber shops for hair dressing and shaving. More than half (65.2%) of the participants reported traditional circumcision, male circumcision, or female genital mutilation (FGM) that was culturally performed at home (98.1%), of which more than half (62.8%) reported sharing circumcision/FGM sharps. All participants reported a heterosexual relationship; however, 85.0% did not respond to the question on the number of sexual partners. Among those who reported a history of alcohol consumption (23.3%), most of them (78.7%) occasionally consume alcohol. A very small proportion (3.8%) reported smoking or using tobacco.
Overall, we observed that the number of health care workers was low (0.9%). Regarding occupational exposure to blood, especially in health care settings, only 4.4% had a history of blood transfusion, 0.4% reported having diabetes, but none of them shared the diabetic sharps, and only 0.9% reported tattoos or scarification. When the participants were asked if they had tested for HIV, more than half (55.8%) knew their HIV status, of whom 3.6% were HIV-positive (Table 3).

Table 3.
Association between Hepatitis B infection and participants’ behavioral, traditional, and cultural practices.
3.5. Bivariable Analysis of Behavioral, Traditional, and Cultural Practices
Bivariable analysis using a logistic regression model revealed that respondents who shared items (cPR = 1.76; 95% CI= 1.28, 2.40), those who reported using other injectable drugs (cPR = 4.02; 95% CI = 1.20, 13.47), those who were circumcised (cPR = 155; 95% CI = 1.13, 2.13), and those who drink alcohol (cOR = 1.42; 95% CI = 1.03–1.97) (cPR = 1.38; 95% CI = 1.03, 1.88) were associated with hepatitis B at a p-value < 0.1. These factors were later subjected to a multivariable analysis (Table 3). Receiving transfused blood, being diabetic or being HIV-positive, hair dressing and shaving hair, being a health care worker, smoking or use of tobacco, and having tattoos or scarification were not associated with hepatitis B in the study population (Table 3).
The results of the multivariable logistic regression suggest that respondents who live more than 5 km away from a health care facility (aPR = 10.44; 95% CI = 2.21–49.37; p = 0.003), those who use other injectable drugs (aPR = 6.71 95% CI = 1.34, 33.67; p = 0.021), those who shared items (aPR = 2.60; 95% CI= 1.54, 4.39; p = <0.001), and those who underwent traditional circumcision (aPR 1.02; 95% CI = 0.56, 1.88; p = 0.040) were significantly associated with higher prevalence rates of hepatitis B virus. (Table 1 and Table 3). On the other hand, education level, being imprisoned or jailed, age category, marital status, number of children, being circumcised, and drinking alcohol, and sharing hygiene items were not associated with hepatitis B infection.
4. Discussion
To our knowledge, this is the first sero-epidemiological study of hepatitis B infection in the rural communities of Kenya. The observed prevalence of 6.33% places the study communities in Kisumu and Baringo, within the WHO-defined intermediate endemicity range (2–7%) [26]. The findings affirm other studies reporting intermediate endemicity [5,9] with variations largely attributed to geographical differences among study populations. Baringo County, which reported a prevalence of >6%, is among other counties in Rift Valley and Northern Kenya that have high endemicity. Kisumu County has a high prevalence of HIV compared to Baringo County. Despite the lower participant turnout observed in Kisumu County compared to Baringo, the prevalence rate was notably lower [5]. HIV and hepatitis share transmission routes, and these two counties exemplify regions where one infection predominates. Integrating HBV into existing HIV services offers a promising approach to strengthening community-based services; however, the uneven distribution of HIV services across regions may limit its implementation and scale-up. These findings highlight the need to strengthen community-based HBV screening and early diagnosis strategies to ensure timely linkage to care and to reduce community-based transmission.
HBV prevalence by gender distribution showed that males had a slightly higher prevalence than females, 6.6% and 6.2%, respectively. This is similar to findings from other studies [27]. Although more males are infected than females, this study also showed that there was a higher proportion of HBsAg+ women at old age compared to men, suggesting that females live with the virus longer compared to males. This observation may be attributed to sexual dimorphism of the liver and androgen response elements, which have a strong influence on HBV infection outcomes, with males more likely to suffer from HBV complications compared to females [28,29,30]. Similarly, females tend to mount stronger immune responses and are likely to develop protective antibodies compared to males [28,31,32,33]. This could explain why no cases of infection were observed among males over 70 years.
Hepatitis B virus progresses to HCC after three to four decades of infection, if there is no intervention [34,35]. The mean age of our study population was 37 ± 5.93 years. We observed a steady increase in infection, with the highest prevalence observed among individuals aged 40–49 years, and several studies have recorded similar observations [27,36]. In our study, age was significantly associated with HBV positivity in the bivariate analysis, as individuals aged 30 years and above were more likely to be infected. On various occasions, the high HBV burden among the elderly largely results from infections acquired perinatally or during early childhood exposure [4,14,37,38]. Such transmissions were due to a lack of immunization, close contact, and sexual activities during adolescence or early adulthood [14]. This study showed that the proportion of infection among young ages was low compared to older participants, most likely due to access to immunization, improved health care, and standards of living that could reduce close contact.
Our findings from community mass testing highlighted that HBV infections were not randomly distributed but tended to cluster within households. There was clear evidence of household clustering, with 15.0% of families reporting two or more infected members and nearly half of the members (48.6%) within the family affected. While transmission appeared particularly pronounced among siblings (56.7%), children and young family members are at risk of transmitting HBV infection to each other, probably through close contact. Other studies have reported similar household transmissions. In India and Tanzania, they reported a household prevalence of 9.2% and 5.4%, respectively [39,40]. In Arak, Iran, they reported a prevalence of 23.3% among HBsAg+ family members, with mothers of index cases having the highest infection rates (46.6%) [41]. A study in Kinshasa had an HBsAg intrafamilial prevalence of 5.0% and revealed that exposed offspring had 3.3 times the prevalence of HBV compared to unexposed offspring [42]. Although the number of children and sharing household sharp items were not significantly associated with HBV transmission at multivariable logistic regression, studies involving different regions and large samples have yielded contrary findings [40]. Identifying the key predictors for interfamilial transmission remains crucial for developing effective prevention strategies.
Married individuals had the highest prevalence (6.95%), but the once-married individuals had the highest prevalence ratio, suggesting they were the most affected group. Participants with an education level above primary had a lower prevalence (5.57%) as compared to those with a primary education or below (7.2%). This may impact the knowledge and awareness of transmission or inadequate health care access that is associated with limited health literacy and access to prevention strategies.
Factors independently associated with HBV infection in this study included residing in Baringo, users of other injectable drugs, those circumcised, and staying >5 km from a health care facility, exhibiting significantly higher ratios of being infected with the hepatitis B virus. The adjusted prevalence ratio of traditionally circumcised individuals (aPR = 1.02; 95% CI = 0.56, 1.88) was close to unity as compared to the other factors. This suggests that it may not be a primary determinant of hepatitis B infection and should be interpreted with caution. HIV positivity, being a health care worker, smoking or use of tobacco, and having tattoos or scarification were not associated with HBV infection; other studies have yielded contrary findings [43,44,45].
The limitation of this study is that it focused on current HBV transmission and did not explore historic exposure to HBV; thus, seropositivity of patients, especially hepatitis B core antibody (HBcAb), was not tested.
5. Conclusions
We identified key transmission predictors of HBV transmission, such as household exposure to infected individuals, traditional and cultural practices like circumcision, unsafe drug injectables, and living in areas that have a high HBV burden. Identifying these exact predisposing factors is a milestone towards transforming public health efforts from broad and reactive to sustainable, precise preventive measures. To reduce the family close-contact transmission, increasing awareness about hepatitis B and vaccination of uninfected family members is essential. These findings highlight the need for timely infant immunization and catch-up vaccine doses to adolescents and adults at increased risk of infection. Administering birth-dose HBV vaccination would provide the much-needed early protection against HBV infection, especially for infants born to households inhabited by infected members.
Recommendations
There is a need to scale up HBV community services to other regions. This can be achieved through integrating HBV services into the existing interventions. We identified key predictors of HBV transmission in endemic regions and recommend their integration into national HBV awareness and prevention campaigns to enhance control efforts. We also recommend future results to consider HBcAb, especially in intrafamilial clusters and the inclusion of different regions in the country. Such information may provide valuable insights into transmission trends and dynamics, thereby strengthening targeted interventions and enhancing prevention strategies.
Author Contributions
Conceptualization: M.O.; methodology, M.O., D.M.-M., J.H.K., S.W.O., L.M.M., E.O., H.M., G.T., R.R., E.S., A.O. and R.O.O.; writing—original draft M.O., D.M.-M. and R.O.O.; software: M.O., H.M., E.O., S.W.O. and L.M.M.; validation, M.O., A.O., E.S., G.T. and R.R.; formal analysis, M.O. and V.W.; investigation, M.O., D.M.-M., J.H.K., S.W.O., L.M.M., E.O., H.M., G.T., R.R., E.S., A.O. and R.O.O.; resources; M.O.; data curation: M.O., D.M.-M., F.O.O. and V.W.; writing—original draft preparation: M.O., F.O.O. and D.M.-M. writing—review and editing, M.O., R.O.O., D.M.-M., J.H.K., S.W.O., L.M.M., E.O., H.M., G.T., R.R., E.S., A.O., F.O.O. and V.W. visualization: E.O., J.H.K. and R.O.O. supervision: A.O., R.R., D.M.-M. and J.H.K.; project administration: M.O., G.T., H.M., S.W.O., L.M.M. and E.O.; funding acquisition: M.O., A.O. and D.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by The Gilead Sciences, Inc. The grant was awarded to Missiani Ochwoto in 2022 under Research Scholar Program (RSP) Award.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by Kenya Medical Research Institute’s (KEMRI’s) Scientific and Ethics Review Unit (SERU) Protocol No. SERU4680, approved on 19 June 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. In addition, informed written consent was sought from all participants by trained personnel collecting the data for storage and publication of their data.
Data Availability Statement
All data generated or analyzed during this study are confidential and are available from the corresponding author on reasonable request.
Acknowledgments
We acknowledge the county research departments of Kisumu and Baringo for allowing us to conduct this research in their counties. We are grateful to the personnel involved in hepatitis B screening and data collection in Kisumu County and Marigat subcounty Hospital. We acknowledge KEMRI management for the support and the ICT department for support in developing data tools used in data collection and training of health workers. We acknowledge the Ministry of Health at Division of National AIDS and STI Control Program (NASCOP) for their support and Mercy Karoney, of Moi University, School of Medicine, for her clinical support. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors have no competing interests to declare that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| aPRs | Prevalence ratios |
| HCC | Hepatocellular carcinoma |
| HBsAg | Hepatitis B surface antigen |
| HBV | Hepatitis B virus |
| KEMRI | Kenya Medical Research Institute’s |
| LMIC | Low- and middle-income countries |
| NASCOP | National AIDS and STI Control Program |
| WHO | World Health Organization |
Appendix A

Table A1.
Distribution of hepatitis B infection by age.
Table A1.
Distribution of hepatitis B infection by age.
| Age Group in Years | No. of Participants n (%) | No. of HBV Infected n | Proportion (%) of HBV Infected Within: | |||
|---|---|---|---|---|---|---|
| Age Group | The Total Population | The Infected Population | ||||
| ≤19 | 167 | (5.6) | 3 | (1.8) | (0.1) | (1.6) |
| 20–24 | 387 | (13.0) | 14 | (3.6) | (0.5) | (7.4) |
| 25–29 | 408 | (13.7) | 23 | (5.6) | (0.8) | (12.2) |
| 30–34 | 366 | (12.3) | 23 | (6.3) | (0.8) | (12.2) |
| 35–39 | 350 | (11.7) | 30 | (8.6) | (10.0) | (15.9) |
| 40–44 | 239 | (8.0) | 20 | (8.4) | (0.7) | (10.6) |
| 45–49 | 176 | (5.9) | 23 | (13.1) | (0.8) | (12.2) |
| 50–54 | 215 | (7.2) | 18 | (8.4) | (0.6) | (9.5) |
| 55–59 | 148 | (5.0) | 8 | (5.4) | (0.3) | (4.2 |
| 60–64 | 163 | (5.5) | 9 | (5.5) | (0.3) | (4.8) |
| 65–69 | 126 | (4.2) | 8 | (6.4) | (0.3) | (4.2) |
| 70–74 | 119 | (4.0) | 6 | (5.0) | (0.2) | (3.2) |
| 75–79 | 61 | (2.0) | 4 | (6.6) | (0.1) | (2.1) |
| 80–84 | 41 | (1.4) | 0 | (0.0) | (0.0) | (0.0) |
| ≥85 | 21 | (0.7) | 0 | (0.0) | (0.0) | (0.0) |
| Total | 2987 | 100.0 | 189 | 6.3 | 6.3 | 100 |
The highest proportion in each category is in bold.
References
- World Health Organization. Global Hepatitis Report 2024: Action for Access in Low-and Middle-Income Countries; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Bigna, J.J.; Kenne, A.M.; Hamroun, A.; Ndangang, M.S.; Foka, A.J.; Tounouga, D.N.; Lenain, R.; Amougou, M.A.; Nansseu, J.R. Gender development and hepatitis B and C infections among pregnant women in Africa: A systematic review and meta-analysis. Infect. Dis. Poverty 2019, 8, 16. [Google Scholar] [CrossRef]
- Al-Busafi, S.A.; Al-Harthi, R.; Al-Naamani, K.; Al-Zuhaibi, H.; Priest, P. Risk Factors for Hepatitis B Virus Transmission in Oman. Oman Med. J. 2021, 36, e287. [Google Scholar] [CrossRef] [PubMed]
- Spearman, C.W.; Afihene, M.; Ally, R.; Apica, B.; Awuku, Y.; Cunha, L.; Dusheiko, G.; Gogela, N.; Kassianides, C.; Kew, M.; et al. Hepatitis B in sub-Saharan Africa: Strategies to achieve the 2030 elimination targets. Lancet Gastroenterol. Hepatol. 2017, 2, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Ondondo, R.O.; Muthusi, J.; Oramisi, V.; Kimani, D.; Ochwoto, M.; Young, P.; Ngugi, C.; Waruru, A.; Mwangi, J.; Chao, A.; et al. Prevalence of hepatitis B virus infection in Kenya: A study nested in the Kenya Population-based HIV Impact Assessment 2018. PLoS ONE 2024, 19, e0310923. [Google Scholar] [CrossRef]
- Kilonzo, S.B.; Gunda, D.W.; Mpondo, B.C.T.; Bakshi, F.A.; Jaka, H. Hepatitis B Virus Infection in Tanzania: Current Status and Challenges. J. Trop. Med. 2018, 2018, 4239646. [Google Scholar] [CrossRef]
- Kafeero, H.M.; Ndagire, D.; Ocama, P.; Kudamba, A.; Walusansa, A.; Sendagire, H. Prevalence and predictors of hepatitis B virus (HBV) infection in east Africa: Evidence from a systematic review and meta-analysis of epidemiological studies published from 2005 to 2020. Arch. Public Health 2021, 79, 167. [Google Scholar] [CrossRef] [PubMed]
- Ochwoto, M.; Kimotho, J.H.; Oyugi, J.; Okoth, F.; Kioko, H.; Mining, S.; Budambula, N.L.M.; Giles, E.; Andonov, A.; Songok, E.; et al. Hepatitis B infection is highly prevalent among patients presenting with jaundice in Kenya. BMC Infect. Dis. 2016, 16, 101. [Google Scholar] [CrossRef]
- Ngaira, J.A.; Kimotho, J.; Mirigi, I.; Osman, S.; Ng’ang’a, Z.; Lwembe, R.; Ochwoto, M. Prevalence, awareness and risk factors associated with Hepatitis B infection among pregnant women attending the antenatal clinic at Mbagathi District Hospital in Nairobi, Kenya. Pan. Afr. Med. J. 2016, 24, 315. [Google Scholar]
- Songtanin, B.; Chaisrimaneepan, N.; Mendóza, R.; Nugent, K. Burden, Outcome, and Comorbidities of Extrahepatic Manifestations in Hepatitis B Virus Infections. Viruses 2024, 16, 618. [Google Scholar] [CrossRef]
- Brook, G.; Chawla, Y. Hepatitis viruses. In Sexually Transmitted Infections-E-Book; Elsevier Health Sciences: Bengaluru, India, 2013; p. 380. [Google Scholar]
- Zoulim, F.; Mason, W.S. Reasons to consider earlier treatment of chronic HBV infections. Gut 2012, 61, 333–336. [Google Scholar] [CrossRef]
- Ishizaki, A.; Bouscaillou, J.; Luhmann, N.; Liu, S.; Chua, R.; Walsh, N.; Hess, S.; Ivanova, E.; Roberts, T.; Easterbrook, P. Survey of programmatic experiences and challenges in delivery of hepatitis B and C testing in low-and middle-income countries. BMC Infect. Dis. 2017, 17, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Vincent, J.P.; Moorhouse, L.; Shimakawa, Y.; Nayagam, S. Risk of early horizontal transmission of hepatitis B virus in children of uninfected mothers in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob. Health 2023, 11, e715–e728. [Google Scholar] [CrossRef]
- Indolfi, G.; Easterbrook, P.; Dusheiko, G.; Siberry, G.; Chang, M.-H.; Thorne, C.; Bulterys, M.; Chan, P.L.; El-Sayed, M.H.; Giaquinto, C.; et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol. Hepatol. 2019, 4, 466–476, Correction in Lancet Gastroenterol. Hepatol. 2020, 5, e4. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Z.; Gu, F. Epidemiology and Prevention of Hepatitis B Virus Infection. Int. J. Med. Sci. 2005, 2, 50–57. [Google Scholar] [CrossRef]
- Shepard, C.W.; Finelli, L.; Fiore, A.E.; Bell, B.P. Epidemiology of Hepatitis B and Hepatitis B Virus Infection in United States Children. Pediatr. Infect. Dis. J. 2005, 24, 755–760. [Google Scholar] [CrossRef]
- Allain, J.-P.; Opare-Sem, O. Screening and diagnosis of HBV in low-income and middle-income countries. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 643–653. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Morikawa, K.; Shimazaki, T.; Takeda, R.; Izumi, T.; Umumura, M.; Sakamoto, N. Hepatitis B: Progress in understanding chronicity, the innate immune response, and cccDNA protection. Ann. Transl. Med. 2016, 4, 337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.; Liu, Z.; Zou, G.; Li, J.; Lu, M. Host Genetic Determinants of Hepatitis B Virus Infection. Front. Genet. 2019, 10, 696. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis Report; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Jaquet, A.; Muula, G.; Ekouevi, D.K.; Wandeler, G. Elimination of viral hepatitis in low and middle-income countries: Epidemiological research gaps. Curr. Epidemiol. Rep. 2021, 8, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mutisya, I.; Muthoni, E.; Ondondo, R.O.; Muthusi, J.; Omoto, L.; Pahe, C.; Katana, A.; Ngugi, E.; Masamaro, K.; Kingwara, L.; et al. A national household survey on HIV prevalence and clinical cascade among children aged ≤15 years in Kenya (2018). PLoS ONE 2022, 17, e0277613. [Google Scholar] [CrossRef]
- Owino, N.; Kamau, G.; Kulundu, J.; Njuguna, A.; Tukei, P.M.; Yano, M.; Naruse, T. KEMRI Hep-cell II hepatitis B surface antigen screening kit. East Afr. Med. J. 1999, 76, 530–532. [Google Scholar] [PubMed]
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.T.; Krause, G.; Ott, J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 2015, 386, 1546–1555. [Google Scholar] [CrossRef]
- Kazmi, S.A.; Rauf, A.; Alshahrani, M.M.; Al Awadh, A.A.; Iqbal, Z.; Soltane, R.; Tag-Eldin, E.; Ahmad, A.; Ansari, Z.; Rehman, Z.U. Hepatitis B among University Population: Prevalence, Associated Risk Factors, Knowledge Assessment, and Treatment Management. Viruses 2022, 14, 1936. [Google Scholar] [CrossRef]
- Brown, R.; Goulder, P.; Matthews, P.C. Sexual Dimorphism in Chronic Hepatitis B Virus (HBV) Infection: Evidence to Inform Elimination Efforts. Wellcome Open Res. 2022, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, L.; Zhao, J.; Ungvari, G.S.; Ng, C.H.; Duan, Z.; Zheng, S.-J.; Xiang, Y.-T. Gender differences in demographic and clinical characteristics in patients with HBV-related liver diseases in China. PeerJ 2022, 10, e13828. [Google Scholar] [CrossRef]
- Ruggieri, A.; Barbati, C.; Malorni, W. Cellular and molecular mechanisms involved in hepatocellular carcinoma gender disparity. Int. J. Cancer 2010, 127, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, B.S. Sex differences in response to hepatitis b virus. Arthritis Rheum. 1979, 22, 1261–1266. [Google Scholar] [CrossRef]
- Xu, X.; Wu, C.; Lou, Z.; Peng, C.; Jiang, L.; Wu, T.; Zeng, T.; Dong, Y.; Ruan, B. Changing incidence of hepatitis B and persistent infection risk in adults: A population-based follow-up study from 2011 in China. BMC Public Health 2023, 23, 256. [Google Scholar] [CrossRef]
- Stroffolini, T.; Esvan, R.; Biliotti, E.; Sagnelli, E.; Gaeta, G.B.; Almasio, P.L. Gender differences in chronic HBsAg carriers in Italy: Evidence for the independent role of male sex in severity of liver disease. J. Med. Virol. 2015, 87, 1899–1903. [Google Scholar] [CrossRef]
- Chu, C.-M.; Sheen, I.-S.; Lin, S.-M.; Liaw, Y.-F. Sex Difference in Chronic Hepatitis B Virus Infection: Studies of Serum HBeAg and Alanine Aminotransferase Levels in 10,431 Asymptomatic Chinese HBsAg Carriers. Clin. Infect. Dis. 1993, 16, 709–713. [Google Scholar] [CrossRef]
- Tang, L.S.Y.; Covert, E.; Wilson, E.; Kottilil, S. Chronic Hepatitis B Infection: A Review. JAMA 2018, 319, 1802–1813. [Google Scholar] [CrossRef]
- Abel, A.G.; Shamarina, S.; Hisham, M.; Hafiz, A.R.A. Prevalence and Associated Risk Factors of Hepatitis B Virus Infection in Lafia Metropolis, Nasarawa State, Nigeria. Afr. J. Infect. Dis. 2025, 19, 45–56. [Google Scholar] [PubMed]
- World Health Organization. Preventing Perinatal Hepatitis B Virus Transmission: A Guide for Introducing and Strengthening Hepatitis B Birth Dose Vaccination; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Seto, W.-K.; Lo, Y.-R.; Pawlotsky, J.-M.; Yuen, M.-F. Chronic hepatitis B virus infection. Lancet 2018, 392, 2313–2324. [Google Scholar] [CrossRef]
- Athalye, S.; Khargekar, N.; Shinde, S.; Parmar, T.; Chavan, S.; Swamidurai, G.; Pujari, V.; Panale, P.; Koli, P.; Shankarkumar, A.; et al. Exploring risk factors and transmission dynamics of Hepatitis B infection among Indian families: Implications and perspective. J. Infect. Public Health 2023, 16, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Mangowi, I.; Mirambo, M.M.; Kilonzo, S.B.; Mlewa, M.; Nyawale, H.; Majinge, D.; Hyera, F.; Jaka, H.; Mtemisika, C.; Michael, F.; et al. Hepatitis B virus infection, associated factors, knowledge and vaccination status among household contacts of hepatitis B index cases in Mwanza, Tanzania. IJID Reg. 2024, 10, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Sofian, M.; Banifazl, M.; Ziai, M.; Aghakhani, A.; Farazi, A.A.; Ramezani, A. Intra-familial Transmission of Hepatitis B Virus Infection in Arak, Central Iran. Iran. J. Pathol. 2016, 11, 328–333. [Google Scholar]
- Morgan, C.E.; Ngimbi, P.; Boisson-Walsh, A.J.N.; Ntambua, S.; Matondo, J.; Tabala, M.; Kashamuka, M.M.; Emch, M.; Edwards, J.K.; Powers, A.K.; et al. Hepatitis B Virus Prevalence and Transmission in the Households of Pregnant Women in Kinshasa, Democratic Republic of Congo. Open Forum Infect. Dis. 2024, 11, ofae150. [Google Scholar] [CrossRef]
- Jafari, S.; Buxton, J.A.; Afshar, K.; Copes, R.; Baharlou, S. Tattooing and risk of hepatitis B: A systematic review and meta-analysis. Can. J. Public Health 2012, 103, 207–212. [Google Scholar] [CrossRef]
- Chuang, S.C.; Lee, Y.C.; Hashibe, M.; Dai, M.; Zheng, T.; Boffetta, P. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1261–1268. [Google Scholar] [CrossRef]
- Liu, X.; Baecker, A.; Wu, M.; Zhou, J.; Yang, J.; Han, R.; Wang, P.; Jin, Z.; Liu, A.; Gu, X.; et al. Interaction between tobacco smoking and hepatitis B virus infection on the risk of liver cancer in a Chinese population. Int. J. Cancer 2018, 142, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.