Abstract
Introduction: Metformin, a commonly used oral antihyperglycemic drug, poses a rare risk related to the development of metformin-associated lactic acidosis (MALA). The Extracorporeal Treatments in Poisoning (EXTRIP) group recommended intermittent haemodialysis (IHD) as a primary elimination therapy in case of severe metformin poisoning. However, in haemodynamically unstable patients, our previous observations suggested that continuous venovenous haemofiltration (CVVH) might be more effective. This retrospective cohort study aimed to contribute evidence on the use of IHD and CVVH in patients with severe MALA, particularly in haemodynamically unstable patients. Methods: Data from January 2015 to December 2020 were collected from the Leuven University Hospital. Two separate search methods, based on hospital activity records and laboratory criteria, were used to identify patients with MALA. Patients diagnosed with MALA, receiving extracorporeal treatment within 24 h of admission, were included. Patients were categorized into the IHD and CVVH groups. Patient characteristics, treatment details, and outcomes were analysed. Results: Among 358,148 patient records, 35 MALA cases were identified. IHD was chosen as the initial elimination technique in 13 cases, whereas 22 patients were first commenced on CVVH. Patients treated with CVVH were sicker, had more comorbidities and had higher ventilation and vasopressor requirements. CVVH group had longer vasopressor use, longer ICU stays, and higher in-hospital mortality. Discussion: CVVH rather than IHD seems to be the preferred elimination technique in the more critically ill patients with MALA. Due to its retrospective design, this study failed to identify the superior elimination technique in terms of efficacy. Poorer outcomes in the CVVH group are likely attributed to the severity of illness rather than the inferiority of the elimination therapy. We acknowledge the diagnostic challenges regarding MALA. Using metformin assays could be beneficial in managing these patients. Conclusions: This study suggests clinicians’ preference for CVVH in severe cases of MALA with haemodynamic instability.
1. Introduction
Metformin is a widely used oral antihyperglycemic drug in the treatment of type 2 diabetes [1,2,3]. It has a complex mechanism of action, including the inhibition of gluconeogenesis, the facilitation of cellular glucose uptake and decreasing insulin resistance [1,2,3,4,5]. The elimination of metformin is performed predominantly by the kidneys [1,2,4,6].
Metformin is known to increase plasma lactate levels by impairing mitochondrial respiration and the inhibition of pyruvate carboxylase [1,2,3,7]. The latter reduces the conversion of lactate to pyruvate. Therapeutic doses of metformin cause minimal (usually less than 1–2 mmol/L) or no elevation in blood lactate levels [2,3]. Metformin toxicity, whether resulting from an acute intentional overdose or chronic exposure in individuals with impaired kidney function, can lead to a condition known as metformin-associated lactic acidosis (MALA). The risk of developing MALA increases further when there are concurrent conditions that elevate lactate levels (e.g., hypotension, sepsis or liver failure) or worsen acidaemia (e.g., hyperuricaemia in acute kidney failure, salicylate toxicity or ketoacidosis) [1,2,3,4,5].
MALA is a rare condition with an estimated prevalence of less than 10 cases/100,000 patient years [1,2,3,4,8]. However, it is associated with high mortality rates (30–50%) [1,2,4].
In 2015, a systematic review was published by the “Extracorporeal Treatments in Poisoning” (EXTRIP) expert group [1]. They advised the use of renal replacement therapy in case of severe metformin poisoning. More specifically, they suggested intermittent haemodialysis (IHD) as the first line elimination therapy and considered continuous venovenous haemofiltration (CVVH) as an acceptable alternative. This systematic review consisted of 175 articles, including 160 case reports, 11 studies of descriptive cohorts and 3 pharmacokinetic studies in end-stage renal disease, therefore yielding a very low quality of evidence for all recommendations [1].
In June 2020, we presented two cases that may indicate that, specifically in haemodynamically unstable patients with severe metabolic acidosis due to metformin poisoning, CVVH is a better therapeutic approach for achieving effective lactate elimination and metabolic correction and thus should be considered as a first-choice elimination technique [9].
The primary objective of this retrospective cohort study was to investigate the clinical outcomes and characteristics of patients diagnosed with MALA who underwent extracorporeal treatment (ECTR) in our centre. Thus, we aimed to contribute additional evidence and insights to the EXTRIP study group’s recommendation to prioritize IHD over CVVH as a first-line treatment for severe metformin poisoning, particularly within the subgroup of patients experiencing haemodynamic instability.
2. Methods
This retrospective single-centre study was conducted at the Leuven University Hospital in Belgium. Data were obtained from electronic medical records of patients admitted to the emergency department (ED) between January 2015 and December 2020. The study population was identified through two separate search methods using predefined criteria. As metformin is not routinely assayed in our clinical laboratory, alternative criteria were used to identify patients with acute metformin toxicity.
The first search method was performed based on certain hospital activity records. Patients were selected if they had any of the following registrations within 72 h after being hospitalized: IHD at the intensive care unit (ICU) or dialysis centre, CVVH or central venous catheter placement.
In the second search method, patients with a history of chronic metformin treatment at the time of admission were selected if they displayed laboratory signs of severe lactic acidosis, characterized by lactate levels > 7 mmol/L and pH < 7.2. To prevent any negative selection bias and in line with EXTRIP’s consensus that certain comorbid conditions (shock, impaired kidney function, liver failure and decreased level of consciousness) should prompt a lower threshold for ECTR, we intentionally opted for a lower threshold than EXTRIP’s standard recommendation of initiating ECTR if pH ≤ 7.0 or lactate concentration > 20 mmol/L [1].
Subsequently, a single investigator (LH) screened the initial search populations in both queries. Patients who were suspected of having MALA and received ECTR within 24 h of admission were included. Duplicate entries were removed, resulting in a final selection of inclusions.
The following patient data were extracted from the medical records: time of emergency admission, gender, age, regular metformin dose, creatinine levels upon admission and baseline, estimated glomerular filtration rates (eGFR) using the chronic kidney disease epidemiology collaboration (CKD-EPI) equation [10] upon admission and baseline, ECTR modality, time of ECTR initiation, blood pH and lactate levels at start of ECTR, mechanical ventilation or vasopressor requirement prior to ECTR initiation, time until metabolic correction (pH > 7.35 and PaCO2 > 35 mmHg) and lactate clearance (lactate ≤ 2 mmol/L), vasopressor treatment duration, ECTR flow rates, length of ICU stay (LOS), in-hospital mortality and secondary admission diagnoses. In cases where the baseline creatinine levels were unavailable, they were estimated using the Modification of Diet in Renal Disease (MDRD) study equation [11].
The choice of ECTR modality was at the treating clinician’s discretion, given the absence of specific guidelines on this matter in our hospital at the time. Depending on the initial ECTR modality, inclusions were categorized into either the IHD or CVVH group. Patient characteristics were compared between both groups. Data analysis was performed with SPSS. In the case of a normal distribution of continuous data, the independent-sample t test was used. Pearson’s chi-square test was used to compare nominal data between groups. In all tests, a p-value < 0.05 was considered statistically significant.
Patients who did not achieve metabolic correction or lactate clearance were excluded from the calculation of the corresponding mean values. The mean duration of vasopressor treatment was calculated by excluding patients who were undergoing vasopressor therapy at the time of their death in hospital. Similarly, when determining the mean length of stay in the ICU, patients who died in hospital were excluded. In both cases, including these patients would have inaccurately reduced the mean values.
3. Results
A total of 649 and 172 patient records were initially retrieved by conducting searches based on hospital activity records and laboratory values, respectively. Subsequent screening of the records and removal of duplicates resulted in 35 patient records being included for further analysis (Figure 1). Patient characteristics are listed in Table 1.
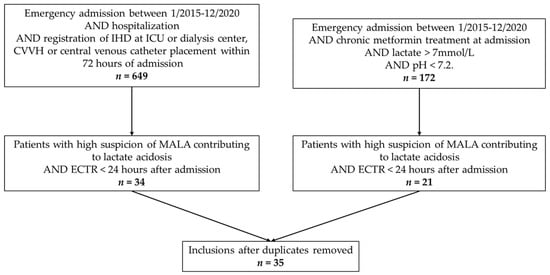
Figure 1.
Flow chart of patient selection. IHD = intermittent haemodialysis; ICU = intensive care unit; CVVH = continuous venovenous haemofiltration; MDLA = metformin-associated lactate acidosis; ECTR = extracorporeal treatment.

Table 1.
Patient characteristics. eGFR = estimated glomerular filtration rate; AKI = Acute Kidney Injury; KDIGO = Kidney Disease Improving Global Outcomes; N/A = not applicable; ECTR = extracorporeal treatment; IHD = intermittent haemodialysis; CVVH = continuous venovenous haemofiltration; ICU = intensive care unit; LOS = length of stay; IHCA = in-hospital cardiac arrest; a = estimated using the CKD-EPI equation; b = estimated using the MDRD study equation; c = daily dose exceeding national recommendations; d = till time of death.
No patients with an intentional or accidental acute metformin overdose were identified. However, almost all patients (n = 33) were diagnosed with acute kidney injury (AKI) upon admission according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria [12]. The majority (n = 26) showed severe stage III kidney injury. As the initiation of ECTR was an inclusion criterion for this study, we omitted this criterion in the KDIGO classification as this would result in all patients being classified as having a stage III kidney injury.
Patients were further categorized according to the initial choice of elimination technique (Table 2). In 13 cases, IHD was the first-choice ECTR modality to treat MALA. Four patients within this group received subsequent CVVH after IHD treatment. Twenty-two patients were treated with CVVH only.

Table 2.
Patient characteristics. IHD versus CVVH group. Data are expressed as the number of patients, n, (%) or the mean (standard deviation, SD). Missing p-values due to insufficient sample sizes for statistical analysis. IHD = intermittent haemodialysis; CVVH = continuous venovenous haemofiltration; AKI = acute kidney injury; KDIGO = Kidney Disease Improving Global Outcomes; ECTR = extracorporeal treatment; ICU LOS = intensive care unit length of stay. * Statistically significant difference.
There were no significant differences in gender, age and daily metformin dose between the IHD and CVVH group. In 12 patients, the daily administration of metformin at the time of admission exceeded the maximum dose recommended by the Belgian Centre for Pharmacotherapeutic Information [5] (Table 1).
Mean blood pH and lactate levels at the start of ECTR and the time to metabolic correction and lactate clearance did not differ significantly between both groups (p = 0.437 and p = 0.929, respectively). Metabolic correction could not be demonstrated in 10 patients due to persistent hyperventilation (PaCO2 < 35 mmHg), the absence of arterial blood gas measurements or in-hospital mortality. Five patients failed to attain lactate clearance due to in-hospital mortality.
Patients in the CVVH group had more comorbidities upon admission, likely contributing to lactate acidosis (sepsis, liver failure, shock and cardiac arrest). They also had significant higher mechanical ventilation and vasopressor requirements prior to ECTR initiation (p = 0.015 and p = 0.006, respectively). All patients receiving CVVH following initial IHD treatment (n = 4) required a vasopressor prior to start of IHD.
The total duration of vasopressor treatment and the ICU LOS were significantly longer in the CVVH group when compared to the IHD group. In-hospital mortality was high overall, reaching 23.1% and 53.8% in the IHD and CVVH group, respectively.
Despite our intention to collect data on ECTR flow rates, there were insufficient data available for analysis.
4. Discussion
From January 2015 to December 2020, we identified 35 cases in which patients strongly suspected of having MALA underwent ECTR within 24 h of their emergency admission. Over this period, the ED treated a total of 358,148 patients. This finding aligns with the perception of MALA being a rare condition [1,2,3,4,8]. Furthermore, the elevated mortality rates observed in the study population closely correspond to the rates reported in the current literature [1,2].
Eighteen patient records were identified in both search methods. Fifteen cases were exclusively selected through the search method based on hospital activity records. In many cases, this can be explained by patients not meeting the laboratory criteria for severe lactate acidosis (lactate levels > 7 mmol/L and pH < 7.2). On the other hand, two cases were solely found by using the search method based on laboratory values, likely due to the lack of registration of IHD, CVVH or central venous catheter placement. Interestingly, some patients did meet the laboratory criteria but were only picked up by the search based on hospital activity records. The reason for this remains unclear.
We did not identify any patient with an intentional or accidental acute metformin overdose. However, a substantial number of patients presented with severe kidney injury, likely leading to metformin toxicity and MALA. It is notable that daily metformin dosing prior to admission exceeded the national recommendations [5] in approximately one-third of the patients (n = 12). This emphasizes the importance of individualizing metformin dosing in accordance with baseline kidney function to mitigate the risk of metformin toxicity.
Patients who were initially treated with CVVH appeared to be sicker compared to patients who received IHD as a first-line therapy. They had more comorbid conditions upon admission and were more likely to require mechanical ventilation and vasopressors prior to the initiation of ECTR. Additionally, all four patients who subsequently underwent CVVH following initial IHD treatment required a vasopressor before the start of IHD. Among the patients solely treated with IHD (n = 9), only three (33.3%) required a vasopressor before starting ECTR, in contrast to all but one (96.2%) of the patients who received CVVH treatment at any point during their hospital admission. The extended vasopressor necessity, longer ICU stay and higher in-hospital mortality in the CVVH group likely resulted from the severity of the patients’ condition rather than the choice of ECTR modality.
This study has several limitations. The retrospective design increases the likelihood of data inaccuracies and diminishes the level of evidence when compared to a prospective study design. Furthermore, only one investigator screened the patient records, which possibly contributed to selection bias.
At the Leuven University Hospital, blood metformin assay data are not routinely available. Metformin was assayed in none of the patients screened for inclusion. The EXTRIP study group noted that there is ongoing debate regarding the clinical applicability of metformin assays, and they did not incorporate metformin concentration into their definition of MALA [1]. In contrast, Lalau et al. [3] identified the absence of metformin assays as a major methodological limitation in most studies on MALA. They argued that metformin assays, along with blood pH, lactate levels and clinical context, are essential for distinguishing MALA from other conditions, such as “lactic acidosis in metformin therapy” (LAMT) or “metformin-unrelated lactic acidosis” (MULA) [3]. In this study, almost all patients (n = 33) presented with one or more comorbid conditions that are known to potentially raise lactate levels or cause acidaemia [1,2,3,4,5]. In many cases, it was impossible to exclude the possibility that one of these comorbid conditions was the primary cause of the hyperlactataemia and acidosis rather than the metformin therapy itself. Only fourteen patients were thought to have metformin toxicity as the primary cause of their lactate acidosis. This diagnostic uncertainty further compounds the issue of selection bias.
IHD sessions were held either in the ED or the ICU. The documentation of the sessions’ start and stop times in the ED was often ambiguous, with slightly varying recorded times for a single session in many cases. CVVH sessions took place in the ICU only. Their start and stop times were consistently recorded within a 2 h window, resulting in a potential 4 h margin of error for each CVVH session. The lack of clear documentation regarding the initiation times of ECTR leads to potential errors in the pH and blood lactate levels at the start of ECTR. Additionally, these values, as well as the time required for metabolic correction and lactate clearance, were influenced by the timing and frequency of blood gas sample collection.
This study failed to provide any evidence supporting the superiority of IHD over CVVH in correcting acidaemia or the removal of lactic acid and metformin, as claimed by the EXTRIP study group [1], particularly within the subgroup of patients with haemodynamic instability. This was anticipated given the retrospective design of this study. Factors such as the absence of a protocol, selection bias, small cohort groups with insufficient statistical power, comorbidities, the lack of metformin assays, poor registration and patients transitioning from IHD to CVVH all contributed to the lack of conclusive evidence regarding the efficacy of these techniques. To address these limitations, a randomised controlled trial with a sufficiently powered sample size should be conducted.
5. Conclusions
The data in this retrospective study suggest that CVVH rather than IHD is the initial ECTR modality of choice in case of severe MALA with haemodynamic instability. Due to its inherent limitations, this study did not offer additional evidence regarding the efficacy of both elimination techniques in correcting metabolic status or eliminating lactate. The CVVH group had a worse prognosis, although this was not statistically significant and was confounded by the patients’ more severe characteristics at baseline and small sample size.
It remains important to titrate metformin doses individually based on renal function, given that a substantial number of patients exceeded the daily recommended doses and almost all suffered from kidney failure at the time of MALA diagnosis.
The high mortality rates in the study underline the need for further research to guide treatment and improve outcomes in patients with MALA.
Author Contributions
Conceptualization, L.H., S.V. and M.S.; methodology, L.H., S.V. and M.S.; formal analysis, D.D.; investigation, L.H. and S.V.; writing—original draft preparation, L.H., S.V. and M.S.; writing—review and editing, L.H., S.V., D.D. and M.S.; visualization, L.H.; supervision, S.V. and M.S.; project administration, L.H., S.V. and M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Leuven University Hospital (protocol code S64978, 15 February 2021).
Informed Consent Statement
Patient consent for this study was waived as it was logistically impossible due to the size of the patient population and the timeframe under examination.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, MS, upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Calello, D.P.; Liu, K.D.; Wiegand, T.J.; Roberts, D.M.; Lavergne, V.; Gosselin, S.; Hoffman, R.S.; Nolin, T.D.; Ghannoum, M. Extracorporeal Treatment for Metformin Poisoning. Crit. Care Med. 2015, 43, 1716–1730. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lalau, J.D.; Kajbaf, F.; Protti, A.; Christensen, M.M.; De Broe, M.E.; Wiernsperger, N. Metformin-associated lactic acidosis (MALA): Moving towards a new paradigm. Diabetes Obes. Metab. 2017, 19, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Turner, R.C. Metformin. N. Engl. J. Med. 1996, 334, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, T.; Maloteaux, J. Belgisch Centrum Voor Farmacotherapeutische Informatie. Gecommentarieerd Geneesmiddelen-Repertorium 2022 [Internet]. 34th ed. Thierry Christiaens. 2022. Available online: https://www.bcfi.be/ggr_pdfs/GGR_NL_2022.pdf (accessed on 5 October 2023).
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Donnino, M.; Montissol, S.; Andersen, L.W.; Chase, M.; Liu, X. Metformin inhibits pyruvate dehydrogenase at high dosages: A potential mechanism for lactic acidosis. Acad. Emerg. Med. 2014, 21, S181. [Google Scholar]
- Stang, M.; Wysowski, D.K.; Butler-Jones, D. Incidence of lactic acidosis in metformin users. Diabetes Care 1999, 22, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Heeren, L.; Sabbe, M.; Christiaen, J.; Verelst, S. Lactate Acidosis Following a Metformin Poisoning: Which Elimination Therapy to use in Case of Haemodynamic Instability? A Case Report. Ann. Case Rep. 2020, 14, 414. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P.; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter. Suppl. 2012, 2, 122–123. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).