Hydrogen-Bond Engineering for Highly Efficient Room-Temperature Phosphorescence with Tunable Multi-Color Emission
Abstract
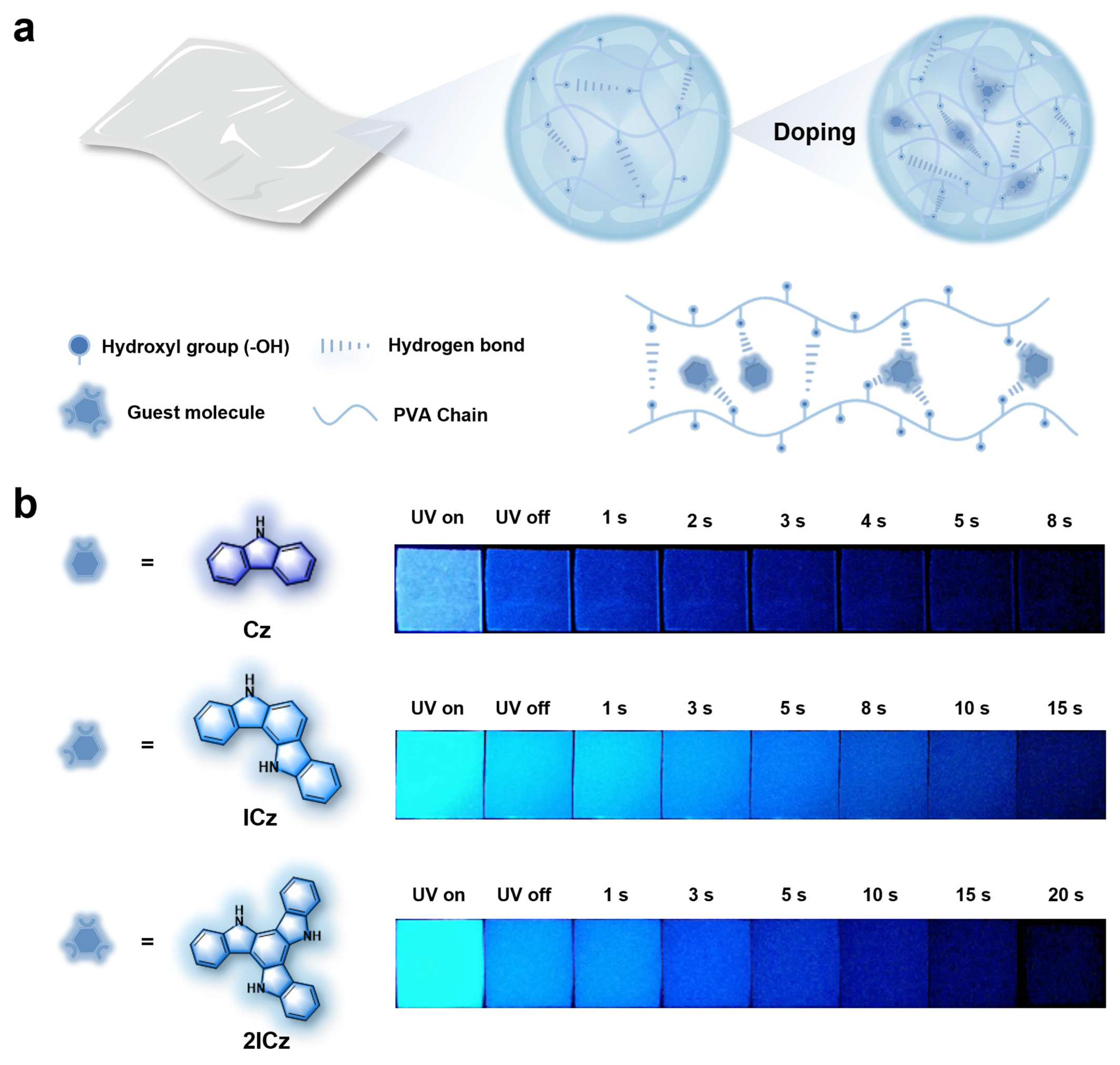
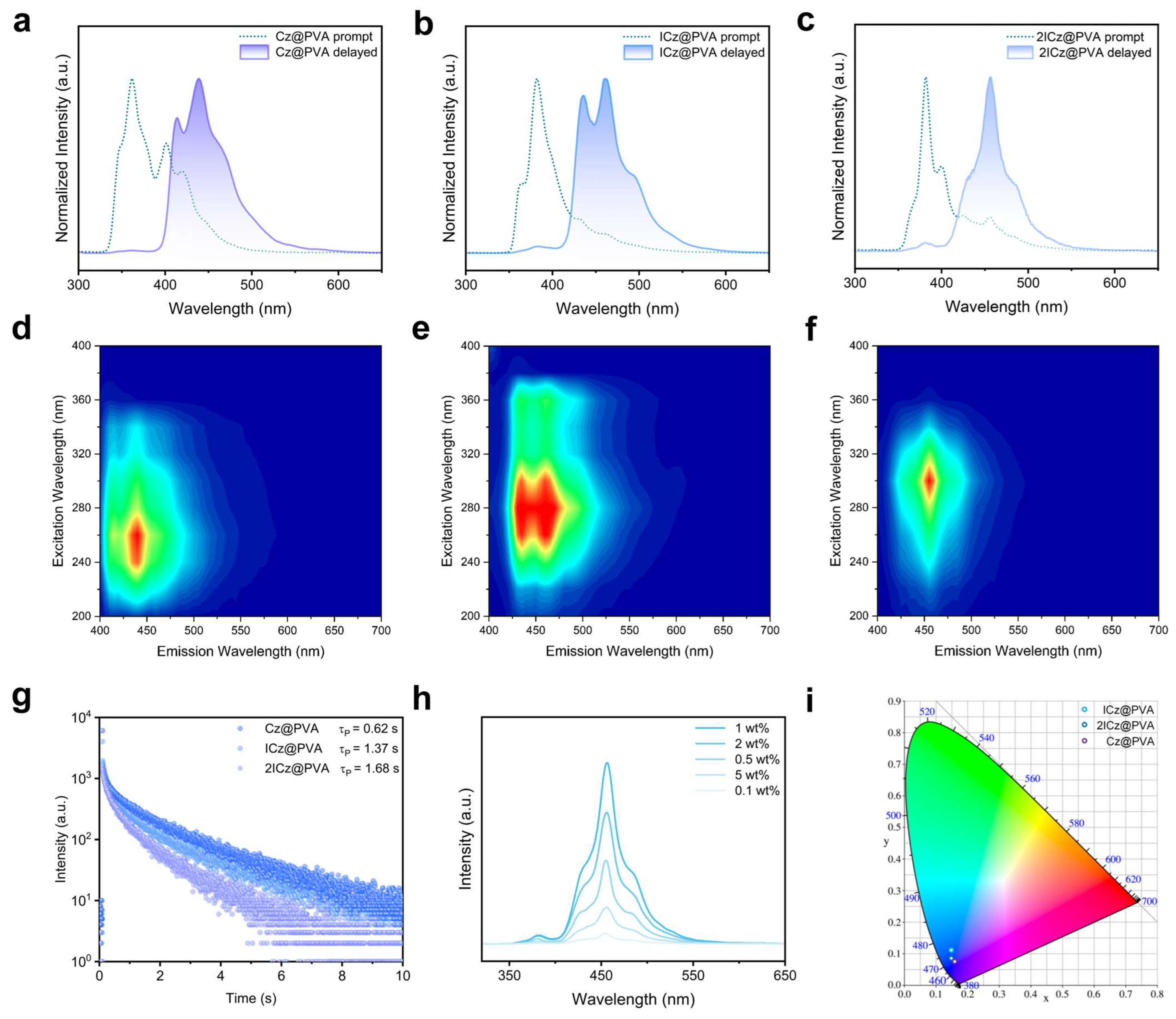
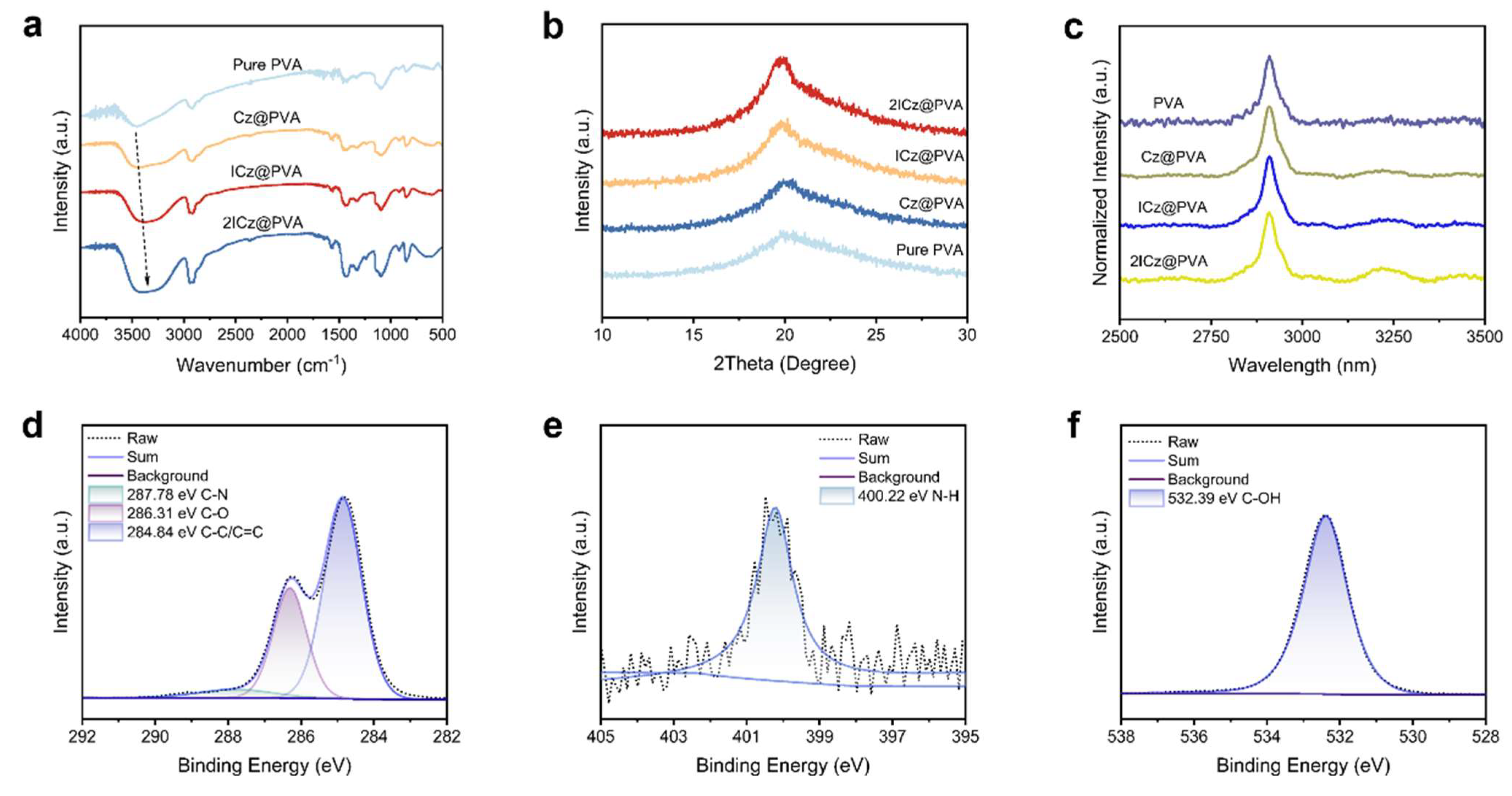
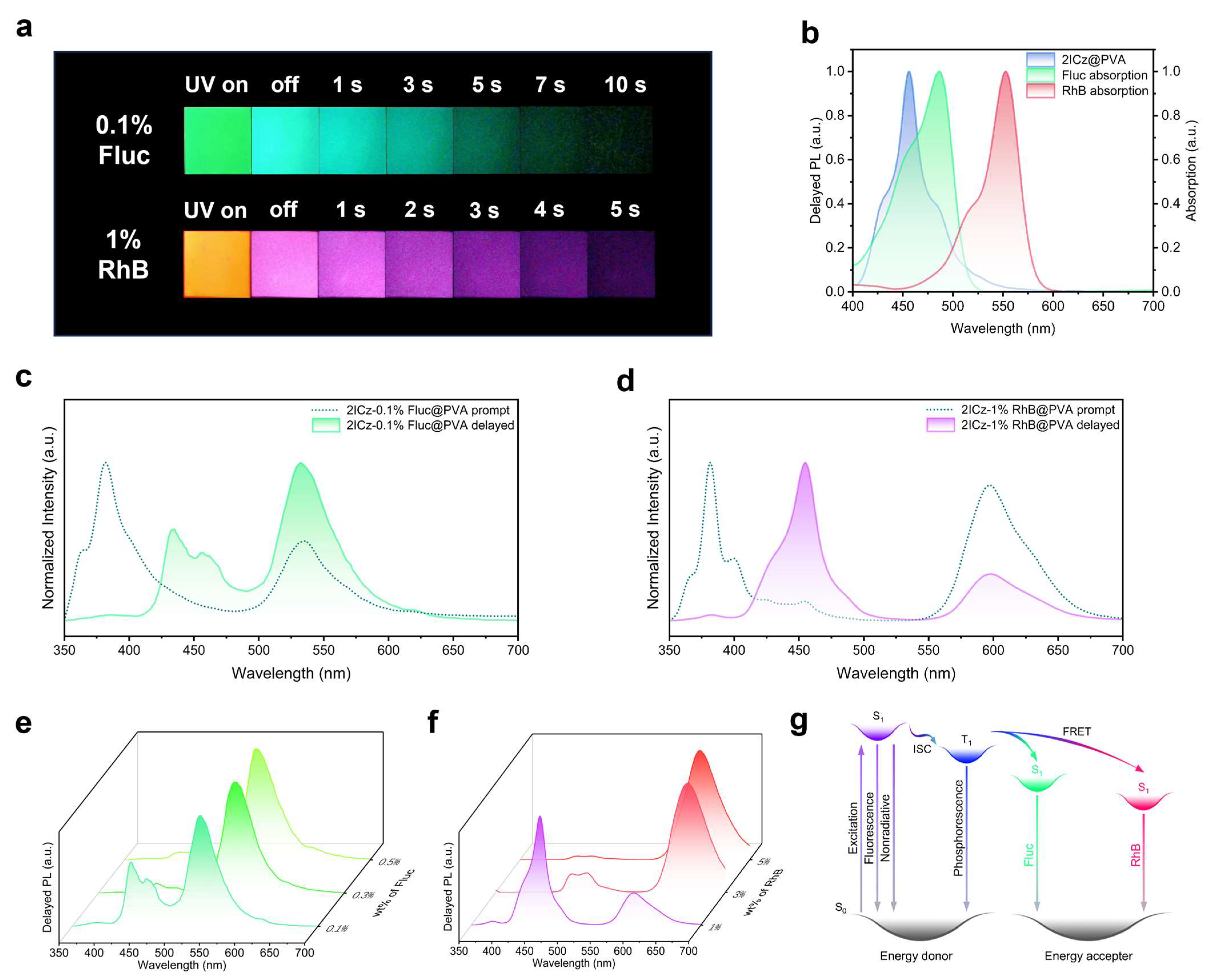
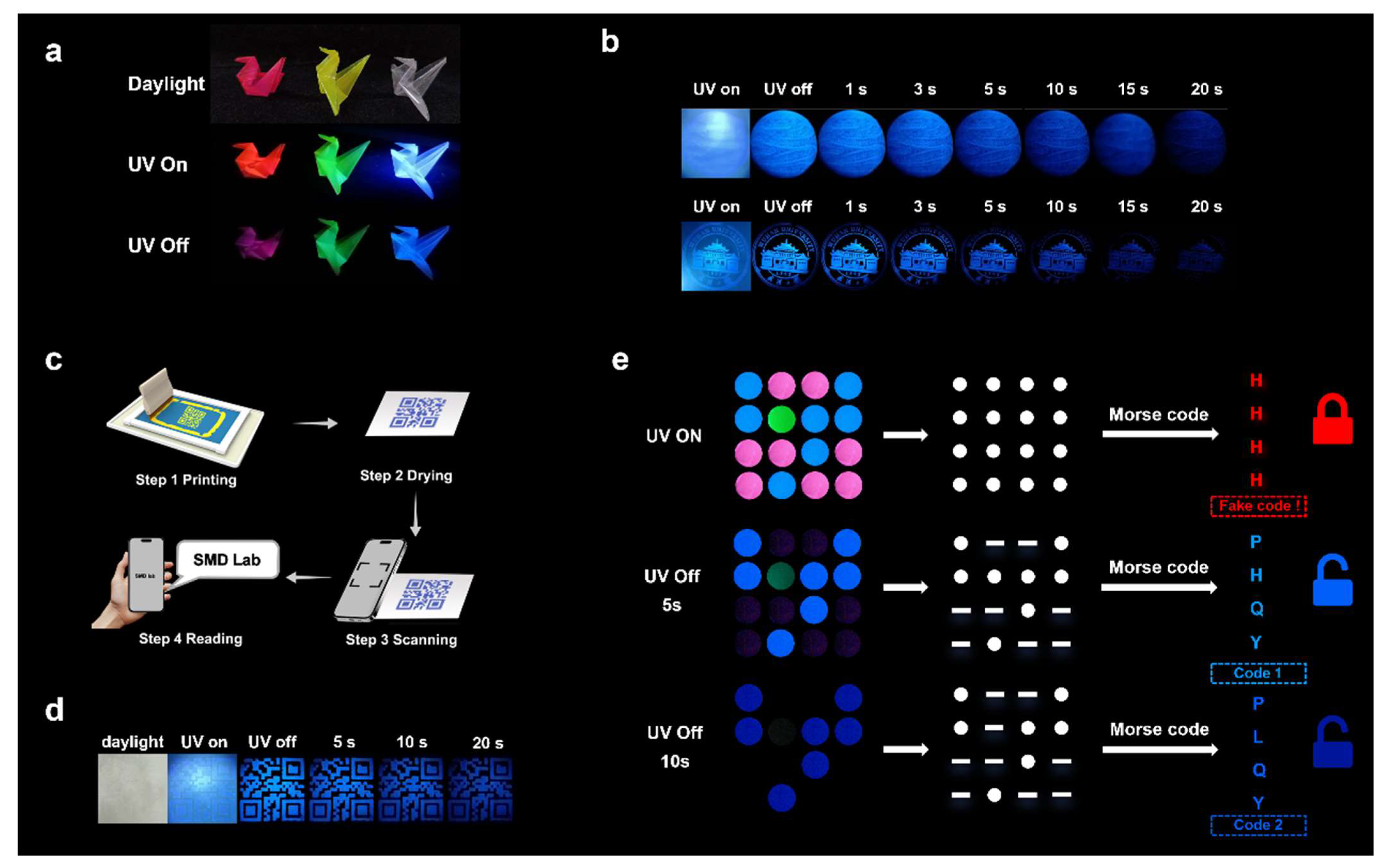
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Cz@PVA
2.1.2. Preparation of ICz@PVA
2.1.3. Preparation of 2ICz@PVA
2.2. Characterization
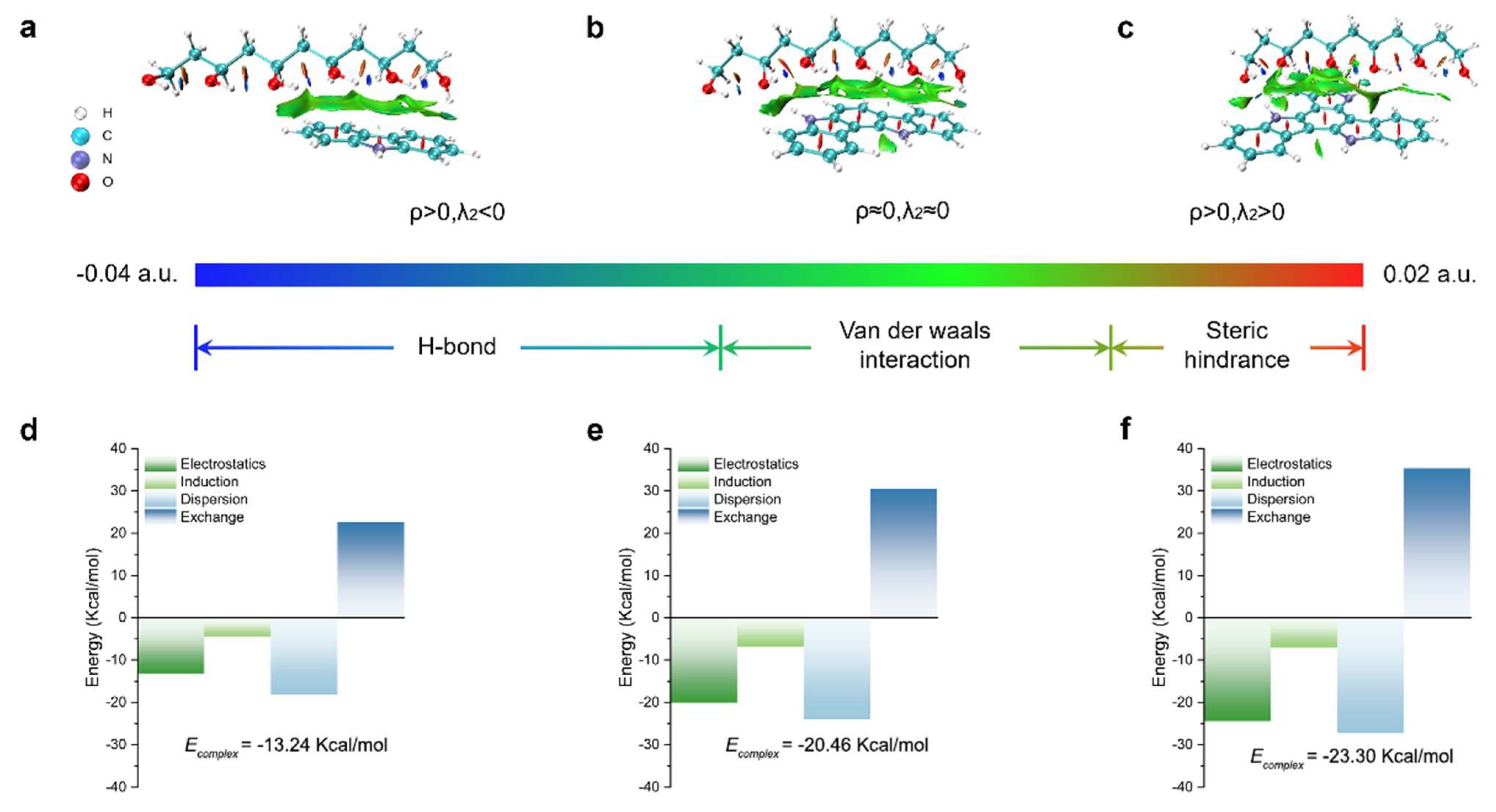
2.3. Theoretical Calculation
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Z.; Zhang, X.; Wang, H.; Huang, C.; Sun, H.; Dong, M.; Ji, L.; An, Z.; Yu, T.; Huang, W. Wide-range lifetime-tunable and responsive ultralong organic phosphorescent multi-host/guest system. Nat. Commun. 2021, 12, 3522. [Google Scholar] [CrossRef]
- Ye, W.; Ma, H.; Shi, H.; Wang, H.; Lv, A.; Bian, L.; Zhang, M.; Ma, C.; Ling, K.; Gu, M.; et al. Confining isolated chromophores for highly efficient blue phosphorescence. Nat. Mater. 2021, 20, 1539–1544. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Yang, M.; Dai, W.; Shi, J.; Tong, B.; Cai, Z.; Wang, Z.; Dong, Y.; Yu, X. Organic room-temperature phosphorescence materials for bioimaging. Chem. Commun. 2023, 59, 5329–5342. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, K.-K.; Liang, Y.-C.; Song, S.-Y.; Deng, Y.; Mao, X.; Wang, Y.; Zhao, W.-B.; Lou, Q.; Shan, C.-X. Brighten Triplet Excitons of Carbon Nanodots for Multicolor Phosphorescence Films. Nano Lett. 2022, 22, 4097–4105. [Google Scholar] [CrossRef]
- Wang, X.; Shi, H.; Ma, H.; Ye, W.; Song, L.; Zan, J.; Yao, X.; Ou, X.; Yang, G.; Zhao, Z.; et al. Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence. Nat. Photonics 2021, 15, 187–192. [Google Scholar] [CrossRef]
- Tian, R.; Gao, S.; Li, K.; Lu, C. Design of mechanical-robust phosphorescence materials through covalent click reaction. Nat. Commun. 2023, 14, 4720. [Google Scholar] [CrossRef] [PubMed]
- Keruckiene, R.; Vekteryte, S.; Urbonas, E.; Guzauskas, M.; Skuodis, E.; Volyniuk, D.; Grazulevicius, J.V. Synthesis and properties of quinazoline-based versatile exciplex-forming compounds. Beilstein J. Org. Chem. 2020, 16, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-K.; Kang, J.-Y.; Wang, Y.-J.; Gao, Y.-J.; Zeng, Y. Theoretical studies on benzonitrile-carbazole-based pure organic molecules with room-temperature phosphorescence. Next Mater. 2025, 7, 100351. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Ding, L.; Yang, Y.; Guan, T.; Qin, C.; Liu, Y.J. Time-resolved excited-state dynamics of star-shaped carbazole-based room temperature phosphorescent molecule by ultrafast absorption spectroscopy. J. Lumin. 2024, 26, 120275. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Y.; Lei, Y.; Dai, W.; Liu, M.; Cai, Z.; Wu, H.; Huang, X.; Ma, X. Twofold rigidity activates ultralong organic high-temperature phosphorescence. Nat. Commun. 2024, 15, 1269. [Google Scholar] [CrossRef]
- Guan, Z.; Tang, Z.; Zeng, J.; Zheng, Y.; Ding, L.; Chen, D.; Li, H.; Liu, X. Stepwise Stiffening Chromophore Strategy Realizes a Series of Ultralong Blue Room-Temperature Phosphorescent Materials. Adv. Sci. 2024, 11, e2402632. [Google Scholar] [CrossRef]
- Wang, D.; Gong, J.; Xiong, Y.; Wu, H.; Zhao, Z.; Wang, D.; Tang, B.Z. Achieving Color-Tunable and Time-Dependent Organic Long Persistent Luminescence via Phosphorescence Energy Transfer for Advanced Anti-Counterfeiting. Adv. Funct. Mater. 2023, 33, 2208895. [Google Scholar] [CrossRef]
- Zhou, L.; Song, J.; He, Z.; Liu, Y.; Jiang, P.; Li, T.; Ma, X. Achieving Efficient Dark Blue Room-Temperature Phosphorescence with Ultra-Wide Range Tunable-Lifetime. Angew. Chem. Int. Ed. 2024, 63, e202403773. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Wang, C.; Zheng, X.; Zheng, Y.; Gao, L.; Yang, C.; Li, Y.; Qu, L.; Zhao, Y. Color-Tunable Polymeric Long-Persistent Luminescence Based on Polyphosphazenes. Adv. Mater. 2020, 32, 1907355. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, Z.; Wang, D.; Deng, G.; Lin, S.; Zhao, Z.; Wang, D.; Xiong, Y.; Tang, B.Z. Positional Isomerism Toward Tunable Organic Afterglow and Reversible Photoactivation Behavior for Anti-Counterfeiting and Encryption. Adv. Funct. Mater. 2025, 35, 2415285. [Google Scholar] [CrossRef]
- Chen, P.; Qie, H.; Yang, X.; Ma, S.; Wang, Z.; Li, N.; Deng, Y.; Bian, F.; Lu, S. Luminous Fish-Inspired Hydrogels with Underwater Long-Lived Room-Temperature Phosphorescence. Adv. Funct. Mater. 2025, 35, 2416430. [Google Scholar] [CrossRef]
- Wang, J.; Gu, X.; Ma, H.; Peng, Q.; Huang, X.; Zheng, X.; Sung, S.H.P.; Shan, G.; Lam, J.W.Y.; Shuai, Z.; et al. A facile strategy for realizing room temperature phosphorescence and single molecule white light emission. Nat. Commun. 2018, 9, 2963. [Google Scholar] [CrossRef]
- Shi, H.; Song, L.; Ma, H.; Sun, C.; Huang, K.; Lv, A.; Ye, W.; Wang, H.; Cai, S.; Yao, W.; et al. Highly Efficient Ultralong Organic Phosphorescence through Intramolecular-Space Heavy-Atom Effect. Phys. Chem. Lett. 2019, 10, 595–600. [Google Scholar] [CrossRef]
- Yan, Z.-A.; Lin, X.; Sun, S.; Ma, X.; Tian, H. Activating Room-Temperature Phosphorescence of Organic Luminophores via External Heavy-Atom Effect and Rigidity of Ionic Polymer Matrix. Angew. Chem. Int. Ed. 2021, 60, 19735–19739. [Google Scholar] [CrossRef]
- Bolton, O.; Lee, K.; Kim, H.-J.; Lin, K.Y.; Kim, J. Activating efficient phosphorescence from purely organic materials by crystal design. Nat. Chem. 2011, 3, 205–210. [Google Scholar] [CrossRef]
- Miao, Y.; Lin, F.; Guo, D.; Chen, J.; Zhang, K.; Wu, T.; Huang, H.; Chi, Z.; Yang, Z. Stable and ultralong room-temperature phosphorescent copolymers with excellent adhesion, resistance, and toughness. Sci. Adv. 2024, 10, eadk3354. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Shi, H.; Ma, H.; Bian, L.; Wu, Q.; Gu, L.; Cai, S.; Wang, X.; Xiong, W.; An, Z.; et al. Ultralong Phosphorescence from Organic Ionic Crystals under Ambient Conditions. Angew. Chem. Int. Ed. 2018, 57, 678–682. [Google Scholar] [CrossRef]
- Ma, L.; Sun, S.; Ding, B.; Ma, X.; Tian, H. Highly Efficient Room-Temperature Phosphorescence Based on Single-Benzene Structure Molecules and Photoactivated Luminescence with Afterglow. Adv. Funct. Mater. 2021, 31, 2010659. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Y.; Wu, H.; Wang, Z.; Wang, C.; Zheng, Y.; Zheng, X.; Gao, L.; Zhou, Q.; Yang, Y.; et al. Large-Area, Flexible, Transparent, and Long-Lived Polymer-Based Phosphorescence Films. Am. Chem. Soc. 2021, 143, 13675–13685. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Fang, M.; Tang, B.Z.; Li, Z. Stimulus-responsive room temperature phosphorescence materials with full-color tunability from pure organic amorphous polymers. Sci. Adv. 2022, 8, eabl8392. [Google Scholar] [CrossRef]
- Wang, K.; Qu, L.; Yang, C. Long-Lived Dynamic Room Temperature Phosphorescence from Carbon Dots Based Materials. Small 2023, 19, 2206429. [Google Scholar] [CrossRef]
- Zhao, W.; He, Z.; Tang, B.Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 2020, 5, 869–885. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Sun, L.; Wu, S.; Hu, G.; Pang, X.; Smith, A.T.; Hu, C.; Zeng, S.; Wang, W.; et al. Ultralong lifetime and efficient room temperature phosphorescent carbon dots through multi-confinement structure design. Nat. Commun. 2020, 11, 5591. [Google Scholar] [CrossRef]
- Zeng, J.; Tang, Z.; Yin, J.; Guan, Z.; Wei, R.; Ke, X.; Liu, X. Time-Dependent Phosphorescence from Carbon Dots Enables Multidimensional Photoactivated Printing and Tunable Molecular Calculations. Chem. Eng. J. 2024, 502, 157819. [Google Scholar] [CrossRef]
- Jia, Q.; Yan, X.; Wang, B.; Li, J.; Xu, W.; Shen, Z.; Bo, C.; Li, Y.; Chen, L. Construction of room temperature phosphorescent materials with ultralong lifetime by in-situ derivation strategy. Nat. Commun. 2023, 14, 4164. [Google Scholar] [CrossRef]
- Huang, A.; Fan, Y.; Wang, K.; Wang, Z.; Wang, X.; Chang, K.; Gao, Y.; Chen, M.; Li, Q.; Li, Z. Organic Persistent RTP Crystals: From Brittle to Flexible by Tunable Self-Partitioned Molecular Packing. Adv. Mater. 2023, 35, 2209166. [Google Scholar] [CrossRef]
- Yao, X.; Ma, H.; Wang, X.; Wang, H.; Wang, Q.; Zou, X.; Song, Z.; Jia, W.; Li, Y.; Mao, Y.; et al. Ultralong organic phosphorescence from isolated molecules with repulsive interactions for multifunctional applications. Nat. Commun. 2022, 13, 4890. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, M.; Yang, M.; Yang, Q.; Zhang, Z.; Shi, J. Induction of long-lived room temperature phosphorescence of carbon dots by water in hydrogen-bonded matrices. Nat. Commun. 2018, 9, 734. [Google Scholar] [CrossRef]
- Guan, Z.; Tang, Z.; Zeng, J.; Deng, J.; Zheng, Y.; Li, H.; Liu, X. Molecular Engineering Enables Multi-Color Room Temperature Phosphorescence of Carbon Dots Composites Derived In Situ, Facilitating their Utilization for Advanced Information Encryption. Adv. Opt. Mater. 2024, 12, 2302820. [Google Scholar] [CrossRef]
- Ma, H.; Yu, H.; Peng, Q.; An, Z.; Wang, D.; Shuai, Z.J. Hydrogen Bonding-Induced Morphology Dependence of Long-Lived Organic Room-Temperature Phosphorescence: A Computational Study. Phys. Chem. Lett. 2019, 10, 6948–6954. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Q.; Qu, D.-H. merging Hydrogen-Bond Design for High-Performance Dynamic Polymeric Materials. ACS Mater. Lett. 2023, 5, 480. [Google Scholar] [CrossRef]
- Yan, S.; Guan, Y.; Zhou, X.; Mei, C.; Mao, H.; Ma, H.; An, Z.; Shi, H.; Huang, W. Highly Efficient Amorphous Polymer-Based Ultralong Phosphorescence Enabled by Intense Repulsive Interactions. Adv. Funct. Mater. 2025, 35, 2413878. [Google Scholar] [CrossRef]
- Gao, L.; Huang, J.; Qu, L.; Chen, X.; Zhu, Y.; Li, C.; Tian, Q.; Zhao, Y.; Yang, C. Stepwise taming of triplet excitons via multiple confinements in intrinsic polymers for long-lived room-temperature phosphorescence. Nat. Commun. 2023, 14, 7252. [Google Scholar] [CrossRef]
- Jiang, J.; Du, X.; Zhang, K.J. Achieving Ultralong Room-Temperature Phosphorescence in Covalent Organic Framework System. Phys. Chem. Lett. 2024, 15, 1658–1667. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q.J. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, H.; Gong, J.; Xiong, Y.; Wu, Q.; Zhao, Z.; Wang, L.; Wang, D.; Tang, B.Z. Unveiling the crucial contributions of electrostatic and dispersion interactions to the ultralong room-temperature phosphorescence of H-bond crosslinked poly(vinyl alcohol) films. Mater. Horiz. 2022, 9, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Xie, G.; Cao, Y.; Zhang, L.; Yan, X.; Zhang, X.; Miao, S.; Tao, Y.; Li, H.; Zheng, C.; et al. On-demand modulating afterglow color of water-soluble polymers through phosphorescence FRET for multicolor security printing. Sci. Adv. 2022, 8, eabk2925. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Tang, Z.; Long, J.; Ke, X.; Peng, R.; Wei, R.; Liu, X. Hydrogen-Bond Engineering for Highly Efficient Room-Temperature Phosphorescence with Tunable Multi-Color Emission. Spectrosc. J. 2025, 3, 28. https://doi.org/10.3390/spectroscj3040028
Ding L, Tang Z, Long J, Ke X, Peng R, Wei R, Liu X. Hydrogen-Bond Engineering for Highly Efficient Room-Temperature Phosphorescence with Tunable Multi-Color Emission. Spectroscopy Journal. 2025; 3(4):28. https://doi.org/10.3390/spectroscj3040028
Chicago/Turabian StyleDing, Lin, Zhaorun Tang, Jiyang Long, Xianwen Ke, Ruqian Peng, Ruyi Wei, and Xinghai Liu. 2025. "Hydrogen-Bond Engineering for Highly Efficient Room-Temperature Phosphorescence with Tunable Multi-Color Emission" Spectroscopy Journal 3, no. 4: 28. https://doi.org/10.3390/spectroscj3040028
APA StyleDing, L., Tang, Z., Long, J., Ke, X., Peng, R., Wei, R., & Liu, X. (2025). Hydrogen-Bond Engineering for Highly Efficient Room-Temperature Phosphorescence with Tunable Multi-Color Emission. Spectroscopy Journal, 3(4), 28. https://doi.org/10.3390/spectroscj3040028





