Abstract
Background: Nodal dendritic cells (DCs) and CD169-positive macrophages, possibly monocyte-derived, cross-present cancer antigens earlier in the proximal node than in the distal node. Methods: We performed immunohistochemical and morphometric analyses to show differences in the distributions of DC-SIGN-, CD68-, and CD169-positive cells in the paratracheal, subcarinal, and hilar nodes from 25 non-small cell lung cancer patients without metastasis. Results: CD169-positive and DC-SIGN-positive cells were colocalized in the subcapsular and paracortical sinuses, whereas CD68-positive, self-renewal alveolar macrophages were present in the medullary sinus. This complementary distribution was more evident in nodes other than hilar nodes. In hilar nodes, the proportion of CD68-positive macrophages usually exceeds 50%. Notably, the proportion of the overlapped cluster between CD169-positive cells and DC-SIGN-positive cells, which likely corresponds to the cross-presentation activity, was almost the same between the hilar and “next-upstream” node (i.e., the paratracheal node for the upper lobe and the subcarinal node for the lower lobe). Monocyte-derived cells occupied a significantly larger area in the hilar nodes of patients with upper lobe cancer than in patients with lower lobe cancer (p = 0.002–0.009). Conclusion: The specific site occupying the lung hilum with collateral vessels seemed to determine the hilar node composite cells.
1. Introduction
Nodal dendritic cells (DCs) and macrophages are key antigen-presenting cells involved in cancer immunity. In this context, this definition did not include follicular dendritic cells. Among lymph node macrophages, two major populations are recognized: self-renewing alveolar macrophages derived from embryonic progenitors and monocyte-derived macrophages that are recruited from circulating monocytes in response to inflammation or cancer [1,2,3]. These two populations exhibit distinct functional roles in cancer immunity—self-renewing macrophages often maintain tissue homeostasis, while monocyte-derived macrophages are more dynamic and can actively participate in antigen presentation. Recent studies have reported that both groups of macrophages are generally immunosuppressive and often favor nodal metastasis [4,5,6,7,8,9,10,11,12,13]. However, CD169 (SIGLEC1 or sialoadhesin)-positive macrophages are believed to cross-present cancer antigens with DCs [12,14,15]. CD169-positive macrophages also enhance the priming of T lymphocytes by DCs, with the localized production of type I interferons in the cancer regional node, resulting in a better prognosis [16,17,18]. CD169-positive sinus macrophages express genes related to antigen presentation and lymphocyte proliferation, and their reduction in elderly patients may contribute to age-related immune dysfunction [19]. In chronic inflammatory diseases, DCs modulate the immune microenvironment in lung small airways [20]. Such compartmentalization may also exist in cancer-draining lymph nodes and affect antitumor immunity.
Either a positive reaction or suppression is likely to require cell–cell interactions, and we believe the latter correlates with the overlapped distribution of different cell populations. The cell–cell interaction between DCs and macrophages likely occurs against tumor antigens early in the “sentinel lymph node”, which is defined as the first draining lymph node or group of nodes that receives lymphatic fluid directly from a primary tumor [21,22,23]. Sentinel node evaluation has been widely used in cancer surgery to assess metastatic spread and guide surgical decisions [22,23,24,25,26]. Based on immunohistochemical staining of DC-SIGN (dendritic cell-specific ICAM-3-grabbing non-integrin; CD209)-positive DCs, Sonoda et al. considered larger overlapped clusters of DC-SIGN- and CD169-positive cells, suggesting greater anticancer activity, and demonstrated morphological differences between the sentinel and non-sentinel nodes of early gastric cancer patients without metastasis [21]. They reported a significantly smaller overlap in sentinel nodes, and the result was consistent with the increased suppression reported in sentinel nodes relative to non-sentinel nodes [22,23,24,25,26]. In the present study, we also hypothesized that the larger overlap corresponded to stronger cross-presentation of cancer antigens between DCs and CD169-positive macrophages.
Anatomical studies and clinical analyses have demonstrated that lymphatic drainage routes differ between lung lobes. Upper lobe lymph often bypasses the hilar node and directly drains into the paratracheal node through collateral subpleural routes, contributing to clinical observations of “skip metastases” [27,28,29,30,31]. In contrast, lower lobe lymphatic flow typically progresses sequentially through the hilar, subcarinal, and paratracheal nodes [32,33,34,35,36]. The lung hilar node is characterized by a consistent morphology containing abundant anthracotic macrophages, without individual differences [37]. It is regarded as the most proximal node along the lymphatic drainage route in lung cancer. However, the hilar node is sometimes free from nodal metastasis in patients with metastasis to the paratracheal node [27].
The hilar node is traditionally considered the most proximal node in the lymphatic drainage route of lung cancer, but paradoxically, it is often spared from metastasis even when more distant, mediastinal nodes are involved [27,33,34]. This raises a critical question: is the hilar node a really proximal or upstream node in the immunological role and functional status? The aim of this study was to investigate the immunological architecture of hilar nodes in comparison with subcarinal and paratracheal nodes in non-metastatic lung cancer patients, using immunohistochemical and morphometric methods. We specifically focused on dendritic cells and CD169-positive macrophages to explore how nodal immune composition may vary by anatomical location and tumor lobe.
2. Results
2.1. Histological Features
For simplification, DC-SIGN-positive cells are called “candidate DCs”. In all nodes examined, the medullary sinus was filled with CD68-positive macrophages containing carbon particles (i.e., anthracotic macrophages), whereas candidate DCs and CD169-positive cells were usually localized in the subcapsular, intermediate, and paracortical sinuses, as well as along arteries and veins (Figure 1, Figure 2 and Figure 3). Outside of the medullary sinus, CD68-positive macrophages tended to contain no or few carbon particles (Figure 1E, Figure 2E and Figure 3E). The complementary distribution tended to be more clearly observed in the paratracheal and subcarinal nodes (Figure 2D–F and Figure 3D–F) relative to the hilar node (Figure 1D–F). Thus, candidate DCs and CD169-positive cells usually coexisted (Figure 2D vs. Figure 2F; Figure 3D vs. Figure 3F), whereas almost all medullary sinus macrophages were CD68-positive and CD169-negative (Figure 1E vs. Figure 1F). The medullary sinus issued protrusions toward the cortex to divide the latter (Figure 2E). The paracortical sinus surrounded one follicle and connected it to another follicle (Figure 3A,D). The intermediate sinus was situated along the trabeculae that originated from the nodal capsule and was inserted into and divided into the superficial cortex (Figure 1D). Overall, the hilar nodes tended to contain fewer CD169-positive cells than the paratracheal and subcarinal nodes. Although we did not perform double staining, CD169-positive and CD68-negative cells were likely present (Figure 3F vs. Figure 3E).
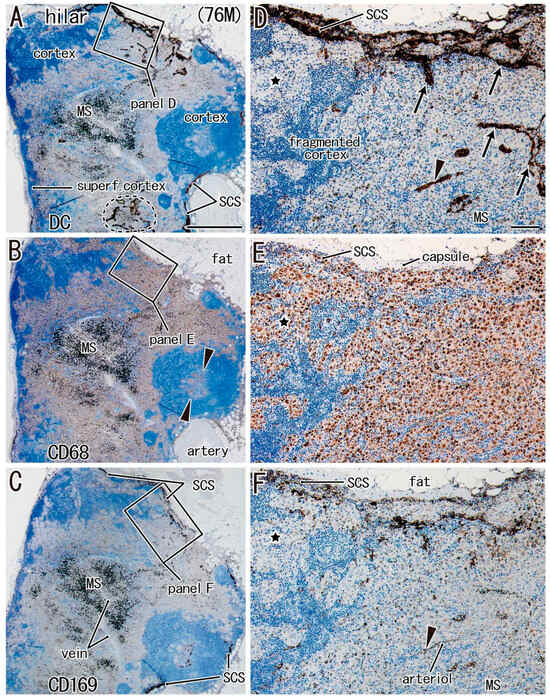
Figure 1.
Hilar node in a male patient with lower lobe cancer (age: 76 years). Three sections located within close proximity to each other. Panels (A,D): immunostaining of DC-SIGN; panels (B,E): immunostaining of CD68; panels (C,F): immunostaining of CD169. Panels (D–F) display higher-magnification views of the squares in panels (A–C), respectively. The superficial cortex (superf. cortex) is thin in panel (A). CD68-positive cells are seen in a follicle (arrowheads in panel B) as well as in the medullary sinus (MS; panel B). Candidate DCs are rich in the subcapsular sinus (SCS in panel D), intermediate sinus (arrows in panel D), and along an arteriole (arrowhead in panel F. The medullary sinus protrudes into the cortex to divide it (fragmented cortex in panel D). Panel (E) shows a large cluster of CD68-positive cells without anthracosis. CD169-positive cells exhibited a distribution similar to that of candidate DCs (panel F vs. panel D). The stars in panels (D–F) show the corresponding sites in the three sections. Panels (A–C) (or panels D–F) were prepared at the same magnification (scale bars: 1 mm in panel A; 0.1 mm in panel D).
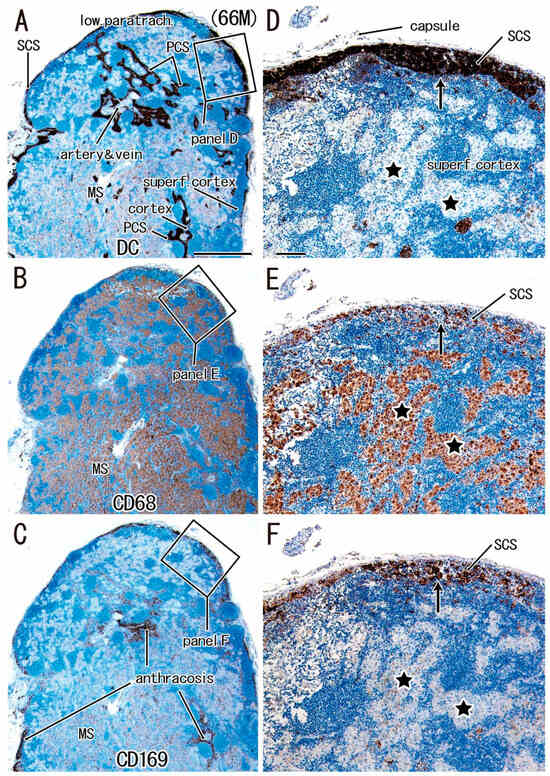
Figure 2.
Lower paratracheal node in a male patient with lower lobe cancer (age: 66 years). Three sections located within close proximity to each other. Panels (A,D): immunostaining of DC-SIGN; panels (B,E): immunostaining of CD68; panels (C,F): immunostaining of CD169. Panels (D–F) display higher-magnification views of the squares in panels (A–C), respectively. The superficial cortex (superf. cortex) is thin in panel (A). Candidate DCs are accumulated in the subcapsular sinus (SCS in panels A and D) and paracortical sinus (PCS in panel A), as well as along the artery and vein (panel A). The medullary sinus (MS), occupied with CD68-positive cells (Panel B), protrudes into the cortex to make “islands” of CD68-positive cells (stars in panel E). CD169-positive cells exhibited a distribution similar to that of candidate DCs (panel F vs. panel D). Stars and arrows in panels (D–F) show the corresponding sites in the three sections. Panels (A–C) (or panels D–F) were prepared at the same magnification (scale bars: 1 mm in panel A; 0.1 mm in panel D).
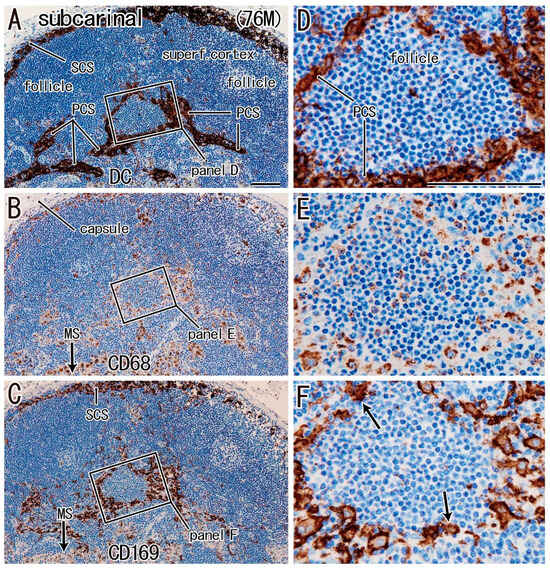
Figure 3.
Subcarinal node in a male patient with lower lobe cancer (age: 76 years). Three sections located within close proximity to each other. Panels (A,D): immunostaining of DC-SIGN; panels (B,E): immunostaining of CD68; panels (C,F): immunostaining of CD169. Panels (D–F) display higher-magnification views of the squares in panels (A–C), respectively. The superficial cortex (superf. cortex) was thick and contained follicles (panel A). Candidate DCs were seen in the subcapsular sinus (SCS in panel A) and paracortical sinus (PCS in panels A and D). CD68-positive cells were observed around the follicle (panel E), as well as in the medullary sinus (MS; arrow in panel B). CD169-positive cells exhibited a distribution similar to that of candidate DCs (panel F vs. panel D). Most of the medullary sinus macrophages were CD169 negative (MS; arrow in panel C). CD169-positive cells indicated by arrows in panel (F) are likely to be CD68-negative. Panels (A–C) (or panels D–F) were prepared at the same magnification (scale bars: 0.1 mm in panels A and D).
For a quick read: DC-SIGN is a reliable marker of dendritic cells and some macrophages. CD68 is a pan-macrophage marker, while CD169 identifies lymph node-resident macrophages involved in cross-presentation and antigen retention. This figure highlights the distinct spatial patterns of immune cell subsets in different regional nodes, particularly the reduced immune cell presence in the hilar node associated with lower lobe tumors.
For a quick read: DC-SIGN is a reliable marker of dendritic cells and some macrophages. CD68 is a pan-macrophage marker, and CD169 identifies lymph node-resident macrophages involved in cross-presentation and antigen retention. This figure illustrates the reduced overlap of CD68- and CD169-positive clusters in hilar nodes, especially in cases of lower lobe tumors, suggesting a relative impairment of macrophage-mediated immune coordination in this region.
For a quick read: DC-SIGN is a reliable marker of dendritic cells and some macrophages. CD68 is a pan-macrophage marker, and CD169 identifies lymph node-resident macrophages involved in cross-presentation and antigen retention. This figure demonstrates a reduced spatial overlap between DC-SIGN-positive cells and CD169- or CD68-positive macrophages in hilar nodes compared to other regional nodes, particularly in patients with lower lobe tumors. This reduction may reflect impaired coordination in antigen presentation and immune activation at the hilar site.
2.2. Quantitative Morphometric Analysis
Table 1 shows the lymph nodes examined, their average sizes and the proportional areas of macrophages and candidate DCs, and Table 2 and Table 3 show overlapping clusters. The size of a node or multiple nodes at a site (=in a nodal group) varied considerably between patients. Thus, relative to the hilar node, a significant difference found was limited to the large paratracheal node in upper lobe cancer patients. In the hilar nodes of all patients, the proportion of CD68-positive macrophages usually exceeded 50%. The complementary distribution between CD68-positive cells and candidate DCs (or CD169-positive cells) was more evident in nodes other than the hilar nodes, especially in patients with lower lobe cancers: CD68-positive clusters in the hilar node were significantly larger than in the paratracheal node (p = 0.041) and the subcarinal node (p = 0.013). However, we did not find the statistical significance in patients with upper lobe cancers. Therefore, hilar node composite cells and their distribution were likely to differ between two groups of patients.

Table 1.
Lymph node examined and the proportional area of macrophages and candidate DCs.

Table 2.
Overlapped clusters in patients with lower lobe cancer.

Table 3.
Overlapped clusters in patients with upper lobe cancer.
2.3. Comparison Between Upper and Lower Lobe Cancers
The key findings were as follows: (1) candidate DCs and CD169-positive cells occupied a significantly smaller area, respectively, in the hilar node of patients with upper lobe cancers, relative to patients with lower lobe cancers (p = 0.002, p = 0.009); (2) the hilar node in patients with lower lobe cancers showed a smaller overlap of CD68-positive cells with candidate DCs (p = 0.004) relative to patients with upper lobe cancers and, in the latter group, the definite complementary distribution of CD68-positive cells was rather rare; (3) the rich CD68-positive macrophages in the hilar node rarely existed with candidate DCs relative to the paratracheal node (p = 0.004) and the subcarinal node (p = 0.003) in the patients with lower lobe cancer.
Although they appeared redundant, the morphometric differences between the cancer sites were most likely to correlate with (1) a similarity in many parameters between the hilar and paratracheal nodes and (2) unique data obtained from the subcarinal node in patients with upper lobe cancers.
2.4. Overlapping Clusters of Immune Cells
Notably, a proportion of the overlapping cluster between CD169-positive cells and candidate DCs, which likely corresponds to activity of the cross-presentation (see Introduction), was similar between the hilar and “next-upstream” nodes (i.e., the paratracheal node for the upper lobe and the subcarinal node for the lower lobe).
In the subcarinal node, immunoreactive cell distributions were also likely to differ between two groups of patients. The subcarinal node of patients with lower lobe cancer carried a significantly smaller overlap between clusters of CD169-positive cells and candidate DCs than the paratracheal node (p = 0.032). Likewise, in patients with upper lobe cancers, the subcarinal node exhibited a significantly smaller overlapped distribution between CD169-positive cells and candidate DCs than the hilar node (p = 0.045) and the paratracheal node (p = 0.004). Thus, in patients with upper lobe cancers, the subcarinal node contained a small overlap of clusters: (1) CD68-positive cell clusters occupied 9.3 ± 6.8% of the DC area; (2) CD169-positive cell clusters accounted for 6.3 ± 5.4% of CD68-positive cell clusters; and (3) CD169-positive cell clusters occupied 21.2 ± 9.8% of the DC area.
2.5. Association with Clinical Variables
Additionally, the smoking index was much higher in men than in women among the patients examined (p < 0.001). The index was strongly correlated with the area of overlap between CD68- and CD169-positive cells (r = 0.411, p = 0.051) and was significantly higher in men than in women (p = 0.04). Whether the primary lesion was an adenocarcinoma or squamous cell carcinoma did not correlate with the parameters examined for nodal macrophages and candidate DCs. Similarly, the latter was not correlated with the tumor size.
3. Discussion
Although the present morphometric data seemed redundant and complex, we were challenged to resolve it according to the general principles of lymphatics and anticancer immunity. A proximal lymph node receives cancer antigens and reacts to them earlier than a distal node, which includes a reconstruction to be tolerable along the lymph flow from the cancer. However, macroscopic collateral lymph vessels are likely to modify anatomical relationships. Either a positive reaction or suppression usually requires cell–cell interactions that are likely to correlate with the overlapping distribution of different cell populations. Likewise, an essential difference in the composite cells in the node, such as monocyte-derived or self-renewal origins, is also connected to the reaction mode.
The present study characterized lung hilar nodes based on the intranodal distribution of macrophages and candidate DCs. Relative to other regional nodes, the hilar node seemed to contain more CD68-positive macrophages and fewer CD169-positive cells and candidate DCs. Notably, this difference was more evident in patients with lower lobe cancer than in those with upper lobe cancer. Against early lower lobe cancers, each of the hilar–subcarinal–paratracheal nodes seemed to take a different “shift” of candidate DCs and macrophages depending on their position along the lymphatic drainage route (see also next paragraph). In contrast, the hilar and paratracheal nodes might play almost the same defensive role against upper lobe cancers, possibly due to collaterals of the upper lobe lymph skipping the hilar node, which is clinically recognized as “skip metastasis” [27].
Skip metastasis refers to the pathological finding in which cancer cells metastasize directly to N2 mediastinal nodes (e.g., paratracheal nodes) without involvement of N1 nodes (e.g., hilar nodes). This phenomenon is observed in approximately 20–30% of patients with non-small cell lung cancer and is particularly common in upper lobe tumors [35,38]. Anatomical studies suggest that this may be due to direct lymphatic drainage routes from the upper lobes to the mediastinum via subpleural or interlobar lymphatic channels, bypassing the hilar station [28,32]. Clinically, skip metastasis has been associated with better prognosis than non-skip N2 involvement, although its implications for treatment decisions remain controversial.
The lower lobe lymph is likely to flow from the ipsilateral hilar node, via the subcarinal node, to the paratracheal node, according to anatomical studies [28,29,30,31,32,33] as well as retrospective analyses of cancer metastasis [34,35,36]. The present study demonstrated that in patients with lower lobe cancer, there was a significantly larger overlap between CD169-positive cells and candidate DCs in the paratracheal node relative to the subcarinal and hilar nodes. The larger overlap seemed to correspond to stronger cross-presentation of cancer antigens between DCs and CD169-positive macrophages. In contrast, the hilar and subcarinal nodes appeared to have already been simultaneously suppressed. Therefore, the hilar node seemed to resemble the “next-upstream node” in status of cancer immunity. This rule was also adapted to upper lobe cancers, a likely result of collateral lymph flow alongside the hilar node [29,30,31].
The hilar node was significantly smaller than the other node in early upper lobe cancers (Table 1). Notably, the hilar node in early upper lobe cancers exhibited a high anticancer reactivity in contrast to the suggested suppressive status in lower lobe cancer. This difference might be explained by an upper lobe-specific lymph flow, such as subpleural collateral lymphatic vessels toward the upper paratracheal node [28,33]. The collaterals might rescue cancer-induced suppression of both hilar and lower paratracheal nodes [38].
The subcarinal node is usually outside the lymphatic route from the upper lobe, as evidenced by the few or no metastases of upper lobe cancer [35,38,39]. Therefore, the data from the subcarinal node in patients with upper lobe cancer were likely to show a minimum or baseline-level activity of lung regional nodes, such as 21.4% (Table 3) of the nodal area corresponding to overlapped clusters of CD169-positive cells and candidate DCs. Nevertheless, in patients with lower lobe cancer, the hilar node exhibited parameters that were much lower than the suggested baseline, with fewer candidate DCs relative to patients with upper lobe cancer (p = 0.004) and fewer CD169-positive cells in the CD68-positive macrophage cluster (p = 0.05). Therefore, the suppression of the hilar node by early cancer is likely to reach a level much lower than the baseline activity. The morphology can accurately be described as truly “pathological”.
From a methodological standpoint, our approach combines immunohistochemical staining with semi-quantitative morphometric analysis based on a clearly defined cluster criterion: 20 or more candidate DCs and macrophages per 100 × 100 µm square. Manual tracing was necessary because, according to our trial, an automatic system was unable to distinguish black-colored deposits in the diaminobenzidine reaction from some or most of the anthracotic macrophages. However, using manually made traces, all quantitative measurements were conducted using ImageJ software (version 1.53), ensuring reproducibility across cases.
The present study and that of Aoki et al. revealed a complementary distribution between candidate DCs and CD68-positive macrophages in the regional nodes of the lung [40]. This was quite different from the gastric regional nodes, in which a small overlap of <10% was unlikely (minimum of 14.4%) [21]. Thoracic nodes generally contain anthracotic alveolar macrophages in both the medullary sinus and cortex [1]. Alveolar macrophages are self-renewing populations that arise from fetal progenitors and require minimal input from adult monocytes in healthy environments [2]. Conversely, CD169-positive and CD68-negative macrophages have recently been reported in the subcapsular sinus of the colic and gastric regional nodes [21,41], and they seemed to be monocyte-derived as moDCs [3]. Due to the space-occupying effect of the advanced accumulation of alveolar macrophages after phagocytosis of carbon particles, hilar nodes in elderly individuals may become more tolerant to the homing of metastatic cancer cells.
Clinically, our study supports the importance of definitive dissection of hilar lymph nodes. Due to the results of a clinical study on non-small cell lung cancer less than 2 cm in size [42], the number of sublobar resections, such as segmentectomy, has been increasing in recent years. However, it has been reported that even small lung cancers less than 2 cm in size have about 10% of patients with hilar lymph node metastasis [43,44]. If the effect of current immunotherapy cannot be expected even if hilar lymph nodes in which the immune cells responsible for cross-presentation are depleted remain, then the hilar lymph nodes should be reliably dissected even when sublobar resection is performed.
Conclusive remark: The lung hilar node was characterized by a rich content of self-renewing alveolar macrophages, in contrast to a likely lower content of monocyte-derived macrophages and DCs. The nodal position nearest to the primary lesion seemed to suppress the specific composite cells earlier in the cancer progression. However, collateral lymph vessels may mask differences from the next-upstream node, such as the paratracheal or subcarinal node. These results support definitive dissection of hilar lymph nodes.
4. Study Limitations
In the present study, we used the term “candidate DCs”. DC-SIGN-positive cells are most likely monocyte-derived DCs [3], but we did not deny the possibility that the positive cells contained a subpopulation of macrophages, such as DC-SIGN-positive and CD169-positive but CD68-negative cells. This was an essential limitation of this study. However, such macrophages seemed to be few in number.
Because hilar nodes tended to attach tightly to the major artery and bronchus, the surgical en masse dissection of multiple nodes in a nodal group along the bronchus was often very difficult. Thus, the nodal capsule was often injured, and the subcapsular sinus was missed. Therefore, available nodes were sometimes limited to 20% or less at a site. The difficulty of avoiding injury to the nodal capsule seemed to be the greatest obstacle for planning a worldwide study including multiple hospitals.
5. Materials and Methods
5.1. Patients and Nodal Specimens
One hundred fourteen thoracic nodes (35 hilar, 35 subcarinal, and 44 lower paratracheal nodes) were examined morphologically and immunohistochemically. Details of the number and location of nodes analyzed per patient are shown in Table S1. All patients were Japanese, and there were no Caucasian Whites or African Blacks. Nodes were surgically obtained from 15 patients with lower lobe cancer and 10 patients with upper lobe cancer who had no signs of metastasis (male, n = 14; female, n = 11; median age, 66 [range: 50–78 years]). Seventeen of the 25 patients were smokers. Patients underwent curative pulmonary resection with lymph node dissection at Kagoshima University Hospital between April 2010 and March 2021. After routine curative resection and nodal dissection (lobectomy, n = 23; bilobectomy, n = 1; pneumonectomy, n = 1). The final pathological examinations of the lung cancer patients were as follows: stage IA (n = 8), stage IB (n = 10), stage IIA (n = 1), stage IIB (n = 2), stage IIIA (n = 3), and stage IIIB (n = 1). The cancers were histopathologically classified as adenocarcinoma (n = 18) or squamous cell carcinoma (n = 7) according to the seventh edition of the TNM classification [45]. All patients survived for more than 5 years without any recurrence. The use of the specimens was approved by the Ethics Committee of Kagoshima University Graduate School of Medicine and Dentistry (No. 210198epi) and conformed to the principles outlined in the Declaration of Helsinki. Research participants and their relatives could opt out by viewing the research content hosted online.
5.2. Immunohistochemistry
Primary antibodies, together with their dilutions and antigen retrieval procedures, are listed in Table 4. Briefly, we used (1) an antibody against DC-SIGN (also known as CD209) as a DC marker, (2) a pan-macrophage marker (CD68), and (3) another marker for the macrophage subpopulation (CD169) (see the Section 1 for more detail). The paracortical lymph sinus endothelium expresses DC-SIGN [46,47,48]. After incubation with the primary antibodies, the sections were incubated for 30 min with horseradish peroxidase-conjugated secondary antibodies (DAKO Envision FLEX HRP; Dako, Denmark A/S) diluted 1:1000. Immunoreactive proteins were detected by incubation with diaminobenzidine for 3–5 min (DAKO Envision FLEX DAB; Dako, Denmark A/S). Each sample was counterstained with hematoxylin, and a negative control without the primary antibody was used for all specimens. Treated sections were counterstained with hematoxylin, dehydrated in ethanol, and cleared in xylene. All histological images were captured using a Nikon Eclipse 80i microscope.

Table 4.
Primary monoclonal antibodies, their dilution and specific treatment.
5.3. Morphometric Analysis of Clusters of DCs and Macrophages
Using stained sections corresponding to the maximum cross-sectional area of the node, we measured three areas: (1) the proportional area of clusters of candidate DCs, (2) the proportional area of CD68-positive macrophage clusters, and (3) the proportional area of CD169-positive macrophage clusters. The areas of candidate DCs are expected to overlap with those of macrophages. We evaluated three types of overlap: (1) the overlapping area between clusters of candidate DCs and CD68-positive macrophages, (2) the overlapping area between clusters of candidate DCs and CD169-positive macrophages, and (3) the overlapping area between clusters of CD68-positive macrophages and CD169-positive macrophages. The methods are shown below.
First, for the analysis, we chose 1–4 node(s) per one site or a nodal group such as the hilar node group. Our targets were 3 sites or 3 nodal groups in one patient, and thus, a total of 75 sites or nodal groups were examined. One nodal group was composed of 1–15 nodes: average numbers of the composite nodes were 3.76 in the hilar node group, 5.88 in the subcarinal group and 4.56 in the lower paratracheal group. Because of tight adhesion of nodes to the bronchi, the nodal capsule and subcapsular sinus had often been injured during surgery: at maximum, 5 nodes in the hilar group and 9 nodes in the paratracheal group had been injured. Thus, available nodes were sometimes limited to almost 10% in number per group. Conversely, 5 or more nodes were available for measurement at a total of 10 sites or groups in 10 patients. When the nodal laminar architecture was similar between these 5 nodes, we chose a single node for measurement, but when the architecture appeared different, multiple nodes were examined to obtain the averaged data among different morphologies.
Second, we prepared three photos of histology (x28 magnification; 1 mm in the histology corresponded to 28 mm on the photo), each of which showed either DCsign-positive cell clusters, CD68-positive macrophage clusters, or CD169-positive cell clusters in the entire section of a node. Next, each photo was covered with tracing paper, and cluster areas were manually delineated using black ink. A cluster was defined as an area containing 20 or more immunoreactive cells within a 100 × 100 µm square. Cluster areas were manually delineated on printed histological images due to the presence of histological artifacts and pigment-laden cells. In particular, surgically resected thoracic lymph nodes often contained anthracotic macrophages laden with carbon particles, which appeared dark brown to black and were difficult to distinguish from black-colored deposits in diaminobenzidine reaction using automated segmentation tools. Therefore, manual tracing was required to ensure the accurate identification of immunoreactive cell clusters. All cluster areas were subsequently quantified using ImageJ software (version 1.53, NIH), with standardized pixel-based area calculation, ensuring semi-quantitative reproducibility.
Candidate DCs likely co-exist with a macrophage subset, and thus, a cluster of DCs likely overlaps a cluster of macrophage subsets (for a definition of the term “cluster”, see above). We therefore evaluated three types of “overlapped clusters”: (1) the overlapping area between clusters of candidate DCs and CD68-positive macrophages, (2) the overlapping area between clusters of candidate DCs and CD169-positive macrophages, and (3) the overlapping area between clusters of CD68-positive macrophages and CD169-positive macrophages. The “overlapped cluster” was measured by a manual tracing of two overlaid translucent tracings, each of which showed spatially intersected clusters of different cell types. Because the shape of the node was slightly different between sections and because sections often contained artifacts during histological procedures, such as folds and tears at various sites, an automatized system had difficulty identifying the fact that “these two sections were adjacent or near”. After manual tracing, the size of each overlapped area was measured using ImageJ and expressed as a percentage of the reference cell cluster (e.g., the CD169 area overlapping with the DC-SIGN area was expressed as % of the total DC-SIGN cluster area). This allowed us to semi-quantitatively assess the potential for cell–cell interaction or immunological collaboration (e.g., cross-presentation) between distinct immune cell types.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/lymphatics3020013/s1, Table S1: Patients’ characteristics.
Author Contributions
Conceptualization, G.M.; methodology, M.A.; software, M.A.; validation, M.A. and G.K.; investigation, M.A., G.K., A.H.-T., T.N. and K.U.; data curation, M.A.; writing—original draft preparation, M.A.; writing—review and editing, G.M.; visualization, G.M.; supervision, K.U.; funding acquisition, M.A. and A.H.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Japan Society for the Promotion of Science. KAKENHI grant numbers 22K08980 (Masaya Aoki) and 22K08981 (Aya Harada-Takeda).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Kagoshima University Graduate School of Medicine and Dentistry (protocol code: 210198epi and date of approval: 13 December 2021).
Informed Consent Statement
Given the retrospective design using existing surgical specimens and de-identified data, the IRB waived the requirement for written informed consent. Study information was publicly disclosed on the institution’s website, and eligible patients and/or their relatives were provided the opportunity to opt out.
Data Availability Statement
All data that support the histological findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Special support for immunostaining experiments in this research was received from Orie Iwaya, a technician belonging to the Department of Pathology, Kagoshima University Graduate School of Medical and Dental Sciences.
Conflicts of Interest
The authors declare no conflicts of interest in association with the present study.
Abbreviations
The following abbreviations are used in this manuscript:
| DCs | dendritic cells |
| DC-SIGN | dendritic cell-specific ICAM-3-grabbing nonintegrin |
References
- Jin, Z.W.; Aoki, M.; Ueda, K.; Kamimura, G.; Takeda-Harada, A.; Murakami, G.; Sato, M. Human lymph node degeneration in the thoracic region: A morphometric and immunohistochemical analysis using surgically-obtained specimens. Front. Physiol. 2022, 13, 990801. [Google Scholar] [CrossRef]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef]
- Kvedaraite, E.; Ginhoux, F. Human dendritic cells in cancer. Sci. Immunol. 2022, 7, eabm9409. [Google Scholar] [CrossRef] [PubMed]
- Commerford, C.D.; Dieterich, L.C.; He, Y.; Hell, T.; Montoya-Zegarra, J.A.; Noerrelykke, S.F.; Russo, E.; Rōcken, M.; Detmar, M. Mechanisms of tumor-induced lymphovascular niche formation in draining lymph nodes. Cell Rep. 2018, 25, 3554–3563. [Google Scholar] [CrossRef] [PubMed]
- van Pul, K.M.; Vuylsteke, R.J.; van de Ven, R.; Te Velde, E.A.; Rutgers, E.J.T.; van den Tol, P.M.; Stockmann, H.B.; de Gruijl, T.D. Selectively hampered activation of lymph node-resident dendritic cells precedes profound T cell suppression and metastatic spread in the breast cancer sentinel lymph node. J. Immunother. Cancer 2019, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Heeren, A.M.; Rotman, J.; Samuels, S.; Zijlmans, H.J.M.A.A.; Fons, G.; van de Vijver, K.K.; Bleeker, M.C.; Kenter, G.G.; Jordanova, E.J.; De Gruijl, T.D. Immune landscape in vulvar cancer-draining lymph nodes indicates distinct immune escape mechanisms in support of metastatic spread and growth. J. Immunother. Cancer 2021, 9, e003623. [Google Scholar] [CrossRef]
- Du, H.; Tang, J.; Li, X.; Wang, X.; Wu, L.; Zhang, R.; Hu, P.; Yang, Y. Siglec-15 is an immune suppressor and potential target for immunotherapy in the pre-metastatic lymph node of colorectal cancer. Front. Cell Dev. Biol. 2021, 9, 691937. [Google Scholar] [CrossRef]
- Wei, W.F.; Chen, X.J.; Liang, L.J.; Yu, L.; Wu, X.G.; Zhou, C.F.; Wang, Z.C.; Fan, L.S.; Hu, Z.; Liang, L.; et al. Periostin+ cancer-associated fibroblasts promote lymph node metastasis by impairing the lymphatic endothelial barriers in cervical squamous cell carcinoma. Mol. Oncol. 2021, 15, 210–227. [Google Scholar] [CrossRef]
- Mehta, A.K.; Kadel, S.; Townsend, M.G.; Oliwa, M.; Guerriero, J.L. Macrophage biology and mechanisms of immune suppression in breast cancer. Front. Immunol. 2021, 12, 643771. [Google Scholar] [CrossRef]
- Blayer, C.; Boyer, T.; Peyraud, F.; Domblides, C.; Larmonier, N. Beyond immunosuppression: The multifaced functions of tumor-promoting myeloid cells in breast cancers. Front. Immunol. 2022, 13, 838040. [Google Scholar] [CrossRef]
- Virgilio, T.; Bordini, J.; Cascione, L.; Sartori, G.; Latino, I.; Molina Romero, D.; Leoni, C.; Akhmedov, M.; Rinaldi, A.; Arribas, A.J.; et al. Subcapsular sinus macrophages promote melanoma metastasis to the sentinel lymph nodes via an IL1a-STAT axis. Cancer Immunol. Res. 2022, 10, 1525–1541. [Google Scholar] [CrossRef]
- Gunnarsdottir, F.B.; Briem, O.; Lindgren, A.Y.; Kăllberg, E.; Andersen, C.; Grenthe, R.; Rosenqvist, C.; Millrud, C.R.; Wallgren, M.; Viklund, H.; et al. Breast cancer associated CD169+ macrophages possess broad immunosuppressive functions but enhance antobody secretion by activated B cells. Front. Immunol. 2023, 14, 1180209. [Google Scholar] [CrossRef]
- Ji, S.; Shi, Y.; Yin, B. Macrophage barrier in the tumor microenvironment and potential clinical applications. Cell Commun. Signal 2024, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, J.; Lopez-Venegas, M.A.; Affandi, A.J.; den Haan, J.M.M. CD169+ macrophages capture and dendritic cell instruct: The interplay of the gatekeeper and the general of the immune system. Frint Immunol. 2018, 9, 2472. [Google Scholar] [CrossRef]
- Reis-Sobreiro, M.; da Mota, A.T.; Jardim, C.; Serre, K. Bringing macrophages to the frontline against cancer: Current immunotherapies targeting macrophages. Cells 2021, 10, 2364. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Yano, H.; Shiota, T.; Komohara, Y. Anticancer immune reaction and lymph node sinus macrophages: A review from human and animal studies. J. Clin. Exp. Hematol. 2024, 64, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, K.; Tasaki, T.; Ohnishi, K.; Shibata, M.; Shimajiri, S.; Harada, M.; Komohara, Y.; Nakayama, T. CD169 expression on lymph node macrophages predicts in patients with gastric cancer. Front. Oncol. 2021, 11, 636751. [Google Scholar] [CrossRef]
- Rakaee, M.; Busund, L.R.; Jamaly, S.; Paulsen, E.E.; Richardsen, E.; Andersen, S.; Al-Saad, S.; Bremnes, R.M.; Donnem, T.; Kilvaer, T.K. Prognostic value of macrophage phenotypes in resectable non-small cell lung cancer associated by multiplex immunohistochemistry. Neoplasia 2019, 21, 282–293. [Google Scholar] [CrossRef]
- Kanemitsu, K.; Yamada, R.; Pan, C.; Tsukamoto, H.; Yano, H.; Shiota, T.; Fujiwara, Y.; Miyamoto, Y.; Mikami, Y.; Baba, H.; et al. Age-associated reduction of sinus macrophages in human mesenteric lymph nodes. J. Clin. Exp. Hematop. 2024, 64, 79–85. [Google Scholar] [CrossRef]
- Van Pottelberge, G.R.; Bracke, K.R.; Demedts, I.K.; De Rijck, K.; Reinartz, S.M.; van Drunen, C.M.; Verleden, G.M.; Vermassen, F.E.; Joos, G.F.; Brusselle, G.G. Selective accumulation of langerhans-type dendritic cells in small airways of patients with COPD. Respir. Res. 2010, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, T.; Arigami, T.; Aoki, M.; Matsushita, D.; Shimonosono, M.; Tsuruda, Y.; Sasaki, K.; Ohtsuka, T.; Murakami, G. Difference between sentinel and non-sentinel lymph nodes in the distribution of dendritic cells and macrophages: An immunohistochemical and morphometric study using gastric regional nodes obtained in sentinel node navigation surgery for early gastric cancer. J. Anat. 2025, 246, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.J.; Morton, D.L.; Stern, S.; Lana, A.M.; Essner, R.; Wen, D.R. Sentinel lymph nodes show profound downregulation of antigen-presenting cells of the paracortex: Implication for tumor biology and treatment. Mod. Pathol. 2001, 4, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Yamaguchi, Y.; Ueno, H.; Osaki, A.; Arihiro, K.; Toge, T. Maturation of dendritic cells and T-cell responses in sentinel lymph nodes from patients with breast carcinoma. Cancer 2006, 106, 1227–1236. [Google Scholar] [CrossRef]
- Botella-Estrada, R.; Dasi, F.; Ramos, D.; Nagore, E.; Herrero, M.J.; Giménez, J.; Fuster, C.; Sanmartín, O.; Guillén, C.; Aliño, S. Cytokine expression and dendritic cell density in melanoma sentinel node. Melanoma Res. 2005, 15, 99–106. [Google Scholar] [CrossRef]
- Otto, B.; Koenig, A.M.; Tolstonog, G.V.; Jeschke, A.; Klaetschke, K.; Vashist, Y.K.; Wicklein, D.; Wagener, C.; Izbicki, J.R.; Streichert, T. Molecular changes in pre-metastatic lymph nodes of esophageal cancer patients. PLoS ONE 2014, 9, e102552. [Google Scholar] [CrossRef] [PubMed]
- van Pul, K.M.; Vuylsteke, R.J.C.L.M.; de Beijer, M.T.A.; van de Ven, R.; van den Tol, M.P.; Stockmann, H.B.A.C.; de Gruijl, T.D. Breast cancer-induced immune suppression in the sentinel lymph node is effectively countered by CpG-B in conjunction with inhibition of the JAK2/STAT3 pathway. J. Immunother. Cancer 2020, 8, e000761. [Google Scholar] [CrossRef]
- Maniwa, T.; Shintani, Y.; Okami, J.; Kadota, Y.; Takeuchi, Y.; Takami, K.; Yokouchi, H.; Kurokawa, E.; Kanzaki, R.; Sakamaki, Y.; et al. Upfront surgery in patients with clinical skip N2 lung cancer based on results of modern radiological examinations. J. Thorac. Dis. 2018, 10, 6828–6837. [Google Scholar] [CrossRef]
- Riquet, M.; Dupont, P.; Hidden, G.; Debesse, B. Mediastinal lymphatic pathway of the azygos and aortic arches. Surg. Radiol. Anat. 1991, 13, 149–154. [Google Scholar] [CrossRef]
- Riquet, M. Anatomic basis of lymphatic spread from carcinoma of the lung to the mediastinum: Surgical and prognostic implications. Surg. Radiol. Anat. 1993, 15, 271–277. [Google Scholar] [CrossRef]
- Murakami, G.; Sato, T.; Takiguchi, T. Topographical anatomy of the bronchomediastinal lymph vessels: Their relationships and formation of the collecting trunks. Arch Histol. Cytol. 1990, 53, 219–235. [Google Scholar] [CrossRef]
- Yano, M. Anatomical study of the last mediastinal lymph nodes in relation to the progress of p-N2 non-small cell lung cancer. J. Jpn. Ass Chest Surg. 1993, 7, 643–654. [Google Scholar] [CrossRef]
- Jossifow, G.M. Das Lymphgefässystem des Menschen; Gustav Fisher: Jena, Germany, 1930. [Google Scholar]
- Riquet, M.; Hidden, G.; Debesse, B. Direct drainage of lung segments to the mediastinal nodes. J. Thorac. Cardiovasc. Surg. 1989, 97, 623–632. [Google Scholar] [CrossRef]
- Naruke, T.; Suemasu, K.; Ishikawa, S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J. Thorac. Cardiovasc. Surg. 1978, 76, 832–839. [Google Scholar] [CrossRef]
- Okada, M.; Tsubota, N.; Yoshimura, M.; Miyamoto, Y. Proposal for reasonable mediastinal lymphadenectomy in bronchogenic carcinomas: Role of subcarinal nodes in selective dissection. J. Thorac. Cardiovasc. Surg. 1998, 116, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Little, A.G.; DeHoyos, A.; Kirgan, D.M.; Arcomano, T.R.; Murray, K.D. Intraoperative lymphatic mapping for non-small-cell lung cancer: The sentinel node technique. J. Thorac. Cardiovasc. Surg. 1999, 117, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Murakami, G.; Taniguchi, I. Histologic heterogeneity and intranodal shunt flow in lymph nodes from elderly subjects: A cadaveric study. Ann. Surg. Oncol. 2004, 11, 279S–284S. [Google Scholar] [CrossRef]
- Asamura, H.; Nakayama, H.; Kondo, H.; Tsuchiya, R.; Naruke, T. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J. Thorac. Cardiovasc. Surg. 1999, 117, 1102–1111. [Google Scholar] [CrossRef]
- Aokage, K.; Yoshida, J.; Ishii, G.; Hishida, T.; Nishimura, M.; Nagai, K. Subcarinal lymph node in upper lobe non-small cell lung cancer patients: Is selective lymph node dissection valid? Lung Cancer 2010, 70, 163–167. [Google Scholar] [CrossRef]
- Aoki, M.; Jin, Z.W.; Ueda, K.; Kamimura, G.; Takeda-Harada, A.; Murakami, G.; Sato, M. Localization of macrophages and dendritic cells in human thoracic lymph node: An immunohistochemical study using surgically-obtained specimens. J. Anat. 2023, 243, 504–516. [Google Scholar] [CrossRef]
- Yamada, R.; Ohnishi, K.; Pan, C.; Yano, H.; Fujiwara, Y.; Shiota, T.; Mikami, Y.; Komohara, Y. Expression of macrophage/dendritic cell-related molecules in lymph node sinus macrophages. Microbiol. Immunol. 2023, 67, 490–500. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Yuan, P.; Yuan, X.; Lv, X.; Wang, Z.; Hu, J. Predictive risk factors for lymph node metastasis in patients with small size non-small cell lung cancer. J. Thorac. Dis. 2014, 6, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.M.; Ilonen, I.K.; Tan, K.S.; Plodkowski, A.J.; Bott, M.; Bains, M.S.; Adusumilli, P.S.; Park, B.J.; Rusch, V.W.; Jones, D.R.; et al. Prevalence of Occult Peribronchial N1 Nodal Metastasis in Peripheral Clinical N0 Small (2 cm) Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2020, 109, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L.; International Association for the Study of Lung Cancer International Staging Committee. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef]
- Engering, A.; van Vliet, S.J.; Hebeda, K.; Jackson, D.G.; Prevo, R.; Singh, S.K.; Geijtenbeek, T.B.; van Krieken, H.; van Kooyk, Y. Dynamic populations of dendritic cell-specific ICAM-3 grabbing nonintegrin-positive immature dendritic cells and liver/lymph node-specific ICAM-3 grabbing nonintegrin-positive endothelial cells in the outer zones of the paracortex of human lymph nodes. Am. J. Pathol. 2004, 164, 1587–1595. [Google Scholar] [CrossRef]
- Lai, W.K.; Sun, P.J.; Zhang, J.; Jennings, A.; Lalor, P.F.; Hubscher, S.; McKeating, J.A.; Adams, D.H. Expression of DC-SIGN and DC-SIGNR on human sinusoidal endothelium. Am. J. Pathol. 2006, 169, 200–208. [Google Scholar] [CrossRef]
- Park, S.M.; Angel, C.E.; McIntosh, J.; Mansell, C.M.; Chen, C.J.J.; Cebon, J.; Dunbar, P.R. Mapping the distinctive populations of lymphatic endothelial cells in different zones of human lymph nodes. PLoS ONE 2014, 9, e94781. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).