Abstract
Non-Hodgkin lymphomas (NHLs) represent a diverse group of hematologic malignancies derived from various cells. B-cell NHLs represent the largest fraction of lymphomas diagnosed and treated in the United States. Standard chemo-immunotherapies with rituximab and multiagent cytotoxic regimens have proven to be effective in the management of these lymphoproliferative neoplasms; nonetheless, a considerable fraction of patients still experience relapse or have treatment-refractory disease. Therapeutic advances using novel immunotherapeutic agents as well as cell-based treatments, such as chimeric antigen receptor (CAR) T-cell therapies, have improved the outcomes of relapsed/refractory (R/R) B-cell NHL. Most of these new treatment strategies are not curative and most patients succumb to R/R disease, leaving this population with an unmet need for effective and well-tolerated therapeutic options. One of these up-and-coming options are bispecific antibodies (BsAb), either as single agent or in combination with other medications. Conclusion: BsAbs offer a novel “off the shelf” chemotherapy-free approach in the management of R/R B-cell NHL. Advancements in antibody construct design along with improved safety profile and clinical effectiveness of the most recent BsAbs suggest that these agents are a promising new option in the management of R/R B-cell NHL.
1. Introduction
Non-Hodgkin lymphomas (NHLs) comprise a diverse group of hematologic malignancies derived from B or T cell progenitors, mature B or T cells or, rarely, natural killer cells. B-cell NHLs are the most prevalent lymphomas, accounting for up to 85–90% of all new diagnoses [1]. Of those, the most common B-cell NHLs in Western countries are large B-cell lymphomas (e.g. diffuse large B-cell lymphoma; DLBCL), accounting for around 31% of adult cases, followed by follicular lymphoma (FL) at 22%, marginal zone lymphoma (MZL) at 8%, mantle cell lymphoma (MCL) at 6%, chronic lymphocytic leukemia/small-cell lymphocytic lymphoma (CLL/SLL) at 6% and lymphoplasmacytic lymphoma (LPL) comprising 1% of cases [2].
Treatments, whenever indicated, for symptomatic indolent B-cell NHL (e.g., FL, MCL, etc.) or aggressive subtypes (e.g., DLBCL) are often built on a backbone of anthracycline-based regimens like CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) [3]. Such regimens were previously noted to have improved progression free survival (PFS) and overall survival (OS) in the management of both indolent and aggressive B-cell NHL. More than 23 years ago, the anti-CD20 monoclonal antibody rituximab (R) proved to be the first active targeted immunotherapy for the treatment of R/R B-cell NHL [4]. Coiffier et al. were among the first to demonstrate the benefit of adding Rituximab (R) to CHOP compared to CHOP in the frontline treatment of DLBCL, noting significantly improvements in both PFS and OS [5]. Similar benefits were seen in other prospective trials and retrospective studies [6,7]. Rituximab also showed improved outcomes in the frontline management of symptomatic indolent B-cell NHL when added to chemotherapy backbones in prospective randomized trials conducted by Hiddemann et al., Luminari et al. and in the STIL trial by Rummel et al. [8,9,10]. While the development of chemo-immunotherapies such as RCHOP has been incredibly important in the field of B-cell NHL, curing 60–65% of DLBCL patients and significantly prolonging the life of patients with low grade B cell lymphomas (10-year OS: 80%), many patients either relapse or experience refractory disease after these frontline therapies [11,12]. Such patients have increased resistance to subsequent lines of treatment, especially in the context of primary refractory and early relapsed disease which render a poor prognosis and higher incidence of early death among this population [13,14,15].
In patients with R/R high grade B-cell NHL, autologous hematopoietic stem cell transplantation (autoHCT) and more recently, chimeric antigen receptor (CAR) T-cell therapy can be curative options in approximately 40–45% of cases; however, a significant fraction of these patients are not candidates for these procedures or experience disease progression after these cellular therapies, thus resulting in dismal prognosis with survival quantified in months [16,17].
Research efforts have attempted to fill the gap for patients who are ineligible for autoHCT or have progressive disease after cellular therapy. Polatuzumab vedotin with bendamustine and rituximab have been approved based on a phase Ib/II study noting higher rates of CR when compared to the control (40% vs. 17%, p = 0.026) and longer OS with a median of 12.4 vs. 4.7 months (HR = 0.42, 95% CI 0.24–0.75, p = 0.002) [18]. Nonetheless, these strategies have not been proven to be curative. Two different anti-CD19 antibody constructs were studies in single arm phase II trials for R/R LBCL patients, obtaining approval for their use in this population. The L-MIND trial tested tafasitamab plus lenalidomide in 80 patients with R/R DLBCL with high-risk features noticing clinical important response rates along with prolong medias PFS and OS in this highly selected population (12.1 months and not reached, respectively) [19]. In the LOTIS2 trial 145 patients with high-risk R/R LBCL were treated with the antibody drug conjugate loncastuximab teserine achieving important ORR and CR (48% and 24%, respectively) paired with PFS and OS (5 months and 10 months respectively) [20,21]. However, none of these approved strategies are consider curable and can have significant side effect that may impair quality of life, reason why more effective and safer treatments are needed.
The introduction of new generation agents aimed at engaging the patient’s immune system (i.e., monoclonal antibodies (MAbs), antibody–drug conjugates (ADCs), immunomodulatory agents (IMiDs) and CAR T-cells) have defined a new era in the management of B-cell NHLs. Although these novel lines of therapy have been proven effective, they face considerable limitations such as availability, durability of response as well as cost of manufacturing and administration. Relapse following treatment with such agents leave limited options for subsequent management and generate a treatment gap for this population of patients.
A new class of therapeutic agents, bispecific antibodies (BsAbs), are carving out a place in the management algorithm of B-cell NHL, particularly in the R/R setting. Such agents have several potential advantages over other T-cell-based therapies such as CAR T-cell products but can also have some drawbacks. BsAbs could have a major impact in the treatment of both aggressive and indolent B-cell NHL patients, and their role in these hematologic malignancies will be reviewed in this manuscript. BsAbs are not limited by elaborate manufacturing processes, nor do they require lymphodepleting therapy prior to administration. Additionally, they do not require administration through central IV lines. Moreover, these agents can fill disease-related gaps in poor prognosis B-cell NHLs.
2. What Are Bispecific Antibodies?
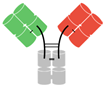
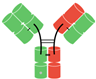
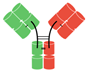
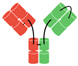
In humans and most mammals, all naturally occurring monomeric immunoglobulins are comprised of four polypeptide chains composed of two identical heavy (H) chains and two identical kappa (κ) or lambda (λ) chains linked together by interchain disulfide bonds [22]. Each heavy chain is in turn comprised of an N-terminal variable domain (VH) and three constant domains (CH1, CH2, CH3) with an additional “hinge region” between CH1 and CH2. The portion comprising the lower hinge region and the CH2 and CH3 segments is collectively known as the fragment crystalline (Fc) and grants the antibody its stability. The light chains are similarly comprised of an N-terminal variable domain (VL) and constant domain (CL). To produce a successful interaction with an antigen, the light chain component interacts with the VH and CH1 components of the heavy chain to form the fragment antibody binding complex (Fab). In nature, these Fab complexes form a bivalent monospecific immunoglobulin that allows the antibody to engage with the same antigen at two sites [22] (see Figure 1).
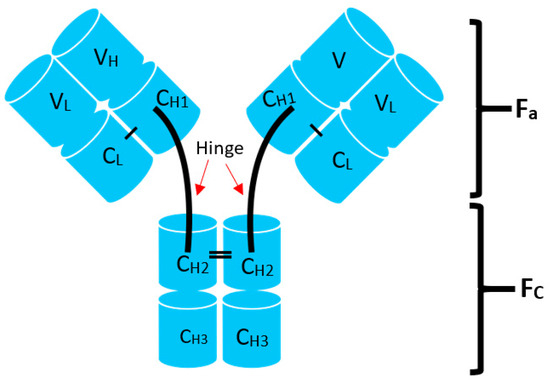
Figure 1.
Structure of typical mammalian IgG immunoglobulin. VH—heavy chain variable domain; VL—light chain variable domain; CH1,2,3—constant domains.
The term bispecific antibody (BsAb) refers to an antibody construct that has binding specificities for two different antigens. When compared to naturally occurring antibodies, BsAbs, consist of two unique single-chain variable fragments (scFVLs) which grant it binding specificity for two different antigens. In the majority of BsAbs, the preferred scFVL T-cell effector target is CD3 while the other molecule has the potential to target different specific antigens (e.g., CD19, BCMA, CD20, etc.). This mechanism engages the patient’s immune system to induce its cytotoxic effect on the target cell through a T-cell-mediated approach [12].
BsAbs can be differentiated into ones with an immunoglobulin-like structure (Fc region) and others without one [23]. First-in-class BsAb constructs belong to the second class and have binding molecules with affinities for CD3 and CD19. These molecules (e.g., blinatumomab) generated clinically relevant T-cell effector responses against the antigen but brought into consideration the practical use of the medication. The lack of an Fc region conferred the antibody a short half-life (1.5–2 h), which required continuous intravenous infusion to achieve its therapeutic effect [24]. The initial studies in patients with R/R B-cell acute lymphoblastic leukemia (ALL) noted that blinatumomab has efficacy at doses >60 µg/m2 with an overall response rate (ORR) and complete response (CR) of 69% and 37%, respectively, and led to the approval of this agent for these patients [25]. A phase I escalation study in R/R DLBCL patients (n = 25) demonstrated an ORR and CR of 43% and 19%, respectively, when the medication was administered at a dose of 112 µg/m2 [26]. The results of the study were encouraging; however, the inability to tolerate the higher dose levels required patients to proceed with lower doses and prolonged courses lasting 2 to 3 weeks, which was challenging in this population with rapidly progressing aggressive tumors [25]. Novel blinatumomab-related adverse events (AEs) such as cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity (ICANS) were also challenging. CRS was reported at an incidence of 24% for grades 1 and 2 and 2% for grades ≥ 3. Neurological AEs were reported in 70% of patients with grades ≥3 accounting for 22% of cases, prompting a 65% drop out from the study after cycle #1 [25].
While further studies have been limited in the application of blinatumomab in B-cell NHL primarily due to the dose-dependent toxicities and cumbersome administration logistics, the medication was also studied in combination with lenalidomide. The result from the phase I trial determined that the combination of blinatumomab and lenalidomide, given at the RP2D dose of 112 micrograms and 20 mg daily, respectively, is safe with mostly manageable toxicity, but moderately limited by neurotoxicity. This regimen demonstrated encouraging efficacy, producing the highest CR rates in this heavily pretreated patient population and provided a promising alternative treatment option for R/R NHL [26]. In recent years, attention has turned to newer generations of BsAbs that attempt to overcome the problems encountered by blinatumomab.
The latter part of the 2010s saw the development of BsAb constructs that sought to correct the shortcomings seen with blinatumomab, including its half-life and tolerance [27,28,29]. Second generation BsAbs maintained the bispecific construct allowing for T-cells to impart their cytotoxic effect via antibody binding to CD3 targeting a different B-cell target: CD20. These new constructs, however, also carry an Fc domain giving them a full-length IgG-like structure, and as such, bestowing similar pharmacokinetic and pharmacodynamic properties as other classic monoclonal antibodies [23]. Of this new wave of BsAbs, there are six agents that are presently under clinical investigation in B-cell NHL and three of them have been approved for the treatment of R/R DLBCL and FL. These agents include: mosunetuzumab (RO7030816), odronextamab (REGN1979), epcortitamab (GEN3013), glofitamab (RG6026), plamotamab (XmAb13676) and IgM2323. (NCT04082936) (see Table 1 and Table 2).

Table 1.
Presently available clinical trials evaluating the use of bispecific antibodies in the management of B-cell NHL.

Table 2.
Bispecific antibody constructs presently under investigation for management of relapsed/refractory DLBCL.
2.1. Mosunetuzumab
Mosunetuzumab is a full-length, humanized, immunoglobulin (IgG1) BsAb and was the first agent targeting CD3 × CD20, developed for B-cell NHL (see Table 2). This construct was tested by Budde et al. in a phase I/IB study of R/R B-cell NHL, which enrolled 98 heavily pretreated patients (median lines of therapy was 3, including 17 prior CAR-T cell treatments) with varying diagnoses: 55 DLBCL, 29 FL, 3 (MCL and 11 other B-cell NHL patients [30]. Mosunetuzumab was given intravenously every 21 days using two strategies of either a full dose on day 1 or dose escalation on days 1, 8 and 15 on cycle 1, and was administered for 8 cycles in patients who achieved a CR or continued for a total of 17 cycles for patients with <CR. Clinical activity was noted at doses >1.2 mg and the clinical activity amongst the 129 evaluable patients treated with the escalated dose strategy (1/2/60/60/30 mg) differed between aggressive and indolent lymphomas. Patients with aggressive B-cell NHL had an ORR and CR of 34.9% and 19.4%, respectively, with a median duration of response (mDOR) for all responders of 7.6 (95% CI, 5.6 to 22.8). In the group of indolent NHL, the ORR, CR and mDOR were 66.2%, 48.5% and 16.8 (95% CI, 11.7 to NE), respectively. The median PFS was 1.4 and 11.8 months for aggressive and indolent histologies, respectively, and the ORR (CR) of the patients that received anti-CD19 CAR-T cell therapy (N = 19) was 36.8% (26.3%). This was remarkable for being the first agent with activity in this high-risk population.
From the 197 treated patients in group B (“safety population”), there were 74.1% treatment-related AEs and 18.3% serious treatment-related AEs. The incidence of CRS as assessed by standardized criteria was 27.4% with 1% being grade ≥3 [35]. Most of the CRS episodes occurred during cycle 1 (D1 and D15), he majority of the cases resolved with supportive care (three patients required tocilizumab), and the dose escalation strategy seemed to further mitigate this T-cell-driven AE. Neurologic AEs were mostly grade 1 and 2 such as headaches, insomnia and dizziness with 4.1% being grade ≥3 and only two were treatment-related; hence, ICANS incidence with mosunetuzumab in this trial was difficult to characterize. Based on the promising results seen in patients with indolent NHL, a phase II trial of mosunetuzumab using the step-up dosing strategy to achieve a target dose of 30 mg was conducted in 90 patients with R/R FL which showed an ORR and CR of 80% and 60%, respectively, with similar AEs as previously reported [30,36]. Both trials led to the FDA approval of mosunetuzumab in November 2022 for the treatment of patients with R/R FL after >3 lines of therapy. The European Union equally granted approval for the management of R/R FL for patients who had received at least two prior lines of therapies.
Further studies into the application of mosunetuzumab prompted work by Olszewski et al. who looked at the utilization of this novel BsAb in the management of elderly, unfit patients with previously untreated DLBCL. This phase I/II multicenter study noted promising durable responses with a manageable safety profile. The updated results noted that among the 54 patients enrolled, ORR and CR rates were 56% (30/54) and 43% (23/54), respectively. The responses following end of treatment (EOT) in both dosing cohorts combined were ORR, 43% (23/54); CR, 35% (19/54); PR, 7% (4/54); and SD, 2% (1/54). The median duration of CR was 15.8 months (95% CI: 8.5–not estimable). These data support the future role of mosunetuzumab as a frontline therapy in elderly/unfit patients and may help identify patients whose tumors respond best to BsAbs [37].
Another avenue to be explored is the application of mosunetuzumab in combination with established lines of therapy including anti-CD20 agents like rituximab. The data thus far have been promising, with retained activity of mosunetuzumab when co-administered with rituximab being noted. The data demonstrate that while rituximab contributed to the occupancy of CD20 receptors, it did not interfere with the cytotoxic activity of mosunetuzumab. This observation suggests that the two medications can be administered alongside one another, allowing for the incorporation of this therapy into previously established frontline regimens [38].
2.2. Epcoritamab
Epicoritamab is yet another BiAb construct that targets CD3 and CD20; but unlike other molecules, this construct is more stable, largely due to point mutations in the sequence of the Fc domain (see Table 2). This stability allows for the medication to be administered subcutaneously as opposed to intravenously. The pharmacodynamics and pharmacokinetics of the subcutaneous injections demonstrated a gradual increase and lower peak levels of inflammatory cytokines which likely contributed to the more favorable toxicity profile. Epcoritamab was well tolerated, noting no grade 3 CRS in cycle 1. Like other BiAbs, epcoritamab had a clinically significant effect in the management of R/R NHL patients. In DLBCL at doses ≥12 mg and ≥48 mg, the ORR (CR) values were 68% (48%) and 91% (55%), respectively. In FL, at doses from 0.76 to 48 mg, the ORR and CR were 90% and 50%, respectively [27,32].
The clinically significant effect of therapy seen in the EPCORE-NHL1 trial as well as the favorable side effect profile has made this BsAb a candidate for further studies. This construct demonstrated compelling evidence for efficacy in highly refractory populations of LBCL as seen in the work of Thieblemont et al. [39]. Data from the subgroup analysis of the efficacy and safety of epcoritamab in the LBCL expansion cohort of the EPCORE NHL-1 phase II trial (NCT03625037) noted deep and durable responses including high MRD negativity rates. The overall response rate (ORR) for the total population of 157 patient was 63%, with a complete response (CR) rate of 39%. The median duration of response (mDOR) was 12 months (95% CI, 6.6–not reached). Of note, these responses were observed early with a mean time to response of 1.4 months. This single-agent therapy also demonstrated a favorable side effect profile. Most treatment related side effects were short-lived and declined markedly after week 12. CRS was reported in 49.7% of the patients in the study population. The events were primarily low grade with only 2.5% of the patients developing grade 3 CRS or greater. Additionally, these side effects were predictable with 42.9% of the events occurring after administration of the first full dose of epcoritamab [40].
The adverse ICANS events were also minimal with only 10 patients reported to have experienced this complication. Nine of these patients were graded as grade 1 or 2 ICANS. Only one grade 5 ICANS event was reported [40].
The clinical effectiveness and manageable safety profile as well as the “off the shelf” availability and ease of administration prompted a phase II trial to evaluate the safety of epcoritamab monotherapy in the outpatient setting without the need for mandatory hospitalization in patients with R/R DLBCL and FL. The study, EPCORE-NHL-6 (NCT05451810), is actively enrolling patients and seeking to allocate approximately 40 patients into each group (R/R DLBCL and R/R FL). The proposed study design would introduce the medication in a step-up fashion with priming and intermediate dosing during cycle 1, days 1 and 8, and a full dose on days 14 and 22. Subsequent cycles would be dosed at the full dose [41].
In May of 2023, the FDA granted accelerated approval to epcoritamab-bysp for R/R DLBCL, including DLBCL arising from indolent lymphoma and high-grade B-cell lymphoma after two or more lines of systemic therapy.
2.3. Glofitamab
Glofitamab is a fully humanized anti-CD20/CD3 BsAb built to have a single CD3-binding site and two binding sites for CD20 (see Table 2). The rationale behind the bivalent CD20 binding is a more stable and deeper anti-tumor activity [33,42]. This unique structure was noted to have increased potency when compared other BsAbs with a 1:1 format [42]. A dose escalation study of 64 patients with aggressive R/R B-NHL and indolent R/R B-NHL demonstrated promising clinical activity in heavily pretreated B-NHL. The best responses were seen at >300 µg, with ORR and CR rates of 38% and 24%, respectively. CRS was noted in 21.8% of patients (grades 1–2, no grade 3 reported) with no neurological toxicity. The study also extrapolated previous data with other BsAb constructs, namely for the ability to co-administer or sequence with anti-CD20 agents without impacting the pharmacologic activity of the BsAb construct. A study by Hutchings et al. pretreated patients with a single dose of obinutuzumab a week prior to administration of glofitamab. This sequencing allowed for the debulking of peripheral B cells and thus reducing CRS confirmed by the lack of f grades ≥3 of CRS [42,43].
Continued studies suggest that the efficacy of this construct is seen in both aggressive and indolent B-NHLs. Phillips et al. has reported the efficacy of glofitamab monotherapy in is a small group of patients with R/R MCL. These patients not only achieved an early response (median time to response: 51 days) but were also noted to have ORR and complete CR rates of 83.8% and 73.0% after a median follow up of 8 months. The median DOR was 12.6 months (95% CI: 10.0–NE) and median duration of CR was 10.0 months (95% CI: 4.9–NE) [44,45].
This construct has demonstrated continued effectiveness in studies, imparting lasting remissions for patients who achieved CR at the end of the fixed course therapy. The typical course of 12 cycles was administered to 214 patients with LBCL who had previously been noted to be refractory to at least one line of therapy. Of those patients, 61 (29%) were noted to have achieved CR as per the investigators’ criteria. The median duration of CR was not reached at the time of analysis and only one patient was noted to have progression of disease, requiring the restarting of treatment. Such data have been particularly encouraging as they demonstrate something that has rarely been observed previously, namely, sustained remission >12 months in patients with heavily pretreated and refractory large B-cell lymphoma [46].
Similar data were presented in a study by Dickinson MJ et al. on 155 patients with R/R DLBCL, with 52 of them having progressive disease after CART therapy. At a median follow-up of 12.6 months, the ORR was 52% with a CR of 39%, along with a median PFS of 5 months (95% CI 3.4-8.1) and a 12-month OS of 11.5 (95% CI 7.9-15.7) The results were consistent among the 52 patients who had previously received CART therapy, 35% of who had achieved a CR. The median time to a complete response was 42 days (95% CI, 42 to 44). The majority (78%) of complete responses were ongoing at 12 months [47]. Glofitmab was noted to have a favorable safety profile as well with only 9% of events leading to discontinuation of therapy with 3% being directly linked to glofitmab. In the study grade 3 or higher adverse events occurred in 62% with most common event being neutropenia. These events however were not severe enough to warrant discontinuation of threapy.
Glofitamab has also been added to conventional therapies in an attempt to improve response rates in patients with previously refractory disease, which has added additional opportunities for therapeutic interventions in patients who otherwise have poor clinical outcomes.
Given the successes noted with the use of glofitamab, the European Medicines Agency recommended the granting of conditional marketing authorization for glofitamab for the treatment of R/R DLBCL in April of 2023. Two months later in June of 2023, the FDA issued accelerated approval to glofitmab for the treatment of R/R DLBCL or large B-cell lymphoma arising from follicular lymphoma, after two or more lines of systemic therapy.
2.4. Odronextamab
Odronextamab is another BsAb construct consisting of an Fc domain as well as bispecific sites directed to CD3 and CD20. What makes this human IgG4-based bispecific product unique is that its hinge is stabilized. This property allows for decreased susceptibility to proteolytic cleavage by proteases present in the malignant microenvironment. As such, odronextamab is expected to have more predictable functional responses. The phase I trial conducted by Bannerji et al. comprised patients with R/R disease including DLBCL, FL and MCL. The patients received IV therapy weekly for 12 weeks followed by administration of 12 doses every two weeks. The study demonstrated that BsAbs do indeed have significant clinical activity in patients. The benefit was seen across all drug doses from 5 to 320 mg in all disease subtypes. Adverse events (AEs) were analyzed, with evidence of CRS, with the incidences of all grade and grade >3 of approximately 61% and 7.3%, respectively. Approximately 3.7% of patients were noted to have ICANS AEs partially attributed to the improved half-life and better-tolerated doses [31].
The ELM-2 study further demonstrated compelling efficacy in patients with FL grade 1–3a having received ≥2 prior lines of therapy, with 75% of patients achieving CR by ICR with an acceptable safety profile. Similar data were seen in patients with diffuse large B-cell lymphomas. The ORR and CR rates were 53% (48/90) and 37% (33/90), respectively, and were consistent across high-risk subgroups and in the subgroup treated with the 0.7/4/20 step-up regimen. CRs were durable; the median duration of CR was not reached (95% CI: 10.2 months-not estimable) and the probability of an ongoing CR at 9 months was 73% [48,49].
2.5. Plamotamab
Plamotamab is an IgG1 bispecific anti-CD20/CD3 antibody. The first in human (FIH) study was reported in 44 patients (36 with B-cell lymphomas and 8 with chronic lymphocytic leukemia that were heavily pretreated (median number of therapies of 3). In the NHL cohort, there was a reported 20% response with 5% CRs at doses >20 micrograms/kg. In DLBCL, the best responses were seen at doses ≥80 micrograms/kg with ORR and CR rates of 38.9% and 27.8%, respectively. CRS, all grades, occurred in 52.8% of cases, with CRS grades ≥3 in 5.7%. Neurological adverse events occurred in 49.1% of patients and involved mainly headaches, paresthesia, lethargy and dizziness. No grade 3 neurological adverse events were reported. At present, the study is ongoing and is in the dose-escalation phase [24,34].
2.6. IgM 2323
IgM2323 is a unique construct which deviates from the previously utilized IgG-based BsAbs. It is a pentameric antibody with expression of CD20 and CD3. The construct allows for a 10 to 1 ratio of binding and subsequent T-cell-mediated cellular toxicity. Preliminary data have suggested that a third of the participants demonstrated a response with 20% achieving CR [50].
3. The Role of BsAbs in the Treatment of B-Cell NHL
The development of bispecific antibody constructs offers another avenue of therapy for patients who are heavily pretreated or have R/R NHL. The phase I and II studies that have explored the effectiveness and safety of BsAbs recruited patients who met such criteria; however, the effectiveness of these treatments has begged the question of the sequencing of BsAbs in the management of NHL. Could these agents be used as an upfront therapy for newly diagnosed disease, or can they be used in combination with other agents such as conventional chemotherapy and immune modulatory agents? Preclinical work in in vitro and murine studies has noted that CD20 × CD3 BsAbs retain their therapeutic effects when administered alongside T cell lymphotoxic agents such as cyclophosphamide and dexamethasone. Despite the decreased pool of T cells, the surviving cells could still be recruited and activated to exert their cytotoxic effect. Two of the current BsAb constructs, glofitamab and epcoritamab, have been tested in the setting of R/R disease in combination with platinum-containing regimens such as R-DHAX and gemcitabine/oxaliplatin with promising results [51,52]. Nonetheless, more studies are needed to elucidate the benefits of this treatment. Similar studies have been conducted with anti-CD79b antibody–drug conjugates noting ORR and CR rates like those seen in single-agent studies [53,54]. Such results need to be investigated further to discern the durability of the responses achieved.
The data have thus far noted that BsAbs such as mosunetuzumab, glofitamab and epcoritamab can be successfully co-administered with agents like rituximab and cyclophosphamide with preserved cytotoxic effects and minimal adverse effects. These observations have prompted two phase I/II studies to combine mosunetuzumab with CHOP with or without rituximab. One study combined epcoritamab with R-CHOP in high-risk patients (defined as international prognostic index of 3–5). The study demonstrated that all 33 participants responded to therapy and 77% achieved complete remission. Further phase III studies are anticipated to commence to further assess BsAb + RCHOP regimens in the upfront therapy of patients with B-cell NHLs.
4. Resistance Mechanisms
The development of novel therapies requires researchers to consider not only how to assess the effectiveness of these new therapies and their safety profiles but also to consider potential pathways of resistance. Three pathways of potential BsAb resistance have been postulated for the resistance to bispecific constructs: (1) selective pressure leading to loss of target CD20 antigen, (2) “down-regulation” of activated T cells via T regulatory cells and (3) recruitment of immunosuppressive myeloid and stromal cells as well as tumor-associated macrophages.
Selective pressure mechanisms have been studied previously in other malignancies which have allowed cancer cells to evade the immune response. Loss of the CD20 target antigen has been reported previously in patients who had received therapy with anti- CD20 antibodies. Given that BsAbs’ mechanism of action depends on CD20 × CD3 interactions, it is conceivable that loss of the CD20 antigen can lead to resistance to bispecific antibodies. Alternatively, activation of CD3+ may lead to indiscriminate activation of T regulatory and T suppressor cells. Such activation may lead to the downregulation of CD3+ effector cells, thus allowing tumor cells to evade immune-mediated destruction. It also worth noting that a major component of the activity of BsAb relies on adequate T cell populations and function. As such, the activity of any BsAb may be hampered by exposure to cytotoxic treatments especially in heavily pretreated patients leading to persistent T-lymphocyte weakening.
Lastly, overactivation of effector T cells can promote the recruitment of myeloid suppressive cells as well as cancer-associated fibroblasts and macrophages which are thought to lead to “exhaustion” of T cells and downregulation of the cells’ cytotoxic ability. This mechanism of resistance is not yet well understood and requires further interrogation in order to better understand its implication as it relates to BsAb use and the management of B-cell NHL.
5. Conclusions
The bispecific antibody constructs have demonstrated promising evidence in the management of relapsed/refractory NHL. The phase I studies of these novel agents have demonstrated good efficacy as well as tolerable adverse effect profiles making them an attractive option for the management of patients. The availability of CAR-T therapy makes the selection of therapy more complicated however, as these modalities have similar mechanisms of action as well as antigen targets. Further studies would need to be performed to establish the sequencing of therapies in the management of R/R NHL, but BsAbs remain an appealing option. The additional benefit of the “off the shelf” design of these unique therapeutic agents would make it available to patients who are not only ineligible for CAR-T therapy or autologous transplantation but who also may not have access to large academic centers who would normally perform these interventions.
Author Contributions
S.I.—completed review of available data on the subject and conceptualized the structure of the publication. J.S.-S.—wrote, reviewed and edited the original paper; M.M.—reviewed and edited original paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the Memorial Cancer Institute as well as the Moffitt Cancer Center at Memorial Healthcare System for the support in the formulation and publication of this original article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- de Leval, L.; Jaffe, E.S. Lymphoma Classification. Cancer J. 2020, 26, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.O.; Weisenburger, D.D. New approach to classifying non-Hodgkin’s lymphomas: Clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J. Clin. Oncol. 1998, 16, 2780–2795. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.; Grillo-López, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab Chimeric Anti-CD20 Monoclonal Antibody Therapy for Relapsed Indolent Lymphoma: Half of Patients Respond to a Four-Dose Treatment Program. J. Clin. Oncol. 2023, 41, 154–162. [Google Scholar] [CrossRef]
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Trümper, L.; Österborg, A.; Pettengell, R.; Trneny, M.; Imrie, K.; Ma, D.; Gill, D.; Walewski, J.; Zinzani, P.-L.; et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the Mab Thera International Trial (MInT) Group. Lancet Oncol. 2006, 7, 379–391. [Google Scholar] [CrossRef]
- Sehn, L.H.; Donaldson, J.; Chhanabhai, M.; Fitzgerald, C.; Gill, K.; Klasa, R.; MacPherson, N.; O’Reilly, S.; Spinelli, J.J.; Sutherland, J.; et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J. Clin. Oncol. 2005, 23, 5027–5033. [Google Scholar] [CrossRef]
- Hiddemann, W.; Kneba, M.; Dreyling, M.; Schmitz, N.; Lengfelder, E.; Schmits, R.; Reiser, M.; Metzner, B.; Harder, H.; Hegewisch-Becker, S.; et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma com- pared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005, 106, 3725–3732. [Google Scholar]
- Luminari, S.; Ferrari, A.; Manni, M.; Dondi, A.; Chiarenza, A.; Merli, F.; Rusconi, C.; Tarantino, V.; Tucci, A.; Vitolo, U.; et al. Long-Term Results of the FOLL05 Trial Comparing R-CVP Versus R-CHOP Versus R-FM for the Initial Treatment of Patients With Advanced-Stage Symptomatic Follicular Lymphoma. J. Clin. Oncol. 2018, 36, 689–696. [Google Scholar] [CrossRef]
- Rummel, M.J.; Niederle, N.; Maschmeyer, G.; Banat, G.A.; von Grünhagen, U.; Losem, C.; Kofahl-Krause, D.; Heil, G.; Welslau, M.; Balser, C.; et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013, 381, 1203–1210, Erratum in Lancet 2013, 381, 1184. [Google Scholar] [CrossRef]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Sarkozy, C.; Maurer, M.J.; Link, B.K.; Ghesquieres, H.; Nicolas, E.; Thompson, C.A.; Traverse-Glehen, A.; Feldman, A.L.; Allmer, C.; Slager, S.L.; et al. Cause of Death in Follicular Lymphoma in the First Decade of the Rituximab Era: A Pooled Analysis of French and US Cohorts. J. Clin. Oncol. 2019, 37, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017, 130, 1800–1808, Erratum in Blood 2018, 131, 587–588. [Google Scholar] [CrossRef]
- Casulo, C.; Byrtek, M.; Dawson, K.L.; Zhou, X.; Farber, C.M.; Flowers, C.R.; Hainsworth, J.D.; Maurer, M.J.; Cerhan, J.R.; Link, B.K.; et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J. Clin. Oncol. 2015, 33, 2516–2522, Erratum in J. Clin. Oncol. 2016, 34, 1430. [Google Scholar] [CrossRef] [PubMed]
- Visco, C.; Di Rocco, A.; Evangelista, A.; Quaglia, F.M.; Tisi, M.C.; Morello, L.; Zilioli, V.R.; Rusconi, C.; Hohaus, S.; Sciarra, R.; et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: Results from the MANTLE-FIRST study. Leukemia 2021, 35, 787–795, Erratum in Leukemia 2021, 35, 932–933. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Jacobson, C.A.; Ghobadi, A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. 5-Year Follow-Up Supports Curative Potential of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1). Blood 2023, 141, 2307–2315. [Google Scholar] [CrossRef]
- Alencar, A.J.; Moskowitz, C.H. Autologous Stem Cell Transplantation in the Management of Relapsed Non-Hodgkin Lymphoma. J. Clin. Oncol. 2021, 39, 467–475. [Google Scholar] [CrossRef]
- Sehn, L.H.; Matasar, M.J.; Flowers, C.R.; Kamdar, M.; McMillan, A.K.; Hertzberg, M.M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab vedotin plus bendamustine with rituximab in relapsed/refractory diffuse large B-cell lymphoma: Updated results of a phase Ib/II randomized study. Blood 2019, 134 (Suppl. S1), 4081. [Google Scholar] [CrossRef]
- Duell, J.; Maddocks, K.J.; González-Barca, E.; Jurczak, W.; Liberati, A.M.; De Vos, S.; Salles, G. Long-term outcomes from the Phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica 2021, 106, 2417–2426. [Google Scholar] [CrossRef]
- Salles, G.; Duell, J.; González Barca, E.; Tournilhac, O.; Jurczak, W.; Liberati, A.M.; Nagy, Z.; Obr, A.; Gaidano, G.; André, M.; et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): A multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020, 21, 978–988. [Google Scholar] [CrossRef]
- Caimi, P.F.; Ai, W.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S.; Baker, D.L. Cellular and Molecular Immunology, 10th ed.; Chapter 5: Antibodies and Antigens; Elsevier: Philadelphia, PA, USA, 2022. [Google Scholar]
- Kontermann, R.E.; Brinkmann, U. Bispecific antibodies. Drug Discov. Today 2015, 20, 838–847, Erratum in Drug Discov. Today 2018, 30, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Puglianini, O.; Chavez, J.C. Bispecific antibodies for non-Hodgkin’s lymphomas and multiple myeloma. Drugs Context 2021, 10, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Coyle, L.; Morley, N.J.; Rambaldi, A.; Mason, K.D.; Verhoef, G.; Furness, C.L.; Zhang, A.; Jung, A.S.; Cohan, D.; Franklin, J.L. Open-label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Leuk. Lymphoma 2020, 61, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.; Frankel, P.; Ruel, C.; Abedi, M.; Schwab, E.; Costello, C.L.; Zain, J.; Budde, L.E.; William, B.M.; Foss, F.M.; et al. Blinatumomab/lenalidomide in relapsed/refractory non-Hodgkin’s lymphoma: A phase I California cancer consortium study of safety, efficacy and immune correlative analysis. Blood 2019, 134 (Suppl. S1), 760. [Google Scholar] [CrossRef]
- Schuster, S.J. Bispecific antibodies for the treatment of lymphomas: Promises and challenges. Hematol. Oncol. 2021, 39, 113–116. [Google Scholar] [CrossRef]
- Goebeler, M.-E.; Knop, S.; Viardot, A.; Kufer, P.; Topp, M.S.; Einsele, H.; Noppeney, R.; Hess, G.; Kallert, S.; Mackensen, A.; et al. Bispecific T-cell engager (bite) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: Final results from a phase I study. J. Clin. Oncol. 2016, 34, 1104–1111. [Google Scholar] [CrossRef]
- Popplewell, L.; Verhoef, G.; Kuruvilla, J.; Tuglus, C.; Kischel, R.; Stieglmaier, J.; Ghobadi, A. A first-in-human study of a half-life extended CD19-targeting bite in relapsed/refractory diffuse large B cell lymphoma, mantle cell lymphoma or follicular lymphoma. Hematol. Oncol. 2019, 37, 566–567. [Google Scholar] [CrossRef]
- Budde, L.E.; Assouline, S.; Sehn, L.H.; Schuster, S.J.; Yoon, S.S.; Yoon, D.H.; Matasar, M.J.; Bosch, F.; Kim, W.S.; Nastoupil, L.J.; et al. Single-Agent Mosunetuzumab Shows Durable Complete Responses in Patients with Relapsed or Refractory B-Cell Lymphomas: Phase I Dose-Escalation Study. J. Clin. Oncol. 2022, 40, 481–491. [Google Scholar] [CrossRef]
- Bannerji, R.; Arnason, J.E.; Advani, R.H.; Brown, J.R.; Allan, J.N.; Ansell, S.M.; Barnes, J.A.; O’Brien, S.M.; Chávez, J.C.; Duell, J.; et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): Results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022, 9, e327–e339. [Google Scholar] [CrossRef]
- Hutchings, M.; Mous, R.; Clausen, M.R.; Johnson, P.; Linton, K.M.; Chamuleau, M.E.D.; Lewis, D.J.; Sureda Balari, A.; Cunningham, D.; Oliveri, R.S.; et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: An open-label, phase 1/2 study. Lancet 2021, 398, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.; Morschhauser, F.; Iacoboni, G.; Carlo-Stella, C.; Offner, F.C.; Sureda, A.; Salles, G.; Martínez-Lopez, J.; Crump, M.; Thomas, D.N.; et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J. Clin. Oncol. 2021, 39, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Michot, J.-M.; Chanan-Khan, A.A.; Salles, G.A.; Cartron, G.; Peyrade, F.; Bouabdallah, R.; Reid, E.G.; Thomas, S.K.; Wierda, W.G.; et al. Preliminary Safety and Anti-Tumor Activity of XmAb13676, an Anti-CD20 x Anti-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Leukemia. Blood 2019, 134 (Suppl. S1), 4079. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Matasar, M.; Bartlett, N.L.; Sehn, L.H.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. P1126: Mosuntuzumab is efficacious and well tolerated in patients aged<65 and ≥ 65 years with relapsed/refractory follicular lymphoma and ≥ 2 prior therapies: Subgroup analysis of a pivotal phase II study. Hemasphere 2022, 6, 1016–1017. [Google Scholar] [CrossRef]
- Olszewski, A.J.; Avigdor, A.; Babu, S.; Levi, I.; Eradat, H.; Abadi, U.; Holmes, H.; McKinney, M.; Woszczyk, D.; Giannopoulos, K.; et al. Mosunetuzumab Monotherapy Continues to Demonstrate Promising Efficacy and Durable Complete Responses in Elderly/Unfit Patients with Previously Untreated Diffuse Large B-Cell Lymphoma. Blood 2022, 140 (Suppl. S1), 1778–1780. [Google Scholar] [CrossRef]
- Sun, L.L.; Ellerman, D.; Mathieu, M.; Hristopoulos, M.; Chen, X.; Li, Y.; Yan, X.; Clark, R.; Reyes, A.; Stefanich, E.; et al. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci. Transl. Med. 2015, 7, 287ra70. [Google Scholar] [CrossRef]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a Novel, Subcutaneous CD3 × CD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J. Clin. Oncol. 2022, 41, 2238–2247. [Google Scholar] [CrossRef]
- Phillips, T.; Thieblemont, C.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.A.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab Monotherapy Provides Deep and Durable Responses Including Minimal Residual Disease (MRD) Negativity: Novel Subgroup Analyses in Patients with Relapsed/Refractory (R/R) Large B-Cell Lymphoma (LBCL). Blood 2022, 140 (Suppl. S1), 9443–9445. [Google Scholar] [CrossRef]
- Sharman, J.P.; Boccia, R.V.; Doerr, T.; Conte, K.; Bai, Y.; Elliott, B.; Andorsky, D.J. Phase 2 Trial to Evaluate Safety of Subcutaneous Epcoritamab Monotherapy in the Outpatient Setting Among Patients with Relapsed or Refractory Diffuse Grade 1-3a Large B-Cell and Follicular Lymphoma (EPCORE NHL-6). Blood 2022, 140 (Suppl. S1), 9491–9492. [Google Scholar] [CrossRef]
- Bacac, M.; Colombetti, S.; Herter, S.; Sam, J.; Perro, M.; Chen, S.; Bianchi, R.; Richard, M.; Schoenle, A.; Nicolini, V.; et al. CD20-TCB with Obinutuzumab Pretreatment as Next-Generation Treatment of Hematologic Malignancies. Clin. Cancer Res. 2018, 24, 4785–4797. [Google Scholar] [CrossRef]
- Hutchings, M.; Iacoboni, G.; Morschhauser, F.; Offner, F.; Sureda, A.; Salles, G.A.; Carlo-Stella, C.; Lopez, J.M.; Thomas, D.; Morcos, P.N.; et al. CD20-Tcb (RG6026), a Novel “2:1” Format T-Cell-Engaging Bispecific Antibody, Induces Complete Remissions in Relapsed/Refractory B-Cell Non-Hodgkin’s Lymphoma: Preliminary Results from a Phase I First in Human Trial. Blood 2018, 132 (Suppl. S1), 226. [Google Scholar] [CrossRef]
- Phillips, T.; Dickinson, M.; Morschhauser, F.; Bachy, E.; Crump, M.; Trněný, M.; Bartlett, N.L.; Zaucha, J.; Humphrey, K.; Perez-Callejo, D.; et al. Glofitamab Step-up Dosing Induces High Response Rates in Patients (pts) with Relapsed or Refractory (R/R) Mantle Cell Lymphoma (MCL), Most of Whom Had Failed Prior Bruton’s Tyrosine Kinase Inhibitor (BTKi) Therapy. Blood 2021, 138 (Suppl. S1), 130. [Google Scholar] [CrossRef]
- Phillips, T.J.; Dickinson, M.; Morschhauser, F.; Bachy, E.; Crump, M.; Trněný, M.; Bartlett, N.L.; Zaucha, J.; Wrobel, T.; Offner, F.; et al. Glofitamab Monotherapy Induces High Complete Response Rates in Patients with Heavily Pretreated Relapsed or Refractory Mantle Cell Lymphoma. Blood 2022, 140 (Suppl. S1), 178–180. [Google Scholar] [CrossRef]
- Hutchings, M.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Patel, K.; Hertzberg, M.; Falchi, L.; et al. Relapse Is Uncommon in Patients with Large B-Cell Lymphoma Who Are in Complete Remission at the End of Fixed-Course Glofitamab Treatment. Blood 2022, 140 (Suppl. S1), 1062–1064. [Google Scholar] [CrossRef]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wróbel, T.; Offner, F.; Trněný, M.; et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar] [CrossRef]
- Kim, T.M.; Taszner, M.; Cho, S.-G.; Novelli, S.; Le Gouill, S.; Poon, M.L.; Villasboas, J.C.; Champion, R.; Bachy, E.; Guidez, S.; et al. Odronextamab in Patients with Relapsed/Refractory (R/R) Follicular Lymphoma (FL) Grade 1-3a: Results from a Prespecified Analysis of the Pivotal Phase II Study ELM-2. Blood 2022, 140 (Suppl. S1), 2280–2282. [Google Scholar] [CrossRef]
- Kim, W.-S.; Kim, T.M.; Cho, S.-G.; Jarque, I.; Iskierka-Jażdżewska, E.; Poon, M.L.; Prince, H.M.; Oh, S.Y.; Lim, F.; Carpio, C.; et al. Odronextamab in Patients with Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL): Results from a Prespecified Analysis of the Pivotal Phase II Study ELM-2. Blood 2022, 140 (Suppl. S1), 1070–1071. [Google Scholar] [CrossRef]
- Budde, E.; Gopal, A.K.; Kim, W.S.; Flinn, I.W.; Cheah, C.Y.Y.; Nastoupil, L.; Matasar, M.J.; Diefenbach, C.S.; Gregory, G.P.; Qazi, I.; et al. A Phase 1 Dose Escalation Study of Igm-2323, a Novel Anti-CD20 × Anti-CD3 IgM T Cell Engager (TCE) in Patients with Advanced B-Cell Malignancies. Blood 2021, 138 (Suppl. S1), 132. [Google Scholar] [CrossRef]
- Abrisqueta, P.; Falchi, L.; Phillips, T.J.; De Vos, S.; Nijland, M.; Offner, F.; Bykhovski, I.; Wu, J.; Wang, L.; Rana, A.; et al. Subcutaneous epcoritamab + R-DHAX/C in patients (pts) with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) eligible for autologous stem cell transplant (ASCT): Preliminary phase 1/2 results. J. Clin. Oncol. 2022, 40, 7528. [Google Scholar] [CrossRef]
- Brody, J.; Wahlin, B.E.; Phillips, T.J.; Costello, R.; Lugtenburg, P.; Cordoba, R.; Wang, L.; Wu, J.; Elliott, B.; Abbas, A.; et al. Epcoritamab (epco) with gemcitabine + oxaliplatin (GemOx) in patients (pts) with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) ineligible for autologous stem cell transplant (ASCT) induces high response rate even in pts failing CAR T therapy. J. Clin. Oncol. 2022, 40, 7527. [Google Scholar]
- Cheson, B.D.; Nowakowski, G.; Salles, G. Diffuse large B-cell lymphoma: New targets and novel therapies. Blood Cancer J. 2021, 11, 68. [Google Scholar] [CrossRef]
- Hess, B.T.; Collins, G.P.; Solh, M.; Gandhi, M.; Wang, Y.; Qin, Y.; Yu, E.; Zinzani, P.L. A Phase 1b Open-Label Study of Loncastuximab Tesirine in Combination with Other Anticancer Agents in Patients with Relapsed or Refractory (R/R) B-Cell Non-Hodgkin Lymphoma (LOTIS-7). Blood 2022, 140, 12079–12080. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).