Abstract
Purpose: To describe the efficacy and safety of surgical treatment in refractory glaucoma using transscleral cyclophotocoagulation with a diode laser (TSCPC). Cyclo-G6 laser with a G-probe delivery system (Iridex, Silicon Valley, CA, USA). Materials and Methods: 134 patients (134 eyes) with refractory glaucoma were included in this study. Patients received transscleral cyclophotocoagulation (TSCPC) using the IRIDEX Cyclo-G6 diode laser equipped with a G-probe delivery system. The eyes were treated with 1250–1500 mW for 3500–4000 ms depending on the iris colour. Intraocular pressure (IOP) and the count of antiglaucoma medications (AGM) were documented at the initial assessment and again at 3, 6, and 12 months following the treatment. Results: A reduction in IOP was observed in 97.73% of eyes at 12 months. At 12 months, 55.97% of the 134 eyes decreased IOP under 21 mmHg. At the 3-month mark, 96.27% of eyes attained an IOP reduction in 30% or more from their initial baseline levels, 85.61% at 6 months, and 82.17% at 12 months. A reduction in AGM was recorded for 86.07% of patients at 6 months and 87.31% at 12 months. There were no instances of severe complications reported, and minor complications were observed in 3% (4 out of 134) of the treated eyes. Conclusions: IRIDEX Cyclo-G6 laser is a surgical method effective in reducing IOP in patients with refractory glaucoma
1. Introduction
Glaucoma stands as a prominent cause of global blindness. This condition encompasses a diverse array of diseases characterized by the cupping of the optic nerve head and associated visual field impairment. It represents an optic neuropathy that leads to gradual and irreversible vision deterioration [1,2].
Elevated intraocular pressure (IOP) serves as the primary risk factor for this condition. It can stem from either an overproduction in aqueous humor or insufficient outflow due to an obstruction [3]. Consequently, the conventional treatment approach for glaucoma focuses on IOP reduction through the use of medications and various surgical methods, spanning from incisional procedures to laser surgery [4].
The rate of glaucoma progression typically decelerates when intraocular pressure is reduced by 30–50% from the initial baseline measurement [1]. Refractory glaucoma, on the other hand, is characterized by unmanageable intraocular pressure, accompanied by evident optic nerve and/or visual field deterioration, even in the presence of the most extensively tolerated topical and/or systemic anti-glaucoma medications. This category may include cases where surgical interventions have been ineffective or a combination of surgical procedures and medications, as well as situations posing a high risk of trabeculectomy failure [5].
Traditional transscleral cyclophotocoagulation (TSCPC) is a laser surgical procedure designed to deliberately induce controlled damage to the ciliary body, the source of aqueous humor, using a diode laser that emits near-infrared light at a wavelength of 810 nm [6,7].
The absorption of laser light by the melanin within the ciliary body triggers the coagulation of both pigmented and non-pigmented epithelium, resulting in a decrease in intraocular pressure (IOP) attributed to reduced aqueous humor secretion [8,9].
The utilization of TSCPC is constrained by the occurrence of post-treatment complications, which can vary in severity. TSCPC is frequently regarded as a final option for managing refractory glaucoma, particularly in cases characterized by limited visual potential or when patients are unsuitable candidates for incisional surgery [6,10].
Common post-treatment complications comprise pain, uveitis, inflammation, eyelid swelling, hyphema, cystoid macular edema, conjunctival scarring, and hypotony [11,12,13,14]. The most critical complications encompass phthisis bulbi, sympathetic ophthalmia, hypotony, scleral burn, scleritis, malignant glaucoma, and perforation [11,12,13,14].
This study aimed to conduct a retrospective assessment of the effectiveness and safety of the conventional TSCPC procedure in patients presenting with various forms of refractory glaucoma.
2. Materials and Methods
This study presents an analysis of data from patients diagnosed with refractory glaucoma who underwent the TSCPC procedure at the Ospedali Riuniti “Villa Sofia-Cervello” (Palermo, Italy) between September 2016 and March 2022. The datasets were obtained from other researchers. These datasets have been appropriately anonymized, and informed consent was obtained during the original data collection process.
The study included 134 patients (134 eyes) with refractory glaucoma. Table 1 represents demographic and preoperative characteristics. Patients underwent TSCPC for one of the following indications: advanced glaucoma after surgery (silicon oil), neovascular, traumatic, uveitis, and aphakic glaucoma (Table 1); 82% of patients underwent trabeculectomy surgery, and 18% of the patients were deemed ineligible for filtering or valvular surgery due to contraindications that emerged following primary surgical interventions.

Table 1.
Demographic data for study eyes.
The study adhered to the principles of the Helsinki Declaration for research ethics. The main inclusion criteria are advanced glaucoma uncontrolled by maximum medical treatments and refractory to surgical or laser treatment, reduced response either to the maximal tolerated local therapy (two to four antiglaucoma agents) or to systemic therapy (acetazolamide) for the target IOP to be reached as well as 12 months as the minimum follow-up period.
All procedures were conducted under monitored anesthesia care, utilizing retrobulbar 2% mepivacaine combined with adrenaline.
Patients underwent TSCPC using the Cyclo G6 laser system (IRIDEX IQ810 Laser Systems, CA) along with the G-probe delivery system, following the manufacturer’s guidelines. The G probe footplate was positioned between the anterior border and the midpoint of the limbus, and the laser applications were distributed across approximately 270° of the limbus (3 quadrants) with 6 to 7 applications per quadrant. Laser settings were adjusted to 1250 mW for 4000 ms for dark brown irises and 1500 mW for 3500 ms for non-dark brown irises, following the manufacturer’s recommendations [15].
After each laser application, the subsequent applications were positioned at intervals equal to half the width of the G-Probe footplate. This was achieved by aligning one side of the probe with the indented center of the previous application. After completing the initial 270° treatment, additional laser applications were administered, if needed, commencing at a 45° angle from the first quadrant and extending to cover half of the untreated quadrant, as well as two and a half quadrants from the previous treatment.
Baseline examinations included a full ophthalmic examination (slit-lamp biomicroscope examination and funduscopy). Goldmann applanation tonometry (GAT), optical coherence tomography (OCT Topcon 3D 2000) of the macula and the optic disc. The postoperative follow-up visits were conducted at 3 months, 6 months, and 12 months after the surgical intervention.
The success of TSCPC treatment was determined by achieving an intraocular pressure (IOP) below 21 mmHg or a 30% reduction from the baseline measurement at the final visit (12 months), without the need for additional glaucoma medication and with no indications of further glaucoma progression [16,17]. Failure was defined as the inability to fulfill the criteria for success or in the event of a severe complication
Statistical Analysis
Preoperative baseline IOP values were compared with postoperative IOP values at 3, 6, and 12 months. Quantitative data were presented as mean ± standard deviation (SD). Statistical analysis of the differences in IOP and AGM between baseline and post-surgery time points was conducted using a paired Student’s t-test. A p-value of less than 0.001 was deemed statistically significant. All statistical analyses were carried out using Microsoft Excel.
3. Results
In this study, a total of 134 eyes (representing 134 patients) were included. Table 1 displays the preoperative characteristics of all patients who completed follow-up examinations at 3, 6, and 12 months after undergoing TSCPC treatment for refractory glaucoma. None of the patients had a baseline IOP lower than 27 mmHg, with the mean baseline IOP measured at 38.87 ± 6.93 mmHg
At 3 months following treatment, all treated eyes experienced a decrease in IOP. This decrease was maintained in 132 eyes (98.51%) at the 6-month follow-up and in 129 eyes (97.73%) at the 12-month follow-up. Table 2 and Table 3 provide the mean values of IOP at the last two follow-up examinations, along with their respective percentage decreases.

Table 2.
Mean values of the intraocular pressure (IOP) at 3. 6 and 12 months of follow-up after treatment compared to the baseline. Student’s t-test for paired data. p-values of 0.001 were considered statistically significant.

Table 3.
Mean values of the intraocular pressure (IOP) at 3. 6 and 12 months of follow-up after treatment compared to the baseline. Student’s t-test for paired data. p-values of 0.001 were considered statistically significant.
Figure 1 illustrates the IOP trends at baseline and during various follow-up periods. Notably, at the 3-month post-treatment mark, the IOP dropped to 14.99 ± 3.88 mmHg (range: 9–29), signifying a remarkable 61.44% reduction compared to the baseline measurements (p < 0.001).
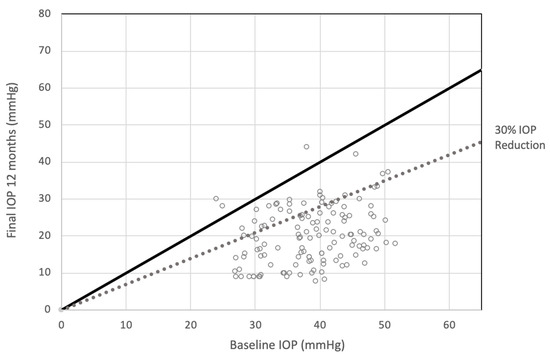
Figure 1.
Scattergram showing intraocular pressure (IOP) at baseline and at the end of follow-up at 12 months. At 12 months after treatment, the 82.17% of patients achieved post-treatment IOP reduction of 30% or more.
By the 6-month mark, there was a gradual increase in IOP, reaching a mean value of 16.78 ± 8.25 mmHg (range: 10–45), which still represented a notable 56.83% reduction compared to the baseline measurements (p < 0.001).
At the 12-month follow-up, the mean IOP was recorded at 19.89 ± 8.6 mmHg, reflecting a decrease of 48.83% compared to the baseline values (p < 0.001)
When considering a post-treatment IOP reduction of 30% or more, it was observed that 96.27% achieved this threshold at the 3-month mark, 85.61% at 6 months, and 82.17% at 12 months after treatment (Table 2—Figure 1 and Figure 2).
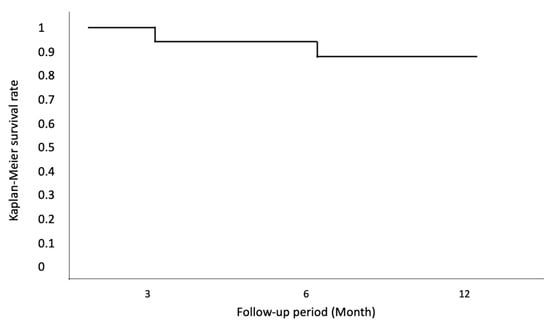
Figure 2.
Kaplan–Meier survival curves were generated to assess surgical success in all eyes, based on the following criteria: achieving an IOP of <21 mmHg or a 30% reduction from baseline at the last visit (12 months), without the need for additional glaucoma medication and in the absence of other indicators of glaucoma progression.
All patients subjected to the treatment did not exhibit enhancements in visual acuity from baseline to 3, 6, and 12 months of follow-up (p-value < 0.001) (Table 3).
Cumulative survival for different types of glaucoma does not show statistically significant differences among the various types of glaucoma. The neovascular type is the one associated with worse long-term effectiveness, even though it maintains a higher cumulative frequency of over 80% (Figure 3).
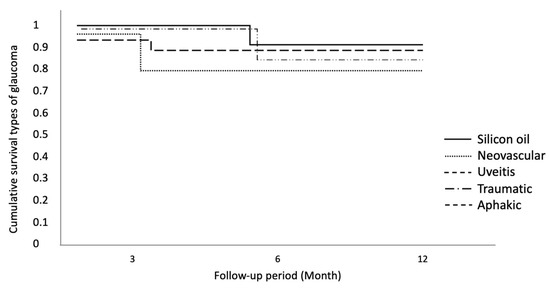
Figure 3.
Cumulative survival for different types of glaucoma.
IOP Baseline > 35 mmHg does not exhibit statistical significance compared to baseline IOP < 35 (Figure 4).
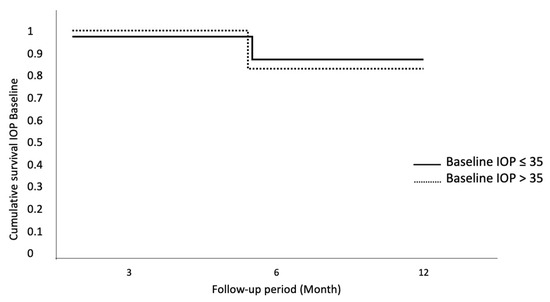
Figure 4.
Cumulative survival for IOP baseline. No statistically significant differences for baseline IOP.
The same result has been observed for different types of surgery (Figure 5).
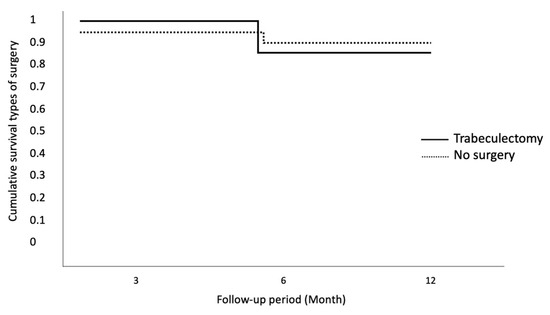
Figure 5.
Cumulative survival for different types of surgery. No statistically significant differences for different types of surgery.
At 12 months, 55.97% of the 134 eyes had decreased IOP under 21 mmHg (Table 2).
When comparing the outcomes between the male and female populations, no statistically significant differences were observed in terms of IOP reduction at 3 months (p = 0.2253), 6 months (p = 0.8855), and 12 months (p = 0.97), as well as in the number of anti-glaucoma medications required at the 12-month mark (p = 0.7499).
In terms of anti-glaucoma medications (AGM), the average number of prescribed medications before TSCPC treatment stood at 4.57 ± 0.75 (range: 3–6), which notably reduced to 2.25 ± 1.60 (p < 0.001, range: 0–6) after 3 months, and further decreased to 2.36 ± 1.7 (p < 0.001) by the 12-month follow-up.
In total, at the 12-month mark, 117 patients (87.31%) exhibited a reduction in the number of prescribed anti-glaucoma medications. Notably, in 30 patients (22%), the number of anti-glaucoma medications prescribed decreased to zero by the 12-month follow-up (Table 4).

Table 4.
Mean AGM baseline and at 3, 6, and 12 months after treatment.
Regarding the frequency of TSCPC surgical treatments, 36 of 134 eyes (26.8%) required retreatment, specifically those with intraocular pressure (IOP) exceeding 21 mmHg 60 days after the initial treatment. Among these, 35 eyes underwent two treatments, while one eye underwent four treatments.
In the comparison between the group of eyes that underwent a single treatment and those that received two treatments, no statistically significant differences were detected concerning pre-treatment IOP values (p = 0.8282), IOP values at 3 months (p = 0.8975), IOP values at 6 months (p = 0.4818), or the number of anti-glaucoma medications (AGM) at 6 months following treatment (p = 0.5953).
At the 6-month mark, an IOP reduction of 20% or more was achieved in 84 out of 97 eyes (87%) in the group that received a single treatment and in 28 out of 35 eyes (80%) in the group that underwent two treatments.
Complications
No severe postoperative complications were documented (i.e., hypotony. phthisis bulbi, or sympathetic ophthalmia). Minor complications were recorded in 4/134 treated eyes (2.98%). In particular, two patients experienced pupil distortion. probably due to incorrect probe positioning. and the other two eyes showed a cystoid macular edema.
Severe postoperative complications like hypotony, phthisis bulbi, or sympathetic ophthalmia were documented. In total, 4 of 134 treated eyes (2.98%) had minor complications. Specifically, two patients experienced pupil distortion, likely attributed to improper probe positioning, while the other two eyes exhibited cystoid macular edema.
4. Discussion
CPC is mainly used for refractory glaucomas, such as neovascular glaucoma, silicone oil glaucoma, traumatic glaucoma, aphakic glaucoma, and advanced developmental glaucoma. and inflammatory glaucoma [13].
CPC is used predominantly for refractory glaucomas, such as neovascular glaucoma, traumatic glaucoma, glaucoma in aphakic eyes, advanced developmental glaucoma, inflammatory glaucoma, glaucoma associated with corneal transplantation, silicone oil-induced glaucoma, and glaucoma in eyes with conjunctival scarring from previous surgery. These conditions are some of the most difficult to control with conventional glaucoma filtration.
The mechanisms of IOP reduction with diode laser include the destruction of ciliary epithelium, ciliary body atrophy, ciliary body vascular reduction, increased uveoscleral outflow, and ciliary body epithelial surface reduction [18].
A review reported that many authors examined 26 to 68 patients with different diagnoses and laser parameters. Laser power was varied from 1.5 to 2.5 watts from 180 to 360 degrees of the limbus, four to seven applications per quadrant. the power was sometimes adjusted for the pop, sometimes not [13].
In our paper. lower power levels were used: 1250 mW for 4000 ms for dark brown iris and 1500 mW for 3500 ms for non-dark brown iris; when the treatment was not effective. rather than increasing the power values. the area of application of the laser was extended.
The other studies considered a satisfying result: the lowering of the IOP, the 30% reduction compared to the baseline pressure, and the IOP post-treatment < 21 mmHg [19].
The Diode Laser Ciliary Ablation Study Group19 undertook a prospective, noncomparative case series study on 27 eyes of 27 patients with no previous ciliary ablation for a follow-up period ranging from 6 weeks to 28 months; the cumulative probability of success was 72% at 1 year and 52% at 2 years [19].
Bloom et al. [20] reported a large retrospective study of 210 eyes: Twenty-eight percent of the patients experienced a decrease in vision. more commonly seen in patients with neovascular and silicone-induced glaucoma.
Brancato et al. [21] reported a prospective, nonrandomized case series with 68 Caucasian patients; the success rate was 70.8%.
Farraová P et al. [22] achieved a reduction in IOP by 9.2 mm Hg (36%) after one month, in which the effect of reduction in IOP persisted also after one year (34%).
In our paper, the percentage of eyes that achieved an IOP < 21 mmHg after treatment is lower than in other works in the literature; however, the percentage of eyes with a reduction greater than 30% after treatment is satisfying; probably, the reason for this result is the elevated baseline IOP.
The IOP trends observed during the follow-up visits were in line with those reported by other authors who utilized TSCPC [23]. However, it’s worth noting that IOP fluctuations in our sample were less pronounced, particularly at the 12-month mark [24].
Complications such as conjunctival surface burns and hypotony have been reported by many authors. but in our study, the rate of complications was low (2.98% or 4 out of 134 eyes). We attribute this to the use of lower power levels and expanding the treatment area instead of increasing the power level in cases of failure.
One of the limitations of this study is that at baseline, the mean visual acuity of the treated eyes is very low. For this reason, many eyes already had severe conditions at the baseline; the TSCPC reduces IOP and pain but it does not improve visual acuity.
Another limitation of the study is its retrospective nature.
5. Conclusions
Cyclophotocoagulation is recommended for patients with refractory glaucoma who have not responded well to trabeculectomy or tube shunt procedures. Our study confirms what has already been reported by other authors in the past and validates the effectiveness of CPC in treating glaucoma that is resistant to filtration surgery. Although complications may arise, they can be minimized by utilizing lower power values and increasing the treatment area.
Author Contributions
Conceptualization, G.L.G., G.M., G.T., A.T. and A.P.; methodology, G.T. and G.L.G.; software, A.T.; validation, A.P. and G.L.G.; formal analysis, G.T.; investigation, G.M.; resources, G.T.; data curation, A.T.; writing—original draft preparation, G.T. and A.P.; writing—review and editing, G.T., G.L.G. and A.P.; visualization, A.T.; supervision, G.M.; project administration, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AGM | antiglaucoma medications |
| CPC | cyclophotocoagulation |
| GAT | Goldmann applanation tonometry |
| IOP | intraocular pressure |
| OCT | Optical coherence tomography |
| TSCPC | transscleral cyclophotocoagulation |
References
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 11, 2183–2193. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Boland, M.V.; Ervin, A.M.; Friedman, D.S.; Jampel, H.D.; Hawkins, B.S.; Vollenweider, D.; Chelladurai, Y.; Ward, D.; Suarez-Cuervo, C.; Robinson, K.A. Comparative effectiveness of treatments for open-angle glaucoma: A systematic review for the US Preventive Services Task Force. Ann. Intern. Med. 2013, 158, 271–279. [Google Scholar] [CrossRef]
- Nassiri, N.; Kamali, G.; Rahnavardi, M.; Mohammadi, B.; Nassiri, S.; Rahmani, L.; Nassiri, N. Ahmed glaucoma valve and single-plate Molteno implants in treatment of refractory glaucoma: A comparative study. Am. J. Ophthalmol. 2010, 149, 893–902. [Google Scholar] [CrossRef]
- Mahmood, K.; Baig, R.A.; Jameel, M.; Waseem, A.; Khan, M.T.; Qazi, Z.A. Transscleral Diode Laser Cyclophotocoagulation for the Treatment of Refractory Glaucoma. Pak. J. Ophthalmol. 2007, 23, 204–208. [Google Scholar]
- Ho, C.L.; Wong, E.Y.; Chew, P.T. Effect of diode laser contact transscleral pars plana photocoagulation on intraocular pressure in glaucoma. Clin. Exp. Ophthalmol. 2002, 30, 343–347. [Google Scholar] [CrossRef]
- Pantcheva, M.B.; Kahook, M.Y.; Schuman, J.S.; Noecker, R.J. Comparison of acute structural and histopathological changes of the porcine ciliary processes after endoscopic cyclophotocoagulation and transscleral cyclophotocoagulation. Clin. Exp. Ophthalmol. 2007, 35, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.M.; El-Harazi, S.M.; LoRusso, F.J.; McCash, C.; Lloyd, W.C., III; Warner, P.A. Histopathologic findings following contact transscleral semiconductor diode laser cyclophotocoagulation in a human eye. J. Glaucoma 1997, 6, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Subrata, M.; Ritu, G.; Jatin, A. Diode laser cyclophotocoagulation. J. Curr. Glaucoma Pract. 2009, 3, 47–59. [Google Scholar]
- Ishida, K. Update on results and complications of cyclophotocoagulation. Curr. Opin. Ophthalmol. 2013, 24, 102–110. [Google Scholar] [CrossRef]
- Vernon, S.A.; Koppens, J.M.; Menon, G.J.; Negi, A.K. Diode laser cycloablation in adult glaucoma: Long-term results of a standard protocol and review of current literature. Clin. Exp. Ophthalmol. 2006, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Pastor, S.A.; Singh, K.; Lee, D.A.; Juzych, M.S.; Lin, S.C.; Netland, P.A.; Nguyen, N.T. Cyclophotocoagulation: A report by the American Academy of Ophthalmology. Ophthalmology 2001, 108, 2130–2138. [Google Scholar] [CrossRef]
- Cheung, J.J.; Li, K.K.; Tang, S.W. Retrospective review on the outcome and safety of transscleral diode laser cyclophotocoagulation in refractory glaucoma in Chinese patients. Int. Ophthalmol. 2019, 39, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Souissi, S.; Le Mer, Y.; Metge, F.; Portmann, A.; Baudouin, C.; Labbé, A.; Hamard, P. An update on continuous-wave cyclophotocoagulation (CW-CPC) and micropulse transscleral laser treatment (MP-TLT) for adult and paediatric refractory glaucoma. Acta Ophthalmol. 2021, 99, e621–e653. [Google Scholar] [CrossRef]
- Anderson, A.P.; Douglas, R. MD Collaborative Normal Tension Glaucoma Study. Curr. Opin. Ophthalmol. 2003, 14, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Frezzotti, P.; Mittica, V.; Martone, G.; Motolese, I.; Lomurno, L.; Peruzzi, S.; Motolese, E. Longterm follow-up of diode laser transscleral cyclophotocoagulation in the treatment of refractory glaucoma. Acta Ophthalmol. 2010, 88, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.B.; Prum, B.E., Jr.; Shields, S.R.; Echelman, D.A.; Shields, M.B. Videographic and histologic comparison of Nd:YAG and diode laser contact transscleral cyclophotocoagulation. Am. J. Ophthalmol. 1994, 117, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Kosoko, O.; Gaasterland, D.E.; Pollack, I.P.; Enger, C.L. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The Diode Laser Ciliary Ablation Study Group. Ophthalmology 1996, 103, 1294–1302. [Google Scholar] [CrossRef]
- Bloom, P.A.; Tsai, J.C.; Sharma, K.; Miller, M.H.; Rice, N.S.; Hitchings, R.A.; Khaw, P.T. “Cyclodiode”. Trans- scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology 1997, 104, 1508–1519, discussion 1519–1520. [Google Scholar] [CrossRef]
- Brancato, R.; Carassa, R.G.; Bettin, P.; Fiori, M.; Trabucchi, G. Contact transscleral cyclophotocoagulation with diode laser in refractory glau-coma. Eur. J. Ophthalmol. 1995, 5, 32–39. [Google Scholar] [CrossRef]
- Farraovà, P.; Ondrejkovà, M.; Demianova, D. Transscleral Diode Cyclophotocoagulation in Treatment Of Glaucoma. Ceska Slov. Oftalmol. 2020, 76, 236–242. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kuchar, S.; Moster, M.R.; Reamer, C.B.; Waisbourd, M. Treatment outcomes of micropulse transscleral cyclophotocoagulation in advanced glaucoma. Lasers Med. Sci. 2016, 31, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, B.; Phan, E.N.; Lin, S.C.; Han, Y. Update on ciliary body laser procedures. Curr. Opin. Ophthalmol. 2017, 28, 181–186. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).