Abstract
Introduction: Acute iron poisoning is a potentially life-threatening condition that primarily affects the gastrointestinal, hepatic and cardiovascular systems. While it most often occurs accidentally in children, intentional overdoses in adolescents and adults remain an important clinical concern. Case description: We report the case of a 14-year-old male patient with a history of depression who intentionally ingested 100 ferrous sulfate tablets (equivalent to 118 mg/kg of elemental iron). The patient was admitted to the emergency department three hours after ingestion. He presented with vomiting tablet remnants, headache, and mild abdominal pain. Supportive measures included intestinal irrigation with polyethylene glycol (PEG), gastric protection, and N-acetylcysteine intravenous administration. The iron chelator therapy with deferoxamine was not possible because the medication was unavailable, so treatment with the oral iron chelator (deferasirox) was initiated. The iron levels gradually decreased, with no evidence of liver or cardiovascular involvement. The patient was discharged on day 20 post-ingestion with outpatient psychiatric follow-up. Discussion: This case highlights the importance of early initiation of gastrointestinal decontamination with PEG to limit systemic iron absorption. The use of deferasirox as an alternative chelating agent in the absence of deferoxamine has been associated with a favorable response. Conclusions: The rational use of oral chelators, gastrointestinal decontamination, and hepatoprotective therapies in acute iron poisoning might prevent major complications and improve prognosis. Alternative therapies can be valuable when an antidote is not immediately available; however, further clinical research is required before making a recommendation.
1. Introduction
Iron is a trace element essential for humans, involved in producing hemoglobin and myoglobin, oxygen transport and uptake, enzyme and deoxyribonucleic Acid (DNA) synthesis, and mitochondrial energy production through the electron transport chain []. Daily iron absorption should be about 1–2 mg and is tightly regulated to maintain iron balance—ensuring enough iron for vital biological functions while preventing excess iron toxicity. When iron intake is excessive, the regulation of absorption by enterocytes becomes saturated, leading to increased passive and paracellular iron absorption [].
After absorption, some iron is stored in epithelial cells; the rest enters the bloodstream and is transported to various organs, where it is used or stored as ferritin and hemosiderin. This primarily occurs in the liver parenchyma and within the reticuloendothelial cells of the bone marrow, spleen, and liver [].
Iron overload occurs when the management system becomes overwhelmed, resulting in excessive iron being stored or transported unsafely. This condition transforms a vital element into a potential cellular toxin.
The primary way iron is toxic is through its ability to catalyze the formation of highly damaging molecules. In a process called the Fenton reaction, excess free divalent iron (Fe2+) reacts with hydrogen peroxide to generate hydroxyl radicals (OH•). This excess of reactive oxygen species (ROS) causes oxidative stress, which damages cellular components, including the lipids in cell membranes, thereby weakening the cell’s structural integrity. Additionally, proteins lose their function as enzymes and structural elements, and DNA is harmed, leading to impaired cell function.
Iron overload can result from hereditary or acquired conditions. Acquired cases often develop due to other underlying health issues. The main cause is long-term transfusion therapy, frequently needed for patients with conditions like thalassemia, where repeated blood transfusions lead to a significant buildup of iron. Additionally, iron is found in supplements and medications commonly used to treat iron deficiency anemia. Oral supplements contain highly absorbable iron preparations, and excessive intake can lead to overload or poisoning. Iron toxicity at high doses primarily affects the gastrointestinal, liver, and cardiovascular systems, with potential complications such as gastrointestinal bleeding, liver failure, cardiogenic or hypovolemic shock, multiorgan failure, and death [,].
Iron poisoning cases are mostly linked to accidental ingestion in children under 5 years old, followed by adults over 20 years old, often with suicidal intent, and this group shows the highest mortality rate. According to the Annual Report of the National Poison Data System, 7767 cases of iron poisoning were reported in the United States in 2023 []. In Colombia, during the eighth epidemiological period of 2024, SIVIGILA, the Colombian Public Health Surveillance System, reported 27,913 cases of chemical poisoning (50% caused by medications, with an incidence of 26.5 per 100,000 inhabitants) and 15,045 cases of suicidal intent, with higher rates among adolescents (121.7 per 100,000). However, there are no specific data on how many of these cases are related to iron poisoning [].
This article presents a case of iron poisoning where a specific antidote was unavailable, prompting the exploration of potentially effective and accessible treatment options. The current literature on managing severe iron poisoning without deferoxamine (DFO) is limited, highlighting the need to report clinical experiences that investigate viable and efficient alternative strategies [].
2. Detailed Description of the Clinical Case
A 14-year-old male patient weighing 55 kg, with a history of depression treated with sertraline (25 mg orally daily), was admitted to the emergency department with his family after intentionally ingesting 100 ferrous sulfate tablets (oral iron) with suicidal intent. The estimated elemental iron dose was 118 mg/kg, which is considered a severe toxic dose. His family brought him in once they realized the problem. On admission, the patient’s vital signs were typical for his age, and his level of consciousness was unchanged. The patient experienced mild generalized abdominal pain associated with three episodes of vomiting, one of which showed evidence of tablets, and a headache rated 7/10 on the analog pain scale (APS). He was treated in the emergency room, receiving a 20 cc/kg bolus of Ringer’s lactate. Serum iron levels, liver function tests, electrocardiogram (ECG), and venous blood gases were obtained. Given the dose ingested, a high risk of liver failure was suspected, so treatment was initiated with N-acetyl cysteine (NAC): 50 mg/kg in the first 4 h, along with gastric irrigation using polyethylene glycol 3350 (50 g/L) administered through a nasogastric tube at 400 mL/h (based on the maximum infusion rate available). Gastric protection was provided with omeprazole, and the antidote DFO was ordered from the pharmacy. The case was discussed with the National Toxicology Line, and the patient was transferred to the pediatric intensive care unit (PICU).
The first report of serum iron levels was 359.28 µg/dL, which falls within the moderate intoxication range. Serum iron levels (Figure 1), coagulation, kidney and liver function tests, and arterial blood gases (Table 1) were obtained. The DFO was not available. At 16 h, the serum iron levels decreased to 272 µg/dL, while liver function remained stable. NAC was continued at 100 mg/kg for 16 h, totaling 150 mg/kg over 24 h. The pharmacy informed the medical team that the antidote had not been obtained; therefore, clinical and laboratory monitoring guided medical decisions. After completing 24 h of irrigation with PEG, feeding was resumed once abdominal pain and vomiting resolved. Subsequently, the patient had black stools, attributed to the ingested iron. There was no anemia or hemodynamic instability. A control ECG was performed at 24 h—this first one—and it was normal. During the second phase of poisoning (48 h post-ingestion, before progressing to the third phase), considering the risk of cardiovascular involvement, an echocardiogram was performed and reported as normal.
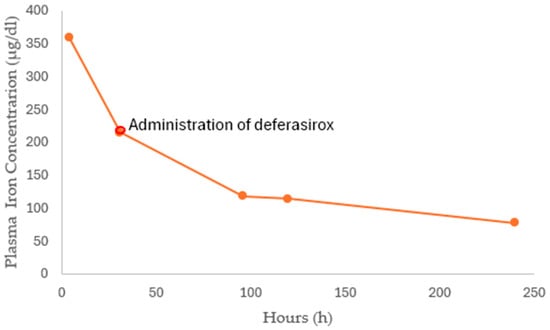
Figure 1.
The plasma iron concentration–time profile. Deferasirox was administered 48 h after ingestion.

Table 1.
Laboratory test reports from day 1 to day 6. The patient initially presented with metabolic acidosis, which resolved with the therapeutic measures implemented. No worsening in serum creatinine, transaminases, or blood gasometry parameters in the following days.
At 48 h, the serum iron concentration was 214.3 µg/dL. Due to the slow decline and ongoing toxicity levels, the iron-chelating agent Deferasirox was initiated at an oral dose of 360 mg every 12 h (13 mg/kg/day) until serum iron dropped below 100 µg/dL, with continued paraclinical follow-up and monitoring in the PICU. Iron levels at 96 h decreased to 118.3 µg/dL, the patient was clinically stable, and liver function remained intact; therefore, inpatient care was maintained.
Five days after intake, the iron level was 114.61 µg/dL, and by day 10, it decreased to 78 µg/dL. At that point, the decision was made to discontinue Deferasirox. The patient remained hemodynamically stable. The only abnormal finding was hyperphosphatemia, with a maximum level of 6.3 mg/dL, which responded to phosphate binders such as sevelamer, calcium carbonate, and aluminum hydroxide. Renal function tests were normal. After the follow-up and continued stability, the plan was to restart treatment for the underlying condition with oral Sertraline (50 mg/day). During hospitalization, the patient was consistently monitored by mental health services, leading to an extended stay until the 20th day after the ferrous sulfate tablets ingestion, with ongoing outpatient follow-up. A timeline outlining the key events of the case was created (Figure 2).
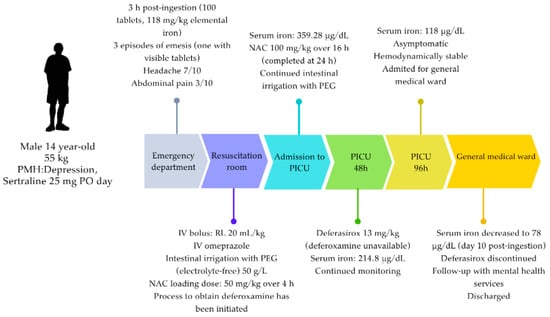
Figure 2.
Timeline showing the patient’s care and the interventions performed. Due to the amount ingested and the acid-base status, deferoxamine (antidote) administration was indicated. However, deferasirox was started during phase II of the intoxication because deferoxamine was unavailable in the country. PMH = previous medical history; RL = Ringer’s lactate solution; NAC = N-acetylcysteine; PEG = polyethylene glycol.
Written informed consent was obtained from the patient’s parents, and verbal assent was obtained from the 14-year-old patient, in accordance with institutional policies, as the current consent format does not explicitly include written assent for minors over 14 years of age.
3. Discussion
Acute iron poisoning remains a serious toxicological emergency, especially in pediatric and adolescent populations, where it can cause rapid systemic toxicity and multiorgan failure. Although the frequency of these cases has decreased worldwide, they still pose a significant clinical concern in areas where access to antidotal therapy is limited or delayed [,]. This report illustrates the clinical reasoning process required in such situations and the potential role of alternative treatments when deferoxamine (DFO) is unavailable.
In the present case, the ingested iron exceeds the threshold for severe toxicity. However, the clinical course was mild, likely due to early vomiting of tablet remnants that limited systemic absorption. This aligns with previous observations that the lack of persistent gastrointestinal symptoms within the first six hours after ingestion correlates with a lower systemic iron burden [,,]. Despite this mitigating factor, serum iron levels reached 272 µg/dL at 16 h, justifying the need for active treatment.
3.1. Pathophysiology and Rationale for Therapeutic Intervention
Iron toxicity occurs when the transferrin-binding capacity becomes saturated, resulting in the formation of non-transferrin-bound iron (NTBI). This free iron promotes redox reactions, such as the Fenton and Haber–Weiss processes, producing reactive oxygen species (ROS) that cause lipid peroxidation, mitochondrial dysfunction, and ferroptosis [,]. These mechanisms explain the hepatotoxicity, metabolic acidosis, and cardiovascular compromise observed in severe poisoning. See Table 2.

Table 2.
Stages of Iron Toxicity. The table was adapted from [,].
Therefore, treatment should focus on (1) preventing further gastrointestinal absorption, (2) removing circulating free iron through chelation and kidney replacement therapy when necessary, and (3) mitigating oxidative tissue injury [,,,,].
Polyethylene Glycol (PEG) as a Strategy to Prevent Gastrointestinal Absorption
Whole-bowel irrigation with polyethylene glycol 3350 played a vital role in decreasing systemic iron absorption in this case. PEG 3350 is a polymer that enables mechanical cleansing of the gastrointestinal tract while keeping electrolyte balance stable. It prevents additional iron absorption, lowers the risk of bezoar formation, and helps remove unabsorbed tablets [,].
This therapy is most effective when started within the first six hours after ingestion and is recommended for large, sustained-release iron tablet overdoses or when tablets are still visible on abdominal radiography [,]. Although PEG is not a specific antidote, its early use can reduce systemic toxicity by limiting continued absorption [,].
In this case, the early use of PEG 3350 contributed to a moderate clinical course and the absence of systemic complications, complementing the chelation and antioxidant strategies employed.
3.2. Role and Limitations of Deferoxamine (DFO)
DFO is the standard and most effective antidote for acute iron poisoning. DFO chelates free iron to form a ferrioxamine complex that is later eliminated through the kidneys. Early administration of DFO has been shown to improve outcomes in severe cases [,,]. However, access to DFO may be limited by logistical, regulatory, or supply issues []. In such cases, clinicians are advised to consider non-standard therapies within an ethically justified and closely monitored framework.
In the absence of a DFO, the medical team considered deferasirox (DFRA), an oral tridentate chelator approved for treating chronic transfusional iron overload [,]. DFRA preferentially chelates tissue iron and eliminates the complex mainly through biliary excretion, as summarized in Table 3. Experimental data in animals have shown that DFRA can reduce iron-induced oxidative damage and mortality when administered shortly after exposure [].

Table 3.
Main available oral chelators, their pharmacological properties, and usual doses for chronic overload, as well as the currently approved setting.
Human evidence, however, remains limited to case reports and small clinical observations. In a randomized crossover study, Griffith et al. [] demonstrated that DFRA reduced systemic iron absorption in models of acute supratherapeutic ingestion, thereby supporting its potential pharmacological plausibility.
In this patient, DFRA was administered under strict supervision and resulted in a gradual decrease in serum iron levels without causing hepatic, cardiac, or neurological complications. However, its use should be considered experimental and justified only when DFO is unavailable, since there is a lack of controlled human data confirming its safety and effectiveness in acute settings. From a pharmacokinetic standpoint, DFRA exhibits good oral bioavailability, enabling rapid systemic exposure, which may help minimize further tissue damage if administered early. Additionally, the ferric iron-DFRA complex is primarily eliminated through the biliary pathway. Therefore, DFRA offers a practical alternative when DFO is not readily accessible [,,,,,].
In clinical studies involving patients with chronic transfusion-related iron overload, DFRA has demonstrated that it effectively reduces serum ferritin concentrations and hepatic iron stores, thereby decreasing the risk of hepatic fibrosis and cardiomyopathy []. Therefore, oral chelators like DFRA might be considered a plausible alternative treatment for acute iron poisoning when standard therapy is unavailable.
Nonetheless, current evidence is limited, and DFO remains the first-line-treatment, particularly in patients with hemodynamic instability or impaired gastrointestinal absorption.
3.3. Adjuvant Therapy with N-Acetylcysteine (NAC) to Mitigate Oxidative Tissue Injury
NAC was given as an adjuvant therapy to reduce oxidative damage and support liver cell function. Besides its well-known role as a glutathione precursor, NAC has been shown to regulate ferroptosis by restoring antioxidant levels within cells and maintaining the activity of glutathione peroxidase 4 (GPX4) []. Gao et al. demonstrated that NAC prevented ferroptosis-related cell damage in multiple organs, including the liver and intestines, in models of oxidative stress and iron overload []. Similarly, Shen et al. [] and Sripetchwandee et al. [] found that combined NAC and oral chelator therapy reversed heart and nerve problems in animals with iron overload, indicating a possible synergistic effect []. Human clinical evidence remains limited. However, the mechanistic reasoning, animal studies, and human experiences support cautious use of NAC as a supportive adjuvant. In this case, intravenous NAC was initiated early and likely contributed to maintaining liver and overall stability. Still, the exact role of NAC in the positive outcome cannot be confirmed, and more research is needed to determine its therapeutic role and proper dosing in acute iron poisoning [,,,].
3.4. Clinical Reflections and Limitations
The positive clinical progress observed in this case likely resulted from a combination of early gastrointestinal decontamination with PEG and additional therapies aimed at reducing systemic iron levels and oxidative stress, along with supportive care. The choice to use DFRA was driven by necessity rather than preference, based on pharmacological reasoning and past case experiences.
However, this report has inherent limitations. As a single observation, it cannot establish causality or overall effectiveness; nonetheless, the information gained from this case could prove valuable in settings where specific antidotes, such as DFO, are unavailable. Potential confounding factors should be recognized as contributors to the positive outcome, such as incomplete iron absorption due to early emesis of tablet remnants, which likely decreased systemic absorption.
Early administration of PEG may have contributed to a more favorable prognosis. Furthermore, although both DFRA and NAC seem biologically plausible, their roles in acute intoxication remain unproven and should not replace DFO when it is available. Figure 3 shows an alternative algorithm that outlines management options for acute iron poisoning in the absence of DFO.
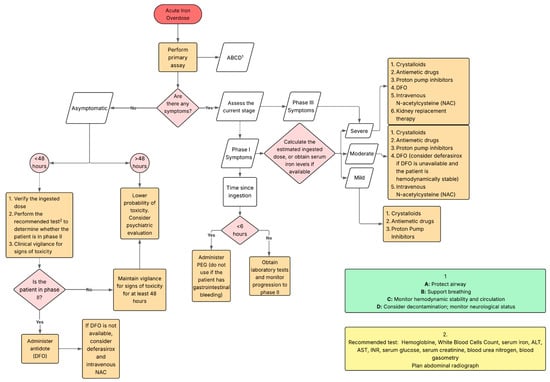
Figure 3.
Clinical decision-making algorithm for acute iron ingestion, outlining alternative therapeutic options in the absence of deferoxamine.
3.5. Perspectives and Clinical Implications
This case emphasizes the importance of flexibility and critical judgment in structured decision-making when conventional therapies are unavailable. It also highlights the need for treatments—such as iron chelators and antioxidant strategies—since current evidence is mostly based on preclinical studies. Future research should focus on clarifying the pharmacokinetics, optimal timing, and safety profile of DFRA in acute poisoning, as well as the possible additive effect of antioxidants like NAC in preventing ferroptosis-related organ damage [,,,,,]. Until such data are available, these methods should be used only in exceptional circumstances and under strict clinical and ethical supervision.
4. Conclusions
This case highlights the difficulties of managing acute iron poisoning when the specific antidote, DFO, is not available. Early gastrointestinal decontamination with polyethylene glycol (PEG) and supportive and adjunct therapies were crucial to the patient’s positive outcome.
The guidelines state that DFO remains the preferred treatment, especially for patients with moderate to severe iron poisoning; however, DFRA may be an alternative in exceptional cases, provided it is administered with strict monitoring and ethical oversight. Its use should be based on pharmacological rationale and limited to situations where DFO is unavailable.
The combined use of N-acetylcysteine (NAC) is not part of standard treatment but may provide antioxidant and liver-protective benefits by affecting ferroptosis and restoring intracellular glutathione levels. However, current evidence for its clinical effectiveness in acute iron poisoning remains limited and experimental.
Overall, this case highlights the importance of early detection and the rational use of alternative therapies in resource-limited settings. It also emphasizes the importance of controlled clinical studies to evaluate the safety, effectiveness, and proper integration of oral chelators and antioxidant agents in cases of acute iron poisoning.
Author Contributions
Conceptualization, M.I.V.-R., M.A.B.-B. and L.C.R.-R.; validation, J.A.A.-M., M.I.V.-R. and L.C.R.-R.; formal analysis, M.I.V.-R., D.P.A.-M. and L.C.R.-R.; investigation, M.I.V.-R., M.A.B.-B., J.A.A.-M., D.P.A.-M. and L.C.R.-R.; resources, D.P.A.-M.; writing—original draft preparation, M.I.V.-R., M.A.B.-B., L.C.R.-R. and J.A.A.-M.; writing—review and editing, D.P.A.-M., J.A.A.-M. and L.C.R.-R.; supervision, L.C.R.-R. and J.A.A.-M.; project administration, L.C.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by Ethics Committee)of HOMI Fundación Hospital Pediátrico La Misericordia (protocol code 102-812-25 and date of approval 26 May 2025).
Informed Consent Statement
Written informed consent to publish this paper has been obtained from the patient.
Data Availability Statement
Restrictions are applied to the availability of these data. Data were obtained from HOMI Fundación Hospital Pediátrico La Misericordia and are available from the authors with the permission of HOMI Fundación Hospital Pediátrico Misericordia.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| APS | Analog Pain Scale |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| ECG | Electrocardiogram |
| NAC | N-acetyl cysteine |
| PEG | Polyethylene glycol |
| DFO | Deferoxamine |
| DFRA | Deferasirox |
| PICU | Pediatric Intensive Care Unit |
| EII | Iron Serum Iron Dose |
| ISL | Iron Serum Levels |
| INR | International Normalized Ratio |
| ROS | Reactive oxygen species |
| RL | Ringer’s Lactate |
| PMH | Previous medical history |
| BID | Bis in die, twice a day |
| TID | Ter in dia, three times a day |
References
- Aisen, P.; Enns, C.; Wessling-Resnick, M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001, 33, 940–959. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Iron Load Toxicity in Medicine: From Molecular and Cellular Aspects to Clinical Implications. Int. J. Mol. Sci. 2023, 24, 12928. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Rivers, L.J.; Feldman, R.; Brown, K.; Pham, N.P.T.; Bronstein, A.C.; DesLauriers, C. 2023 Annual Report of the National Poison Data System® (NPDS) from America’s Poison Centers®: 41st Annual Report. Clin. Toxicol. 2024, 62, 793–1027. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Salud. Comportamiento de las intoxicaciones agudas por sustancias químicas en Colombia a periodo epidemiológico VIII de 2024 [Acute Chemical Poisonings in Colombia at Epidemiological Period VIII, 2024]. In Boletín Epidemiológico Semanal; INS: Bogotá, Colombia, 2024; Epidemiological Week 35, 3–4. Available online: https://www.ins.gov.co/buscador-eventos/Paginas/Vista-Boletin-Epidemilogico.aspx (accessed on 1 July 2025).
- Kontoghiorghes, G.; Kontoghiorghe, C. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- JIron, P. Goldfrank’s Toxicologic Emergencies, 11th ed.; Nelson, L.S., Howland, M.A., Lewin, N.A., Smith, S.W., Goldfrank, L.R., Hoffman, R.S., Eds.; McGraw-Hill Education: New York, NY, USA, 2019; Available online: https://accesspharmacy.mhmedical.com/content.aspx?aid=1163011509 (accessed on 10 June 2025).
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des. Devel. Ther. 2016, 10, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Wang, Y.; Min, J.; Wang, F. Iron metabolism and ferroptosis in human health and disease. BMC Biol. 2025, 23, 263. [Google Scholar] [CrossRef] [PubMed]
- Halil, H.; Tuygun, N.; Polat, E.; Karacan, C.D. Minimum ingested iron cut-off triggering serious iron toxicity in children. Pediatr. Int. 2019, 61, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, L.; Fu, K.; Cheng, S.; Wang, S.; Feng, Z.; Yu, S.; Yang, Z. N-Acetylcysteine Alleviates Necrotizing Enterocolitis by Depressing SESN2 Expression to Inhibit Ferroptosis in Intestinal Epithelial Cells. Inflammation 2024, 48, 464–482. [Google Scholar] [CrossRef] [PubMed]
- The Royal Children’s Hospital Melbourne. Clinical Practice Guidelines. Iron Poisoning. Available online: https://www.rch.org.au/clinicalguide/guideline_index/iron_poisoning/ (accessed on 9 July 2025).
- Tenenbein, M. The role of whole bowel irrigation in the treatment of toxic ingestions. Br. J. Clin. Pharmacol. 2023, 89, 2359–2361. [Google Scholar] [CrossRef] [PubMed]
- Hoegberg, L.C.G.; Shepherd, G.; Wood, D.M.; Johnson, J.; Hoffman, R.S.; Caravati, E.M.; Chan, W.L.; Smith, S.W.; Olson, K.R.; Gosselin, S. Systematic review on the use of activated charcoal for gastrointestinal decontamination following acute oral overdose. Clin. Toxicol. 2021, 59, 1196–1227. [Google Scholar] [CrossRef] [PubMed]
- Gumber, M.R.; Kute, V.B.; Shah, P.R.; Vanikar, A.V.; Patel, H.V.; Balwani, M.R.; Ghuge, P.P.; Trivedi, H.L. Successful treatment of severe iron intoxication with gastrointestinal decontamination, deferoxamine, and hemodialysis. Ren Fail. 2013, 35, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.R.; Ghaemi Amiri, M.; Moghadamnia, A.A. Iron; Benefits or threatens (with emphasis on mechanism and treatment of its poisoning). Hum. Exp. Toxicol. 2023, 42, 09603271231192361. [Google Scholar] [CrossRef] [PubMed]
- Berkovitch, M.; Livne, A.; Lushkov, G.; Segal, M.; Talmor, C.; Bentur, Y.; Klein, J.; Koren, G. The efficacy of oral deferiprone in acute iron poisoning. Am. J. Emerg. Med. 2000, 18, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Griffith, E.A.; Fallgatter, K.C.; Tantama, S.S.; Tanen, D.A.; Matteucci, M.J. Effect of Deferasirox on Iron Absorption in a Randomized, Placebo-Controlled, Crossover Study in a Human Model of Acute Supratherapeutic Iron Ingestion. Ann. Emerg. Med. 2011, 58, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, W.S.; Shaikh, A.; Sasane, S.; Al Rais, Z.; Baqer, M.A. Near-fatal ferrous sulfate poisoning: A case report of successful conservative management. J. Acute Dis. 2022, 11, 247–250. [Google Scholar] [CrossRef]
- Pongamnuaykrit, M.; Tantiworawit, A.; Niprapan, P.; Punnachet, T.; Hantrakun, N.; Piriyakhuntorn, P.; Rattanathammethee, T.; Hantrakool, S.; Chai-Adisaksopha, C.; Rattarittamrong, E.; et al. The Efficacy and Safety of Deferasirox Monotherapy as a Second-Line Treatment in Transfusion-Dependent Thalassemia with Iron Overload. J. Clin. Med. 2025, 14, 6212. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.C.; Zhang, Y.C.; Zhao, M.F. Protective effects of deferasirox and N-acetyl-L-cysteine on iron overload-injured bone marrow. Braz. J. Med. Biol. Res. 2017, 50, e6087. [Google Scholar] [CrossRef] [PubMed]
- Sripetchwandee, J.; Pipatpiboon, N.; Chattipakorn, N.; Chattipakorn, S. Combined Therapy of Iron Chelator and Antioxidant Completely Restores Brain Dysfunction Induced by Iron Toxicity. PLoS ONE 2014, 9, e85115. [Google Scholar] [CrossRef] [PubMed]
- Wongjaikam, S.; Kumfu, S.; Khamseekaew, J.; Chattipakorn, S.C.; Chattipakorn, N. Restoring the impaired cardiac calcium homeostasis and cardiac function in iron overload rats by the combined deferiprone and N-acetyl cysteine. Sci. Rep. 2017, 7, 44460. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).