Abstract
The oyster shell is a valuable calcium resource; however, its application is limited by its high NaCl content. Therefore, to establish the use of oyster shells as a viable resource, conditional experiments were conducted to select optimum parameters for NaCl removal. For this purpose, we compared leaching methods with batch and sequential procedures, determined the volume of water used for washing, and evaluated the mixing speed. The batch system removed NaCl when washed for >24 h over a shell to water ratio of 1:5. Results from the batch experiments confirmed that washing twice can completely remove NaCl from the shells on a like-for-like basis. Additionally, the efficiency of washing was sequentially evaluated in terms of the number of washing cycles. Compared to batch experiments, continuous washing could remove NaCl in approximately 10 min at a shell to water ratio of 1:4. We found that regardless of the washing methods, the volume of water used for washing is key for enhancing NaCl removal. Consequently, increasing the volume of water used for washing coupled with a proper sorting of fine particles can help enhance the purity of calcium, which will enable the use of oyster shell as an alternate Ca-resource.
1. Introduction
Oysters are widely used as part of a healthy diet, as they help improve immunity and in the rapid absorption of zinc and iron; moreover, increased awareness of their health benefits has led to an increase in the consumption of oysters []. As of 2017, 639 tons per year of Pacific oysters are produced in Asia, which constitutes approximately 92% of the global oyster market []. An oyster comprises about 14% oyster meat and 86% shell; during oyster processing, the shells are disposed as waste into the sea or buried into the ground [,]. Shells buried into the ground pose problems, as they produce leachates and odors. Furthermore, the organic matter in the shells discarded into the sea may pollute the ocean ecosystem. Hence, there is a need for a rapid establishment of policy support to address the substantial increase in the number of shells generated at present and in the future. Moreover, it is desirable to utilize shells as resources without disposing them as waste, as they can contribute to an environmental and economic circular economy [,]. Both Korea and Southern Brazil have suggested recycling of shells for environmental benefits; as of 2018 [,], Japan has recycled 140,000 tons of oyster shells to improve the environment of fishing farms and to enhance the sediment quality by establishing underwater plants []. Taiwan generates 34,000 tons of oyster shells, whose disposal into the environment causes sanitation, pollution, and protection issues [].
Oyster shells have been disposed as waste along the coast of Korea []; however, after the amendment of the Framework Act on Resources Circulation in 2022, the status of oyster shells has been changed from waste to resources to help control the influx of impurities such as organic residues. Nevertheless, there are two challenges associated with the application of oyster shells as an alternative to industrial material such as limestone: (i) the levels of impurities equivalent to those of limestone and (ii) the demand for a massive quantity [,,].
Oyster shells are subjected to physical and chemical treatments before they can be used as high value-added resources []. However, their consumption as a calcium substitute is minimal, because only a meagre percentage is applied during the dark fermentation of cement waste [,]. Previous studies indicate that the oyster shell content in concrete cannot exceed 15% []; the suggested concentration in dark fermentation is less than 8% []. Thus, the limited application of oyster shells in the construction industry cannot fundamentally help in the recycling of the neglected oyster shells (NOSs). Oyster shells have also been used in water treatment, as they are considered as absorbents that help remove phosphorus and nitrogen compounds [,,]. However, their absorption capacity is minimal at ~228.15 mg-P/kg-oyster shell, and the recovered absorptive materials are generated as secondary waste [].
There is a massive demand for calcium compounds such as CaCO3 and Ca(OH)2 as they are commonly used for acid gas treatment; moreover, several studies have been conducted on SOx removal using CaCO3 [,]. This process involves two stages where CaCO3 reacts with SO4 to produce gypsum (CaSO4·2H2O) as the final product, as shown in Equation (1). Oyster shells can be used as a substitute for limestone, which would effectively address the over-exploitation of limestone in the environment.
H2SO4 + CaCO3 + 0.5O2 + H2O ↔ CO2 + CaSO4 + 2H2O
Interestingly, Lim et al. [] found that the efficiency of flue gas desulfurization (FGD) increased with the addition of NaCl. SO2 removal increased up to 52% under Ca/S = 2 with the addition of 3.2% of NaCl, whereas it was relatively low at 32% in the absence of NaCl []. The sulfidation of CaO with alkali NaCl was much higher than that of CaO without alkali NaCl, as shown in Equation (2) []:
H2SO4 + CaCO3 + 2NaCl ↔ Na2SO4 + CaCl2+ H2O + CO2
However, when the sulfidation temperature was over 750 °C with the inclusion of sodium, the higher sodium concentration led to a greater spreading resistance ratio for sulfidation. As NaCl-CaSO4 partially melts under high temperature, the liquid phase present locally blocks the pores. In other words, a higher sodium concentration increases gas spreading resistance in the pores []. Furthermore, the gypsum (CaSO4·2H2O) recovered after FGD is high in sodium, which limits its conversion into viable products. Additionally, previous studies have reported that NaCl impurities affect the degree of limestone calcination [,,,]. Therefore, the use of oyster shells as a viable resource requires the removal of NaCl.
To overcome the existing limitations, in the present study, we investigated washing processes of oyster shells to convert them into useful resources.
2. Materials and Methods
2.1. Oyster Shell Sample Collection
Oyster shells were collected from an open-air yard in Tongyeong, South Korea, where shells are stored outdoors for over six months to generate shell fertilizer; this facilitates the natural washing out of NaCl from the shells. The shell separates the luggage rope using a cylindrical centrifuge, and in this process, some of the shell is crushed. The speed of the centrifuge is about 250 rpm, and the size of the crushed shell is about 5 cm or less. The NaCl content in these shells is expected to be lower than in those generated freshly at the shucking place.
2.2. Oyster Shell Samples Subjected to Washing Process
2.2.1. Batch Washing
To identify the characteristics of NaCl removal from the oyster shells, an elution experiment was conducted using batch as well as sequential reactions. The batch experiment was conducted with 500 g of shells in a container by washing them with tap water, varying the ratio of the volume of tap water from one to six times the weight of the shells; the different ratios of 1:1, 1:3, 1:4, 1:5, and 1:6 were denoted as B-OWR1 to B-OWR6 (B-OWR, batch-oyster shell to tap water ratio). Each oyster shell sample was agitated with the corresponding volume of tap water at 100 rpm at room temperature. Most of the experiments were conducted at 100 rpm, but the effect of rpm was investigated by changing the rpm to 200.
2.2.2. Continuous Washing
In the continuous washing process, oyster shells were washed once, and the tap water used for elution of NaCl from the shells was removed, followed by the addition of new tap water. The washing and elution steps were repeated six times per sample. The samples were agitated for 1 min; the reacted solution was recovered after solid–liquid separation by filtration. The ratios of tap water used for the washing steps were maintained the same as those used for batch washing; the different ratios were denoted as C-OWR1 to C-OWR6 (C-OWR, continuous-oyster shell to tap water ratio). The number of elution processes was determined based on the concentration of NaCl obtained with each wash.
2.3. Estimation of NaCl Leachates
Considering that the oyster shells were not washed with tap water during shucking, the concentrations of leached dissolved ions from the shell could be associated with those of seawater components. The NaCl content of the tap water eluted after batch or continuous washing was estimated indirectly using electrical conductivity (EC) (Pro 30, YSI, Yellow Springs, Ohio, USA). From the measured EC values, the NaCl content in the shells was calculated according to Equation (3) []:
where k is conversion of NaCl from the observed TDS value, that is 0.064, and SSR is the solution to solid ratio.
Total dissolved solids (mg/L) = k × Electrical conductivity (liquid − tap water) (μS/cm) × SSR
2.4. Analysis of Oyster Shell Particle Size and Chemical Composition
Shells recovered after washing were classified based on their particle size and chemical composition. The particle-size of the shells that ranged from 0.2 mm to 5 mm were classified using standard sieves. Chemical compositions of the shells before and after washing were identified using X-ray fluorescence spectroscopy (XRF-1800, Shimadzu, Kyoto, Japan), and an X-ray diffractometer (XRD-6100, Shimadzu, Kyoto, Japan) was used to analyze the mineral phases.
3. Results and Discussion
3.1. Physical Properties of Neglected Oyster Shells
This study evaluated the basic characteristics of NOSs near Tongyeong, South Korea. The NaCl content of NOSs was measured considering the number of repeated experiments, which were repeated more than six times at B-OWR6 for 24 h. As shown in Figure 1, the NaCl content of shells varied depending on the duration. From the result values, the average of the measured values repeated six times was used and the moisture content of sampled shells was measured as 1.22% ± 0.03 on average.
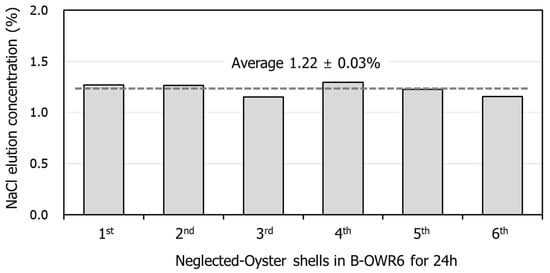
Figure 1.
NaCl content of neglected oyster shells in B-OWR6 for 24 h.
Chemical analysis of the oyster shell samples using XRF indicated CaO, SiO2, Na2O, SO3, and MgO as the major constituents, and the average of the measured values repeated six times was used. As shown in Table 1, the presence of Na, Mg, and Mg in the shells suggests that major elements of the seawater could be captured when oysters grow in their habitat. Meanwhile, Al, Fe, and Si were believed to have originated from marine sediments [,].

Table 1.
Chemical composition of neglected oyster shells (NOSs).
However, Cl was not detected in the XRF analysis. Because NaCl was regarded as the major salt, the XRF results suggest that NaCl was possibly washed out owing to its high water solubility (up to 36%). In fact, oyster shells in this study were obtained from a fertilizer production facility, where shells were left outdoors for more than six months, indicating that the high level of Cl in the shells had been washed away naturally, and hence, it was not detected.
3.2. Removal of NaCl Using Washing Methods
Since washing efficiencies could be attributed to the physical properties of the oyster shells, the washing method was tested based on the volume of water used, washing duration, and mixing speeds.
3.2.1. Batch Washing
As previously mentioned, NaCl is highly soluble in water; therefore, its leaching would depend on the volume of water used in the washing step. Figure 2 illustrates the leaching properties of NaCl from the shells with elapsed time depending on the volume of water, and the average of the measured values repeated three times was used.
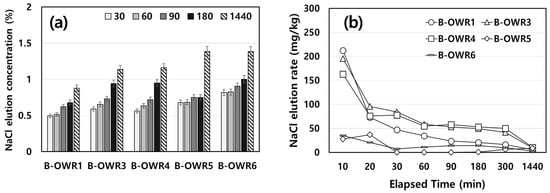
Figure 2.
Neglected-oyster shell to water ratio (OWR) depending on elution time. (a) NaCl elution concentration and (b) NaCl elution rate. B-OWR, batch-oyster shell to water ratio.
With respect to the volume of water, the absolute value of eluted NaCl was relatively higher when the water to shell ratio was higher (Figure 2a). However, it was observed that the elution volume of NaCl increased among samples with a smaller ratio of water used for washing (OWR < 4.0). In addition, the leaching of NaCl increased with an increase in elution time in general, except for the OWR of 5. However, no substantial effect was observed when a large ratio of water (OWRs of 5 and 6) was used. We observed a relatively higher absolute elution value when shells were washed for a relatively longer time at OWRs > 5 than that when washed at lower OWRs (Figure 2b). NaCl was eluted into the water during the initial reaction, which indicates that the concentration of eluted NaCl depends on the volume of water used for washing. Hence, it could be inferred that continuous washing for shorter periods was more effective as it enhances NaCl removal from the shells. The average NaCl content in the shells was 1.22 wt.%, and the removal efficiencies are shown in Table 2. The average of the measured values repeated three times was used. The batch system sufficiently removed NaCl residues from the shells under a B-OWR of ≥5. However, some differences persisted depending on the volume of washing water used.

Table 2.
Maximum NaCl elution volume depending on water.
Figure 3 shows leaching of NaCl from the shells based on the mixing speed and the average of the measured values repeated three times was used. Leaching patterns of NaCl were very similar regardless of mixing speeds of 100 and 200 rpm. However, the amount of NaCl leached from the shell at 200 rpm was approximately 20% higher than that at 100 rpm. Leaching concentrations of NaCl at 200 rpm increased from the start of the reaction and eventually reached up to 80%. This indicates that over 90% of NaCl removal efficiency relies on the volume of water, even though leaching properties primarily rely on the mixing speed.
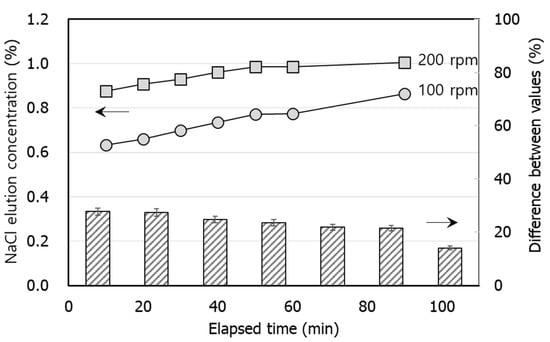
Figure 3.
NaCl elution concentration (left arrow) with elapsed time at B-OWR3 with different mixing speeds. The bar chart is difference between values 100 rpm and 200 rpm (right arrow) with elapsed time at B-OWR3.
These results indicate that the volume of water used for washing is key for effective removal of NaCl from the surface of oyster shells. The removal efficiency of NaCl from the shells reached over 90% when the OWR was >5.0 (Table 2).
3.2.2. Continuous Washing
Figure 4 shows the washing efficiencies for each washing step by continuous washing of oyster shell samples and the average of the measured values repeated three times was used. Leaching of NaCl from the shell was proportionate to the number of washing steps. The amount of leached NaCl at the first step was over 63–96% depending on the OWR. As shown in Figure 2 and Table 2, over 90% leaching was observed when the OWR was >5. Considering that the NaCl removal efficiency reached 90% by sequentially washing the shells twice even at a first OWR, a continuous washing method would help reduce the volume of water used for washing.
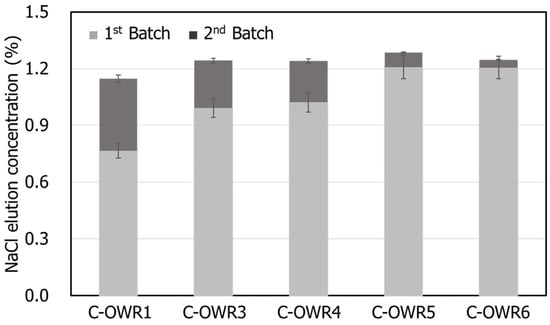
Figure 4.
NaCl elution concentration via first and second batch system with various OWRs.
In the present study, we comparatively performed shell washing under different OWRs to reduce the reaction time. OWRs were chosen at two, three, and four times water volumes. The washing steps were repeated 10 times sequentially, as shown in Figure 5a, which also shows the unit mass of leached NaCl from the shells at every single step. As confirmed by batch experiments (Figure 2), a high concentration of eluted NaCl was observed during the early stages of washing steps (up to three times) and reduced gradually reaching nearly zero after six washes. The leaching patterns observed were similar regardless of OWRs; however, the total amount of leached NaCl differed largely under varied OWRs. The average of measured values repeated three times was used, as shown in Figure 5b. C-OWR4 had the highest value of 11.1 g/kg-OS among the three samples, which is 2.2-fold higher than that of C-OWR2, implying that volume of water is the key factor that controls washing properties.
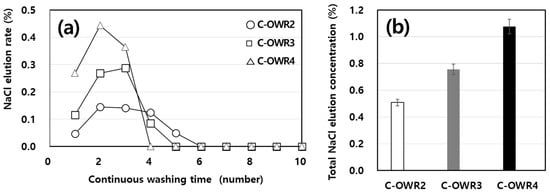
Figure 5.
Amount of NaCl elution with continuous washing steps at different OWRs. (a) Changes in NaCl concentration as a function of washing steps. (b) Total NaCl elution concentration after 10 times of sequential washing.
We observed a substantial difference in NaCl leaching properties between the batch and continuous experiments. Assuming that the percentage of maximum eluted NaCl could be approximately 1.2%, leaching rates of NaCl at B-OWR5, B-OWR6, and C-OWR4 are repeated three time and indicated in Table 3. The maximum values (1.2%) of C-OWR4 were reached at 10 min, whereas batch experiments (B-OWR5 and B-OWR6) were 15 times more time consuming and utilized larger volumes of water to achieve the maximum elution value. As a result, the leaching rates of NaCl from the shells seem to be more affected by the volume of water than the duration of washing periods.

Table 3.
Comparison of the optimum conditions between the batch and continuous systems.
3.3. Particle Size of Washed Shells and Changes in Mineral Composition
The particle size distributions are depicted in a parabolic shape, accounting for 70% of coarse grains over 5.0 mm, and the average of measured values repeated three times was used in Figure 6a. The particle size for raw shells indicated that particles ≥1 mm accounted for 78% and those ≥5 mm in the coarse-grains accounted for 70%.
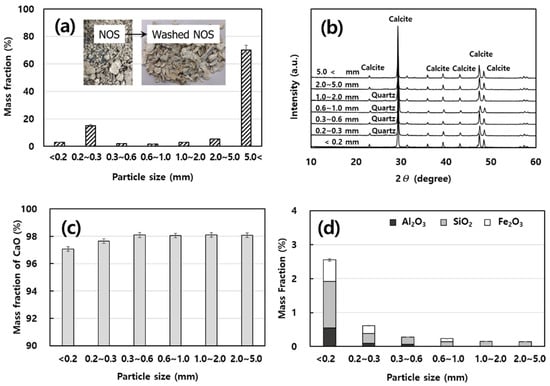
Figure 6.
Properties of particle size of washed neglected oyster shells (NOSs). (a) Particle size distribution. (b) Results of X-ray diffractometer (XRD) analysis of washed NOSs with different particle sizes. (c) CaO contents of washed NOSs with different particle sizes. (d) Al2O3, SiO2, and Fe2O3 contents of washed NOS with different particle sizes.
The NOSs were assessed using an XRD, and the patterns are represented in Figure 6b. Mineral composition of separated shells analyzed by XRD analysis confirmed that the main mineral was CaCO3 regardless of the particle size; some quartz was observed in the fine-grained particles []. These results were averaged three times and agreed with those of XRF; the main element found in particle sizes of over 0.2 mm was Ca, which accounted for >98% (Figure 6c). The content of CaO increased as the particles became coarser, and Si, Fe, and Al were relatively more enriched in the finer particles. Generally, these elements are the main components (Al2O3, SiO2, and Fe2O3) of the soil, suggesting that marine sediments could be recovered together with oyster shells from oyster aquacultural points. In Figure 6d, these results were averaged three times, the composition of Si, Fe, and Al were relatively more concentrated in the finer particles, and the content of these impurities clearly increased in particles ≤2 mm.
3.4. Leaching Experiment
Total component analysis results by elution test were compared for the washed and unwashed shells (Table 4). Although some differences were observed depending on the particle size, Na and Cl contents in washed shells clearly decreased by 77–93%. With the exception of Zn, heavy metals were not detected and decreased with increasing particle size. Some Na content remained after washing, which is likely because some seawater components are captured when oysters grow. Nonetheless, given that impurities in shells were relatively concentrated in particles, it is important to apply washing conditions considering the particle size to improve washing efficiency. Therefore, the sorting of particle size helps to remove impurities and NaCl simultaneously, as most of the impurities are confined to the finer particles. These results indicate that it is important to remove the impurities along with salts to use shells as Ca resources and selectively utilize fine-grained shells of ≥1.0 mm.

Table 4.
NOSs and washed NOS components under different particle size by elution test.
4. Conclusions
The results of our study indicate the elution efficiency of NaCl with varied OWRs under the batch and continuous systems and different washing speeds and particle sizes. To completely elute out NaCl in one wash, at least five times as much water and 24 h mixing is required under the batch system. The NaCl elution is effective under a higher OWR where elution time can be minimized. The optimum NaCl elution efficiency of NOSs was predicted at an OWR of over 5, with an agitation time of 10 min and under a continuous washing system. Furthermore, because the NaCl proportion mainly constitutes fine particles, selective washing of particles ≥ 1.0 mm would increase washing efficiency. Larger particles of NOSs may benefit and be enhanced by the elution efficiency of NaCl. The washing speed is proportional to washing efficiency; otherwise, the elution efficiency according to the washing speed has a limitation in that the elution time would be longer in a batch system than in a continuous system. In conclusion, the washing process is essential for use neglected-oyster shells as a resource and materialize, and the use of the neglected oyster shells is a very important consideration for solve environmental problems.
Author Contributions
Conceptualization, S.K. and J.E.P.; formal analysis, data curation, S.E.L. and J.E.P.; writing—original draft preparation, writing—review and editing, J.E.P.; supervision, project administration, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Environment Industry & Technology Institute (KEITI) through the Project to develop eco-friendly new materials and processing technology derived from wildlife Project, funded by Korea Ministry of Environment (MOE) (2021003280003).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, Q.; Wang, Q.; Xu, S.; Mao, J.; Li, X.; Zhao, Y. Properties of water-repellent concrete mortar containing superhydrophobic oyster shell powder. Constr. Build. Mater. 2022, 337, 127423. [Google Scholar] [CrossRef]
- Chairopoulou, M.A.; Garcia-Trinanes, P.; Teipel, U. Oyster shell reuse: A particle engineering perspective for the use as emulsion stabilizers. Powder Technol. 2022, 408, 117721. [Google Scholar] [CrossRef]
- Yoon, G.-L.; Kim, B.-T.; Kim, B.-O.; Han, S.-H. Chemical-mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Ruslan, H.N.; Muthusamy, K.; Mohsin, S.M.S.; Jose, R.; Omar, R. Oyster shell waste as a concrete ingredient: A review. Mater. Today Proc. 2022, 48, 713–719. [Google Scholar] [CrossRef]
- Ubachukwu, O.A.; Okafor, F.O. Towards green concrete: Response of oyster shell powder-cement concrete to splitting tensile load. Niger. J. Technol. 2020, 39, 363–368. [Google Scholar] [CrossRef]
- Alvarenga, R.A.F.; Galindro, B.M.; Helpa, C.F.; Soares, S.R. The recycling of oyster shells: An environmental analysis using Life Cycle Assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef]
- Baek, E.-Y.; Lee, W.-G. A study on the Rational Recycling of Oyster-shell. J. Fish. Bus. Adm. 2020, 51, 71–87. [Google Scholar] [CrossRef]
- Baek, E.-Y. Oyster Shell Recycling and Marine Ecosystems: A Comparative Analysis in the Republic of Korea and Japan. J. Coast. Res. 2021, 114, 350–354. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Kuo, W.-T.; Lin, C.-C.; Chen, P.-Y. Study of the material properties of fly ash added to oyster cement mortar. Constr. Build. Mater. 2013, 41, 532–537. [Google Scholar] [CrossRef]
- Framework Act on Resource Circulation. Available online: https://elaw.klri.re.kr/kor_mobile/viewer.do?hseq=51210&type=sogan&key=16 (accessed on 4 October 2022).
- Lu, M.; Shi, X.; Feng, Q.; Li, X.; Lian, S.; Zhang, M.; Guo, R. Effects of humic acid modified oyster shell addition on lignocellulose degradation and nitrogen transformation during digestate composting. Bioresour. Technol. 2021, 329, 124834. [Google Scholar] [CrossRef]
- Yen, L.-T.; Wang, C.-H.; Than, N.A.T.; Tzeng, J.-H.; Jacobson, A.R.; Iamsaard, K.; Dang, V.D.; Lin, Y.-T. Mode of inactivation of Staphylococcus aureus and Escherichia coli by heated oyster-shell powder. Chem. Eng. J. 2022, 432, 134386. [Google Scholar] [CrossRef]
- Park, K.; Sadeghi, K.; Panda, P.K.; Seo, J.; Seo, J. Ethylene vinyl acetate/low-density polyethylene/oyster shell powder composite films: Preparation, characterization, and antimicrobial properties for biomedical applications. J. Taiwan Inst. Chem. Eng. 2022, 134, 104301. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, C.; Qiu, Q.; Guo, S.; Zhang, Y.; Mu, H. Oyster shell waste as potential co-substrate for enhancing methanogenesis of starch wastewater at low inoculation ratio. Bioresour. Technol. 2022, 361, 127689. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, L.; Yuan, H.; Li, X.; Chang, Y.; Zuo, X. Oyster shells improve anaerobic dark fermentation performances of food waste: Hydrogen production, acidification performances, and microbial community characteristics. Bioresour. Technol. 2021, 335, 125268. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-K.; Chang, W.-L. The effect of water purification by oyster shell contact bed. Ecol. Eng. 2015, 77, 382–390. [Google Scholar] [CrossRef]
- Lee, J.-I.; Kang, J.-K.; Oh, J.-S.; Yoo, S.-C.; Lee, C.-G.; Jho, E.H.; Park, S.-J. New insight to the use of oyster shell for removing phosphorus from aqueous solutions and fertilizing rice growth. J. Clean. Prod. 2021, 328, 129536. [Google Scholar] [CrossRef]
- Lim, J.; Cho, H.; Kim, J. Optimization of wet flue gas desulfurization system using recycled waste oyster shell as high-grade limestone substitutes. J. Clean. Prod. 2021, 318, 128492. [Google Scholar] [CrossRef]
- Lim, J.; Kim, J. Optimization of a wet flue gas desulfurization system considering low-grade limestone and waste oyster shell. J. Korea Soc. Waste Manag. 2020, 37, 263–274. [Google Scholar] [CrossRef]
- Liu, Y.; Che, D.; Xu, T. Effects of NaCl on the capture of SO2 by CaCO3 during coal combustion. Fuel 2006, 85, 524–531. [Google Scholar] [CrossRef]
- Cho, S.; Lim, J.; Cho, H.; Yoo, Y.; Kang, D.; Kim, H. Novel process design of desalination wastewater recovery for CO2 and SOx utilization. Chem. Eng. J. 2022, 433, 133602. [Google Scholar] [CrossRef]
- Koralegedara, N.H.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Recent advances in flue gas desulfurization gypsum processes and applications—A review. J. Environ. Manag. 2019, 251, 109572. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.-H.; Kim, S.-H.; Cha, W.-S.; Kim, K.; Moon, B.-K. Mineralogical Changes of Oyster Shells by Calcination: A Comparative Study with Limestone. Econ. Environ. Geol. 2018, 51, 485–492. [Google Scholar] [CrossRef]
- Ha, S.H.; Kim, K.; Kim, S.-H.; Kim, Y. The effects of marine sediments and NaCl as Impurities on the Calcination of Oyster Shells. Econ. Environ. Geol. 2019, 52, 223–230. [Google Scholar] [CrossRef]
- Laursen, K.; Grace, J.R.; Lim, C.J. Enhancement of the Sulfur Capture Capacity of Limestones by the Addition of Na2CO3 and NaCl. Environ. Sci. Technol. 2001, 35, 4384–4389. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.A.; Johnson, L.; Turner, C.B. Effects of sodium chloride on limestone calcination and sulfation in fluidized-bed combustion. Environ. Sci. Technol. 1979, 13, 1113–1118. [Google Scholar] [CrossRef]
- Rusydi, A.F. Correlation between conductivity and total dissolved solid in various type of water: A review. Earth Environ. Sci. 2018, 118, 012019. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Nam, S.Y.; Kim, C.; Ahn, J.W. Oyster Shell Waste is alternative source for Calcium carbonate (CaCO3) instead of Natural limestone. J. Energy Eng. 2018, 27, 59–64. [Google Scholar] [CrossRef]
- Prusty, J.K.; Patro, S.K.; Basarkar, S.S. Concrete using agro-waste as fine aggregate for sustainable built environment—A review. Int. J. Sustain. Built Environ. 2016, 5, 312–333. [Google Scholar] [CrossRef]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).